Fatty Acid Ratios Versus Conventional Risk Factors in Stroke: Insights into Severe Disability and Mortality Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
- Patients aged ≥ 18 years old;
- Acute ischemic stroke confirmed by clinical evaluation and imaging;
- Admission within 72 h from symptom onset.
- Hemorrhagic strokes;
- Stroke mimics diagnosed after admission;
- Lack of consent for data collection and/or blood sampling.
2.2. Data Collection
2.3. Fatty Acid Analysis Process
2.4. Graphics
2.5. Statistical Analysis
3. Results
3.1. Demographic, Risk Factors, Disability, and Mortality—Population Analysis
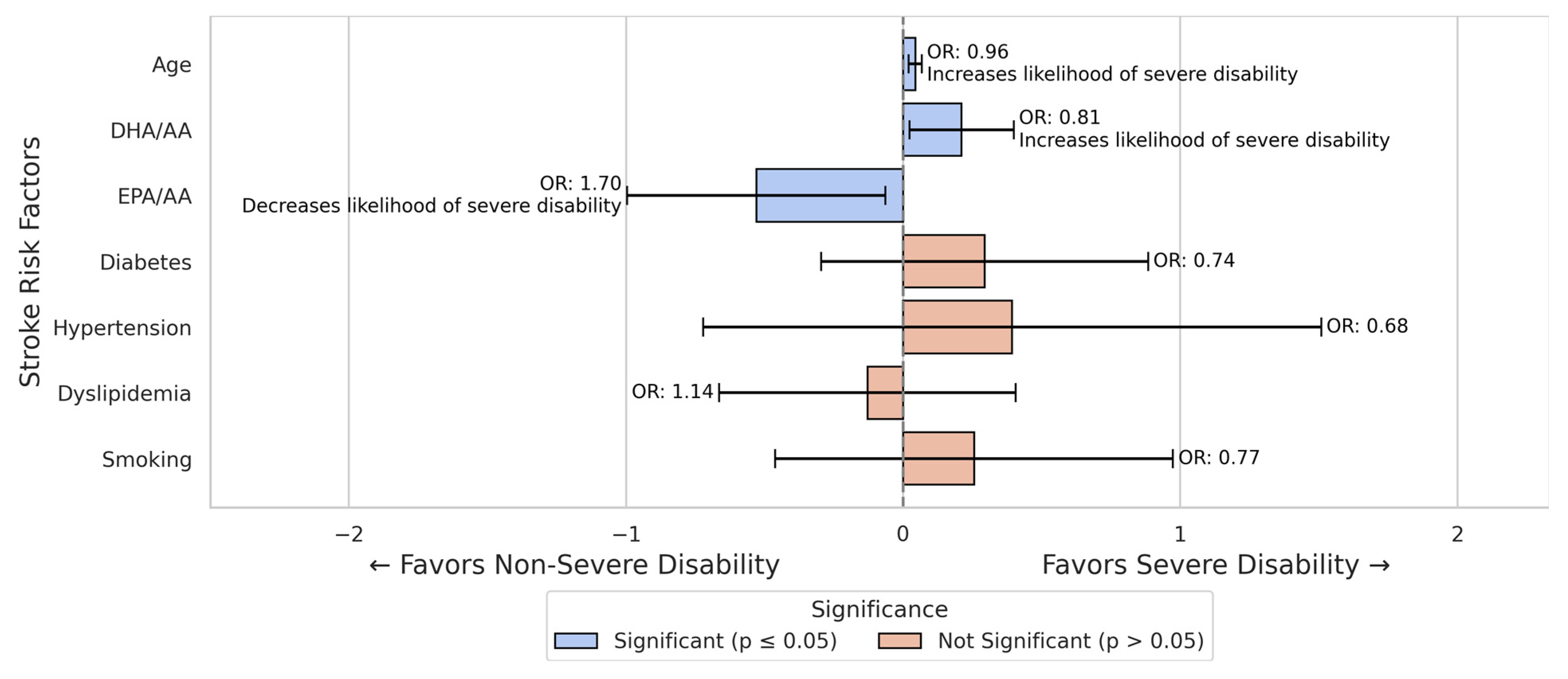
3.2. Stroke Severe Disability Prediction Models
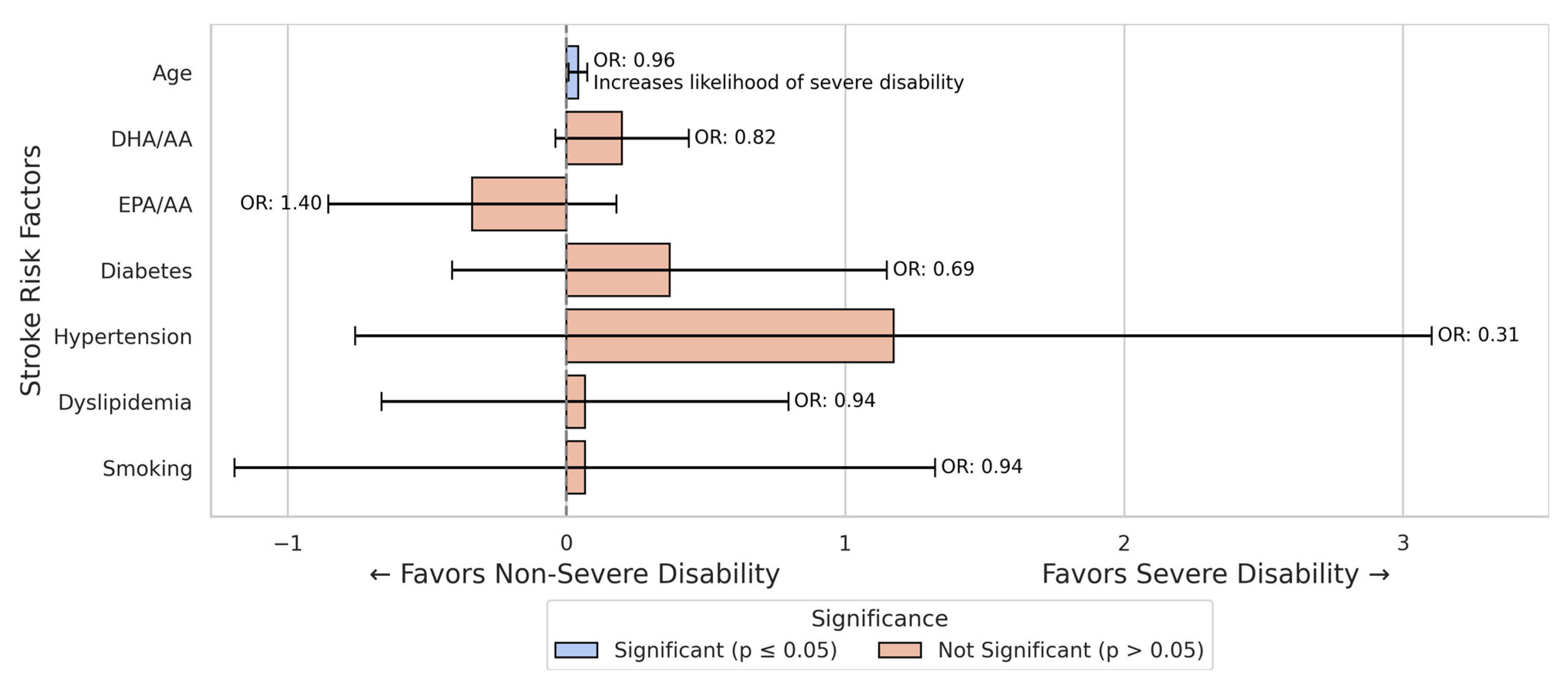
3.3. Stroke Mortality Prediction Models
4. Discussion
4.1. Sex-Specific Differences
4.2. Fatty Acids—Biological Mechanisms
4.3. Omega-3 PUFAs and Stroke: Clinical Evidence
4.4. Fatty Acids vs. Conventional Risk Factors
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Wafa, H.A.; Wolfe, C.D.A.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef]
- Ananth, C.V.; Brandt, J.S.; Keyes, K.M.; Graham, H.L.; Kostis, J.B.; Kostis, W.J. Epidemiology and Trends in Stroke Mortality in the USA, 1975–2019. Int. J. Epidemiol. 2023, 52, 858–866. Available online: https://academic.oup.com/ije/article/52/3/858/6809033 (accessed on 21 December 2024). [CrossRef]
- Soto, Á.; Guillén-Grima, F.; Morales, G.; Muñoz, S.; Aguinaga-Ontoso, I. Trends in Mortality from Stroke in the European Union, 1996–2015. Eur. J. Neurol. 2021, 28, 182–191. [Google Scholar] [CrossRef]
- Seminog, O.O.; Scarborough, P.; Wright, F.L.; Rayner, M.; Goldacre, M.J. Determinants of the Decline in Mortality from Acute Stroke in England: Linked National Database Study of 795,869 Adults. BMJ 2019, 365, l1778. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Zhou, G. Extended Risk Factors for Stroke Prevention. J. Natl. Med. Assoc. 2019, 111, 447–456. [Google Scholar] [CrossRef]
- Potter, T.B.H.; Tannous, J.; Vahidy, F.S. A Contemporary Review of Epidemiology, Risk Factors, Etiology, and Outcomes of Premature Stroke. Curr. Atheroscler. Rep. 2022, 24, 939–948. [Google Scholar] [CrossRef]
- Di Legge, S.; Koch, G.; Diomedi, M.; Stanzione, P.; Sallustio, F. Stroke Prevention: Managing Modifiable Risk Factors. Stroke Res. Treat 2012, 2012, 391538. [Google Scholar] [CrossRef]
- Juli, C.; Heryaman, H.; Arnengsih; Ang, E.-T.; Defi, I.R.; Gamayani, U.; Atik, N. The Number of Risk Factors Increases the Recurrence Events in Ischemic Stroke. Eur. J. Med. Res. 2022, 27, 138. [Google Scholar] [CrossRef]
- Cupino, A.; Fraser, G.; Knutsen, S.; Knutsen, R.; Heskey, C.; Sabaté, J.; Shavlik, D. Are Total Omega-3 and Omega-6 Polyunsaturated Fatty Acids Predictors of Fatal Stroke in the Adventist Health Study 2 Prospective Cohort? PLoS ONE 2022, 17, e0274109. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0274109 (accessed on 16 April 2025). [CrossRef] [PubMed]
- Song, T.-J.; Cho, H.-J.; Chang, Y.; Choi, K.; Jung, A.-R.; Youn, M.; Shin, M.-J.; Kim, Y.-J. Low Plasma Proportion of Omega 3-Polyunsaturated Fatty Acids Predicts Poor Outcome in Acute Non-Cardiogenic Ischemic Stroke Patients. J. Stroke 2015, 17, 168–176. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Neuroprotectin D1-Mediated Anti-Inflammatory and Survival Signaling in Stroke, Retinal Degenerations, and Alzheimer’s Disease. J. Lipid Res. 2009, 50, S400–S405. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Tanaka, K.; Farooqui, A.A.; Siddiqi, N.J.; Alhomida, A.S.; Ong, W.-Y. Effects of Docosahexaenoic Acid on Neurotransmission. Biomol. Ther. 2012, 20, 152–157. [Google Scholar] [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef]
- Kousparou, C.; Fyrilla, M.; Stephanou, A.; Patrikios, I. DHA/EPA (Omega-3) and LA/GLA (Omega-6) as Bioactive Molecules in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 10717. [Google Scholar] [CrossRef]
- Mori, T.; Yoshioka, K.; Tanno, Y.; Kasakura, S. Association of Serum Fatty Acids at Admission with the Age of Onset of Acute Ischemic Stroke. Nutrients 2020, 12, 2411. [Google Scholar] [CrossRef]
- Farczádi, L.; Dobreanu, M.; Huțanu, A.; Imre, S. Development and Validation of an LC-MS/MS Method for the Determination of Plasma and Red Blood Cell Omega Fatty Acids: A Useful Diagnostic Tool. Separations 2024, 11, 140. [Google Scholar] [CrossRef]
- Ali, M.; van Os, H.J.A.; van der Weerd, N.; Schoones, J.W.; Heymans, M.W.; Kruyt, N.D.; Visser, M.C.; Wermer, M.J.H. Sex Differences in Presentation of Stroke: A Systematic Review and Meta-Analysis. Stroke 2022, 53, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Barker-Collo, S.; Bennett, D.A.; Krishnamurthi, R.V.; Parmar, P.; Feigin, V.L.; Naghavi, M.; Forouzanfar, M.H.; Johnson, C.O.; Nguyen, G.; Mensah, G.A.; et al. Sex Differences in Stroke Incidence, Prevalence, Mortality and Disability-Adjusted Life Years: Results from the Global Burden of Disease Study 2013. Neuroepidemiology 2015, 45, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, A.K.; Schirmer, M.D.; Bretzner, M.; Hong, S.; Regenhardt, R.W.; Brudfors, M.; Donahue, K.L.; Nardin, M.J.; Dalca, A.V.; Giese, A.-K.; et al. Outcome after Acute Ischemic Stroke Is Linked to Sex-Specific Lesion Patterns. Nat. Commun. 2021, 12, 3289. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Shajahan, S.; Liu, X.; Sun, L.; Carcel, C.; Harris, K.; Anderson, C.S.; Woodward, M.; Wang, X. Sex Differences in the Utilization and Outcomes of Endovascular Treatment after Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Front. Glob. Womens Health 2023, 3, 1032592. [Google Scholar] [CrossRef]
- Phan, H.T.; Blizzard, C.L.; Reeves, M.J.; Thrift, A.G.; Cadilhac, D.A.; Sturm, J.; Heeley, E.; Otahal, P.; Vemmos, K.; Anderson, C.; et al. Factors Contributing to Sex Differences in Functional Outcomes and Participation after Stroke. Neurology 2018, 90, e1945–e1953. [Google Scholar] [CrossRef]
- Guo, X.; Xiong, Y.; Huang, X.; Pan, Z.; Kang, X.; Chen, C.; Zhou, J.; Zheng, H.; Chen, Y.; Hu, W.; et al. Sex-Based Differences in Long-Term Outcomes after Stroke: A Meta-Analysis. PLoS ONE 2023, 18, e0283204. [Google Scholar] [CrossRef]
- Ryu, W.-S.; Chung, J.; Schellingerhout, D.; Jeong, S.-W.; Kim, H.-R.; Park, J.E.; Kim, B.J.; Kim, J.-T.; Hong, K.-S.; Lee, K.; et al. Biological Mechanism of Sex Difference in Stroke Manifestation and Outcomes. Neurology 2023, 100, e2490–e2503. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, H.; Yang, X.; Luo, X.; Wang, X.; Shao, C.; He, J. Fish Consumption and Stroke Risk: A Meta-Analysis of Prospective Cohort Studies. J. Stroke Cerebrovasc. Dis. 2019, 28, 604–611. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wootton, S.A. Conversion of Alpha-Linolenic Acid to Eicosapentaenoic, Docosapentaenoic and Docosahexaenoic Acids in Young Women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.E.; Romeu-Nadal, M.; Burdge, G.C.; Calder, P.C. Gender Differences in the N-3 Fatty Acid Content of Tissues. Proc. Nutr. Soc. 2008, 67, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.J.; Bushnell, C.D.; Howard, G.; Gargano, J.W.; Duncan, P.W.; Lynch, G.; Khatiwoda, A.; Lisabeth, L. Sex Differences in Stroke: Epidemiology, Clinical Presentation, Medical Care, and Outcomes. Lancet Neurol. 2008, 7, 915–926. [Google Scholar] [CrossRef]
- McCullough, L.D.; Hurn, P.D. Estrogen and Ischemic Neuroprotection: An Integrated View. Trends Endocrinol. Metab. 2003, 14, 228–235. [Google Scholar] [CrossRef]
- Belayev, L.; Hong, S.-H.; Menghani, H.; Marcell, S.J.; Obenaus, A.; Freitas, R.S.; Khoutorova, L.; Balaszczuk, V.; Jun, B.; Oriá, R.B.; et al. Docosanoids Promote Neurogenesis and Angiogenesis, Blood-Brain Barrier Integrity, Penumbra Protection, and Neurobehavioral Recovery After Experimental Ischemic Stroke. Mol Neurobiol 2018, 55, 7090–7106. [Google Scholar] [CrossRef]
- Suda, S.; Katsumata, T.; Okubo, S.; Kanamaru, T.; Suzuki, K.; Watanabe, Y.; Katsura, K.; Katayama, Y. Low Serum N-3 Polyunsaturated Fatty Acid/n-6 Polyunsaturated Fatty Acid Ratio Predicts Neurological Deterioration in Japanese Patients with Acute Ischemic Stroke. Cerebrovasc. Dis. 2013, 36, 388–393. [Google Scholar] [CrossRef]
- Bu, J.; Dou, Y.; Tian, X.; Wang, Z.; Chen, G. The Role of Omega-3 Polyunsaturated Fatty Acids in Stroke. Oxid. Med. Cell. Longev. 2016, 2016, 6906712. [Google Scholar] [CrossRef]
- Bazan, N.G. Docosanoids and Elovanoids from Omega-3 Fatty Acids Are pro-Homeostatic Modulators of Inflammatory Responses, Cell Damage and Neuroprotection. Mol. Asp. Med. 2018, 64, 18–33. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-Chain Omega-3 Fatty Acids and the Brain: A Review of the Independent and Shared Effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Tintle, N.L.; Harris, W.S.; O’Keefe, E.L.; Sala-Vila, A.; Attia, J.; Garg, G.M.; Hure, A.; Bork, C.S.; Schmidt, E.B.; et al. Omega-3 Blood Levels and Stroke Risk: A Pooled and Harmonized Analysis of 183 291 Participants From 29 Prospective Studies. Stroke 2024, 55, 50–58. [Google Scholar] [CrossRef]
- Jiang, X.; Pu, H.; Hu, X.; Wei, Z.; Hong, D.; Zhang, W.; Gao, Y.; Chen, J.; Shi, Y. A Post-Stroke Therapeutic Regimen with Omega-3 Polyunsaturated Fatty Acids That Promotes White Matter Integrity and Beneficial Microglial Responses after Cerebral Ischemia. Transl. Stroke Res. 2016, 7, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Suenaga, J.; Pu, H.; Wei, Z.; Smith, A.D.; Hu, X.; Shi, Y.; Chen, J. Post-Stroke Administration of Omega-3 Polyunsaturated Fatty Acids Promotes Neurovascular Restoration after Ischemic Stroke in Mice: Efficacy Declines with Aging. Neurobiol. Dis. 2019, 126, 62–75. [Google Scholar] [CrossRef]
- Ueno, Y.; Miyamoto, N.; Yamashiro, K.; Tanaka, R.; Hattori, N. Omega-3 Polyunsaturated Fatty Acids and Stroke Burden. Int. J. Mol. Sci. 2019, 20, 5549. [Google Scholar] [CrossRef]
- Arboix, A.; Alió, J. Cardioembolic Stroke: Clinical Features, Specific Cardiac Disorders and Prognosis. Curr. Cardiol. Rev. 2010, 6, 150–161. [Google Scholar] [CrossRef]
- Andone, S.; Farczádi, L.; Imre, S.; Bajko, Z.; Moțățăianu, A.; Maier, S.; Bărcuțean, L.; Bălașa, R. Serum Fatty Acids Are Associated with a Higher Risk of Ischemic Stroke. Nutrients 2023, 15, 585. [Google Scholar] [CrossRef]
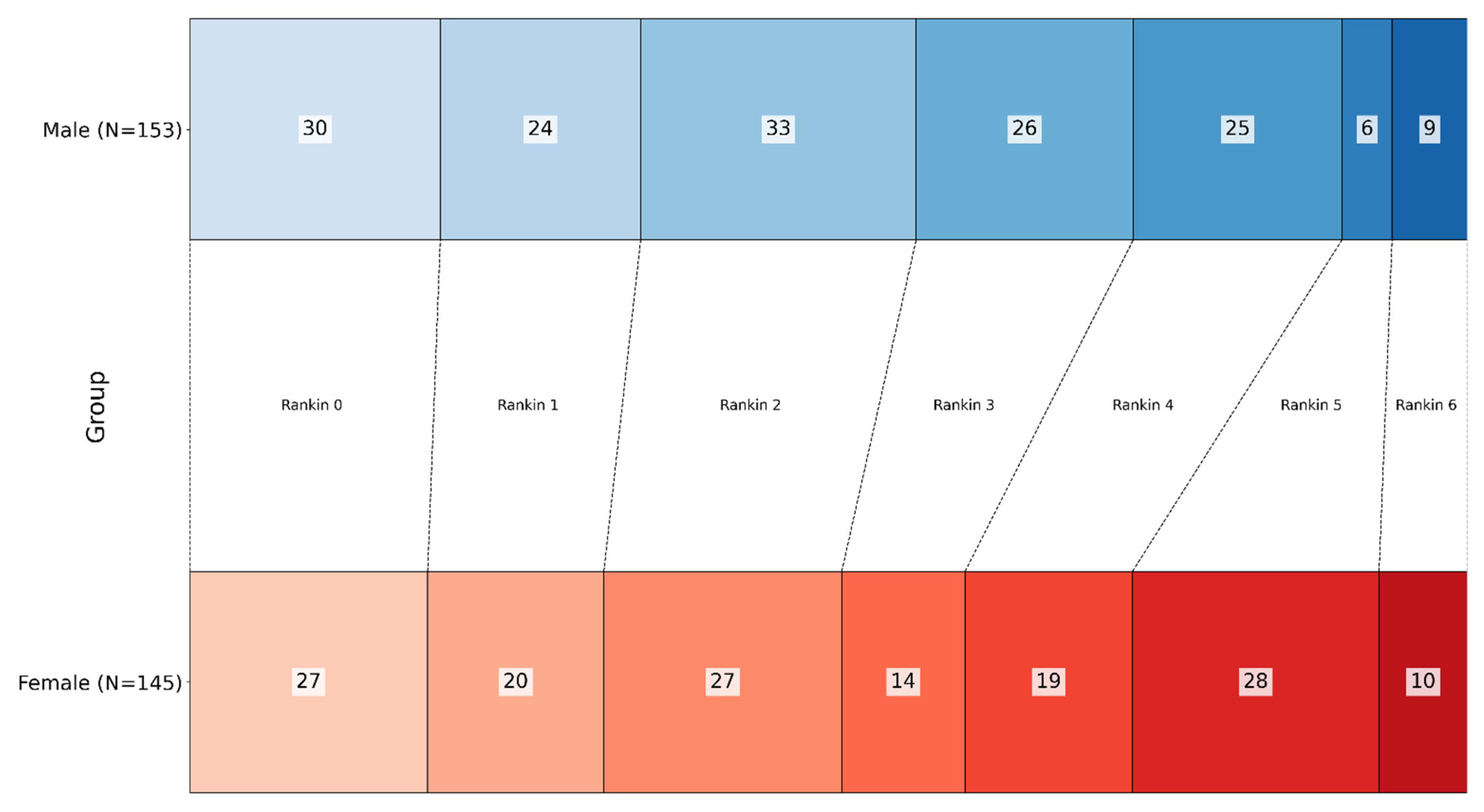


| n = 298 | Male (n = 153) | Female (n = 145) | |
|---|---|---|---|
| Age | 67.27 ± 14.14 (25–100) | 72.74 ± 12.11 (32–94) | p < 0.001 |
| Rankin at discharge | 2.30 ± 1.73 | 2.70 ± 1.70 | p = 0.061 |
| 0 | 30 | 27 | |
| 1 | 24 | 20 | |
| 2 | 33 | 27 | |
| 3 | 26 | 14 | |
| 4 | 25 | 19 | |
| 5 | 6 | 28 | |
| 6 (Dead) | 9 | 10 | |
| Disability | |||
| Low–moderate (Rankin 0–3) | 113 | 88 | p = 0.007 |
| Severe (Rankin 4–6) | 40 | 57 | |
| Mortality | |||
| Alive at discharge | 144 | 135 | p = 0.814 |
| Died during hospitalization | 9 | 10 | |
| Fatty acid ratios | |||
| DHA/AA | 4.96 ± 2.31 | 5.04 ± 2.13 | p = 0.179 |
| EPA/AA | 1.79 ± 1.13 | 1.75 ± 1.19 | p = 0.773 |
| Conventional risk factors | Yes/No | Yes/No | |
| Hypertension | 136/17 | 139/6 | p = 0.029 |
| Diabetes | 36/117 | 43/102 | p = 0.240 |
| Dyslipidemia | 77/76 | 90/55 | p = 0.047 |
| Smoking | 57/96 | 16/129 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andone, S.; Lénárd, F.; Imre, S.; Dumitreasa, M.; Bălașa, R. Fatty Acid Ratios Versus Conventional Risk Factors in Stroke: Insights into Severe Disability and Mortality Outcomes. Nutrients 2025, 17, 1518. https://doi.org/10.3390/nu17091518
Andone S, Lénárd F, Imre S, Dumitreasa M, Bălașa R. Fatty Acid Ratios Versus Conventional Risk Factors in Stroke: Insights into Severe Disability and Mortality Outcomes. Nutrients. 2025; 17(9):1518. https://doi.org/10.3390/nu17091518
Chicago/Turabian StyleAndone, Sebastian, Farczádi Lénárd, Silvia Imre, Mihai Dumitreasa, and Rodica Bălașa. 2025. "Fatty Acid Ratios Versus Conventional Risk Factors in Stroke: Insights into Severe Disability and Mortality Outcomes" Nutrients 17, no. 9: 1518. https://doi.org/10.3390/nu17091518
APA StyleAndone, S., Lénárd, F., Imre, S., Dumitreasa, M., & Bălașa, R. (2025). Fatty Acid Ratios Versus Conventional Risk Factors in Stroke: Insights into Severe Disability and Mortality Outcomes. Nutrients, 17(9), 1518. https://doi.org/10.3390/nu17091518







