Arabinoxylan Concentrate from Wheat as a Functional Food Ingredient to Improve Glucose Homeostasis
Abstract
1. Introduction
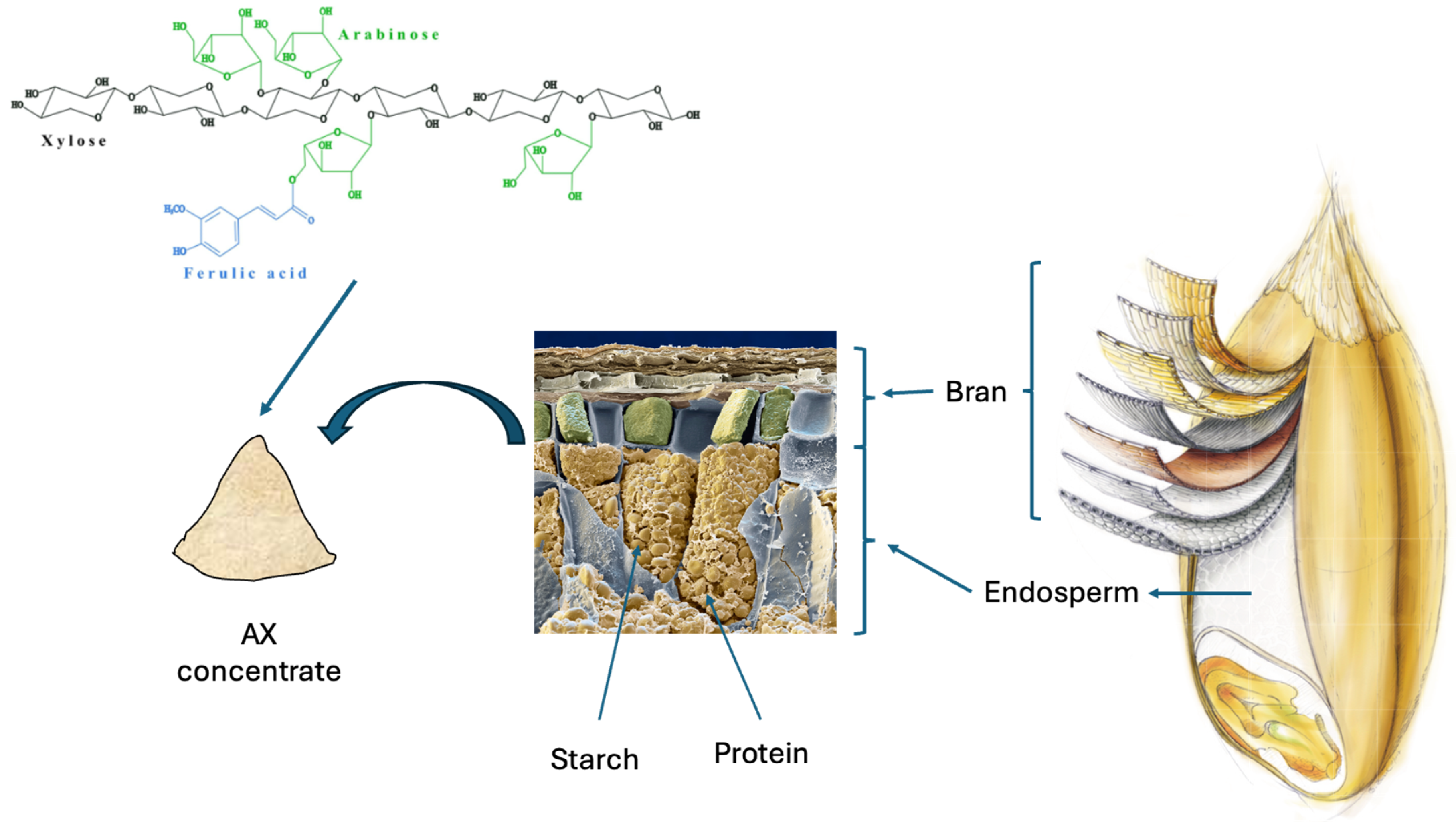
2. Arabinoxylan as an Ingredient and Its Incorporation in Breads
| Whole Grain Wheat | AX-Rich Fraction I | AX-Rich Fraction II | AX-Rich Fraction III | |
|---|---|---|---|---|
| Wheat processing | Starch and gluten | Starch | Starch and gluten | |
| Ash | 18 | nm | 13 | 72 |
| Protein | 138 | 101 | 170 | 397 |
| Fat | 29 | Trace | 4 | 2 |
| Starch | 649 | 156 | nm | 40 |
| Total NDC | 131 | nm | nm | 464 |
| Total NSP | 115 | 742 | nm | 312 |
| Soluble:total NSP | 0.26 | 0.62 | nm | 0.91 |
| AX | 75 | 668 | 566 | 234 |
| Arabinose:xylose | 0.62 | 0.66 | 0.80 | 0.94 |
| AX-oligosaccharides | nm | nm | nm | 95 |
| Reference | # | [28] | [29] | [30] |
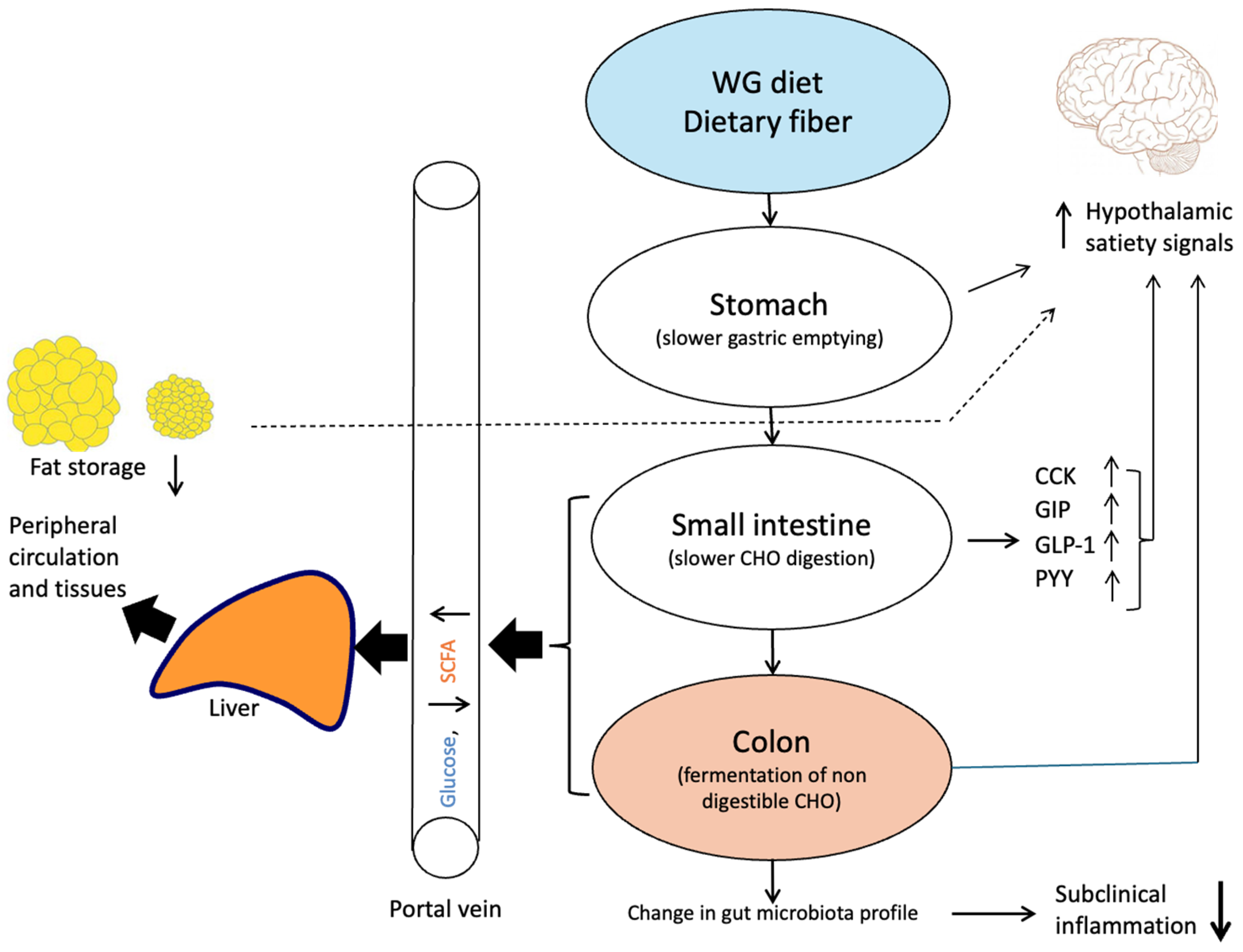
3. Functional Properties of AX in the Gastrointestinal Tract and Influence on Digestion
4. Influence of AX on Glucose Absorption and Regulation
4.1. Acute Studies
| AX Source | AX Dose and Control | Species | Parameters Studied | Observed Effect | Reference |
|---|---|---|---|---|---|
| AX extracted from wheat co-products after starch and gluten extraction | 6 or 12 g of AX provided in breads and compared with white wheat bread as a control | Human normoglycemic subjects | Plasma glucose, insulin, and iAUC | Dose-dependent effects of AX on iAUC glucose and iAUC insulin in normoglycemic subjects | [28] |
| AX concentrate from the soluble fraction after wheat starch and gluten extraction | 7 g of AX provided in bread and compared to white wheat bread, whole grain rye bread with kernels, and β-glucan-rich bread | Human subjects with metabolic syndrome | Plasma glucose, insulin, GLP-1, GIP, ghrelin, iAUC, and appetite score | AX reduced peak glucose but neither the initial glycemic response nor insulin response. AX increased satiety compared to the white wheat bread but did not result in a significant difference in subsequent ad libitum energy intake after 270 min. | [38] |
| AX concentrate from the soluble fraction after wheat starch and gluten extraction | 3.5 g of AX provided alone or in combination with whole rye kernels (4.4 g AX) compared to semolina porridge | Human subjects with metabolic syndrome | Plasma glucose, insulin, GLP-1, ghrelin, iAUC, breast hydrogen, plasma short-chain fatty acids, and appetite score | AX combined with rye kernels reduced acute glucose and insulin responses and feelings of hunger compared with the control meal. AX alone and in combination with rye kernels increased butyrate and acetate concentrations after 6 h compared to control but with no differences in the second meal response for glucose, insulin, free fatty acids, glucagon-like peptide-1, or ghrelin | [50] |
| AX-enriched white bread flour | 3.2 g of AX-enriched bread compared with white wheat bread | Human normoglycemic subjects | Plasma glucose | The 30 min peak plasma glucose concentration after AX-enriched meals was significantly lower than that for control white wheat bread | [51] |
| AX concentrate from the soluble fraction after wheat starch and gluten extraction | AX provided in bread and compared to white wheat bread, whole grain rye bread, whole grain rye bread with kernels, and β-glucan-rich bread | Portal vein-catheterized pigs | Plasma glucose, insulin, glucose absorption, GLP-1, and GIP | Net portal glucose absorption was reduced in pigs fed the AX bread at 60 min and insulin secretion was lowered at 30 min with AX bread and whole grain rye bread compared to white wheat bread | [37] |
| AX | iAUC | Concentration | |||||||
|---|---|---|---|---|---|---|---|---|---|
| g or % of DM | Glucose | Insulin | Glucose | Insulin | GLP-1 | GIP | Ghrelin | Ref. | |
| AX extracted from wheat co-products after starch and gluten extraction | 6 g | ↓ | ↓ | ↓ | → | [28] | |||
| 12 g | ↓↓ | ↓ | ↓↓ | ↓ | |||||
| AX concentrate from the soluble fraction after wheat starch and gluten extraction | 7 g | → | → | ↓ | ↑ | → | → | → | [38] |
| AX concentrate from the soluble fraction after wheat starch and gluten extraction | 3.5 g | ↓ | → | → | → | → | → | [50] | |
| 4.4 g | → | ↓ | ↓ | ↓ | → | → | |||
| AX-enriched white bread flour | 3.2 g | ↓ | [51] | ||||||
| AX concentrate from the soluble fraction after wheat starch and gluten extraction | 7.8% vs. 1.7% 1 | → | → | ↓ 2 | ↓ 2 | → | → | [37] | |
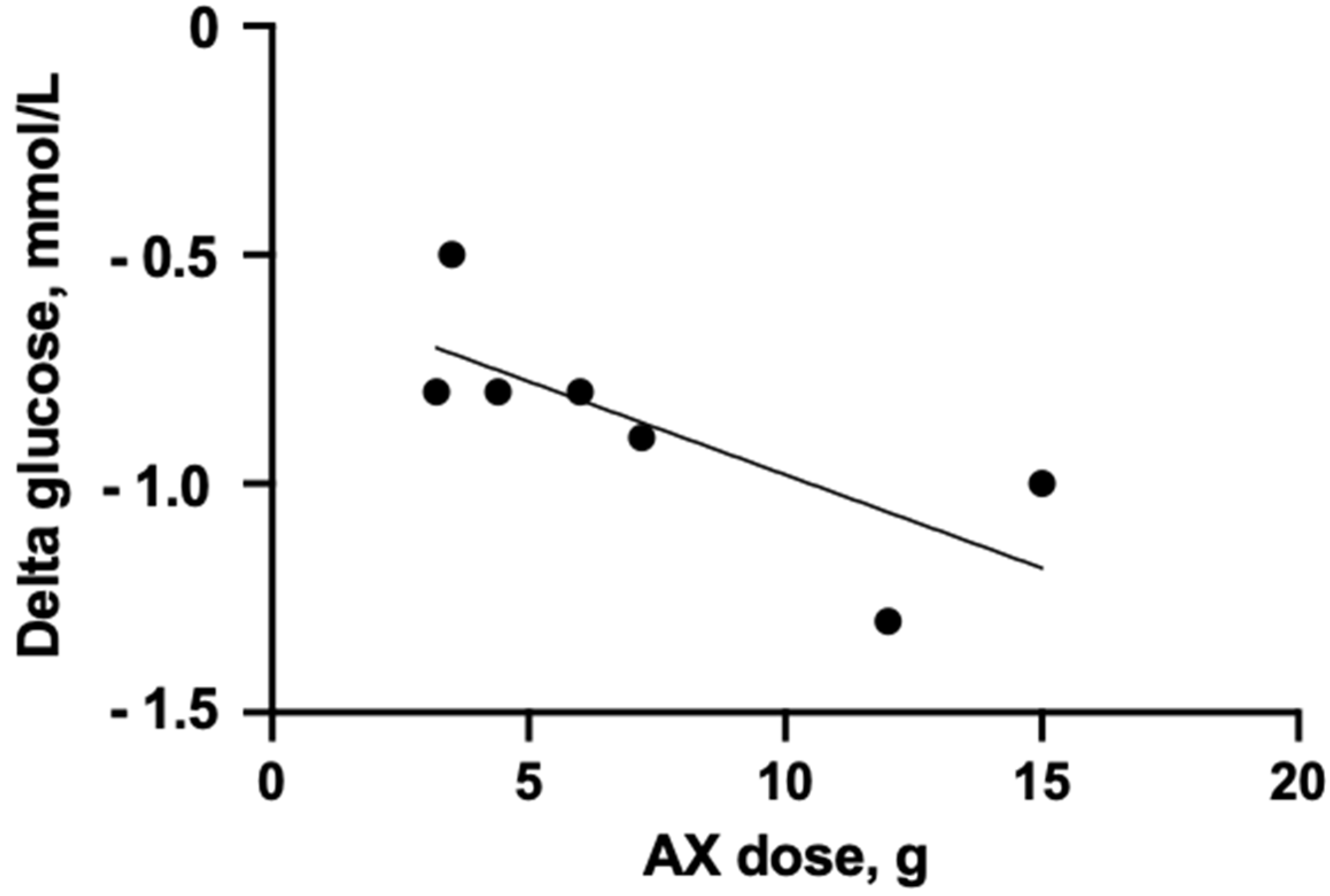
4.2. Medium- and Long-Term Intervention Studies
| AX Source | AX Dose and Control | Species and Duration | Parameters Studied | Observed Effect | Reference |
|---|---|---|---|---|---|
| AX extracted from wheat co-products after starch and gluten extraction | 15 g of AX-rich fiber provided through bread and muffins compared to a whole grain white flour (1:1) | Human subjects with type 2 diabetes. Intervention period: 5 weeks | Plasma glucose, insulin, fructosamine, blood lipids, blood pressure, OGTT, and fecal output | AX consumption resulted in significantly lower fasting and 2 h plasma glucose, lower 2 h insulin and serum fructosamine, and increased fecal output | [58] |
| AX concentrated from process water after wheat starch extraction | 15 g of AX provided in breads and as powder compared to white wheat bread | Human subjects with impaired glucose tolerance. Intervention period: 6 weeks | Serum glucose, insulin, and triglycerides, and plasma total and acetylated ghrelin | AX consumption resulted in lower postprandial responses in serum glucose, insulin, and triglycerides. Compared to the placebo, total plasma ghrelin was also reduced, but acetylated ghrelin was not | [29,31] |
| AX concentrate from wheat by-products after gluten extraction | AX (10%) added to a wheat starch-based diet alone or combined with β-glucan (5% AX+5% β-glucan) and compared with a low-DF wheat starch diet, a wheat starch diet with added β-glucan (10%), or a whole wheat flour diet | Male normoglycemic pigs. Intervention period: 3 weeks | Plasma glucose, insulin, NEFA, GIP, GLP-1, PYY, ghrelin, glucagon, cortisol concentrations, and OGTT | AX had no effect on glycemic response following the feed challenge or the oral glucose tolerance test as determined by the area under the curve. A biphasic glucose and insulin response was detected for all pigs following the OGTT. Pigs fed the combination of AX and β-glucan had a reduced GIP response and delayed insulin peak following the feed challenge. Incretin (GLP-1 and GIP) secretion appeared asynchronous, reflecting their different enteroendocrine cell locations and responses to nutrient absorption | [65] |
| AX concentrate from the soluble fraction after wheat starch and gluten extraction | Pelleted diets prepared from ground and dried AX bread, white wheat bread, whole grain rye bread, whole grain rye bread with kernels, and β-glucan-rich bread | Zucker diabetic fatty (ZDF) rats. Intervention period: 7 weeks | Plasma glucose, insulin, OGTT, glucagon, triglycerides, cholesterol, HDL cholesterol, free fatty acids, HbA1c, and key genes related to insulin signaling cascade, glucose metabolism, and inflammation | AX added to wheat bread had similar beneficial effects on glycemic control according to the oral glucose tolerance test and in changing the expression of key adipose and hepatic genes to AX-rich rye breads without and with kernels | [63] |
| AX | Concentration | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| g/d or % of DM | OGTT | Glucose | Insulin | Fructosamine/HbA1c | GLP-1 | GIP | Ghrelin | Ref. | |
| AX extracted from wheat co-products after starch and gluten extraction | 15 g | ↓ | ↓ | ↓ | ↓ | [58] | |||
| AX concentrated from process water after wheat starch extraction | 15 g | ↓ | ↓ | → | [29,31] | ||||
| AX concentrate from wheat by-products after gluten extraction | 10% vs. 0% | → | → | → | → | → | → | [65] | |
| AX concentrate from the soluble fraction after wheat starch and gluten extraction | 7.1% vs. 2.2% | ↓ | ↓ | ↓ | ↓ | [63] | |||
5. Influence of AX on Insulin and Incretins
6. Influence of AX on the Plasma Metabolome
7. Influence of AX in Diets on Satiety and Plasma Ghrelin
8. Conclusions
9. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AX | Arabinoxylan |
| A:X | Arabinose-to-xylose ratio |
| DF | Dietary fiber |
| NSP | Non-starch polysaccharides |
| NDC | Non-digestible carbohydrate |
| LMW | Low molecular weight |
| DM | Dry matter |
| Mw | Molecular weight |
| SCFA | Short-chain fatty acids |
| GIP | Glucose-dependent insulinotropic peptide |
| GLP-1 | Glucagon-like peptide-1 |
| HbA1c | Glycated hemoglobin A1c |
| GI | Glycemic index |
| iAUC | Incremental area under the curve |
| OGTT | Oral glucose tolerance test |
| ZDF | Zucker diabetic fatty rats |
| T2DM | Type 2 diabetes mellitus |
| STZ | Streptozotocin |
References
- Russell, W.R.; Baka, A.; Bjorck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Hadjilucas, E.; Juvonen, K.; Lahtinen, S.; Lansink, M.; et al. Impact of Diet Composition on Blood Glucose Regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef]
- Peronnet, F.; Meynier, A.; Sauvinet, V.; Normand, S.; Bourdon, E.; Mignault, D.; St-Pierre, D.H.; Laville, M.; Rabasa-Lhoret, R.; Vinoy, S. Plasma glucose kinetics and response of insulin and GIP following a cereal breakfast in female subjects: Effect of starch digestibility. Eur. J. Clin. Nutr. 2015, 69, 740–745. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E.J. Metabolic syndrome. Medicine 2015, 43, 80–87. [Google Scholar] [CrossRef]
- Tosh, S. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur. J. Clin. Nutr. 2013, 67, 310–317. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Tosh, S.M.; Spruill, S.E.; Jenkins, A.L.; Ezatagha, A.; Duss, R.; Johnson, J.; Chu, Y.; Steinert, R.E. Increasing oat beta-glucan viscosity in a breakfast meal slows gastric emptying and reduces glycemic and insulinemic responses but has no effect on appetite, food intake, or plasma ghrelin and PYY responses in healthy humans: A randomized, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2020, 111, 319–328. [Google Scholar] [CrossRef]
- Holst, J.J. The incretin system in healthy humans: The role of GIP and GLP-1. Metabolism 2019, 96, 46–55. [Google Scholar] [CrossRef]
- Karhunen, L.J.; Juvonen, K.R.; Huotari, A.; Purhonen, A.K.; Herzig, K.H. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul. Pept. 2008, 149, 70–78. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 5–21. [Google Scholar] [CrossRef]
- Everard, A.; Cani, P.D. Gut microbiota and GLP-1. Rev. Endocr. Metab. Dis. 2014, 15, 189–196. [Google Scholar] [CrossRef]
- Muller, M.; Hernandez, M.A.G.; Goossens, G.H.; Reijnders, D.; Holst, J.J.; Jocken, J.W.E.; van Eijk, H.; Canfora, E.E.; Blaak, E.E. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 2019, 9, 12515. [Google Scholar] [CrossRef] [PubMed]
- Greiner, T.U.; Bäckhed, F. Microbial regulation of GLP-1 and L-cell biology. Mol. Metab. 2016, 5, 753–758. [Google Scholar] [CrossRef]
- Mio, K.; Ogawa, R.; Tadenuma, N.; Aoe, S. Arabinoxylan as well as beta-glucan in barley promotes GLP-1 secretion by increasing short-chain fatty acids production. Biochem. Biophys. Rep. 2022, 32, 101343. [Google Scholar] [CrossRef]
- Sørensen, A.-M.; Fagt, S.; Møller, A. Dietary fibre intake in Denmark. In Dietary Fibre Intakes in Europe; Cummings, J.H., Frølich, W., Eds.; Directorate-General Science, Research and Development: Brussel, Belgium, 1993; pp. 37–39. [Google Scholar]
- Bach Knudsen, K.E.; Norskov, N.P.; Bolvig, A.K.; Hedemann, M.S.; Laerke, H.N. Dietary fibers and associated phytochemicals in cereals. Mol. Nutr. Food. Res. 2017, 61, 1600518. [Google Scholar] [CrossRef]
- van der Kamp, J.W.; Jones, J.M.; Miller, K.B.; Ross, A.B.; Seal, C.J.; Tan, B.; Beck, E.J. Consensus, Global Definitions of Whole Grain as a Food Ingredient and of Whole-Grain Foods Presented on Behalf of the Whole Grain Initiative. Nutrients 2022, 14, 138. [Google Scholar] [CrossRef]
- Carlsen, H.; Pajari, A.M. Dietary fiber—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 10-29219. [Google Scholar] [CrossRef]
- Hemdane, S.; Jacobs, P.J.; Dornez, E.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Wheat (Triticum aestivum L.) Bran in Bread Making: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 28–42. [Google Scholar] [CrossRef]
- Zannini, E.; Bravo Nunez, A.; Sahin, A.W.; Arendt, E.K. Arabinoxylans as Functional Food Ingredients: A Review. Foods 2022, 11, 1026. [Google Scholar] [CrossRef]
- Wood, P.J.; Beer, M.U.; Butler, G. Evaluation of role of concentration and molecular weight of oat beta-glucan in determining effect of viscosity on plasma glucose and insulin following an oral glucose load. Br. J. Nutr. 2000, 84, 19–23. [Google Scholar] [CrossRef]
- Mathews, R.; Kamil, A.; Chu, Y.F. Global review of heart health claims for oat beta-glucan products. Nutr. Rev. 2020, 78, 78–97. [Google Scholar] [CrossRef] [PubMed]
- European-Food-Safety-Authority. Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and ‘digestive function’ (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207–2228. [Google Scholar]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Gruppen, H.; Verbruggen, M.A.; Viëtor, R.J. Characterization of cereal arabinoxylans. In Xylans and Xylanases; Visser, J., Beldman, G., Someren, M.A.K.-V., Voragen, A.G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 51–67. [Google Scholar]
- Dervilly, G.; Saulnier, L.; Roger, P.; Thibault, J.F. Isolation of Homogeneous Fractions from Wheat Water-Soluble Arabinoxylans. Influence of the Structure on Their Macromolecular Characteristics. J. Agric. Food Chem. 2000, 48, 270–278. [Google Scholar] [CrossRef]
- Gruppen, H.; Hamer, R.J.; Voragen, A.G.J. Barium hydroxide as a tool to extract pure arabinoxylans from water-insoluble cell wall material of wheat flour. J. Cereal Sci. 1991, 13, 275–290. [Google Scholar] [CrossRef]
- Surget, A.; Barron, C. Histologie du grain de blé (histology of the wheat grain). Ind. Cér. 2005, 145, 3–7. [Google Scholar]
- Zhang, Z.X.; Smith, C.; Li, W.L. Extraction and modification technology of arabinoxylans from cereal by-products: A critical review. Food Res. Int. 2014, 65, 423–436. [Google Scholar] [CrossRef]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; Mascara, T.; O’Dea, K. Arabinoxylan fiber, a byproduct of wheat flour processing, reduces the postprandial glucose response in normoglycemic subjects. Am. J. Clin. Nutr. 2000, 71, 1123–1128. [Google Scholar] [CrossRef]
- Garcia, A.L.; Steiniger, J.; Reich, S.C.; Weickert, M.O.; Harsch, I.; Machowetz, A.; Mohlig, M.; Spranger, J.; Rudovich, N.N.; Meuser, F.; et al. Arabinoxylan fibre consumption improved glucose metabolism, but did not affect serum adipokines in subjects with impaired glucose tolerance. Horm. Metab. Res. 2006, 38, 761–766. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Lærke, H.N.; Bach Knudsen, K.E. Effects of Isolated and Complex Dietary Fiber Matrices in Breads on Carbohydrate Digestibility and Physicochemical Properties of Ileal Effluent from Pigs. J. Agric. Food. Chem. 2012, 60, 12469–12476. [Google Scholar] [CrossRef]
- Garcia, A.L.; Otto, B.; Reich, S.C.; Weickert, M.O.; Steiniger, J.; Machowetz, A.; Rudovich, N.N.; Mohlig, M.; Katz, N.; Speth, M.; et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur. J. Clin. Nutr. 2007, 61, 334–341. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Laerke, H.N.; Knudsen, K.E. Changes in molecular characteristics of cereal carbohydrates after processing and digestion. Int. J. Mol. Sci. 2012, 13, 16833–16852. [Google Scholar] [CrossRef]
- Saulnier, L.; Sado, P.-E.; Branlard, G.; Charmet, G.; Guillon, F. Wheat arabinoxylans: Exploiting variation in amount and composition to develop enhanced varieties. J. Cereal Sci. 2007, 46, 261–281. [Google Scholar] [CrossRef]
- Åman, P.; Rimsten, L.; Andersson, R. Molecular weight distribution of β-glucan in oat-based foods. Cereal. Chem. 2004, 81, 356–360. [Google Scholar] [CrossRef]
- Luo, D.; Li, X.; Geng, M.; Zhang, Y.; Lan, H.; Li, J.; Qi, C.; Bai, Z.; Huang, J. Effect of Arabinoxylan from Wastewater Generated during Vital Wheat Gluten Production on Liver Metabolism in Type 2 Diabetic Mice. Foods 2023, 12, 2640. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Christensen, K.L.; Hedemann, M.S.; Laerke, H.N.; Jorgensen, H.; Mutt, S.J.; Herzig, K.H.; Bach Knudsen, K.E. Concentrated arabinoxylan but not concentrated beta-glucan in wheat bread has similar effects on postprandial insulin as whole-grain rye in porto-arterial catheterized pigs. J. Agric. Food. Chem. 2013, 61, 7760–7768. [Google Scholar] [CrossRef]
- Hartvigsen, M.L.; Gregersen, S.; Laerke, H.N.; Holst, J.J.; Bach Knudsen, K.E.; Hermansen, K. Effects of concentrated arabinoxylan and beta-glucan compared with refined wheat and whole grain rye on glucose and appetite in subjects with the metabolic syndrome: A randomized study. Eur. J. Clin. Nutr. 2014, 68, 84–90. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007, 46, 101–118. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef]
- Glitsø, L.V.; Gruppen, H.; Schols, H.A.; Højsgaard, S.; Sandström, B.; Bach Knudsen, K.E. Degradation of rye arabinoxylans in the large intestine of pigs. J. Sci. Food Agric. 1999, 79, 961–969. [Google Scholar] [CrossRef]
- Xu, Y.; Curtasu, M.V.; Bendiks, Z.; Marco, M.L.; Nørskov, N.P.; Knudsen, K.E.B.; Hedemann, M.S.; Laerke, H.N. Effects of dietary fibre and protein content on intestinal fibre degradation, short-chain fatty acid and microbiota composition in a high-fat fructose-rich diet induced obese Gottingen Minipig model. Food Funct. 2020, 11, 10758–10773. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Bird, A.R.; Conlon, M.A.; Williams, B.A.; Kang, S.; McSweeney, C.S.; Zhang, D.; Bryden, W.L.; Gidley, M.J.; Topping, D.L. An arabinoxylan-rich fraction from wheat enhances caecal fermentation and protects colonocyte DNA against diet-induced damage in pigs. Br. J. Nutr. 2012, 107, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.X.; Hu, J.L.; Chen, H.H.; Geng, F.; Nie, S.P. Arabinoxylan ameliorates type 2 diabetes by regulating the gut microbiota and metabolites. Food Chem. 2022, 371, 131106. [Google Scholar] [CrossRef]
- Lancaster, S.M.; Lee-McMullen, B.; Abbott, C.W.; Quijada, J.V.; Hornburg, D.; Park, H.; Perelman, D.; Peterson, D.J.; Tang, M.; Robinson, A.; et al. Global, distinctive, and personal changes in molecular and microbial profiles by specific fibers in humans. Cell Host Microbe 2022, 30, 848–862.e847. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Paez, A.; Kjolbaek, L.; Gomez Del Pulgar, E.M.; Brahe, L.K.; Astrup, A.; Matysik, S.; Schott, H.F.; Krautbauer, S.; Liebisch, G.; Boberska, J.; et al. A Multi-omics Approach to Unraveling the Microbiome-Mediated Effects of Arabinoxylan Oligosaccharides in Overweight Humans. mSystems 2019, 4, e00209-19. [Google Scholar] [CrossRef]
- Hald, S.; Schioldan, A.G.; Moore, M.E.; Dige, A.; Laerke, H.N.; Agnholt, J.; Knudsen, K.E.B.; Hermansen, K.; Marco, M.L.; Gregersen, S.; et al. Effects of Arabinoxylan and Resistant Starch on Intestinal Microbiota and Short-Chain Fatty Acids in Subjects with Metabolic Syndrome: A Randomised Crossover Study. PLoS ONE 2016, 11, e0159223. [Google Scholar] [CrossRef]
- Ellis, P.R.; Roberts, F.G.; Low, A.G.; Morgan, L.M. The effect of high-molecular-weight guar gum on net apparent glucose absorption and net apparent insulin and gastric inhibitory polypeptide production in the growing pig: Relationship to rheological changes in jejunal digesta. Br. J. Nutr. 1995, 74, 539–556. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef]
- Hartvigsen, M.L.; Laerke, H.N.; Overgaard, A.; Holst, J.J.; Bach Knudsen, K.E.; Hermansen, K. Postprandial effects of test meals including concentrated arabinoxylan and whole grain rye in subjects with the metabolic syndrome: A randomised study. Eur. J. Clin. Nutr. 2014, 68, 567–574. [Google Scholar] [CrossRef]
- Falchi, A.G.; Grecchi, I.; Muggia, C.; Palladini, G.; Perlini, S. Effects of a bioavalable arabinoxylan-enriched white bread flour on postprandial glicose response in normoglycemic subjects. J. Diet Suppl. 2016, 13, 626–633. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Hartvigsen, M.L.; Hedemann, M.S.; Hermansen, K. Mechanisms whereby whole grain cereals modulate the prevention of type 2 diabetes. In Molecular Nutrition and Diabetes; Mauricia, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; p. 87103. [Google Scholar]
- Fardet, A.; Leenhardt, F.; Lioger, D.; Scalbert, A.; Remesy, C. Parameters controlling the glycaemic response to breads. Nutr. Res. Rev. 2006, 19, 18–25. [Google Scholar] [CrossRef]
- Rojas-Bonzi, P.; Vangsoe, C.T.; Nielsen, K.L.; Laerke, H.N.; Hedemann, M.S.; Knudsen, K.E.B. The Relationship between In Vitro and In Vivo Starch Digestion Kinetics of Breads Varying in Dietary Fibre. Foods 2020, 9, 1337. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Vangsøe, C.T.; Ingerslev, A.K.; Theil, P.K.; Hedemann, M.S.; Lærke, H.N.; Bach Knudsen, K.E. In vitro starch digestion kinetics of diets varying in resistant starch and arabinoxylan compared with in vivo portal appearance of glucose in pigs. Food Res. Inter. 2016, 88, 199–206. [Google Scholar] [CrossRef]
- Rérat, A.A.; Vaissade, P.; Vaugelade, P. Absorption kinetics of some carbohydrates in conscious pigs. 2. Quantitative aspects. Br. J. Nutr. 1984, 51, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; O’Dea, K. Arabinoxylan fibre improves metabolic control in people with Type II diabetes. Eur. J. Clin. Nutr. 2004, 58, 621–628. [Google Scholar] [CrossRef]
- Schweizer, T.F.; Wursch, P. The physiological and nutritional importance of dietary fibre. Experientia 1991, 47, 181–186. [Google Scholar] [CrossRef]
- Nie, Q.; Xing, M.; Chen, H.; Hu, J.; Nie, S. Metabolomics and Lipidomics Profiling Reveals Hypocholesterolemic and Hypolipidemic Effects of Arabinoxylan on Type 2 Diabetic Rats. J. Agric. Food Chem. 2019, 67, 10614–10623. [Google Scholar] [CrossRef]
- Singh, J.; Metrani, R.; Shivanagoudra, S.R.; Jayaprakasha, G.K.; Patil, B.S. Review on Bile Acids: Effects of the Gut Microbiome, Interactions with Dietary Fiber, and Alterations in the Bioaccessibility of Bioactive Compounds. J. Agric. Food Chem. 2019, 67, 9124–9138. [Google Scholar] [CrossRef]
- Hanai, H.; Ikuma, M.; Sato, Y.; Iida, T.; Hosoda, Y.; Matsushita, I.; Nogaki, A.; Yamada, M.; Kaneko, E. Long-term effects of water-soluble corn bran hemicellulose on glucose tolerance in obese and non-obese patients: Improved insulin sensitivity and glucose metabolism in obese subjects. Biosci. Biotechnol. Biochem. 1997, 61, 1358–1361. [Google Scholar] [CrossRef]
- Hartvigsen, M.L.; Jeppesen, P.B.; Laerke, H.N.; Njabe, E.N.; Knudsen, K.E.; Hermansen, K. Concentrated arabinoxylan in wheat bread has beneficial effects as rye breads on glucose and changes in gene expressions in insulin-sensitive tissues of Zucker diabetic fatty (ZDF) rats. J. Agric. Food Chem. 2013, 61, 5054–5063. [Google Scholar] [CrossRef]
- Nielsen, K.L.; Hartvigsen, M.L.; Hedemann, M.S.; Laerke, H.N.; Hermansen, K.; Bach Knudsen, K.E. Similar metabolic responses in pigs and humans to breads with different contents and compositions of dietary fibers: A metabolomics study. Am. J. Clin. Nutr. 2014, 99, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Pluschke, A.M.; Williams, B.A.; Zhang, D.; Anderson, S.T.; Roura, E.; Gidley, M.J. Male grower pigs fed cereal soluble dietary fibres display biphasic glucose response and delayed glycaemic response after an oral glucose tolerance test. PLoS ONE 2018, 13, e0193137. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Christensen, E.G.; Licht, T.R.; Kristensen, M.; Bahl, M.I. Bifidogenic effect of whole-grain wheat during a 12-week energy-restricted dietary intervention in postmenopausal women. Eur. J. Clin. Nutr. 2013, 67, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Schioldan, A.G.; Gregersen, S.; Hald, S.; Bjornshave, A.; Bohl, M.; Hartmann, B.; Holst, J.J.; Stodkilde-Jorgensen, H.; Hermansen, K. Effects of a diet rich in arabinoxylan and resistant starch compared with a diet rich in refined carbohydrates on postprandial metabolism and features of the metabolic syndrome. Eur. J. Nutr. 2018, 57, 795–807. [Google Scholar] [CrossRef]
- Furness, J.B.; Rivera, L.R.; Cho, H.J.; Bravo, D.M.; Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Hu, J.; Gao, H.; Li, M.; Sun, Y.; Chen, H.; Zuo, S.; Fang, Q.; Huang, X.; Yin, J.; et al. Bioactive Dietary Fibers Selectively Promote Gut Microbiota to Exert Antidiabetic Effects. J. Agric. Food Chem. 2021, 69, 7000–7015. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, J.H.; Jiang, X.L.; Tang, J.N.; Zhu, C.L.; Chen, H.; Laghi, L. Metabolomic Characteristics of Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Different Fibers. Foods 2023, 12, 1403. [Google Scholar] [CrossRef]
- Salleh, S.N.; Fairus, A.A.H.; Zahary, M.N.; Bhaskar Raj, N.; Mhd Jalil, A.M. Unravelling the Effects of Soluble Dietary Fibre Supplementation on Energy Intake and Perceived Satiety in Healthy Adults: Evidence from Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Foods 2019, 8, 15. [Google Scholar] [CrossRef]
- Asakawa, A.; Inui, A.; Fujimiya, M.; Sakamaki, R.; Shinfuku, N.; Ueta, Y.; Meguid, M.M.; Kasuga, M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 2005, 54, 18–24. [Google Scholar] [CrossRef]
- Chen, C.Y.; Inui, A.; Asakawa, A.; Fujino, K.; Kato, I.; Chen, C.C.; Ueno, N.; Fujimiya, M. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology 2005, 129, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Möhling, M.; Koebnick, C.; Weickert, M.O.; Lueder, W.; Otto, B.; Steiniger, J.; Twilfert, M.; Meuser, F.; Pfeiffer, A.F.H.; Zunft, H.J. Arabinoxylan-enriched meal increases serum ghrelin levels in healthy humans. Horm. Metab. Res. 2005, 37, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Zhang, Z.; Riva, A.; Armet, A.M.; Perez-Munoz, M.E.; Nguyen, N.K.; Krysa, J.A.; Seethaler, B.; Zhao, Y.Y.; Cole, J.; et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome 2022, 10, 77. [Google Scholar] [CrossRef]
- Collins, S.M.; Gibson, G.R.; Stainton, G.N.; Bertocco, A.; Kennedy, O.B.; Walton, G.E.; Commane, D.M. Chronic consumption of a blend of inulin and arabinoxylan reduces energy intake in an ad libitum meal but does not influence perceptions of appetite and satiety: A randomised control-controlled crossover trial. Eur. J. Nutr. 2023, 62, 2205–2215. [Google Scholar] [CrossRef]

 , white wheat bread (WF);
, white wheat bread (WF);  , rye bread with kernels (RK);
, rye bread with kernels (RK);  , arabinoxylan bread (AX);
, arabinoxylan bread (AX);  , β-glucan bread (BG). Amount of total variation accounted for by the principal components is shown in brackets. Data from [64].
, β-glucan bread (BG). Amount of total variation accounted for by the principal components is shown in brackets. Data from [64].
 , white wheat bread (WF);
, white wheat bread (WF);  , rye bread with kernels (RK);
, rye bread with kernels (RK);  , arabinoxylan bread (AX);
, arabinoxylan bread (AX);  , β-glucan bread (BG). Amount of total variation accounted for by the principal components is shown in brackets. Data from [64].
, β-glucan bread (BG). Amount of total variation accounted for by the principal components is shown in brackets. Data from [64].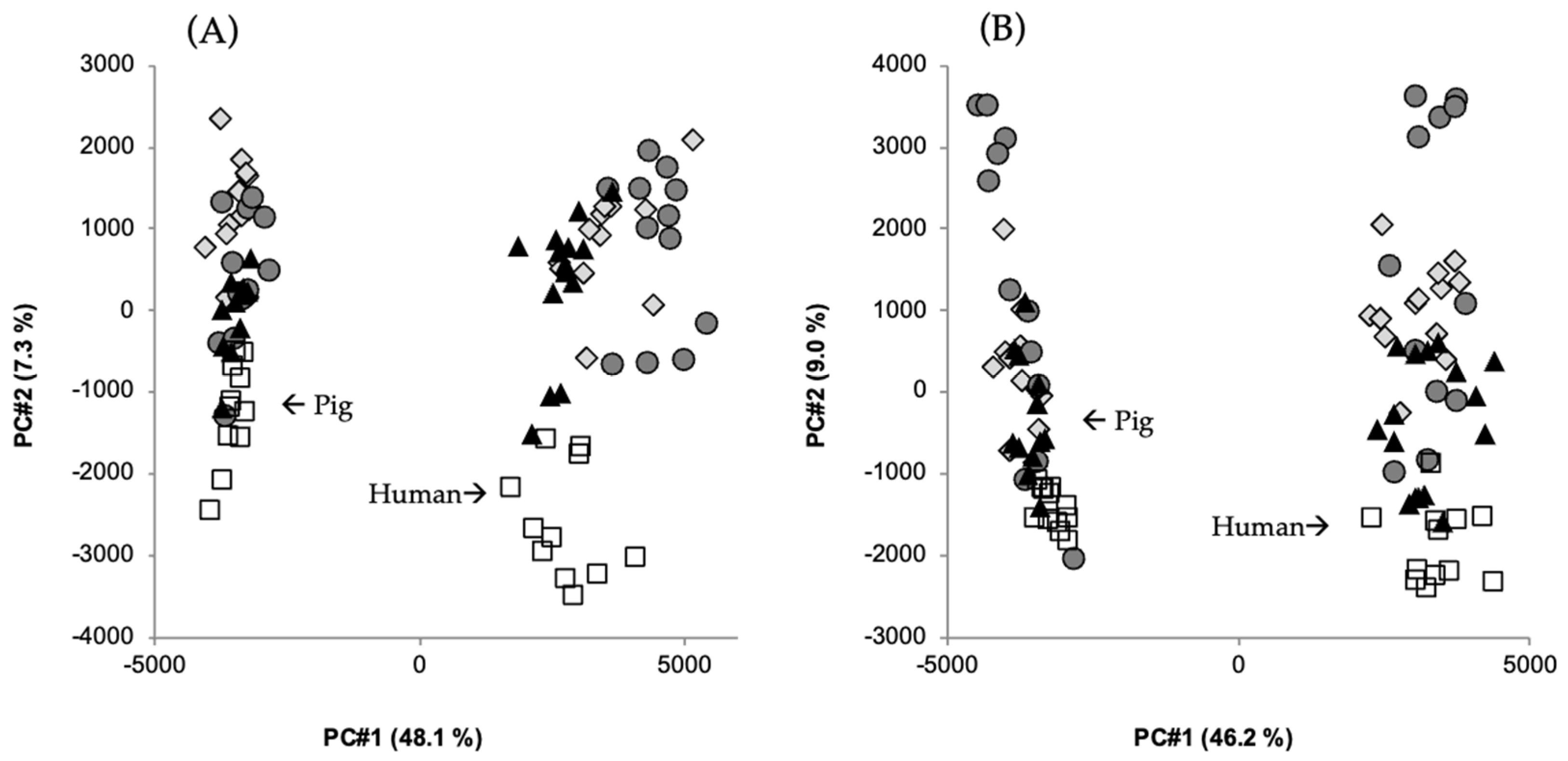
| Chemical Composition | WWB | WRB | WRBK | AXB | BGB |
|---|---|---|---|---|---|
| Dry matter (g/kg as is) | 634 | 520 | 543 | 708 | 615 |
| Total starch | 711 | 588 | 608 | 514 | 612 |
| Total sugars | 47 | 32 | 23 | 39 | 58 |
| Dietary fiber | |||||
| LMW non-digestible carbohydrates | 30 | 52 | 53 | 81 | 10 |
| Resistant starch | 4 | 9 | 14 | 7 | 18 |
| Total NSP (soluble NSP) | 35 (17) | 134 (53) | 139 (50) | 116 (86) | 163 (54) |
| Cellulose | 6 | 19 | 18 | 6 | 53 |
| β-Glucan (soluble β-glucan) | 3 (2) | 21 (7) | 19 (4) | 3 (2) | 52 (40) |
| AX (soluble AX) | 17 (13) | 76 (36) | 77 (37) | 78 (66) | 32 (9) |
| Total non-digestible carbohydrates a | 69 | 195 | 206 | 204 | 190 |
| Klason lignin | 8 | 14 | 14 | 8 | 9 |
| Total dietary fiber b | 77 | 209 | 220 | 212 | 199 |
| Diet | ||||||
|---|---|---|---|---|---|---|
| WWB | WRB | WRBK | AXB | BGB | SE | |
| Starch, % | 99 a | 98 b | 97 c | 99 a | 96 c | 0.2 |
| AX, % | 28 | 27 | 32 | 11 | 31 | 6.7 |
| NSP, % | 17 b,c | 27 a,b,c | 29 a,b | 13 c | 38 a | 5.4 |
| AX, g/kg DM | 108 c | 212 b | 190 b | 278 a | 95 c | 8.9 |
| NSP, g/kg DM | 256 d | 375 bc | 354 c | 416 a,b | 424 a | 13.6 |
| Viscosity (mean and 95% CI, mPa.S) | 5.9 (3.3–10.5) b | 8.4 (4.7–15.0) b | 7.4 (4.2–13.3) b | 15.5 (8.6–27.6) a | 2.6 (1.5–4.7) c | |
| Breads | |||||||
|---|---|---|---|---|---|---|---|
| WWB | WRB | WRBK | AXB | BGB | SEM | p-Value | |
| In vitro | |||||||
| k, % hydr./min | 0.1595 a | 0.1462 ab | 0.1048 ab | 0.1000 ab | 0.0744 b | 0.02 | 0.06 |
| Asymptote, % | 89.8 | 89.6 | 89.5 | 94.4 | 90.2 | 3.1 | 0.75 |
| In vivo | |||||||
| k, % absorption/min | 0.0227 a | 0.0195 a | 0.0210 a | 0.0191 a | 0.0167 a | 0.023 | 0.085 |
| Inflection point | 55.4 | 66.0 | 66.0 | 60.6 | 59.8 | 7.3 | 0.82 |
| Asymptote, % | 71.8 | 56.8 | 62.6 | 68.5 | 72.2 | 10.4 | 0.85 |
| kin vitro/kin vivo | 7.02 | 7.50 | 4.99 | 5.23 | 4.45 | ||
| iAUC | 100 | 70 | 83 | 74 | 89 | 12 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knudsen, K.E.B.; Lærke, H.N.; Hedemann, M.S.; Nielsen, K.L.; Kasprzak, M.M.; Jeppesen, P.B.; Hartvigsen, M.L.; Hermansen, K. Arabinoxylan Concentrate from Wheat as a Functional Food Ingredient to Improve Glucose Homeostasis. Nutrients 2025, 17, 1561. https://doi.org/10.3390/nu17091561
Knudsen KEB, Lærke HN, Hedemann MS, Nielsen KL, Kasprzak MM, Jeppesen PB, Hartvigsen ML, Hermansen K. Arabinoxylan Concentrate from Wheat as a Functional Food Ingredient to Improve Glucose Homeostasis. Nutrients. 2025; 17(9):1561. https://doi.org/10.3390/nu17091561
Chicago/Turabian StyleKnudsen, Knud Erik Bach, Helle Nygaard Lærke, Mette Skou Hedemann, Kirstine Lykke Nielsen, Mirosław Marek Kasprzak, Per Bendix Jeppesen, Merete Lindberg Hartvigsen, and Kjeld Hermansen. 2025. "Arabinoxylan Concentrate from Wheat as a Functional Food Ingredient to Improve Glucose Homeostasis" Nutrients 17, no. 9: 1561. https://doi.org/10.3390/nu17091561
APA StyleKnudsen, K. E. B., Lærke, H. N., Hedemann, M. S., Nielsen, K. L., Kasprzak, M. M., Jeppesen, P. B., Hartvigsen, M. L., & Hermansen, K. (2025). Arabinoxylan Concentrate from Wheat as a Functional Food Ingredient to Improve Glucose Homeostasis. Nutrients, 17(9), 1561. https://doi.org/10.3390/nu17091561







