Small Intestinal Bacterial Overgrowth and Pediatric Obesity—A Systematic Review
Abstract
1. Introduction
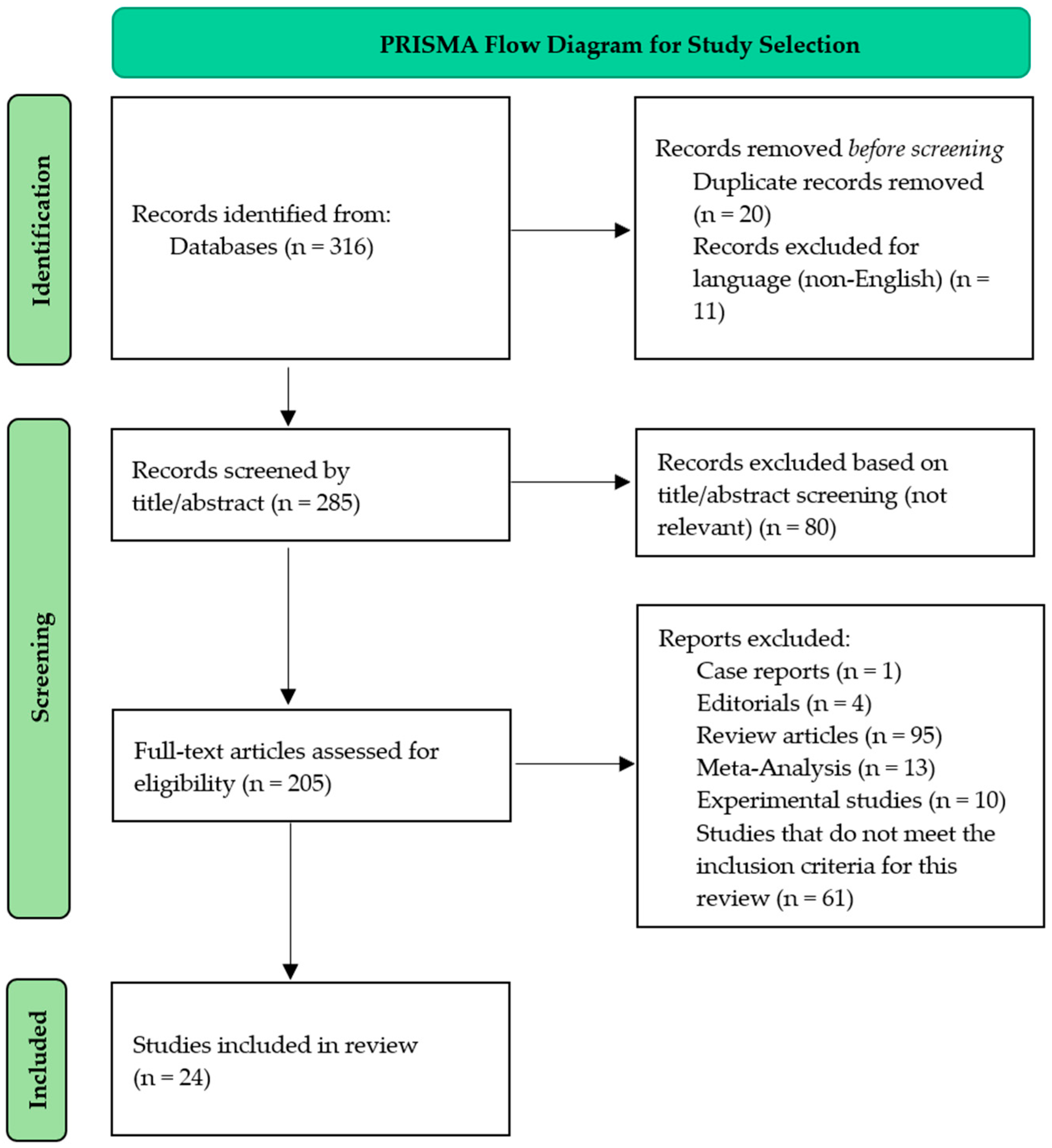
2. Materials and Methods
3. Results
3.1. Correlation Between SIBO and Obesity in Pediatric Populations
3.2. The Factors Involved in Offspring’ Gut Microbiota
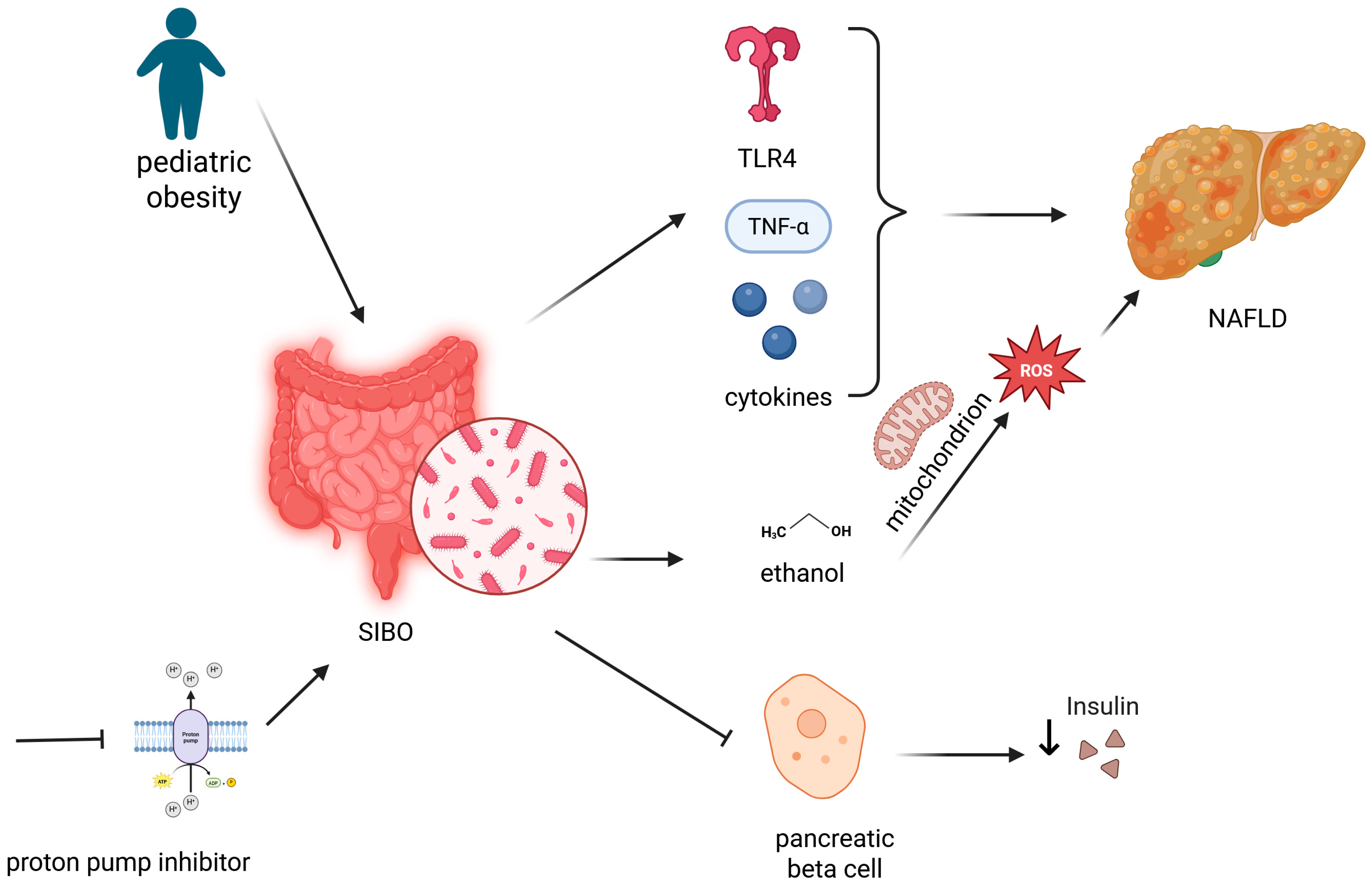
3.3. NAFLD in Children—Risks and Prevention
| Study (Year) | Study Type | Population | Diagnostic Tool (s) | Main Outcome (s) |
|---|---|---|---|---|
| Belei et al., 2017 [29] | 2-year prospective case-control study | 125 overweight/obese children (10–18 years) and 120 healthy controls | GHBT (SIBO), abdominal ultrasound and liver enzymes (NAFLD), metabolic syndrome parameters | SIBO prevalence was 37.6% in obese children vs. 3.3% in controls. SIBO-positive obese children had a significantly higher NAFLD prevalence (59.5% vs. 10.2%) and more frequent elevated ALT/AST, hypertension, and metabolic syndrome. |
| Browning et al., 2011 [46] | Randomized dietary intervention (2-week trial) | 18 adults with obesity and NAFLD (mean age 45; BMI ~35) | MRI spectroscopy (hepatic fat content) | Both low-carb and low-calorie diets reduced liver fat (~42%), but a low-carb diet led to a greater reduction (−55% vs. −28%; p = 0.008). AST decreased with weight loss; ALT remained unchanged. |
| Abdelmalek et al., 2010 [42] | Multicenter cross-sectional observational study | 427 adults with biopsy-confirmed NAFLD | Food-frequency questionnaire (fructose intake); liver histology (steatosis, inflammation, fibrosis); metabolic syndrome parameters | Daily fructose intake was linked to younger age, higher BMI and triglycerides, lower steatosis grade, but more advanced fibrosis. Daily consumers had ~2.6× higher odds of advanced fibrosis (p = 0.004). |
| Guercio Nuzio et al., 2017 [35] | Cross-sectional case-control study | 23 obese children (mean age 11 years) and 9 healthy controls | Lactulose: mannitol test (intestinal permeability), LHBT (SIBO), serum endotoxin, blood ethanol, fecal calprotectin, ultrasound and ALT (steatosis) | 48% of obese children had increased intestinal permeability. SIBO was only detected in the obese group. Elevated permeability was associated with higher endotoxin (r = 0.48) and ethanol and was a risk factor for steatosis (p < 0.002). |
| Shanab et al., 2010 [94] | Comparative case-control study | 18 adults with biopsy-confirmed NASH and 16 age- and sex-matched healthy controls | LHBT (SIBO), LBP (endotoxemia), TLR-4 (monocytes), plasma cytokines (IL-1β, IL-6, IL-8, TNF-α) | SIBO prevalence was higher in NASH (77.8%) vs. controls (31.3%). Only IL-8 was significantly elevated (p = 0.04) and correlated with TLR-4 expression (r = 0.51). No differences in LBP or other cytokines. |
3.4. Impact of Proton Pump Inhibitors, Probiotics, Prebiotics, and Antibiotics on Obese Children with SIBO
3.4.1. The Role of PPIs in Pediatric Obesity and SIBO
3.4.2. Probiotics as a Mitigating Strategy for PPI-Induced SIBO
3.4.3. Antibiotics and Their Impact on Gut Dysbiosis in SIBO Management
3.4.4. The Role of Prebiotics in Restoring Gut Microbial Balance
3.5. SIBO Diagnosis—Methods and Pediatric Considerations
4. Discussion
4.1. Interpreting Diagnostic Complexities
4.2. Disparities in Prevalence Reports
4.3. Evaluating Inflammation and Metabolic Consequences
4.4. Microbiota Profiles in the Context of Obesity
4.5. Implications for Management and Treatment
4.6. Persistent Limitations and Areas for Advancement
- Standardize SIBO diagnostic criteria and testing protocols
- Include longitudinal, multicenter cohorts to establish causality
- Control for confounders such as diet, medication, and socioeconomic status
- Investigate microbiota-based biomarkers for early detection and therapeutic targeting
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lobstein, T.; Baur, L.; Uauy, R. IASO International Obesity TaskForce Obesity in children and young people: A crisis in public health. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2004, 5 (Suppl. S1), 4–104. [Google Scholar] [CrossRef] [PubMed]
- Bibbins-Domingo, K.; Coxson, P.; Pletcher, M.J.; Lightwood, J.; Goldman, L. Adolescent overweight and future adult coronary heart disease. N. Engl. J. Med. 2007, 357, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-J.; Park, S.-G.; Jang, H.B.; Choi, M.-G.; Park, K.-H.; Kang, J.H.; Park, S.I.; Lee, H.-J.; Cho, S.-H. Obesity Alters the Microbial Community Profile in Korean Adolescents. PLoS ONE 2015, 10, e0134333. [Google Scholar] [CrossRef] [PubMed]
- James, W.P.T. The epidemiology of obesity: The size of the problem. J. Intern. Med. 2008, 263, 336–352. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Holub, C.K.; Elder, J.P.; Arredondo, E.M.; Barquera, S.; Eisenberg, C.M.; Sánchez Romero, L.M.; Rivera, J.; Lobelo, F.; Simoes, E.J. Obesity control in Latin American and U.S. Latinos: A systematic review. Am. J. Prev. Med. 2013, 44, 529–537. [Google Scholar] [CrossRef]
- Murugesan, S.; Ulloa-Martínez, M.; Martínez-Rojano, H.; Galván-Rodríguez, F.M.; Miranda-Brito, C.; Romano, M.C.; Piña-Escobedo, A.; Pizano-Zárate, M.L.; Hoyo-Vadillo, C.; García-Mena, J. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1337–1346. [Google Scholar] [CrossRef]
- Di Stefano, M.; Quigley, E.M.M. The diagnosis of small intestinal bacterial overgrowth: Two steps forward, one step backwards? Neurogastroenterol. Motil. 2018, 30, e13494. [Google Scholar] [CrossRef]
- Sieczkowska, A.; Landowski, P.; Kamińska, B.; Lifschitz, C. Small Bowel Bacterial Overgrowth in Children. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 196–207. [Google Scholar] [CrossRef]
- Sabaté, J.-M.; Jouët, P.; Harnois, F.; Mechler, C.; Msika, S.; Grossin, M.; Coffin, B. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: A contributor to severe hepatic steatosis. Obes. Surg. 2008, 18, 371–377. [Google Scholar] [CrossRef]
- Madrid, A.M.; Poniachik, J.; Quera, R.; Defilippi, C. Small intestinal clustered contractions and bacterial overgrowth: A frequent finding in obese patients. Dig. Dis. Sci. 2011, 56, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Roland, B.C.; Lee, D.; Miller, L.S.; Vegesna, A.; Yolken, R.; Severance, E.; Prandovszky, E.; Zheng, X.E.; Mullin, G.E. Obesity increases the risk of small intestinal bacterial overgrowth (SIBO). Neurogastroenterol. Motil. 2018, 30, e13199. [Google Scholar] [CrossRef] [PubMed]
- Geurts, L.; Neyrinck, A.M.; Delzenne, N.M.; Knauf, C.; Cani, P.D. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: Novel insights into molecular targets and interventions using prebiotics. Benef. Microbes 2014, 5, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Burcelin, R.; Garidou, L.; Pomié, C. Immuno-microbiota cross and talk: The new paradigm of metabolic diseases. Semin. Immunol. 2012, 24, 67–74. [Google Scholar] [CrossRef]
- Debnath, M.; Agrawal, S.; Agrawal, A.; Dubey, G.P. Metaflammatory responses during obesity: Pathomechanism and treatment. Obes. Res. Clin. Pract. 2016, 10, 103–113. [Google Scholar] [CrossRef]
- Karelis, A.D.; Rabasa-Lhoret, R. Obesity: Can inflammatory status define metabolic health? Nat. Rev. Endocrinol. 2013, 9, 694–695. [Google Scholar] [CrossRef]
- Hamer, M.; Stamatakis, E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J. Clin. Endocrinol. Metab. 2012, 97, 2482–2488. [Google Scholar] [CrossRef]
- Roth, C.L.; Kratz, M.; Ralston, M.M.; Reinehr, T. Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism 2011, 60, 445–452. [Google Scholar] [CrossRef]
- Genest, J. C-reactive protein: Risk factor, biomarker and/or therapeutic target? Can. J. Cardiol. 2010, 26 (Suppl. SA), 41A–44A. [Google Scholar] [CrossRef]
- Brun, P.; Castagliuolo, I.; Di Leo, V.; Buda, A.; Pinzani, M.; Palù, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef]
- Lo, W.-K.; Chan, W.W. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: A meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 483–490. [Google Scholar] [CrossRef]
- Chandra, S.; Dutta, U.; Noor, M.T.; Taneja, N.; Kochhar, R.; Sharma, M.; Singh, K. Endoscopic jejunal biopsy culture: A simple and effective method to study jejunal microflora. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2010, 29, 226–230. [Google Scholar] [CrossRef]
- Theisen, J.; Nehra, D.; Citron, D.; Johansson, J.; Hagen, J.A.; Crookes, P.F.; DeMeester, S.R.; Bremner, C.G.; DeMeester, T.R.; Peters, J.H. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2000, 4, 50–54. [Google Scholar] [CrossRef]
- Compare, D.; Pica, L.; Rocco, A.; De Giorgi, F.; Cuomo, R.; Sarnelli, G.; Romano, M.; Nardone, G. Effects of long-term PPI treatment on producing bowel symptoms and SIBO. Eur. J. Clin. Investig. 2011, 41, 380–386. [Google Scholar] [CrossRef]
- Bures, J.; Cyrany, J.; Kohoutova, D.; Förstl, M.; Rejchrt, S.; Kvetina, J.; Vorisek, V.; Kopacova, M. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 2010, 16, 2978–2990. [Google Scholar] [CrossRef]
- Miazga, A.; Osiński, M.; Cichy, W.; Żaba, R. Current views on the etiopathogenesis, clinical manifestation, diagnostics, treatment and correlation with other nosological entities of SIBO. Adv. Med. Sci. 2015, 60, 118–124. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 22 April 2025).
- Sieczkowska, A.; Landowski, P.; Zagozdzon, P.; Kaminska, B.; Lifschitz, C. Small Bowel Bacterial Overgrowth Associated with Persistence of Abdominal Symptoms in Children Treated with a Proton Pump Inhibitor. J. Pediatr. 2015, 166, 1310–1312.e1. [Google Scholar] [CrossRef]
- Belei, O.; Olariu, L.; Dobrescu, A.; Marcovici, T.; Marginean, O. The relationship between non-alcoholic fatty liver disease and small intestinal bacterial overgrowth among overweight and obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2017, 30, 1161–1168. [Google Scholar] [CrossRef]
- Belei, O.; Olariu, L.; Dobrescu, A.; Marcovici, T.; Marginean, O. Is It Useful to Administer Probiotics Together With Proton Pump Inhibitors in Children With Gastroesophageal Reflux? J. Neurogastroenterol. Motil. 2018, 24, 51–57. [Google Scholar] [CrossRef]
- Tapiainen, T.; Koivusaari, P.; Brinkac, L.; Lorenzi, H.A.; Salo, J.; Renko, M.; Pruikkonen, H.; Pokka, T.; Li, W.; Nelson, K.; et al. Impact of intrapartum and postnatal antibiotics on the gut microbiome and emergence of antimicrobial resistance in infants. Sci. Rep. 2019, 9, 10635. [Google Scholar] [CrossRef]
- Manzoni, P.; García Sánchez, R.; Meyer, M.; Stolfi, I.; Pugni, L.; Messner, H.; Cattani, S.; Betta, P.M.; Memo, L.; Decembrino, L.; et al. Exposure to Gastric Acid Inhibitors Increases the Risk of Infection in Preterm Very Low Birth Weight Infants but Concomitant Administration of Lactoferrin Counteracts This Effect. J. Pediatr. 2018, 193, 62–67.e1. [Google Scholar] [CrossRef]
- Scott, F.I.; Horton, D.B.; Mamtani, R.; Haynes, K.; Goldberg, D.S.; Lee, D.Y.; Lewis, J.D. Administration of Antibiotics to Children Before Age 2 Years Increases Risk for Childhood Obesity. Gastroenterology 2016, 151, 120–129.e5. [Google Scholar] [CrossRef]
- Todendi, P.F.; Possuelo, L.G.; Klinger, E.I.; Reuter, C.P.; Burgos, M.S.; Moura, D.J.; Fiegenbaum, M.; Valim, A.R.D.M. Low-grade inflammation markers in children and adolescents: Influence of anthropometric characteristics and CRP and IL6 polymorphisms. Cytokine 2016, 88, 177–183. [Google Scholar] [CrossRef]
- Guercio Nuzio, S.; Di Stasi, M.; Pierri, L.; Troisi, J.; Poeta, M.; Bisogno, A.; Belmonte, F.; Tripodi, M.; Di Salvio, D.; Massa, G.; et al. Multiple gut–liver axis abnormalities in children with obesity with and without hepatic involvement. Pediatr. Obes. 2017, 12, 446–452. [Google Scholar] [CrossRef]
- Lai, H.-H.; Chiu, C.-H.; Kong, M.-S.; Chang, C.-J.; Chen, C.-C. Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients 2019, 11, 1150. [Google Scholar] [CrossRef]
- Korterink, J.J.; Benninga, M.A.; Van Wering, H.M.; Deckers-Kocken, J.M. Glucose Hydrogen Breath Test for Small Intestinal Bacterial Overgrowth in Children With Abdominal Pain–Related Functional Gastrointestinal Disorders. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 498–502. [Google Scholar] [CrossRef]
- Galley, J.D.; Bailey, M.; Kamp Dush, C.; Schoppe-Sullivan, S.; Christian, L.M. Maternal Obesity Is Associated with Alterations in the Gut Microbiome in Toddlers. PLoS ONE 2014, 9, e113026. [Google Scholar] [CrossRef]
- McCann, A.; Ryan, F.J.; Stockdale, S.R.; Dalmasso, M.; Blake, T.; Ryan, C.A.; Stanton, C.; Mills, S.; Ross, P.R.; Hill, C. Viromes of one year old infants reveal the impact of birth mode on microbiome diversity. PeerJ 2018, 6, e4694. [Google Scholar] [CrossRef]
- Ignacio, A.; Fernandes, M.R.; Rodrigues, V.A.A.; Groppo, F.C.; Cardoso, A.L.; Avila-Campos, M.J.; Nakano, V. Correlation between body mass index and faecal microbiota from children. Clin. Microbiol. Infect. 2016, 22, 258.e1–258.e8. [Google Scholar] [CrossRef]
- Esposito, S.; Biscarini, A.; Federici, B.; Cofini, M.; Argentiero, A.; Neglia, C.; Lanciotti, L.; De’ Angelis, G.L.; Principi, N. Role of Small Intestinal Bacterial Overgrowth (SIBO) and Inflammation in Obese Children. Front. Pediatr. 2020, 8, 369. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Suzuki, A.; Guy, C.; Unalp-Arida, A.; Colvin, R.; Johnson, R.J.; Diehl, A.M.; Nonalcoholic Steatohepatitis Clinical Research Network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010, 51, 1961–1971. [Google Scholar] [CrossRef]
- Shanab, A.A.; Scully, P.; Crosbie, O.; Buckley, M.; O’Mahony, L.; Shanahan, F.; Gazareen, S.; Murphy, E.; Quigley, E.M.M. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: Association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig. Dis. Sci. 2011, 56, 1524–1534. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef]
- Revaiah, P.C.; Kochhar, R.; Rana, S.V.; Berry, N.; Ashat, M.; Dhaka, N.; Rami Reddy, Y.; Sinha, S.K. Risk of small intestinal bacterial overgrowth in patients receiving proton pump inhibitors versus proton pump inhibitors plus prokinetics. JGH Open 2018, 2, 47–53. [Google Scholar] [CrossRef]
- Browning, J.D.; Baker, J.A.; Rogers, T.; Davis, J.; Satapati, S.; Burgess, S.C. Short-term weight loss and hepatic triglyceride reduction: Evidence of a metabolic advantage with dietary carbohydrate restriction. Am. J. Clin. Nutr. 2011, 93, 1048–1052. [Google Scholar] [CrossRef]
- Van Rossum, T.; Haiß, A.; Knoll, R.L.; Marißen, J.; Podlesny, D.; Pagel, J.; Bleskina, M.; Vens, M.; Fortmann, I.; Siller, B.; et al. Bifidobacterium and Lactobacillus Probiotics and Gut Dysbiosis in Preterm Infants: The PRIMAL Randomized Clinical Trial. JAMA Pediatr. 2024, 178, 985–995. [Google Scholar] [CrossRef]
- Atazadegan, M.A.; Heidari-Beni, M.; Entezari, M.H.; Sharifianjazi, F.; Kelishadi, R. Effects of synbiotic supplementation on anthropometric indices and body composition in overweight or obese children and adolescents: A randomized, double-blind, placebo-controlled clinical trial. World J. Pediatr. WJP 2023, 19, 356–365. [Google Scholar] [CrossRef]
- Rahkola, E.-N.; Rautava, S.; Hiltunen, H.; Ross, C.; Lahti, L.; Isolauri, E. The preterm gut microbiota and administration routes of different probiotics: A randomized controlled trial. Pediatr. Res. 2023, 94, 1480–1487. [Google Scholar] [CrossRef]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, M.; Xue, J.; Huang, J.; Zhuang, R.; Zhou, X.; Zhang, H.; Fu, Q.; Hao, Y. Body Mass Index Differences in the Gut Microbiota Are Gender Specific. Front. Microbiol. 2018, 9, 1250. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Balamurugan, R.; George, G.; Kabeerdoss, J.; Hepsiba, J.; Chandragunasekaran, A.M.S.; Ramakrishna, B.S. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br. J. Nutr. 2010, 103, 335–338. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Ross, R.P.; O’Toole, P.W.; Shanahan, F.; Cotter, P.D. Targeting the microbiota to address diet-induced obesity: A time dependent challenge. PLoS ONE 2013, 8, e65790. [Google Scholar] [CrossRef]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, R.; Zhang, Y.; Lin, X.; Yang, X. Sexual dimorphism of gut microbiota at different pubertal status. Microb. Cell Factories 2020, 19, 152. [Google Scholar] [CrossRef]
- Korpela, K.; Kallio, S.; Salonen, A.; Hero, M.; Kukkonen, A.K.; Miettinen, P.J.; Savilahti, E.; Kohva, E.; Kariola, L.; Suutela, M.; et al. Gut microbiota develop towards an adult profile in a sex-specific manner during puberty. Sci. Rep. 2021, 11, 23297. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, N. Roles of Sex Hormones and Gender in the Gut Microbiota. J. Neurogastroenterol. Motil. 2021, 27, 314–325. [Google Scholar] [CrossRef]
- Del Castillo-Izquierdo, Á.; Mayneris-Perxachs, J.; Fernández-Real, J.M. Bidirectional relationships between the gut microbiome and sexual traits. Am. J. Physiol. Cell Physiol. 2022, 322, C1223–C1229. [Google Scholar] [CrossRef]
- Leao, L.; Miri, S.; Hammami, R. Gut feeling: Exploring the intertwined trilateral nexus of gut microbiota, sex hormones, and mental health. Front. Neuroendocrinol. 2025, 76, 101173. [Google Scholar] [CrossRef]
- Sisk-Hackworth, L.; Kelley, S.T.; Thackray, V.G. Sex, puberty, and the gut microbiome. Reproduction 2023, 165, R61–R74. [Google Scholar] [CrossRef]
- Yue, M.; Zhang, L. Exploring the Mechanistic Interplay between Gut Microbiota and Precocious Puberty: A Narrative Review. Microorganisms 2024, 12, 323. [Google Scholar] [CrossRef]
- Valeri, F.; Endres, K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocrinol. 2021, 61, 100912. [Google Scholar] [CrossRef]
- Lim, M.Y.; Rho, M.; Song, Y.-M.; Lee, K.; Sung, J.; Ko, G. Stability of gut enterotypes in Korean monozygotic twins and their association with biomarkers and diet. Sci. Rep. 2014, 4, 7348. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kompoti, M. Obesity and infection. Lancet Infect. Dis. 2006, 6, 438–446. [Google Scholar] [CrossRef]
- Makino, H.; Kushiro, A.; Ishikawa, E.; Kubota, H.; Gawad, A.; Sakai, T.; Oishi, K.; Martin, R.; Ben-Amor, K.; Knol, J.; et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS ONE 2013, 8, e78331. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1030. [Google Scholar] [CrossRef]
- Meliț, L.E.; Mărginean, C.O.; Săsăran, M.O. The Yin-Yang Concept of Pediatric Obesity and Gut Microbiota. Biomedicines 2022, 10, 645. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4578–4585. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. MMBR 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Mencin, A.A.; Lavine, J.E. Nonalcoholic fatty liver disease in children. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 151–157. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef]
- Roberts, E.A. Pediatric nonalcoholic fatty liver disease (NAFLD): A “growing” problem? J. Hepatol. 2007, 46, 1133–1142. [Google Scholar] [CrossRef]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; McCall, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef]
- OʼSullivan, T.A.; Oddy, W.H.; Bremner, A.P.; Sherriff, J.L.; Ayonrinde, O.T.; Olynyk, J.K.; Beilin, L.J.; Mori, T.A.; Adams, L.A. Lower fructose intake may help protect against development of nonalcoholic fatty liver in adolescents with obesity. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 624–631. [Google Scholar] [CrossRef]
- Wigg, A.J.; Roberts-Thomson, I.C.; Dymock, R.B.; McCarthy, P.J.; Grose, R.H.; Cummins, A.G. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 2001, 48, 206–211. [Google Scholar] [CrossRef]
- Rofsky, N.M.; Fleishaker, H. CT and MRI of diffuse liver disease. Semin. Ultrasound. CT MR 1995, 16, 16–33. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M.S. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846–858. [Google Scholar] [CrossRef]
- Ruiz, A.G.; Casafont, F.; Crespo, J.; Cayón, A.; Mayorga, M.; Estebanez, A.; Fernadez-Escalante, J.C.; Pons-Romero, F. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: Evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes. Surg. 2007, 17, 1374–1380. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Fialho, A.; Fialho, A.; Thota, P.; McCullough, A.J.; Shen, B. Small Intestinal Bacterial Overgrowth Is Associated with Non-Alcoholic Fatty Liver Disease. J. Gastrointest. Liver Dis. JGLD 2016, 25, 159–165. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Donald, I.P.; Kitchingmam, G.; Donald, F.; Kupfer, R.M. The diagnosis of small bowel bacterial overgrowth in elderly patients. J. Am. Geriatr. Soc. 1992, 40, 692–696. [Google Scholar] [CrossRef]
- Vajro, P.; Paolella, G.; Fasano, A. Microbiota and gut-liver axis: Their influences on obesity and obesity-related liver disease. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 461–468. [Google Scholar] [CrossRef]
- Mitchel, E.B.; Lavine, J.E. Review article: The management of paediatric nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2014, 40, 1155–1170. [Google Scholar] [CrossRef]
- Solga, S.F.; Diehl, A.M. Non-alcoholic fatty liver disease: Lumen-liver interactions and possible role for probiotics. J. Hepatol. 2003, 38, 681–687. [Google Scholar] [CrossRef]
- Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; De Maria, S.; Cartenì, M.; Nardone, G. Gut--liver axis: The impact of gut microbiota on non alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. NMCD 2012, 22, 471–476. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Abu-Shanab, A.; Quigley, E.M.M. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 691–701. [Google Scholar] [CrossRef]
- Edholm, D.; Kullberg, J.; Haenni, A.; Karlsson, F.A.; Ahlström, A.; Hedberg, J.; Ahlström, H.; Sundbom, M. Preoperative 4-week low-calorie diet reduces liver volume and intrahepatic fat, and facilitates laparoscopic gastric bypass in morbidly obese. Obes. Surg. 2011, 21, 345–350. [Google Scholar] [CrossRef]
- Yang, M.; Gong, S.; Ye, S.Q.; Lyman, B.; Geng, L.; Chen, P.; Li, D.-Y. Non-alcoholic fatty liver disease in children: Focus on nutritional interventions. Nutrients 2014, 6, 4691–4705. [Google Scholar] [CrossRef]
- Davies, I.; Burman-Roy, S.; Murphy, M.S. Guideline Development Group Gastro-oesophageal reflux disease in children: NICE guidance. BMJ 2015, 350, g7703. [Google Scholar] [CrossRef]
- Pereira, S.P.; Gainsborough, N.; Dowling, R.H. Drug-induced hypochlorhydria causes high duodenal bacterial counts in the elderly. Aliment. Pharmacol. Ther. 1998, 12, 99–104. [Google Scholar] [CrossRef]
- Cares, K.; Al-Ansari, N.; Macha, S.; Zoubi, N.; Zaghloul, H.; Thomas, R.; Lalinsky, P.; El-Baba, M. Short article: Risk of small intestinal bacterial overgrowth with chronic use of proton pump inhibitors in children. Eur. J. Gastroenterol. Hepatol. 2017, 29, 396–399. [Google Scholar] [CrossRef]
- Reilly, J.P. Safety profile of the proton-pump inhibitors. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 1999, 56, S11–S17. [Google Scholar] [CrossRef]
- Rosen, R.; Amirault, J.; Liu, H.; Mitchell, P.; Hu, L.; Khatwa, U.; Onderdonk, A. Changes in Gastric and Lung Microflora With Acid Suppression: Acid Suppression and Bacterial Growth. JAMA Pediatr. 2014, 168, 932. [Google Scholar] [CrossRef]
- Liang, S.; Xu, L.; Zhang, D.; Wu, Z. Effect of probiotics on small intestinal bacterial overgrowth in patients with gastric and colorectal cancer. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2016, 27, 227–232. [Google Scholar] [CrossRef]
- Lombardo, L.; Foti, M.; Ruggia, O.; Chiecchio, A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2010, 8, 504–508. [Google Scholar] [CrossRef]
- Choung, R.S.; Ruff, K.C.; Malhotra, A.; Herrick, L.; Locke, G.R.; Harmsen, W.S.; Zinsmeister, A.R.; Talley, N.J.; Saito, Y.A. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment. Pharmacol. Ther. 2011, 33, 1059–1067. [Google Scholar] [CrossRef]
- Guillet, R.; Stoll, B.J.; Cotten, C.M.; Gantz, M.; McDonald, S.; Poole, W.K.; Phelps, D.L.; National Institute of Child Health and Human Development Neonatal Research Network. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2006, 117, e137–e142. [Google Scholar] [CrossRef]
- Kaufman, D.A.; Manzoni, P. Strategies to prevent invasive candidal infection in extremely preterm infants. Clin. Perinatol. 2010, 37, 611–628. [Google Scholar] [CrossRef]
- Manzoni, P.; Stolfi, I.; Messner, H.; Cattani, S.; Laforgia, N.; Romeo, M.G.; Bollani, L.; Rinaldi, M.; Gallo, E.; Quercia, M.; et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: A randomized controlled trial. Pediatrics 2012, 129, 116–123. [Google Scholar] [CrossRef]
- Pammi, M.; Abrams, S.A. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2015, 20, CD007137. [Google Scholar]
- Mastromarino, P.; Capobianco, D.; Campagna, G.; Laforgia, N.; Drimaco, P.; Dileone, A.; Baldassarre, M.E. Correlation between lactoferrin and beneficial microbiota in breast milk and infant’s feces. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2014, 27, 1077–1086. [Google Scholar] [CrossRef]
- Littman, D.R.; Pamer, E.G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 2011, 10, 311–323. [Google Scholar] [CrossRef]
- Buffie, C.G.; Jarchum, I.; Equinda, M.; Lipuma, L.; Gobourne, A.; Viale, A.; Ubeda, C.; Xavier, J.; Pamer, E.G. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect. Immun. 2012, 80, 62–73. [Google Scholar] [CrossRef]
- Antunes, L.C.M.; Han, J.; Ferreira, R.B.R.; Lolić, P.; Borchers, C.H.; Finlay, B.B. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob. Agents Chemother. 2011, 55, 1494–1503. [Google Scholar] [CrossRef]
- Kariv, R.; Navaneethan, U.; Venkatesh, P.G.K.; Lopez, R.; Shen, B. Impact of Clostridium difficile infection in patients with ulcerative colitis. J. Crohns Colitis 2011, 5, 34–40. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4554–4561. [Google Scholar] [CrossRef]
- Jacobs, C.; Coss Adame, E.; Attaluri, A.; Valestin, J.; Rao, S.S.C. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment. Pharmacol. Ther. 2013, 37, 1103–1111. [Google Scholar] [CrossRef]
- Krajicek, E.J.; Hansel, S.L. Small Intestinal Bacterial Overgrowth: A Primary Care Review. Mayo Clin. Proc. 2016, 91, 1828–1833. [Google Scholar] [CrossRef]
- Lewis, S.J.; Franco, S.; Young, G.; O’Keefe, S.J. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment. Pharmacol. Ther. 1996, 10, 557–561. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Prokinetics in the Management of Functional Gastrointestinal Disorders. J. Neurogastroenterol. Motil. 2015, 21, 330–336. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Ghoshal, U.; Das, K.; Misra, A. Utility of hydrogen breath tests in diagnosis of small intestinal bacterial overgrowth in malabsorption syndrome and its relationship with oro-cecal transit time. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2006, 25, 6–10. [Google Scholar]
- Pande, C.; Kumar, A.; Sarin, S.K. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment. Pharmacol. Ther. 2009, 29, 1273–1281. [Google Scholar] [CrossRef]
- Schneider, A.R.J.; Klueber, S.; Posselt, H.-G.; Funk, B.; Murzynski, L.; Caspary, W.F.; Stein, J. Application of the glucose hydrogen breath test for the detection of bacterial overgrowth in patients with cystic fibrosis—A reliable method? Dig. Dis. Sci. 2009, 54, 1730–1735. [Google Scholar] [CrossRef]
- Perman, J.A.; Modler, S.; Barr, R.G.; Rosenthal, P. Fasting breath hydrogen concentration: Normal values and clinical application. Gastroenterology 1984, 87, 1358–1363. [Google Scholar] [CrossRef]
- Rana, S.V.; Sharma, S.; Kaur, J.; Sinha, S.K.; Singh, K. Comparison of lactulose and glucose breath test for diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Digestion 2012, 85, 243–247. [Google Scholar] [CrossRef]
- Yu, D.; Cheeseman, F.; Vanner, S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut 2011, 60, 334–340. [Google Scholar] [CrossRef]
- de Boissieu, D.; Chaussain, M.; Badoual, J.; Raymond, J.; Dupont, C. Small-bowel bacterial overgrowth in children with chronic diarrhea, abdominal pain, or both. J. Pediatr. 1996, 128, 203–207. [Google Scholar] [CrossRef]
- Rigsbee, L.; Agans, R.; Shankar, V.; Kenche, H.; Khamis, H.J.; Michail, S.; Paliy, O. Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am. J. Gastroenterol. 2012, 107, 1740–1751. [Google Scholar] [CrossRef]
- Leiby, A.; Mehta, D.; Gopalareddy, V.; Jackson-Walker, S.; Horvath, K. Bacterial overgrowth and methane production in children with encopresis. J. Pediatr. 2010, 156, 766–770, 770.e1. [Google Scholar] [CrossRef]
- Mello, C.S.; Rodrigues, M.S.D.C.; Filho, H.B.D.A.; Melli, L.C.F.L.; Tahan, S.; Pignatari, A.C.C.; De Morais, M.B. Fecal microbiota analysis of children with small intestinal bacterial overgrowth among residents of an urban slum in Brazil. J. Pediatr. (Rio J.) 2018, 94, 483–490. [Google Scholar] [CrossRef]
- Mello, C.S.; Tahan, S.; Melli, L.C.F.L.; Rodrigues, M.S.d.C.; de Mello, R.M.P.; Scaletsky, I.C.A.; de Morais, M.B. Methane production and small intestinal bacterial overgrowth in children living in a slum. World J. Gastroenterol. 2012, 18, 5932–5939. [Google Scholar] [CrossRef]
- Hwang, L.; Low, K.; Khoshini, R.; Melmed, G.; Sahakian, A.; Makhani, M.; Pokkunuri, V.; Pimentel, M. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig. Dis. Sci. 2010, 55, 398–403. [Google Scholar] [CrossRef]
- Schatz, R.A.; Zhang, Q.; Lodhia, N.; Shuster, J.; Toskes, P.P.; Moshiree, B. Predisposing factors for positive D-Xylose breath test for evaluation of small intestinal bacterial overgrowth: A retrospective study of 932 patients. World J. Gastroenterol. 2015, 21, 4574–4582. [Google Scholar] [CrossRef]
- Parodi, A.; Dulbecco, P.; Savarino, E.; Giannini, E.G.; Bodini, G.; Corbo, M.; Isola, L.; De Conca, S.; Marabotto, E.; Savarino, V. Positive glucose breath testing is more prevalent in patients with IBS-like symptoms compared with controls of similar age and gender distribution. J. Clin. Gastroenterol. 2009, 43, 962–966. [Google Scholar] [CrossRef]
- Soykan, I.; Sivri, B.; Sarosiek, I.; Kiernan, B.; McCallum, R.W. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig. Dis. Sci. 1998, 43, 2398–2404. [Google Scholar] [CrossRef]
- Collins, B.S.; Lin, H.C. Chronic abdominal pain in children is associated with high prevalence of abnormal microbial fermentation. Dig. Dis. Sci. 2010, 55, 124–130. [Google Scholar] [CrossRef]
- Rana, S.V.; Sharma, S.; Kaur, J.; Prasad, K.K.; Sinha, S.K.; Kochhar, R.; Malik, A.; Morya, R.K. Relationship of cytokines, oxidative stress and GI motility with bacterial overgrowth in ulcerative colitis patients. J. Crohns Colitis 2014, 8, 859–865. [Google Scholar] [CrossRef]
- Wira, C.R.; Sandoe, C.P.; Steele, M.G. Glucocorticoid regulation of the humoral immune system. I. In vivo effects of dexamethasone on IgA and IgG in serum and at mucosal surfaces. J. Immunol. 1990, 144, 142–146. [Google Scholar] [CrossRef]
- Klaus, J.; Spaniol, U.; Adler, G.; Mason, R.A.; Reinshagen, M.; von Tirpitz, C.C. Small intestinal bacterial overgrowth mimicking acute flare as a pitfall in patients with Crohn’s Disease. BMC Gastroenterol. 2009, 9, 61. [Google Scholar] [CrossRef]
- Roland, B.C.; Ciarleglio, M.M.; Clarke, J.O.; Semler, J.R.; Tomakin, E.; Mullin, G.E.; Pasricha, P.J. Low ileocecal valve pressure is significantly associated with small intestinal bacterial overgrowth (SIBO). Dig. Dis. Sci. 2014, 59, 1269–1277. [Google Scholar] [CrossRef]
- Neut, C.; Bulois, P.; Desreumaux, P.; Membré, J.-M.; Lederman, E.; Gambiez, L.; Cortot, A.; Quandalle, P.; van Kruiningen, H.; Colombel, J.-F. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 939–946. [Google Scholar] [CrossRef]
- Posserud, I.; Stotzer, P.-O.; Björnsson, E.S.; Abrahamsson, H.; Simrén, M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007, 56, 802–808. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.-M.; Zhang, Y.; Zhang, J.; Lu, N.; Liu, N. Hydrogen breath test to detect small intestinal bacterial overgrowth: A prevalence case–control study in autism. Eur. Child Adolesc. Psychiatry 2018, 27, 233–240. [Google Scholar] [CrossRef]
- Simrén, M.; Stotzer, P.-O. Use and abuse of hydrogen breath tests. Gut 2006, 55, 297–303. [Google Scholar] [CrossRef]
- Bratten, J.R.; Spanier, J.; Jones, M.P. Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am. J. Gastroenterol. 2008, 103, 958–963. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Lamousé-Smith, E.S.; Lightdale, J.R.; Jin, Z.; Yang, Y.-X.; Abrams, J.A. Use of Acid Suppression Medication is Associated With Risk for C. difficile Infection in Infants and Children: A Population-based Study. Clin. Infect. Dis. 2015, 61, 912–917. [Google Scholar] [CrossRef]
- Patil, U.P.; Bailey, S.M.; Wachtel, E.V.; Orosz, E.; Zarchin, R.; Mally, P.V. Efficacy of and potential morbidities associated with the use of antacid medications in preterm neonates. J. Perinat. Med. 2017, 45, 947–952. [Google Scholar] [CrossRef]
- Cheng, W.; Lu, J.; Lin, W.; Wei, X.; Li, H.; Zhao, X.; Jiang, A.; Yuan, J. Effects of a galacto-oligosaccharide-rich diet on fecal microbiota and metabolite profiles in mice. Food Funct. 2018, 9, 1612–1620. [Google Scholar] [CrossRef]
- Biagi, E.; Candela, M.; Franceschi, C.; Brigidi, P. The aging gut microbiota: New perspectives. Ageing Res. Rev. 2011, 10, 428–429. [Google Scholar] [CrossRef]
- Tims, S.; Derom, C.; Jonkers, D.M.; Vlietinck, R.; Saris, W.H.; Kleerebezem, M.; de Vos, W.M.; Zoetendal, E.G. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013, 7, 707–717. [Google Scholar] [CrossRef]
- Babio, N.; Toledo, E.; Estruch, R.; Ros, E.; Martínez-González, M.A.; Castañer, O.; Bulló, M.; Corella, D.; Arós, F.; Gómez-Gracia, E.; et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ Can. Med. Assoc. J. 2014, 186, E649–E657. [Google Scholar] [CrossRef]
- Liong, M.-T. Safety of probiotics: Translocation and infection. Nutr. Rev. 2008, 66, 192–202. [Google Scholar] [CrossRef]
| Component | Details |
|---|---|
| Keywords | “small intestinal bacterial overgrowth” OR “SIBO” AND “prevalence” AND “low-grade inflammatory markers” OR “metabolic status” AND “gut microbiome” AND “dysbiosis” AND “obese children” |
| Databases Searched | PubMed, Scopus, Web of Science |
| Database-Specific Search Details | PubMed: (“small intestinal bacterial overgrowth” OR “SIBO”) AND “prevalence” AND “gut microbiome” AND “obese children” Scopus: (“SIBO” OR “small intestinal bacterial overgrowth”) AND “low-grade inflammatory markers” AND “metabolic status” Web of Science: (“small intestinal bacterial overgrowth” OR “SIBO”) AND “obesity” AND “children” AND “gut microbiota” |
| Timeframe | January 2010–Present |
| Language | English only |
| Study Types Included | Randomized controlled trials, cohort studies, cross-sectional studies, longitudinal studies |
| Additional Sources | References cited in included articles and selected reviews |
| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Study Categories |
|
| Author (Year) | Study Design | Risk of Bias | Justification |
|---|---|---|---|
| Sieczkowska et al. (2015) [28] | Prospective cohort | Moderate | Small sample size; limited confounder control; no blinding. |
| Belei et al. (2017) [29] | Cross-sectional | Moderate | Good comparison groups but observational; potential residual confounding. |
| Belei et al. (2018) [30] | RCT | Low | Randomization, placebo group, and outcome measurement are well-described. |
| Tapiainen et al. (2019) [31] | Prospective controlled cohort | Low | A well-designed prospective study with exposure/outcome measures clearly defined. |
| Manzoni et al. (2018) [32] | Secondary analysis of RCT | Moderate | Non-randomized comparison between exposure groups; secondary analysis. |
| Scott et al. (2016) [33] | Retrospective cohort | Moderate | Large sample but risk of misclassification bias from administrative data. |
| Todendi et al. (2016) [34] | Cross-sectional | Moderate | No causal inference; confounding variables are only partially controlled. |
| Murugesan et al. (2015) [7] | Cross-sectional | Moderate | Descriptive study with compositional analysis; limited generalizability. |
| Guercio Nuzio et al. (2017) [35] | Cross-sectional | Moderate | Small sample size; associations only. |
| Lai et al. (2019) [36] | RCT | Low | Placebo-controlled RCT with microbiota and clinical outcomes measured. |
| Korterink et al. (2015) [37] | Cross-sectional | Moderate | Adequate outcome measurement; confounding and selection bias likely. |
| Galley et al. (2014) [38] | Cross-sectional | Moderate | Microbiome analysis is valid but limited in controlling for SES and other variables. |
| McCann et al. (2018) [39] | Cross-sectional | Moderate | Small sample size; descriptive viral diversity analysis. |
| Ignacio et al. (2016) [40] | Cross-sectional | Moderate | Limited sample; single center; descriptive analysis. |
| Esposito et al. (2020) [41] | Cross-sectional | Moderate | Well-defined population, but no causal conclusions. |
| Hu et al. (2015) [3] | Cross-sectional | Moderate | Associative findings without adjustment for all relevant confounders. |
| Abdelmalek et al. (2010) [42] | Cross-sectional | Moderate | Liver biopsy adds rigor; dietary self-report introduces potential bias. |
| Abu-Shanab et al. (2011) [43] | Case-control | Moderate | Matched controls; potential recall bias; small sample. |
| Boursier et al. (2016) [44] | Cross-sectional | Moderate | Includes histological confirmation; possible confounding bias. |
| Revaiah et al. (2018) [45] | Cross-sectional | Moderate | Good comparison; non-randomized groups; confounders not fully adjusted. |
| Browning et al. (2011) [46] | RCT | Low | Controlled intervention with biological outcomes; small sample size noted. |
| Van Rossum et al. (2024) [47] | RCT | Low | Large multicenter RCT with microbiome and clinical endpoints. |
| Atazadegan et al. (2023) [48] | RCT | Low | Double-blinded RCT with objective outcomes. |
| Rahkola et al. (2023) [49] | RCT | Low | Randomized, placebo-controlled microbiota outcomes were measured; a small sample was acknowledged. |
| Author (Year) | Study Design | Population | Sample Size | Key Outcomes |
|---|---|---|---|---|
| Sieczkowska et al. (2015) [28] | Prospective cohort | Children (3–18 y) on PPI for esophagitis | 40 | 22.5% developed SIBO after 3 months of omeprazole. GI symptoms are more frequent in SIBO-positive cases. |
| Belei et al. (2017) [29] | Cross-sectional case-control | Obese/overweight vs. lean children | 245 | SIBO prevalence is 37.6% in obese vs. 3.3% controls. The strong link between SIBO and NAFLD in obese children. |
| Belei et al. (2018) [30] | RCT | Children with GERD treated with PPI | 128 | Probiotic + PPI reduced SIBO (6.2% vs. 56.2%). Fewer GI side effects in the probiotic group. |
| Tapiainen et al. (2019) [31] | Prospective controlled cohort | Newborns (with/without antibiotics) | 149 | Antibiotic exposure altered microbiota. Dysbiosis persisted despite probiotics. ↑ Antimicrobial resistance genes. |
| Manzoni et al. (2018) [32] | Secondary analysis of RCT | Preterm VLBW infants | 743 | Acid-suppressants ↑ LOS risk. Lactoferrin co-admin mitigated risk. |
| Scott et al. (2016) [33] | Retrospective cohort | UK children (birth to 4 years) | 21,714 | ≥3 antibiotic courses before age 2 ↑ obesity risk at age 4 (OR~1.47). |
| Todendi et al. (2016) [34] | Cross-sectional | Brazilian children/adolescents | 470 | Obesity associated with ↑ CRP and IL-6. Central adiposity and CRP gene variant influenced inflammation. |
| Murugesan et al. (2015) [7] | Cross-sectional | Mexican schoolchildren (5–11 y) | 190 | Obese children had ↓ butyrate ↑ propionate. Specific microbial shifts, no major dysbiosis. |
| Guercio Nuzio et al. (2017) [35] | Cross-sectional | Obese children (±NAFLD) | 32 | ↑ Gut permeability, endotoxemia. SIBO only in obese. No mucosal inflammation. |
| Lai et al. (2019) [36] | RCT | Children (6 mo–6 y) with diarrhea | 81 | L. casei improved symptoms, ↓ inflammatory markers, ↑ and beneficial bacteria. |
| Korterink et al. (2015) [37] | Cross-sectional | Children (6–18 y) with FGIDs | 161 | SIBO in 14.3%. Strong link with IBS, symptoms predictive. |
| Galley et al. (2014) [38] | Cross-sectional | Toddlers born to obese vs. normal-weight mothers | 77 | Obese mothers → infants with altered microbiota (↑ Faecalibacterium, Blautia). |
| McCann et al. (2018) [39] | Cross-sectional (metagenomic) | Infants (1 y) by birth mode | 20 | Vaginal birth → ↑ virome diversity. Virome clustered by birth mode. |
| Ignacio et al. (2016) [40] | Cross-sectional | Brazilian children (5–9 y) | 51 | Obese children had ↑ Lactobacillus ↓ Bifidobacterium. ↑ Bacteroides fragilis group. |
| Esposito et al. (2020) [41] | Cross-sectional | Obese children with/without SIBO | 50 | 72% had SIBO. ↑ IL-6, TNF-α, IL-8. No correlation with SIBO severity. |
| Hu et al. (2015) [3] | Cross-sectional | Chinese adolescents (13–17 y) | 81 | ↑ Prevotella, ↓ Bacteroides in obese. Microbial changes linked to inflammation. |
| Abdelmalek et al. (2010) [42] | Cross-sectional | Adults with NAFLD | 341 | Daily fructose intake linked to ↑ fibrosis and inflammation in NAFLD. |
| Abu-Shanab et al. (2011) [43] | Case-control | Adults with NASH vs. controls | 34 | SIBO 77.8% in NASH vs. 31.3% controls. ↑ IL-8, TLR-4. Suggests microbial role in NASH. |
| Boursier et al. (2016) [44] | Cross-sectional | Adults with NAFLD | 57 | ↑ Bacteroides, ↓ Prevotella in NASH. ↓ Ruminococcus in fibrosis. Microbiota predicts NAFLD severity. |
| Revaiah et al. (2018) [45] | Cross-sectional | Adults on PPIs | 147 | SIBO: 13.2% on PPI vs. 1.8% with prokinetic. Linked to slower gut transit. |
| Browning et al. (2011) [46] | Randomized intervention trial | Adults with NAFLD | 18 | 2-week low-carb vs. calorie restriction. Both ↓ liver fat and carb-restricted diets led to greater ↓ (55% vs. 28%). Linked to ↑ketones, fat oxidation. |
| Van Rossum et al. (2024) [47] | Randomized clinical trial (PRIMAL study) | Preterm infants (28–32 weeks GA) | 618 | Multistrain probiotics (B. infantis, BB-12, L. acidophilus) did not reduce MDRO+ colonization by day 30 but improved eubiosis scores, aligning the microbiome with that of healthy term infants. |
| Atazadegan et al. (2023) [48] | RCT | Overweight/obese children (8–18 y) | 60 | Synbiotic supplementation (L. coagulans, L. indicus + FOS) significantly reduced waist-to-height ratio but not other body metrics. |
| Rahkola et al. (2023) [49] | RCT | Preterm neonates (25–35 weeks GA) | 68 | Direct LGG + Bb12 supplementation significantly increased B. animalis and Lactobacillales in preterms, while maternal delivery was less effective. Early direct use shapes microbiota composition. |
| Comparison | Dominant Taxa | Key Characteristics |
|---|---|---|
| Obese Children | Lactobacillus, Prevotella, E. coli, Clostridium | ↑ Energy harvest, ↑ inflammation, ↓ butyrate (functional dysbiosis) |
| Lean Children | Bifidobacterium, Bacteroides | ↑ Microbial diversity, ↓ inflammatory markers |
| Obese Females | ↑ Lactobacillus, Bifidobacterium, Alistipes | Higher microbial diversity, influenced by estrogen |
| Obese Males | ↑ Bilophila, Phascolarctobacterium | Lower diversity, delayed adult-like microbiota maturation |
| High-Fiber Diet (e.g., rural) | ↑ Bacteroidetes (e.g., Prevotella) | Anti-inflammatory profile, ↑ SCFAs |
| Western Diet (high-fat) | ↑ Firmicutes, ↓ Bacteroidetes | Pro-inflammatory taxa, metabolic disruption |
| Pediatrics Population Studies PPI Only | |||||
|---|---|---|---|---|---|
| Study | Design and Population | PPI Exposure | SIBO Diagnostic Method | SIBO Prevalence | Key Findings |
| Sieczkowska et al., 2015 [28] | Prospective cohort; 40 children (3–18 y) with peptic esophagitis | Omeprazole 1 mg/kg/day for 3 months | GHBT | 0% → 22.5% after 3 months | 3-month PPI therapy induced SIBO in 22.5% of children. SIBO-positive children had more abdominal pain, bloating, and flatulence. |
| Cares et al., 2017 [99] | Prospective cohort; 83 children (3–17 y)—PPI users (n = 56) vs. controls (n = 27) | ≥6 months on PPI vs. no PPI | GHBT | 8.9% (PPI) vs. 3.7% (control); p = 0.359 | Higher SIBO was found in the PPI group, but it was not statistically significant. No definitive increased risk of SIBO from PPI use. |
| PPI + Probiotic | |||||
| Belei et al., 2018 [30] | RCT; 128 children (1–18 y) with GERD on PPI; 120 controls | 12-week PPI therapy + placebo (n = 64) vs. PPI + L. reuteri (n = 64) | GHBT before/after treatment | PPI + placebo: 56.2% vs. PPI + probiotic: 6.2%; Control: 5% | Probiotic co-administration reduced SIBO to control levels. Significant protective effect against PPI-induced SIBO (p < 0.001). |
| Adult Population Studies PPI Only vs. PPI + Prokinetic | |||||
| Revaiah et al., 2018 [45] | Cross-sectional: 147 patients (>12 y) with GERD/FD on long-term PPI | Group A: PPI only vs. Group B: PPI + prokinetic (levosulpiride 75 mg/day) | GHBT | PPI only: 13.2% vs. PPI + prokinetic: 1.8%; p = 0.018 | Adding a prokinetic significantly reduced SIBO risk compared to PPI alone. Suggests the benefit of motility agents in SIBO prevention. |
| Feature | GHBT | LHBT |
|---|---|---|
| Substrate | Glucose | Lactulose |
| Site of Action | Proximal small intestine | Whole gut |
| Sensitivity | 62–91% | ~63.6% |
| Specificity | 75–100% | ~67.7% |
| False Positives | Low | Higher (especially with fast transit) |
| False Negatives | May miss distal SIBO | Less common |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koller, A.M.; Săsăran, M.O.; Mărginean, C.O. Small Intestinal Bacterial Overgrowth and Pediatric Obesity—A Systematic Review. Nutrients 2025, 17, 1499. https://doi.org/10.3390/nu17091499
Koller AM, Săsăran MO, Mărginean CO. Small Intestinal Bacterial Overgrowth and Pediatric Obesity—A Systematic Review. Nutrients. 2025; 17(9):1499. https://doi.org/10.3390/nu17091499
Chicago/Turabian StyleKoller, Ana Maria, Maria Oana Săsăran, and Cristina Oana Mărginean. 2025. "Small Intestinal Bacterial Overgrowth and Pediatric Obesity—A Systematic Review" Nutrients 17, no. 9: 1499. https://doi.org/10.3390/nu17091499
APA StyleKoller, A. M., Săsăran, M. O., & Mărginean, C. O. (2025). Small Intestinal Bacterial Overgrowth and Pediatric Obesity—A Systematic Review. Nutrients, 17(9), 1499. https://doi.org/10.3390/nu17091499






