Bioactive Components, Pharmacological Properties, and Applications of Cistanche deserticola Y. C. Ma: A Comprehensive Review
Highlights
- Identifies over 20 phenylethanol glycosides, polysaccharides, iridoids, and lignans in C. deserticola, possessing anti-inflammatory, antioxidant, and neuroprotective properties.
- Elucidates anti-fatigue mechanisms via the AMPK/energy metabolism pathway and immune-enhancing effects through the TLR4/NF-κB pathway.
- Highlights China’s 2020 approval of C. deserticola as a dual-use (medicinal–food) resource, facilitating functional food innovation and the establishment of standardized safety protocols.
- Proposes sustainable cultivation strategies and prioritizes clinical validation to bridge the gap between ethnopharmacology and modern therapeutics.
Abstract
1. Introduction
2. Methodology
3. Bioactive Constituents of C. deserticola
3.1. Phenylethanol Glycosides
3.2. Iridoid
3.3. Lignans
3.4. Cistanche Polysaccharides
| Solvent Extraction Methods | Monosaccharide Composition | Bioactivity | References |
|---|---|---|---|
| Ethanol soaking, grinding, water dissolution, enzyme treatment, dialysis, concentration, precipitation, washing, drying, and DEAE agarose gel column elution | Glucose and galactose | Promote the health of gut microbiota | [35] |
| Ultrasonic cellulase-assisted extraction, followed by ethanol precipitation. | Ribose, mannose, glucose, and galactose | Antioxidant | [32] |
| Hot water extraction, followed by ethanol precipitation and column chromatography for purification. | Glucose, galactose, rhamnose, arabinose, and fructose. | Antioxidant | [31] |
| Cold water extraction, the hexadecanol precipitation method, and ion exchange chromatography for separation, followed by gel filtration purification. | Arabinose, D-galactose, L-rhamnose, D-galacturonic acid, and trace amounts of D-xylose and D-glucose. | Immune regulatory function | [33] |
| Soxhlet extraction, air drying, distilled water extraction, concentration dialysis, ethanol precipitation, washing and drying, DEAE cellulose column elution, and Sephacryl S-300 purification. | CDA-1A (homogeneous chitosan) is composed of glucose. CDA-3B (homogeneous polysaccharide) is composed of rhamnose, arabinose, galactose, glucose, and galacturonic acid. | Immune regulatory function | [7] |
| Ethanol extraction, hot water extraction, filtration centrifugation, ethanol precipitation, protein removal, crude polysaccharide freeze-drying, DEAE column purification, and Sephadry S-300 purification, Sephadex G-25 desalination. | Arabinose, galactose, and glucose, as well as trace amounts of rhamnose and mannose. | Immune regulatory function | [34] |
4. The Main Pharmacological Functions
4.1. Antifatigue Effects of Bioactive Substances
4.2. The Immune Regulatory and Mechanisms of Bioactive Substances
4.3. Antioxidant Effects
4.4. Antitumor Effects
4.5. Anti-Inflammatory Effects
5. Medicinal and Dietary Applications of C. deserticola
5.1. Medicinal Applications of C. deserticola
5.1.1. Tonifying Kidney Yang
5.1.2. Nourishing Essence and Blood
5.1.3. Regulating Intestinal Function and Promoting Defecation
5.2. The Dietary Application of Bioactive Substances in C. deserticola
5.2.1. The Application of CPs in the Food Industry
5.2.2. Utilization as a Drug Stabilizer
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, W.; Jiang, S.; Zhao, Y.; Zhu, G. Echinacoside: A promising active natural products and pharmacological agents. Pharmacol. Res. 2023, 197, 106951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, Y.; Chen, J.; Yu, M.; Zhao, Q.; Jing, B.; Yang, N.; Ma, X.; Wang, Y. Chemical composition, pharmacological effects, and parasitic mechanisms of Cistanche deserticola: An update. Phytomedicine 2024, 132, 155808. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Yang, X.; Huang, L. Cistanches herba: A Neuropharmacology Review. Front. Pharmacol. 2016, 7, 289. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Xie, W. Cistanche deserticola Y. C. Ma, “Desert Ginseng”: A Review. Am. J. Chin. Med. 2012, 40, 1123–1141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Ma, R.-A.; Ji, F.-L.; Liu, Y.; Wang, B.; Fu, S.-Q.; Ma, L.-S.; Wang, S.; Liu, C.-X.; Guo, Z.; et al. Structure characterization of polysaccharides from Cistanche deserticola and their neuroprotective effects against oxidative stress in slow transit constipation mice. Int. J. Biol. Macromol. 2024, 260, 129527. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Gu, L. The Antidepressant and Cognitive Improvement Activities of the Traditional Chinese Herb Cistanche. Evid.-Based Complement. Altern. Med. 2017, 2017, 3925903. [Google Scholar] [CrossRef]
- Wang, L.; Jia, J.-X.; Zhang, S.-B.; Song, W.; Yan, X.-S.; Huo, D.-S.; Wang, H.; Wu, L.-E.; Yang, Z.-J. The protective effect and mechanism of glycosides of Cistanche deserticola on rats in middle cerebral artery occlusion (MCAO) model. J. Toxicol. Environ. Health Part A 2024, 87, 448–456. [Google Scholar] [CrossRef]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.; Tian, T.; Wei, X.; Chou, G. Phenylethanoid Glycosides from Caryopteris aureoglandulosa and Their Biological Activities. Chem. Biodivers. 2023, 20, e202201037. [Google Scholar] [CrossRef]
- Nyamsuren, E.; Odontuya, G. Chemical structures of phenylethanoid glycosides from Pedicularis species, their biological and pharmacological activities. Bull. Inst. Chem. Chem. Technol. Mong. Acad. Sci. 2019, 7, 54–60. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Flores-Félix, J.D.; Alves, G.; Silva, L.R. Cherries and Blueberries-Based Beverages: Functional Foods with Antidiabetic and Immune Booster Properties. Molecules 2022, 27, 3294. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, Y.; Jiang, Y.; Huang, L.; Liu, Q.; Ouyang, D. Eucommia lignans alleviate the progression of diabetic nephropathy through mediating the AR/Nrf2/HO-1/AMPK axis in vivo and in vitro. Chin. J. Nat. Med. 2023, 21, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Han, L.; Zhang, P.; Mao, H.; Zhang, H.; Wang, Y.; Gao, X.; Liu, E. Cistanche polysaccharides enhance echinacoside absorption in vivo and affect the gut microbiota. Int. J. Biol. Macromol. 2020, 149, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Lin, P.; He, Y.; Lu, D.; Li, Z.; Liang, Y.; Ma, Y.; Cairang, N.; Zuo, M.; Bao, Y.; et al. Echinacoside prevents hypoxic pulmonary hypertension by regulating the pulmonary artery function. J. Pharmacol. Sci. 2020, 144, 237–244. [Google Scholar] [CrossRef]
- Wang, C.; Li, F.; Li, Y.; Feng, H.; Zhao, M.-W.; Tu, P.-F.; Tian, H. Cistanche deserticola for regulation of bone metabolism: Therapeutic potential and molecular mechanisms on postmenopausal osteoporosis. Chin. J. Integr. Med. 2022, 29, 74–80. [Google Scholar] [CrossRef]
- Tureyen, A.; Demirel, H.H.; Demirkapi, E.N.; Eryavuz, A.M.; Ince, S.; Tubuloside, A. A phenylethanoid glycoside, alleviates diclofenac induced hepato-nephro oxidative injury via Nrf2/HO-1. J. Cell. Mol. Med. 2023, 27, 3404–3413. [Google Scholar] [CrossRef]
- Yao, J.; Wan, H.; Zhang, J.; Shen, W.; Wei, X.; Shi, C.; Ou, B.; Liu, D.; Ge, L.; Fei, J.; et al. Tubuloside B, a major constituent of Cistanche deserticola, inhibits migration of hepatocellular carcinoma by inhibiting Hippo-YAP pathway. Phytomedicine 2024, 129, 155552. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, W.; Xu, T.; Wu, L.; Chen, Q.; Peng, J.; Liu, X.; Lu, B. Natural P-gp inhibitor EGCG improves the acteoside absorption in Caco-2 cell monolayers and increases the oral bioavailability of acteoside in rats. Food Chem. Toxicol. 2020, 146, 111827. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.-H.; Song, Z.; Li, M.-L.; Wu, X.-W.; Guo, M.-X.; Zhang, X.-H.; Zou, X.-P. Isoacteoside attenuates acute kidney injury induced by severe acute pancreatitis. Mol. Med. Rep. 2021, 23, 287. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Zhao, X.; Zhang, B.; Yang, L.; Liu, J.; Snooks, H.; Hu, C.; Ma, X. Beneficial effect of 2’-acetylacteoside on ovariectomized mice via modulating the function of bone resorption. Biomed. Pharmacother. 2020, 131, 110747. [Google Scholar] [CrossRef]
- Li, L.; Tsao, R.; Yang, R.; Liu, C.; Young, J.C.; Zhu, H. Isolation and purification of phenylethanoid glycosides from Cistanche deserticola by high-speed counter-current chromatography. Food Chem. 2008, 108, 702–710. [Google Scholar] [CrossRef]
- Le, D.D.; Han, S.; Yu, J.; Ahn, J.; Kim, C.-K.; Lee, M. Iridoid derivatives from Vitex rotundifolia L. f. with their anti-inflammatory activity. Phytochemistry 2023, 210, 113649. [Google Scholar] [CrossRef]
- Nan, Z.; Zhao, M.; Zeng, K.; Tian, S.; Wang, W.; Jiang, Y.; Tu, P. Anti-inflammatory iridoids from the stems of Cistanche deserticola cultured in Tarim Desert. Chin. J. Nat. Med. 2016, 141, 61–65. [Google Scholar]
- Liu, M.; Zhu, D.; Jin, F.; Li, S.; Liu, X.; Wang, X. Peptide modified geniposidic acid targets bone and effectively promotes osteogenesis. J. Orthop. Transl. 2022, 38, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Huong, T.T.; Tram, L.H.; Van Thong, N.; Minh, N.H.; Minh, T.T.; Quang, T.T.; Hung, N.D.; Van Don, D.; Nguyen, T.M.N.; Park, J. Furofuran lignans from Valeriana jatamansi with their antioxidant and anticancer properties. Vietnam. J. Chem. 2022, 60, 157–163. [Google Scholar] [CrossRef]
- Piao, X.; Feng, M.; Zhao, W.; Wu, Z.; Zhang, W.; Hou, H.; Wang, J.; Wang, L.; Huang, J.; Zhang, Y. Dendrocandin U from Dendrobium officinale Kimura et Migo Inhibits M1 Polarization in Alveolar Macrophage by Suppressing NF-κB Signaling Pathway. Chem. Biodivers. 2023, 20, e202300999. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, X.; Li, X.; Yang, J.; Liu, W.; Yang, L.; Liu, B. Study on the protective effect and mechanism of Liriodendrin on radiation enteritis in mice. J. Radiat. Res. 2022, 63, 213–220. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Wang, B.; Zhang, L.; Zhang, Q.; Li, D.; Zhang, S.; Gao, H.; Wang, X. Protective role of liriodendrin in mice with dextran sulphate sodium-induced ulcerative colitis. Int. Immunopharmacol. 2017, 52, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Nan, Z.-D.; Zhao, M.-B.; Jiang, Y.; Tu, P.-F. Lignans from stems of Cistanche deserticola cultured in Tarim desert. China J. Chin. Mater. Medica 2015, 40, 463–468. [Google Scholar]
- Trang, D.T.H.; Viet, P.H.; Anh, D.H.; Tai, B.H.; Anh, N.Q.; Nhiem, N.X.; Van Kiem, P. Lignans and other compounds from the roots of Pandanus tonkinensis and their lipid peroxidation inhibitory activity. Nat. Prod. Commun. 2022, 17, 1934578X221088372. [Google Scholar] [CrossRef]
- Xue, T.; Zheng, D.; Hou, Q.; Wen, L.; Wang, B.; Geng, R.; Wang, Q.; Dai, W.; Tian, L.; He, S.; et al. Optimization of Extraction Process, Structural Characterization, and Antioxidant and Hypoglycemic Activity Evaluation of Polysaccharides from the Medicinal and Edible Plant: Cistanche deserticola Ma. Phytochem. Anal. 2025, 0, 1–18. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Wang, W.; Li, Q.; Chen, Y.; Feng, W.; Zheng, D.; Zhao, T.; Mao, G.; Yang, L.; et al. Extraction, purification, characterization and antioxidant activities of polysaccharides from Cistanche tubulosa. Int. J. Biol. Macromol. 2016, 93, 448–458. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, X.; Li, Q.; Yang, Y.; Zhao, G.; Wang, B.; Wu, D. Immunostimulatory activity of water-extractable polysaccharides from Cistanche deserticola as a plant adjuvant in vitro and in vivo. PLoS ONE 2018, 13, e0191356. [Google Scholar] [CrossRef]
- Wu, X.M.; Gao, X.M.; Tsim, K.W.; Tu, P.F. An arabinogalactan isolated from the stems of Cistanche deserticola induces the proliferation of cultured lymphocytes. Int. J. Biol. Macromol. 2005, 37, 278–282. [Google Scholar] [CrossRef]
- Zeng, H.; Huang, L.; Zhou, L.; Wang, P.; Chen, X.; Ding, K. A galactoglucan isolated from of Cistanche deserticola Y. C. Ma. and its bioactivity on intestinal bacteria strains. Carbohydr. Polym. 2019, 223, 115038. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Shen, X.; Liu, Y. The use of traditional Chinese medicines in relieving exercise-induced fatigue. Front. Pharmacol. 2022, 13, 969827. [Google Scholar] [CrossRef]
- Luo, C.; Xu, X.; Wei, X.; Feng, W.; Huang, H.; Liu, H.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 2019, 148, 104409. [Google Scholar] [CrossRef]
- Dong, J.; Li, J.; Liu, Y.; Cui, L.; Liu, X.; Wang, G.; Wang, Q.; Criddle, D.N.; Tu, P.; Li, C. A comparative study of the anti-fatigue activity of extracts from different parts of Cistanche tubulosa (Schenk) Wight. J. Tradit. Chin. Med. Sci. 2024, 11, 222–231. [Google Scholar] [CrossRef]
- Lan, T.; Yu, Q. Cistanches deserticola PhG-RE through inhibiting ERS apoptosis mechanism to protect myocardial cell apoptosis from H2O2-Induced endoplasmic reticulum stress. Evid.-Based Complement. Altern. Med. 2020, 2020, 8219296. [Google Scholar] [CrossRef]
- Takaya, K.; Asou, T.; Kishi, K. Cistanche deserticola Polysaccharide Reduces Inflammation and Aging Phenotypes in the Dermal Fibroblasts through the Activation of the NRF2/HO-1 Pathway. Int. J. Mol. Sci. 2023, 24, 15704. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, L.; Hu, K.; Yang, Y.; Ma, J.; Zhai, Y.; Jiang, Y.; Zhang, D. Anti-Fatigue Effects of Lycium barbarum Polysaccharide and Effervescent Tablets by Regulating Oxidative Stress and Energy Metabolism in Rats. Int. J. Mol. Sci. 2022, 23, 10920. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, F.; Liu, J.; You, S.; Liu, T.; Yang, J.; Hu, J. Effects of acteoside from Cistanche tubulosa on the plasma metabolome of cancer-related fatigue mice inoculated with colon cancer cells. Front. Pharmacol. 2025, 15, 1370264. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, D.; Zhou, Y.; Duan, H.; Jiang, Y.; Yan, W. Analysis of the active ingredients and health applications of cistanche. Front. Nutr. 2023, 10, 1101182. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Li, X.; Mao, T.; Liu, T. Anti-fatigue activity of gardenia yellow pigment and Cistanche phenylethanol glycosides mixture in hypoxia. Food Biosci. 2021, 40, 100902. [Google Scholar] [CrossRef]
- Jia, X.; Liu, B.; Xue, J.; Liu, Y.; Zhang, J.; Qin, S.; Zhang, Y. Phenylethanoid glycosides extract from Cistanche deserticola ameliorates atherosclerosis in apolipoprotein E-deficient mice and regulates intestinal PPARγ-LXRα-ABCA1 pathway. J. Pharm. Pharmacol. 2023, 75, 677–685. [Google Scholar] [CrossRef]
- Wang, F.; Li, R.; Tu, P.; Chen, J.; Zeng, K.; Jiang, Y. Total glycosides of Cistanche deserticola promote neurological function recovery by inducing neurovascular regeneration via Nrf-2/Keap-1 pathway in MCAO/R rats. Front. Pharmacol. 2020, 11, 236. [Google Scholar] [CrossRef]
- Feng, S.; Yang, X.; Weng, X.; Wang, B.; Zhang, A. Aqueous extracts from cultivated Cistanche deserticola Y.C. Ma as polysaccharide adjuvant promote immune responses via facilitating dendritic cell activation. J. Ethnopharmacol. 2021, 277, 114256. [Google Scholar] [CrossRef]
- Li, Q.; Ba, X.; Cao, H.; Weng, X.; Yang, Y.; Wang, B.; Zhang, A. Crude polysaccharides from Cistanche deserticola Y.C. Ma as an immunoregulator and an adjuvant for foot-and-mouth disease vaccine. J. Funct. Foods 2021, 87, 104800. [Google Scholar] [CrossRef]
- Weng, X.; Zhao, B.; Feng, S.; Yang, Y.; Zhang, A. Chemical composition and adjuvant properties of the macromolecules from cultivated Cistanche deserticola Y. C. Ma as an immunopotentiator. Int. J. Biol. Macromol. 2022, 220, 638–658. [Google Scholar] [CrossRef]
- Chen, W.; Lin, H.R.; Wei, C.M.; Luo, X.H.; Sun, M.L.; Yang, Z.Z.; Chen, X.Y.; Wang, H.B. Echinacoside, a phenylethanoid glycoside from Cistanche deserticola, extends lifespan of Caenorhabditis elegans and protects from Aβ-induced toxicity. Biogerontology 2018, 19, 47–65. [Google Scholar] [CrossRef]
- Hemmami, H.; Ben Seghir, B.; Zeghoud, S.; Ben Amor, I.; Kouadri, I.; Rebiai, A.; Zaater, A.; Messaoudi, M.; Benchikha, N.; Sawicka, B.; et al. Desert Endemic Plants in Algeria: A Review on Traditional Uses, Phytochemistry, Polyphenolic Compounds and Pharmacological Activities. Molecules 2023, 28, 1834. [Google Scholar] [CrossRef]
- Morikawa, T.; Xie, H.; Pan, Y.; Ninomiya, K.; Yuan, D.; Jia, X.; Yoshikawa, M.; Nakamura, S.; Matsuda, H.; Muraoka, O. A Review of Biologically Active Natural Products from a Desert Plant Cistanche tubulosa. Chem. Pharm. Bull. 2019, 67, 675–689. [Google Scholar] [CrossRef]
- Delicato, A.; Masi, M.; de Lara, F.; Rubiales, D.; Paolillo, I.; Lucci, V.; Falco, G.; Calabrò, V.; Evidente, A. In vitro characterization of iridoid and phenylethanoid glycosides from Cistanche phelypaea for nutraceutical and pharmacological applications. Phytother. Res. 2022, 36, 4155–4166. [Google Scholar] [CrossRef]
- Zhang, H.; Xiang, Z.; Duan, X.; Jiang, J.L.; Xing, Y.M.; Zhu, C.; Song, Q.; Yu, Q.R. Antitumor and anti-inflammatory effects of oligosaccharides from Cistanche deserticola extract on spinal cord injury. Int. J. Biol. Macromol. 2019, 124, 360–367. [Google Scholar] [CrossRef]
- Muhtar, E.; Ylham, G.; Tiemuer, A.; Edirs, S. Unraveling the Dual Anti-Inflammatory and Antioxidant Mechanisms of Acteoside: Computational Insights and Experimental Validation. Chem. Biodivers. 2024, 22, e202401564. [Google Scholar] [CrossRef]
- He, H.; Osire, T.; Zhang, X.; Zheng, Q.; Ramonova, A.; Arkhipova, A. Antioxidant Activity of Aqueous Extracts from Eucommia ulmoides and Cistanche deserticola: An In Vitro Study. Microsc. Microanal. 2023, 29, 1111–1112. [Google Scholar] [CrossRef]
- Thida, M.; Li, B.; Zhang, X.; Chen, C.; Zhang, X. Echinacoside alleviates acetaminophen-induced liver injury by attenuating oxidative stress and inflammatory cytokines in mice. J. Appl. Biomed. 2021, 19, 105–112. [Google Scholar] [CrossRef]
- Wei, W.; Lan, X.-B.; Liu, N.; Yang, J.-M.; Du, J.; Ma, L.; Zhang, W.-J.; Niu, J.-G.; Sun, T.; Yu, J.-Q. Echinacoside alleviates hypoxic-ischemic brain injury in neonatal rat by enhancing antioxidant capacity and inhibiting apoptosis. Neurochem. Res. 2019, 44, 1582–1592. [Google Scholar] [CrossRef]
- Peng, F.; Chen, J.; Wang, X.; Xu, C.; Liu, T.; Xu, R. Changes in levels of phenylethanoid glycosides, antioxidant activity, and other quality traits in Cistanche deserticola slices by steam processing. Chem. Pharm. Bull. 2016, 64, 1024–1030. [Google Scholar] [CrossRef]
- Du, B.; Xu, B. Natural Bioactive Compounds Exerting Health-Promoting Effects by Ameliorating Oxidative Stress. Antioxidants 2025, 14, 85. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Razis, A.F.A.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Chaudhary, N.; Roy, Z.; Bansal, R.; Siddiqui, L. Understanding the Role of Free Radicals and Antioxidant Enzymes in Human Diseases. Curr. Pharm. Biotechnol. 2023, 24, 1265–1276. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, F.; Zhang, X.; Niu, Y.; Liu, X.; Bu, R.; Ma, Y.; Wu, G.; Li, B.; Yang, H.; et al. Cistanche phenylethanoid glycosides induce apoptosis and pyroptosis in T-cell lymphoma. Am. J. Cancer Res. 2024, 14, 1338–1352. [Google Scholar] [CrossRef]
- Fan, Q.-L.; Wang, J.-W.; Zhang, S.-L.; Liu, T.; Zhao, J.; You, S.-P. Phenylethanol glycosides protect myocardial hypertrophy induced by abdominal aortic constriction via ECE-1 demethylation inhibition and PI3K/PKB/eNOS pathway enhancement. Evid.-Based Complement. Altern. Med. 2020, 2020, 2957094. [Google Scholar] [CrossRef]
- Feng, D.; Zhou, S.-Q.; Zhou, Y.-X.; Jiang, Y.-J.; Sun, Q.-D.; Song, W.; Cui, Q.-Q.; Yan, W.-J.; Wang, J. Effect of total glycosides of Cistanche deserticola on the energy metabolism of human HepG2 cells. Front. Nutr. 2023, 10, 1117364. [Google Scholar] [CrossRef]
- Wen, L.; Hu, J.; Zhang, J.; Yang, J. Phenylethanol glycosides from Herba Cistanche improve the hypoxic tumor microenvironment and enhance the effects of oxaliplatin via the HIF-1α signaling pathway. Mol. Med. Rep. 2021, 24, 517. [Google Scholar] [CrossRef]
- Ong, W.-Y.; Herr, D.R.; Sun, G.Y.; Lin, T.-N. Anti-inflammatory effects of phytochemical components of Clinacanthus nutans. Molecules 2022, 27, 3607. [Google Scholar] [CrossRef]
- Bai, R.; Fan, J.; Wang, Y.; Wang, Y.; Li, X.; Hu, F. Protective effect of Cistanche deserticola on gentamicin-induced nephrotoxicity in rats. Chin. Herb. Med. 2022, 15, 102–109. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Li, Y.; Guo, L.; Xu, P.; Du, H.; Lin, N.; Xu, Y. The role of Cistanches Herba and its ingredients in improving reproductive outcomes: A comprehensive review. Phytomedicine 2024, 129, 155681. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, J.; Li, Y.; Jiang, L.; Ouyang, Y.; Li, Y.; Yang, L.; Zhao, X.; Huang, L.; Xiang, H.; et al. Cistanche deserticola polysaccharide induces melanogenesis in melanocytes and reduces oxidative stress via activating NRF2/HO-1 pathway. J. Cell. Mol. Med. 2020, 24, 4023–4035. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, Z.; Shi, X.; Wang, T.; Huang, M.; Chen, G.; Ye, X.; Hou, C.; Liu, W.; Dong, W.; et al. Comparison between tonifying kidney yang and yin in treating segmental bone defects based on the induced membrane technique: An experimental study in a rat model. Evid.-Based Complement. Altern. Med. 2020, 2020, 6575127. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Yang, S.; Xiao, Y.; Guan, H.; Yue, X.; Wang, X.; Li, X. The Difference of chemical components and biological activities of the raw products slices and the wine steam-processed product from Cistanche deserticola. Evid.-Based Complement. Altern. Med. 2019, 2019, 2167947. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Li, Q.; Zhao, C.; He, P.; Ma, X. Enhancement of kidney invigorating function in mouse model by Cistanches herba dried rapidly at a medium high temperature. J. Med. Food 2019, 22, 1246–1253. [Google Scholar] [CrossRef]
- Li, Z.; Ryenchindorj, L.; Liu, B.; Shi, J.; Zhang, C.; Hua, Y.; Liu, P.; Shan, G.; Jia, T. Chemical profiles and metabolite study of raw and processed Cistanche deserticola in rats by UPLC-Q-TOF-MSE. Chin. Med. 2021, 16, 95. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, F.-J.; Zhang, Z. Therapeutic effect of Cistanche deserticola on defecation in senile constipation rat model through stem cell factor/C-kit signaling pathway. World J. Gastroenterol. 2021, 27, 5392–5403. [Google Scholar] [CrossRef]
- Lu, X.; Chen, J.; Guo, Z.; Zheng, Y.; Rea, M.C.; Su, H.; Zheng, X.; Zheng, B.; Miao, S. Using polysaccharides for the enhancement of functionality of foods: A review. Trends Food Sci. Technol. 2019, 86, 311–327. [Google Scholar] [CrossRef]
- Hou, S.; Tan, M.; Chang, S.; Zhu, Y.; Rong, G.; Wei, G.; Zhang, J.; Zhao, B.; Zhao, Q.-S. Effects of different processing (Paozhi) on structural characterization and antioxidant activities of polysaccharides from Cistanche deserticola. Int. J. Biol. Macromol. 2023, 245, 125507. [Google Scholar] [CrossRef]
- Deore, U.V.; Mahajan, H.S. Isolation and characterization of natural polysaccharide from Cassia Obtustifolia seed mucilage as film forming material for drug delivery. Int. J. Biol. Macromol. 2018, 115, 1071–1078. [Google Scholar] [CrossRef]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Yu, X.; Wang, X.; Zheng, Y.; Hu, X.; Zhang, P.; Sun, Q.; Wang, Q.; Li, N. Effect of polysaccharide addition on food physical properties: A review. Food Chem. 2023, 431, 137099. [Google Scholar] [CrossRef]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Balan, M.; Bernic, M.; Ţislinscaia, N. Drying installation for granular products in the suspension layer. J. Eng. Sci. 2020, 1, 64–68. [Google Scholar]
- Wang, X.; Wang, J.; Guan, H.; Xu, R.; Luo, X.; Su, M.; Chang, X.; Tan, W.; Chen, J.; Shi, Y. Comparison of the Chemical Profiles and Antioxidant Activities of Different Parts of Cultivated Cistanche deserticola Using Ultra Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry and a 1,1-Diphenyl-2-picrylhydrazyl-Based Assay. Molecules 2017, 22, 2011. [Google Scholar] [CrossRef]
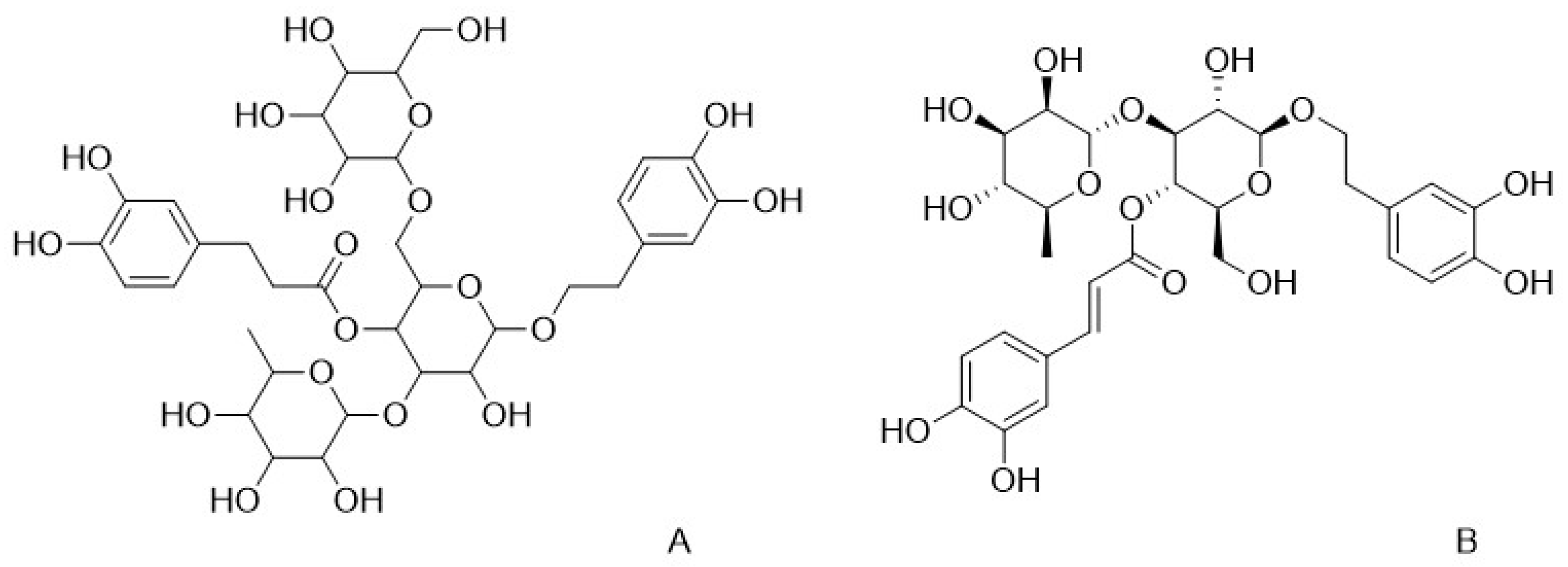

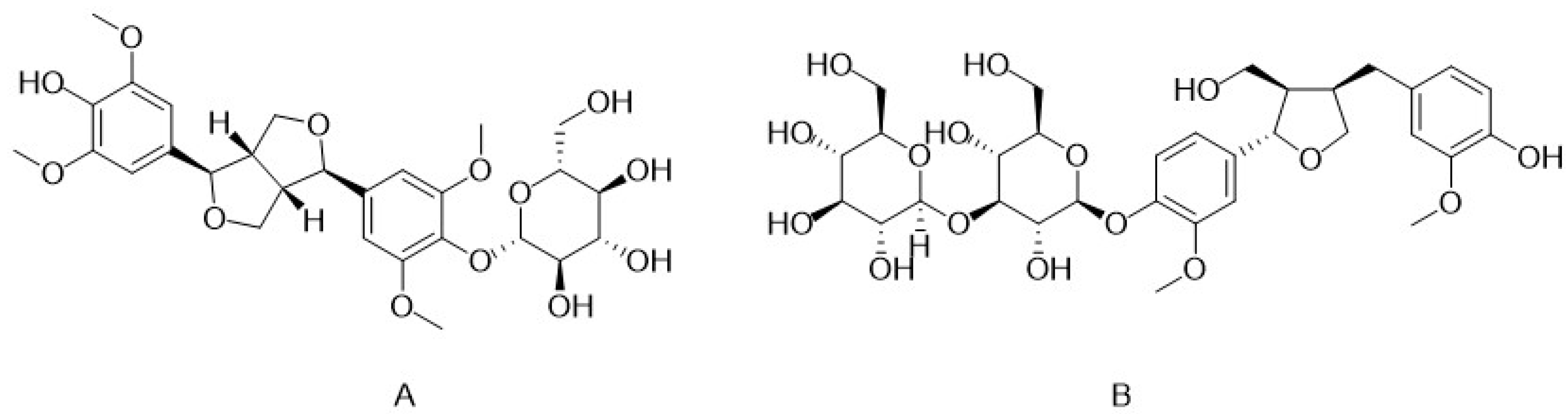
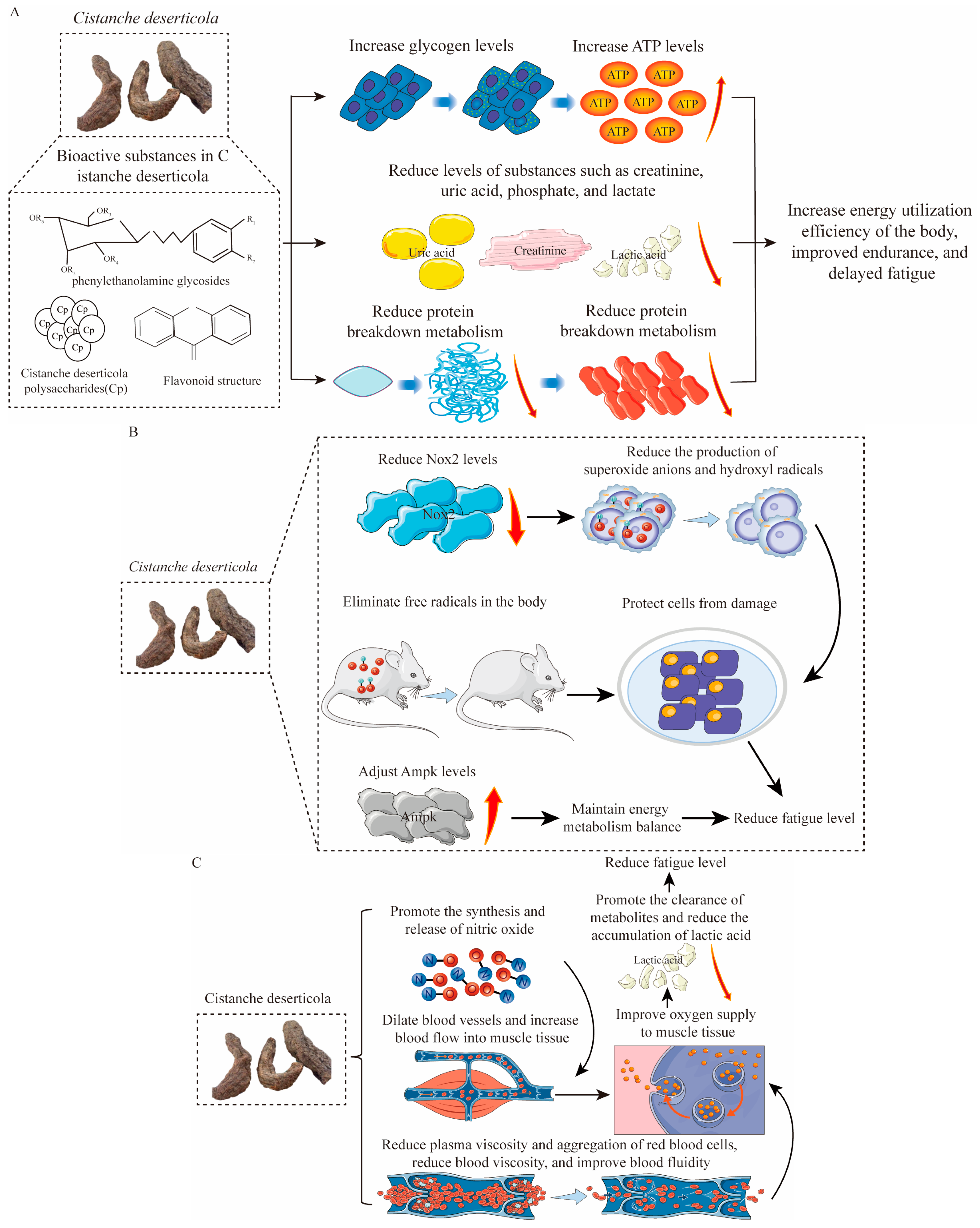
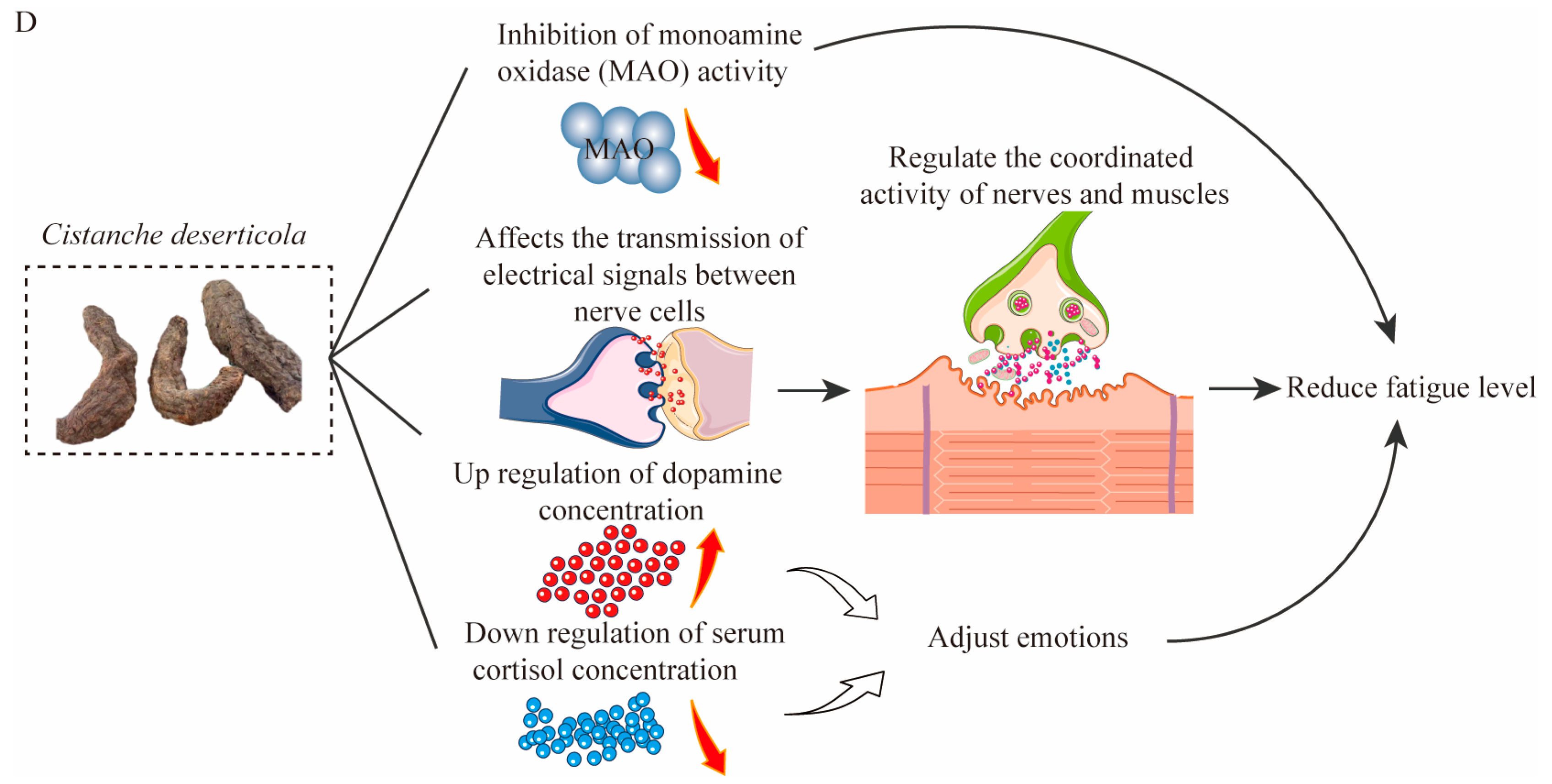

| Compounds | Active Action | References |
|---|---|---|
| Phenylethanol glycosides | Anti-inflammatory, antioxidant, regulates osteoblast differentiation and mineralization, anti-hypoxic myocardial injury, improves renal injury, anti-osteoclastogenesis, anti-ischemia, hepatoprotective, anti-viral, improves glucose tolerance, regulates blood glucose, neuroprotective, inhibits postprandial glucose elevation, improves glucose tolerance, inhibits vasodilatation, suppresses appetite, antidiabetic, anticancer, antidiabetic vascular complication | [8,9,10] |
| Iridoid | Anti-inflammatory, antioxidant, antibacterial, antithrombotic, antidepressant, antidiabetic, anti-kidney injury, hepatoprotective, estrogenomimetic, inhibits postprandial glucose elevation, neuroprotective | [11] |
| Lignans | Antivasorelaxant, hepatoprotective, anti-inflammatory, antimicrobial, anti-kidney injury, neuroprotective, antioxidant, inhibit postprandial glucose elevation | [2,12] |
| Cistanche polysaccharides | Anti-fatigue, laxative, energizing, anti-viral, anti-inflammatory | [13] |
| Compounds | Active Action | References |
|---|---|---|
| Echinacoside | Anti-cerebral ischemia, liver protection, and anti-inflammatory | [14,15] |
| Acteoside | Hypoxia-resistant myocardial injury and anti-vascular dementia | [18] |
| Tubuloside A | Anti-aging and liver protection | [16] |
| Tubuloside B | Protect the nerves and protect the liver | [17] |
| 2′-Acetylacteoside | Antibone resorption cell generation | [20] |
| Isoacteoside | Improve kidney injury | [19] |
| Compounds | Bioactivity | References |
|---|---|---|
| (+)-Pinoresinol | Vasodilator | [29] |
| (+)-Pinoresinol-O-β-D-glucopyranoside | Liver protection | [25] |
| (+)-Syringaresinol-O-β-D-glucopyranoside | Liver protection | [26] |
| Liriodendrin | Anti-inflammatory and liver protection | [27,28] |
| Eucommin A | Antibacterial | [30] |
| Application Area | Application Methods | Mechanism of Action | Application Prospect | References |
|---|---|---|---|---|
| Food industry | Thickener, coagulant, stabilizer | Adjust viscosity, improve product stability, and improve taste | Can be widely used in fields such as pastries, candies, and health foods to meet consumer needs | [76,77] |
| Medical field | Capsules, tablets, injections | Adjust microstructure and enhance drug stability | Can be used for the development of various drug formulations, improving efficacy and safety | [78] |
| Functional foods | Extracts | Increase the nutritional value of products | Improve product competitiveness and adapt to consumer demand for healthy food | [76] |
| Deeply processed products | Extracts, facial mask, health products | Develop more products with special functions | Meet the needs of different demand groups | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yang, Z.; Han, M.; Zhang, Y.; Muhammad, H.; Zhong, H.; Guan, R. Bioactive Components, Pharmacological Properties, and Applications of Cistanche deserticola Y. C. Ma: A Comprehensive Review. Nutrients 2025, 17, 1501. https://doi.org/10.3390/nu17091501
Liu X, Yang Z, Han M, Zhang Y, Muhammad H, Zhong H, Guan R. Bioactive Components, Pharmacological Properties, and Applications of Cistanche deserticola Y. C. Ma: A Comprehensive Review. Nutrients. 2025; 17(9):1501. https://doi.org/10.3390/nu17091501
Chicago/Turabian StyleLiu, Xiaofeng, Zichao Yang, Minjun Han, Yao Zhang, Hussain Muhammad, Hao Zhong, and Rongfa Guan. 2025. "Bioactive Components, Pharmacological Properties, and Applications of Cistanche deserticola Y. C. Ma: A Comprehensive Review" Nutrients 17, no. 9: 1501. https://doi.org/10.3390/nu17091501
APA StyleLiu, X., Yang, Z., Han, M., Zhang, Y., Muhammad, H., Zhong, H., & Guan, R. (2025). Bioactive Components, Pharmacological Properties, and Applications of Cistanche deserticola Y. C. Ma: A Comprehensive Review. Nutrients, 17(9), 1501. https://doi.org/10.3390/nu17091501






