Weissella viridescens Attenuates Hepatic Injury, Oxidative Stress, and Inflammation in a Rat Model of High-Fat Diet-Induced MASLD
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Strains and Growth Conditions
2.2. Animals Model
2.3. Biochemical Parameters of Serum and Liver
2.4. Histological Analyses
2.5. Transcriptome Analysis
2.6. Real-Time Quantitative PCR (RT-qPCR)
2.7. 16S rRNA Sequencing and Gut Microbiota Analysis
2.8. Metabolites Analysis
2.9. Statistical Analysis
3. Results
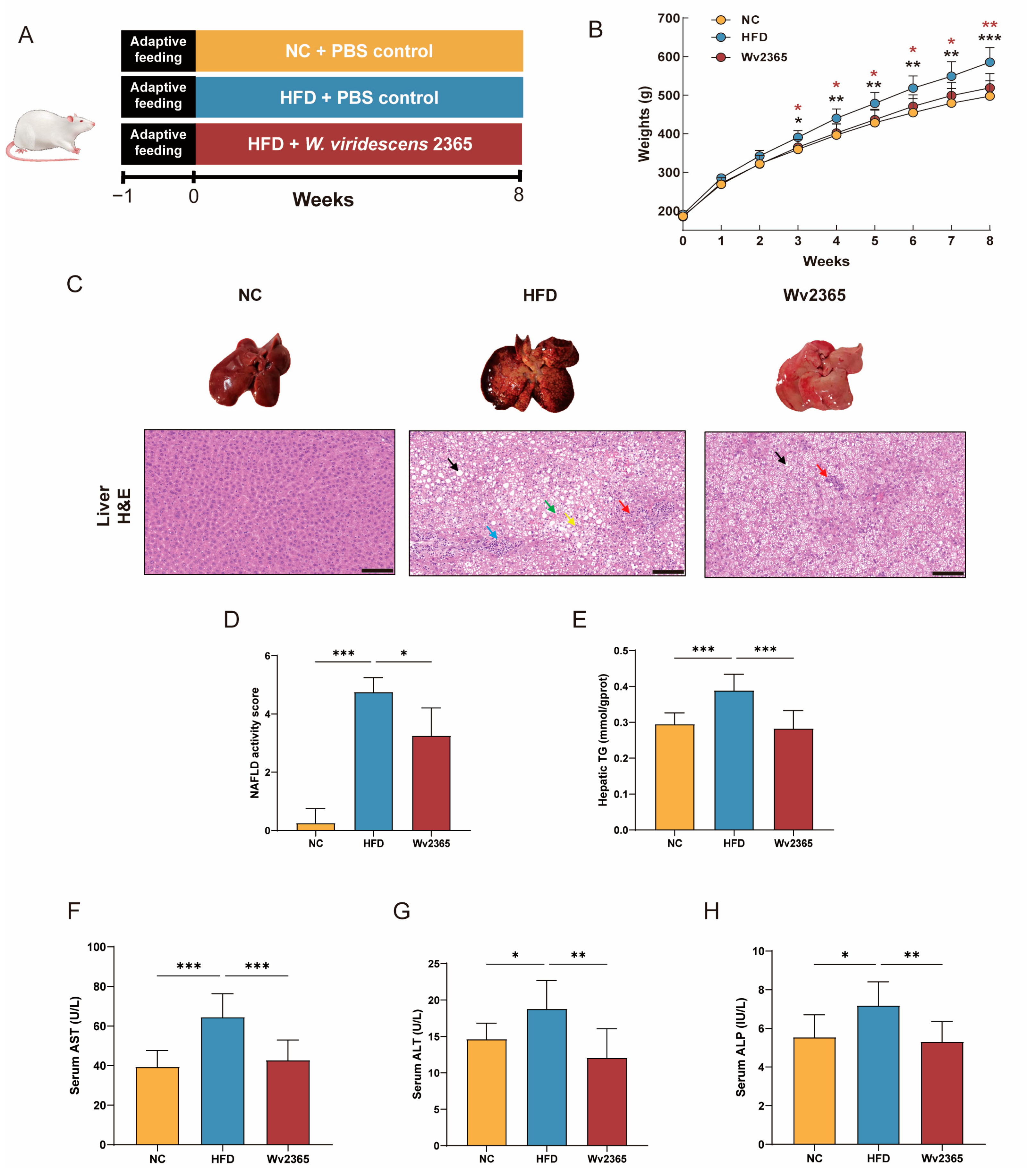
3.1. Effects of Wv2365 on Body Weight, Food Intake, and Hepatic Parameters in MASLD Rats
3.2. Effects of Wv2365 on Serum Lipid and Adipocyte Morphology in MASLD Rats
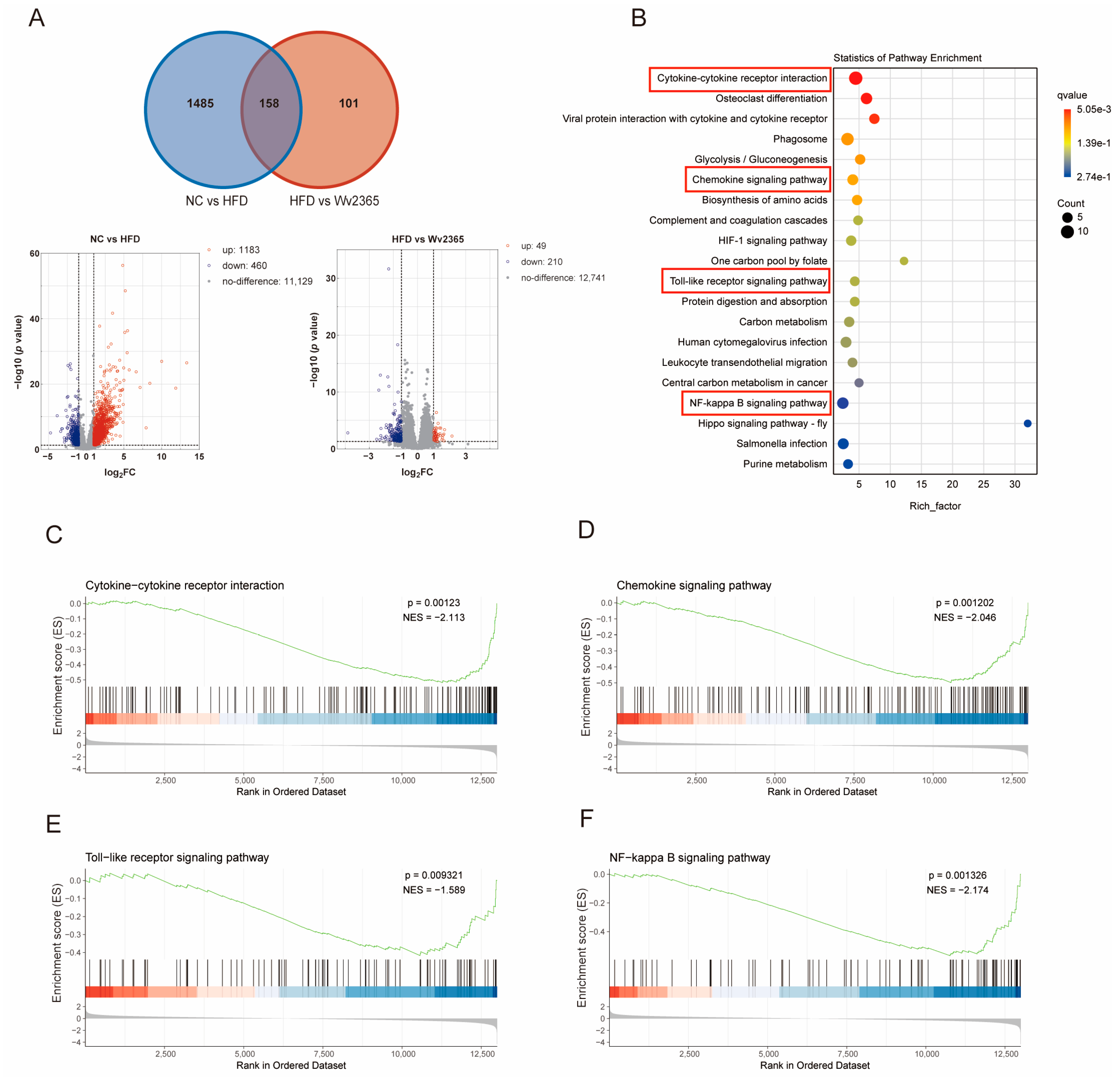
3.3. Wv2365 Modulates Inflammation-Related Hepatic Transcriptomic Profiles in MASLD Rats
3.4. Effects of Wv2365 on Serum Inflammation in MASLD Rats
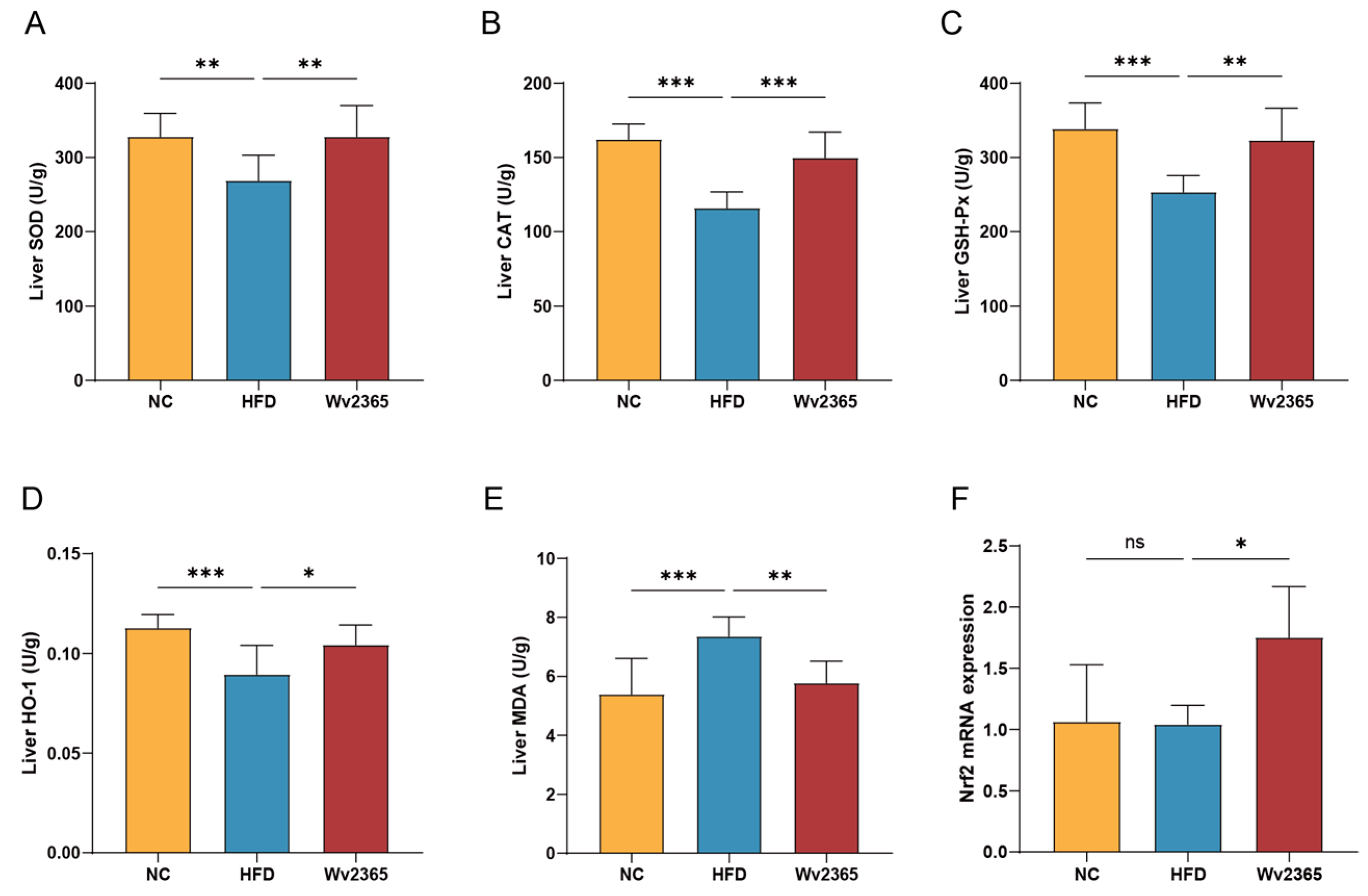
3.5. Effects of Wv2365 on Oxidative Stress in MASLD Rats
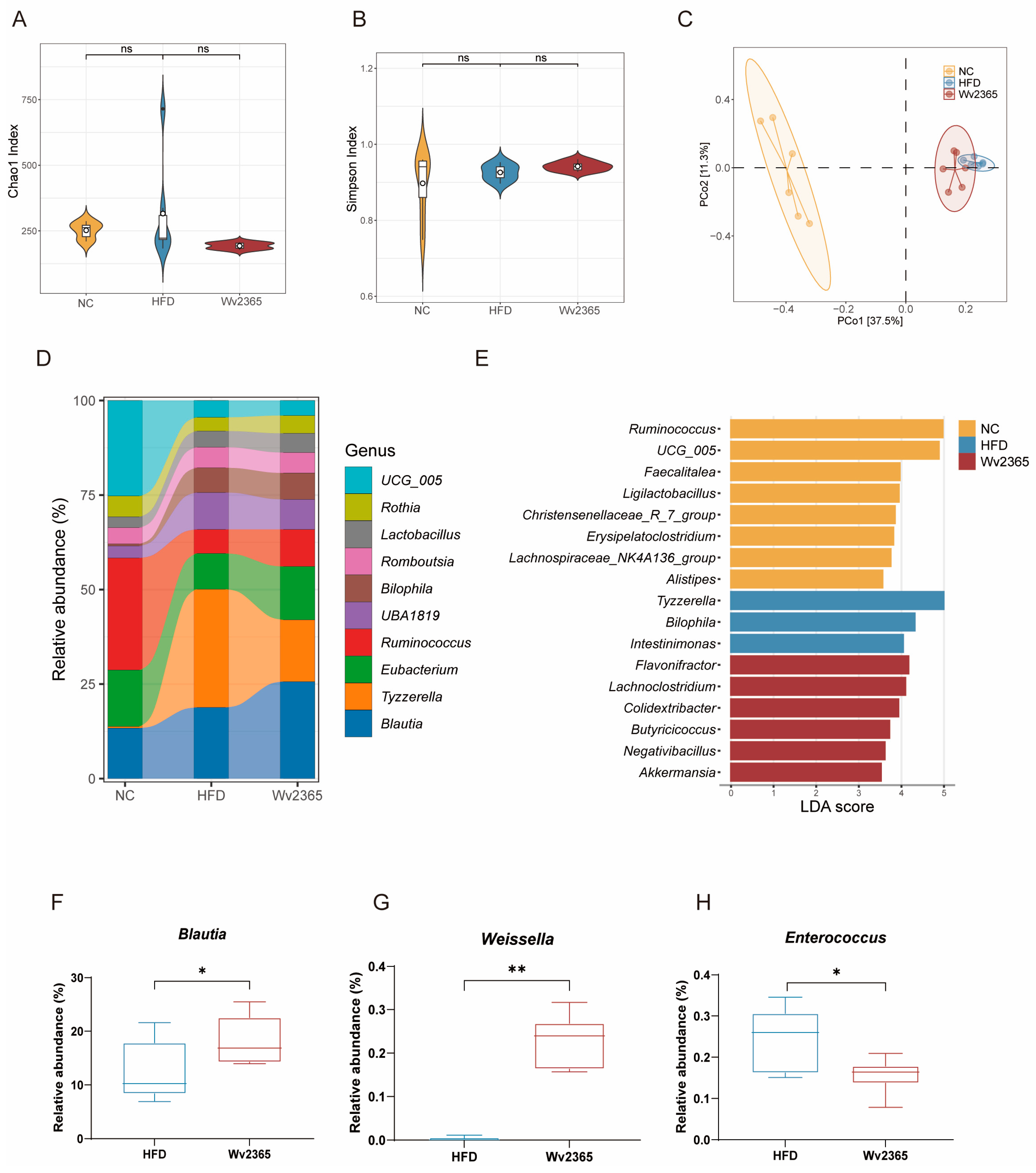
3.6. Wv2365 Induces Gut Microbiota Alterations in MASLD Rats
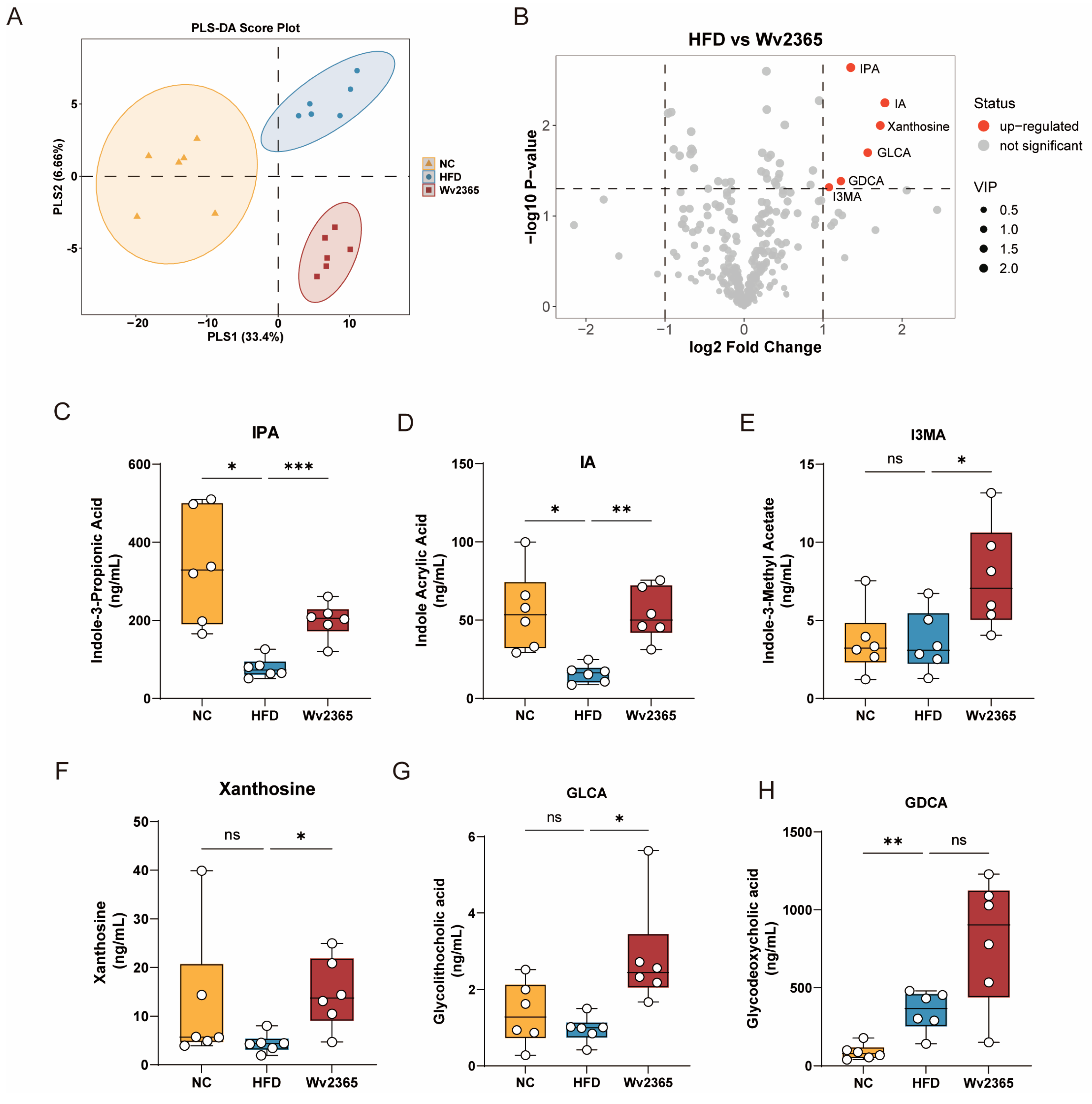
3.7. Wv2365 Altered Serum Metabolites in MASLD Rats
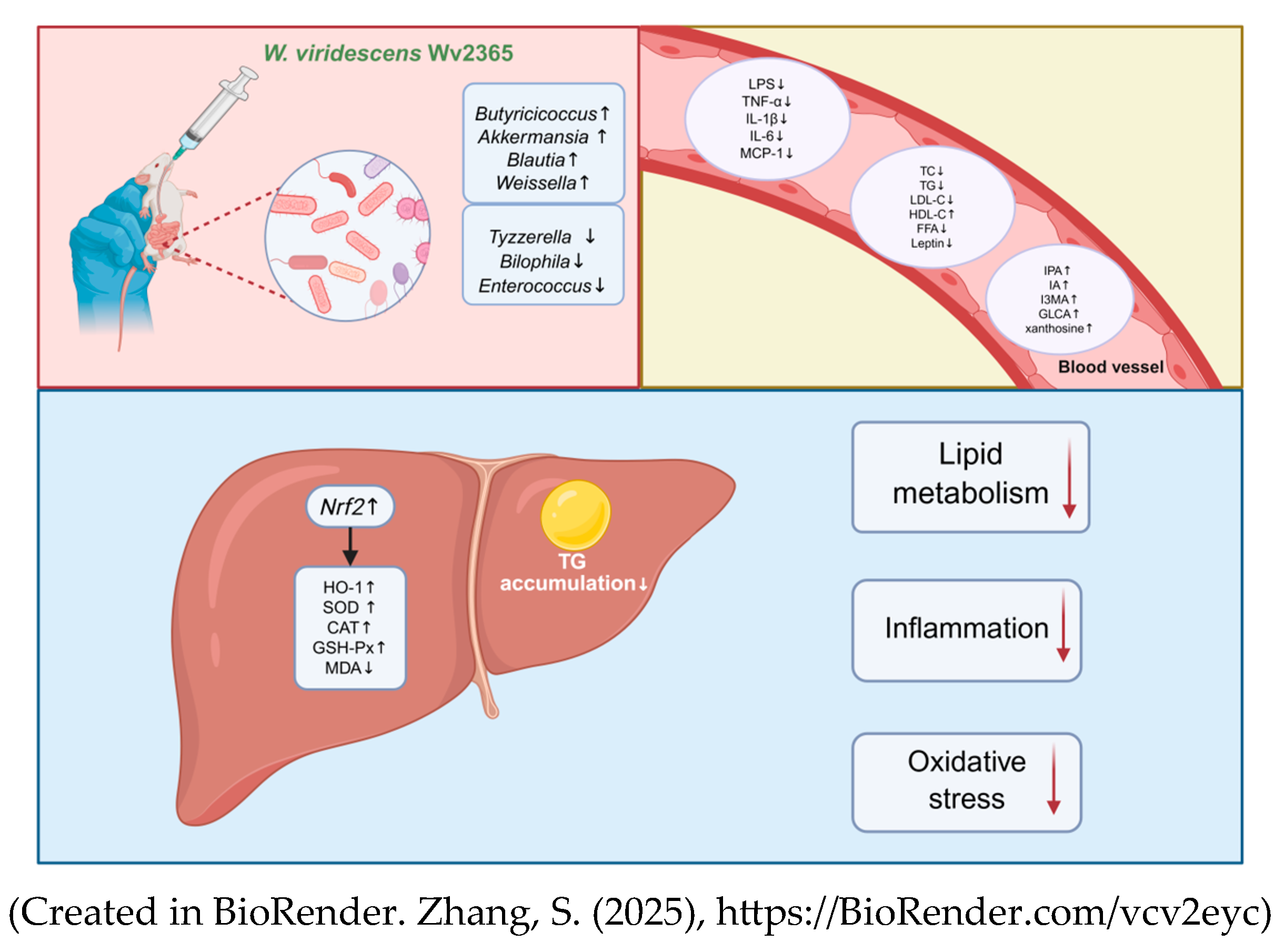
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MASLD | Metabolic-dysfunction-associated steatotic liver disease |
| NAS | NAFLD Activity Score |
| NC | Normal chow diet |
| HFD | High-fat diet |
| PBS | Phosphate-buffered saline |
| TC | Total cholesterol |
| TGs | Triglycerides |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| FFAs | Free fatty acids |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| LPSs | Lipopolysaccharides |
| TNF-α | Tumor necrosis factor-alpha |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GSH-Px | Glutathione peroxidase |
| HO-1 | Heme oxygenase-1 |
| MDA | Malondialdehyde |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| IPA | Indole-3-propionic acid |
| IA | Indoleacrylic acid |
| GLCA | Glycolithocholic acid |
References
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic Dysfunction–Associated Steatotic Liver Disease: Update and Impact of New Nomenclature on the American Association for the Study of Liver Diseases Practice Guidance on Nonalcoholic Fatty Liver Disease. Hepatology 2024, 79, 1212. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-Alcoholic Fatty Liver Disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, F.; Wang, W.; Zhang, X.-J.; Ji, Y.-X.; Zhang, P.; She, Z.-G.; Zhu, L.; Cai, J.; Li, H. Epidemiological Features of NAFLD from 1999 to 2018 in China. Hepatology 2020, 71, 1851. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 321–350. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD Development and Therapeutic Strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolis 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Paternostro, R.; Trauner, M. Current Treatment of Non-Alcoholic Fatty Liver Disease. J. Intern. Med. 2022, 292, 190–204. [Google Scholar] [CrossRef]
- Li, H.; Cheng, S.; Wang, Y.; Sun, Y.; Zhang, J.; Sun, M.; Man, C.; Zhang, Y.; Jiang, Y. Lactobacillus Plantarum J26 Alleviates Alcohol-Induced Oxidative Liver Injury by Regulating the Nrf2 Signaling Pathway. Food Sci. Hum. Wellness 2024, 13, 2068–2078. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The Role of the Gut Microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The Role of the Microbiome in NAFLD and NASH. Embo Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef]
- Long, Q.; Luo, F.; Li, B.; Li, Z.; Guo, Z.; Chen, Z.; Wu, W.; Hu, M. Gut Microbiota and Metabolic Biomarkers in Metabolic Dysfunction–Associated Steatotic Liver Disease. Hepatol. Commun. 2024, 8, e0310. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, Y.; Li, D.; Zhang, S.; Wu, Y.; Zhang, Q.; Bai, W. New Insights into the Mechanisms of High-fat Diet Mediated Gut Microbiota in Chronic Diseases. iMeta 2023, 2, e69. [Google Scholar] [CrossRef] [PubMed]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Zeng, X.-F.; Varady, K.A.; Wang, X.-D.; Targher, G.; Byrne, C.D.; Tayyem, R.; Latella, G.; Bergheim, I.; Valenzuela, R.; George, J.; et al. The Role of Dietary Modification in the Prevention and Management of Metabolic Dysfunction-Associated Fatty Liver Disease: An International Multidisciplinary Expert Consensus. Metabolism 2024, 161, 156028. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Zelber-Sagi, S.; Henry, L.; Gerber, L.H. Lifestyle Interventions in Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 708–722. [Google Scholar] [CrossRef]
- Dang, D.; Li, B.; Ding, M.; Paul Ross, R.; Stanton, C.; Zhao, J.; Yang, B.; Chen, W. Limosilactobacillus Mucosae FZJTZ26M3 Prevents NAFLD in Mice through Modulation of Lipid Metabolism and Gut Microbiota Dysbiosis. Food Sci. Hum. Wellness 2024, 13, 1589–1601. [Google Scholar] [CrossRef]
- Mijangos-Trejo, A.; Nuño-Lambarri, N.; Barbero-Becerra, V.; Uribe-Esquivel, M.; Vidal-Cevallos, P.; Chávez-Tapia, N. Prebiotics and Probiotics: Therapeutic Tools for Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 14918. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, W.; Shan, M.; Lu, Z.; Lu, Y. Yogurt-Derived Lactobacillus Plantarum Q16 Alleviated High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice. Food Sci. Hum. Wellness 2022, 11, 1428–1439. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.-S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The Genus Weissella: Taxonomy, Ecology and Biotechnological Potential. Front Microbiol 2015, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Benhouna, I.S.; Heumann, A.; Rieu, A.; Guzzo, J.; Kihal, M.; Bettache, G.; Champion, D.; Coelho, C.; Weidmann, S. Exopolysaccharide Produced by Weissella Confusa: Chemical Characterisation, Rheology and Bioactivity. Int. Dairy J. 2019, 90, 88–94. [Google Scholar] [CrossRef]
- Teixeira, C.G.; Fusieger, A.; Miliao, G.L.; Martins, E.; Drider, D.; Nero, L.A.; de Carvalho, A.F. Weissella: An Emerging Bacterium with Promising Health Benefits. Probiotics Antimicrob. Proteins 2021, 13, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Chieffi, D.; Fanelli, F.; Montemurro, M.; Rizzello, C.G.; Franz, C.M.A.P. The Weissella and Periweissella Genera: Up-to-Date Taxonomy, Ecology, Safety, Biotechnological, and Probiotic Potential. Front. Microbiol. 2023, 14, 1289937. [Google Scholar] [CrossRef]
- Liu, C.; Xue, W.-J.; Ding, H.; An, C.; Ma, S.-J.; Liu, Y. Probiotic Potential of Lactobacillus Strains Isolated From Fermented Vegetables in Shaanxi, China. Front. Microbiol. 2021, 12, 774903. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Monje, M.; Campos, J.; Alvarez Villamil, E.; Jerez, A.; Dentice Maidana, S.; Elean, M.; Salva, S.; Kitazawa, H.; Villena, J.; García-Cancino, A. Characterization of Weissella Viridescens UCO-SMC3 as a Potential Probiotic for the Skin: Its Beneficial Role in the Pathogenesis of Acne Vulgaris. Microorganisms 2021, 9, 1486. [Google Scholar] [CrossRef]
- Yang, J.; Pu, J.; Lu, S.; Bai, X.; Wu, Y.; Jin, D.; Cheng, Y.; Zhang, G.; Zhu, W.; Luo, X.; et al. Species-Level Analysis of Human Gut Microbiota With Metataxonomics. Front. Microbiol. 2020, 11, 2029. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; NASH Clinical Research Network (CRN). Nonalcoholic Fatty Liver Disease (NAFLD) Activity Score and the Histopathologic Diagnosis in NAFLD: Distinct Clinicopathologic Meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Lee, S.M.; Koh, D.H.; Jun, D.W.; Roh, Y.J.; Kang, H.T.; Oh, J.H.; Kim, H.S. Auranofin Attenuates Hepatic Steatosis and Fibrosis in Nonalcoholic Fatty Liver Disease via NRF2 and NF- κB Signaling Pathways. Clin. Mol. Hepatol. 2022, 28, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-I.; You, S.; Kim, S.; Won, G.; Kang, C.-H.; Kim, G.-H. Weissella Cibaria MG5285 and Lactobacillus Reuteri MG5149 Attenuated Fat Accumulation in Adipose and Hepatic Steatosis in High-Fat Diet-Induced C57BL/6J Obese Mice. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Lian, C.Y.; Zhai, Z.Z.; Li, Z.F.; Wang, L. High Fat Diet-Triggered Non-Alcoholic Fatty Liver Disease: A Review of Proposed Mechanisms. Chem.-Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef]
- Chen, Y.; Varghese, Z.; Ruan, X.Z. The Molecular Pathogenic Role of Inflammatory Stress in Dysregulation of Lipid Homeostasis and Hepatic Steatosis. Genes Dis. 2014, 1, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Duan, Y.; Yang, L.; Schnabl, B. Small Metabolites, Possible Big Changes: A Microbiota-Centered View of Non-Alcoholic Fatty Liver Disease. Gut 2019, 68, 359–370. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular Pathways of Nonalcoholic Fatty Liver Disease Development and Progression. Cell. Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Naomi, R.; Teoh, S.H.; Embong, H.; Balan, S.S.; Othman, F.; Bahari, H.; Yazid, M.D. The Role of Oxidative Stress and Inflammation in Obesity and Its Impact on Cognitive Impairments—A Narrative Review. Antioxidants 2023, 12, 1071. [Google Scholar] [CrossRef]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2–ARE Pathway: An Emerging Target against Oxidative Stress and Neuroinflammation in Neurodegenerative Diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, S.; Jiang, X.; Wang, Y.-H.; Li, F.; Wang, Y.-G.; Zheng, Y.; Cai, L. The Role of the Nrf2/Keap1 Pathway in Obesity and Metabolic Syndrome. Rev. Endocr. Metab. Disord. 2015, 16, 35–45. [Google Scholar] [CrossRef]
- Ajuwon, O.R.; Marnewick, J.L.; Oguntibeju, O.O.; Davids, L.M. Red Palm Oil Ameliorates Oxidative Challenge and Inflammatory Responses Associated with Lipopolysaccharide-Induced Hepatic Injury by Modulating NF-Κβ and Nrf2/GCL/HO-1 Signaling Pathways in Rats. Antioxidants 2022, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Understanding Mechanisms of Antioxidant Action in Health and Disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. Available online: https://www.nature.com/articles/s41580-023-00645-4 (accessed on 2 January 2024).
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of Inflammation by the Antioxidant Haem Oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The Multifaceted Role of Nrf2 in Mitochondrial Function. Curr. Opin Toxicol. 2016, 1, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.K.; Bansal, M.B. Pathogenesis of MASLD and MASH—Role of Insulin Resistance and Lipotoxicity. Aliment. Pharmacol. Ther. 2024, 59, S10–S22. [Google Scholar] [CrossRef]
- Hu, W.; Gao, W.; Liu, Z.; Fang, Z.; Wang, H.; Zhao, J.; Zhang, H.; Lu, W.; Chen, W. Specific Strains of Faecalibacterium Prausnitzii Ameliorate Nonalcoholic Fatty Liver Disease in Mice in Association with Gut Microbiota Regulation. Nutrients 2022, 14, 2945. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, J.K.; Liu, J.; Goon, J.A. Roles of Traditional and Next-Generation Probiotics on Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review and Network Meta-Analysis. Antioxidants 2024, 13, 329. [Google Scholar] [CrossRef]
- Higarza, S.G.; Arboleya, S.; Arias, J.L.; Gueimonde, M.; Arias, N. Akkermansia Muciniphila and Environmental Enrichment Reverse Cognitive Impairment Associated with High-Fat High-Cholesterol Consumption in Rats. Gut Microbes 2021, 13, 1880240. [Google Scholar] [CrossRef]
- Ioannou, A.; Berkhout, M.D.; Geerlings, S.Y.; Belzer, C. Akkermansia Muciniphila: Biology, Microbial Ecology, Host Interactions and Therapeutic Potential. Nat. Rev. Microbiol. 2025, 23, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Kuang, Z.; Li, C.; Guo, S.; Xu, Y.; Zhao, D.; Hu, Y.; Song, B.; Jiang, Z.; Ge, Z.; et al. Gut Akkermansia Muciniphila Ameliorates Metabolic Dysfunction-Associated Fatty Liver Disease by Regulating the Metabolism of L-Aspartate via Gut-Liver Axis. Gut Microbes 2021, 13, 1927633. [Google Scholar] [CrossRef] [PubMed]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van de Wiele, T. Butyrate-Producing Bacteria Supplemented in Vitro to Crohn’s Disease Patient Microbiota Increased Butyrate Production and Enhanced Intestinal Epithelial Barrier Integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral Administration of Blautia Wexlerae Ameliorates Obesity and Type 2 Diabetes via Metabolic Remodeling of the Gut Microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Hu, X.; Song, Y.; Wang, C.; Luo, P.; Ni, S.; Jiao, F.; Qiu, J.; Jiang, W.; Yang, S.; et al. Blautia Coccoides Is a Newly Identified Bacterium Increased by Leucine Deprivation and Has a Novel Function in Improving Metabolic Disorders. Adv. Sci. 2024, 11, 2309255. [Google Scholar] [CrossRef]
- Dietary Fibre Directs Microbial Tryptophan Metabolism via Metabolic Interactions in the Gut Microbiota | Nature Microbiology. Available online: https://www.nature.com/articles/s41564-024-01737-3 (accessed on 26 November 2024).
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan Metabolism in Health and Disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Xin, F.-Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.-L.; Cui, A.; Liu, Z.; et al. Indole-3-Propionic Acid Inhibits Gut Dysbiosis and Endotoxin Leakage to Attenuate Steatohepatitis in Rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Min, B.H.; Devi, S.; Kwon, G.H.; Gupta, H.; Jeong, J.-J.; Sharma, S.P.; Won, S.-M.; Oh, K.-K.; Yoon, S.J.; Park, H.J.; et al. Gut Microbiota-Derived Indole Compounds Attenuate Metabolic Dysfunction-Associated Steatotic Liver Disease by Improving Fat Metabolism and Inflammation. Gut Microbes 2024, 16, 2307568. [Google Scholar] [CrossRef]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37.e6. [Google Scholar] [CrossRef]
- Stoll, M.L.; Kumar, R.; Lefkowitz, E.J.; Cron, R.Q.; Morrow, C.D.; Barnes, S. Fecal Metabolomics in Pediatric Spondyloarthritis Implicate Decreased Metabolic Diversity and Altered Tryptophan Metabolism as Pathogenic Factors. Genes Immun. 2016, 17, 400–405. Available online: https://www.nature.com/articles/gene201638 (accessed on 30 March 2025).
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Keitel, V.; Gertzen, C.G.W.; Schäfer, S.; Klindt, C.; Wöhler, C.; Deutschmann, K.; Reich, M.; Gohlke, H.; Häussinger, D. Bile Acids and TGR5 (Gpbar1) Signaling. In Mammalian Sterols; Rozman, D., Gebhardt, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 81–100. ISBN 978-3-030-39683-1. [Google Scholar]
- Ahmed, S.A.; Sarma, P.; Barge, S.R.; Swargiary, D.; Devi, G.S.; Borah, J.C. Xanthosine, a Purine Glycoside Mediates Hepatic Glucose Homeostasis through Inhibition of Gluconeogenesis and Activation of Glycogenesis via Regulating the AMPK/ FoxO1/AKT/GSK3β Signaling Cascade. Chem.-Biol. Interact. 2023, 371, 110347. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhao, R.; Wang, R.; Lu, Y.; Xu, M.; Lin, X.; Lan, R.; Zhang, S.; Tang, H.; Fan, Q.; et al. Weissella viridescens Attenuates Hepatic Injury, Oxidative Stress, and Inflammation in a Rat Model of High-Fat Diet-Induced MASLD. Nutrients 2025, 17, 1585. https://doi.org/10.3390/nu17091585
Zhang S, Zhao R, Wang R, Lu Y, Xu M, Lin X, Lan R, Zhang S, Tang H, Fan Q, et al. Weissella viridescens Attenuates Hepatic Injury, Oxidative Stress, and Inflammation in a Rat Model of High-Fat Diet-Induced MASLD. Nutrients. 2025; 17(9):1585. https://doi.org/10.3390/nu17091585
Chicago/Turabian StyleZhang, Shuwei, Ruiqing Zhao, Ruoshi Wang, Yao Lu, Mingchao Xu, Xiaoying Lin, Ruiting Lan, Suping Zhang, Huijing Tang, Qianhua Fan, and et al. 2025. "Weissella viridescens Attenuates Hepatic Injury, Oxidative Stress, and Inflammation in a Rat Model of High-Fat Diet-Induced MASLD" Nutrients 17, no. 9: 1585. https://doi.org/10.3390/nu17091585
APA StyleZhang, S., Zhao, R., Wang, R., Lu, Y., Xu, M., Lin, X., Lan, R., Zhang, S., Tang, H., Fan, Q., Yang, J., Liu, L., & Xu, J. (2025). Weissella viridescens Attenuates Hepatic Injury, Oxidative Stress, and Inflammation in a Rat Model of High-Fat Diet-Induced MASLD. Nutrients, 17(9), 1585. https://doi.org/10.3390/nu17091585




