Carbohydrate Quality Is Independently Associated with Cardiometabolic Risk in Chinese Individuals with Impaired Glucose Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Anthropometrics and Body Composition
2.4. Biochemical Profiles
2.5. Hepatic Parameters Measurements:
2.6. Dietary Evaluation and Physical Activity
2.7. Statistical Analysis
3. Results
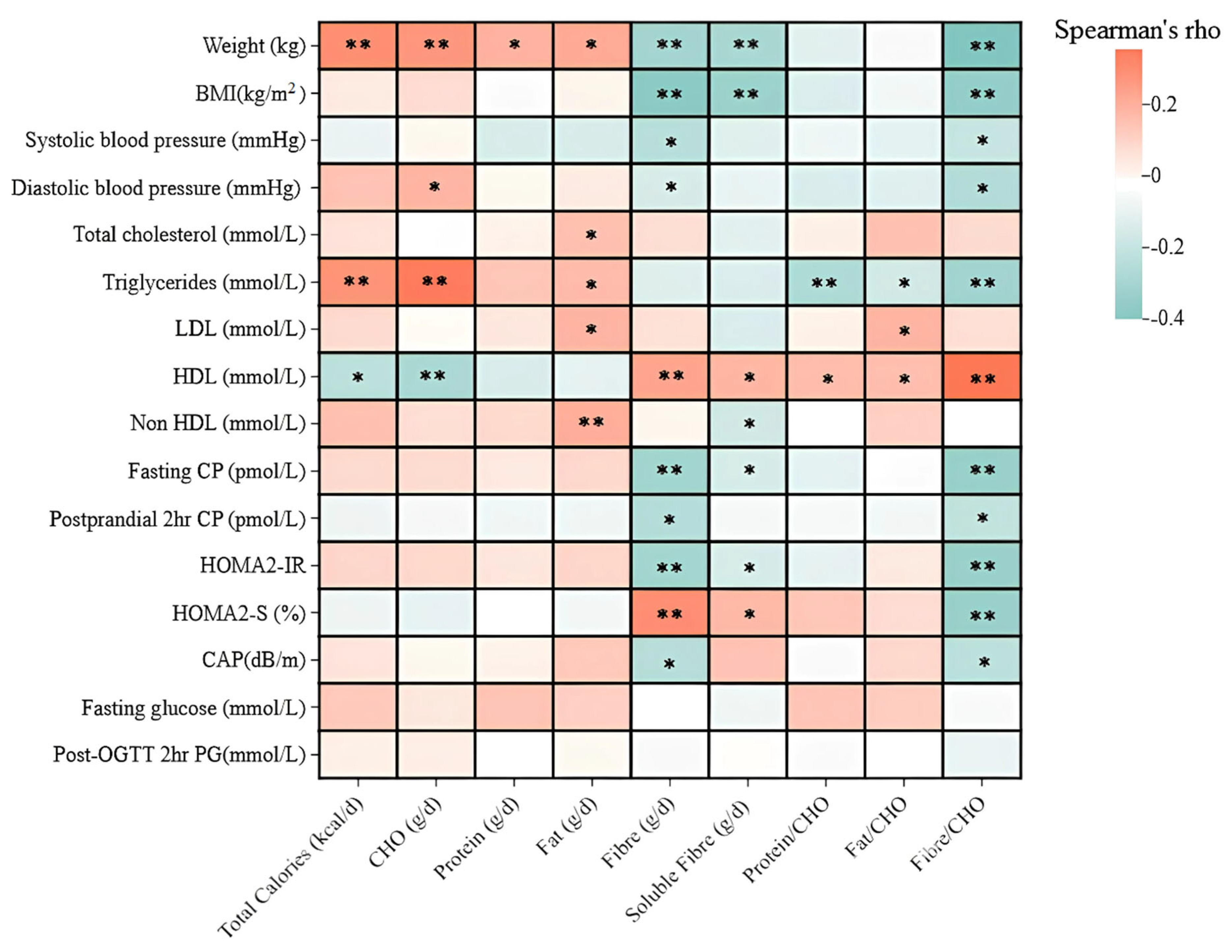
3.1. Correlations Between Nutritional Index, Lipid Profiles, and Liver Fat
3.2. Correlations Between Nutritional Index and Insulin Response
4. Discussion
4.1. Impact of Carbohydrate Quantity on Lipid Profiles and Liver Fat
4.2. Potential Mechanisms by Which Higher Dietary Fibre/CHO Ratio May Be Associated with Improved Cardiometabolic Parameters
4.3. Associatios Between Protein/CHO and Fat/CHO Ratios with Lipid Profiles
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parks, E.J. Effect of dietary carbohydrate on triglyceride metabolism in humans. J. Nutr. 2001, 131, 2772S–2774S. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-H.; Choi, K.M. Impact of high-carbohydrate diet on metabolic parameters in patients with type 2 diabetes. Nutrients 2017, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Orliacq, J.; Pérez-Cornago, A.; Parry, S.A.; Kelly, R.K.; Koutoukidis, D.A.; Carter, J.L. Associations between types and sources of dietary carbohydrates and liver fat: A UK Biobank study. BMC Med. 2023, 21, 444. [Google Scholar] [CrossRef] [PubMed]
- Churuangsuk, C.; Lean, M.E.; Combet, E. Low and reduced carbohydrate diets: Challenges and opportunities for type 2 diabetes management and prevention. Proc. Nutr. Soc. 2020, 79, 498–513. [Google Scholar] [CrossRef]
- Choi, J.H.; Cho, Y.J.; Kim, H.-J.; Ko, S.-H.; Chon, S.; Kang, J.-H.; Kim, K.-K.; Kim, E.M.; Kim, H.J.; Song, K.-H. Effect of carbohydrate-restricted diets and intermittent fasting on obesity, type 2 diabetes mellitus, and hypertension management: Consensus statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hypertension. Clin. Hypertens. 2022, 28, 26. [Google Scholar]
- Saslow, L.R.; Daubenmier, J.J.; Moskowitz, J.T.; Kim, S.; Murphy, E.J.; Phinney, S.D.; Ploutz-Snyder, R.; Goldman, V.; Cox, R.M.; Mason, A.E. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr. Diabetes 2017, 7, 304. [Google Scholar] [CrossRef]
- Hansen, C.D.; Gram-Kampmann, E.-M.; Hansen, J.K.; Hugger, M.B.; Madsen, B.S.; Jensen, J.M.; Olesen, S.; Torp, N.; Rasmussen, D.N.; Kjærgaard, M. Effect of calorie-unrestricted low-carbohydrate, high-fat diet versus high-carbohydrate, low-fat diet on type 2 diabetes and nonalcoholic fatty liver disease: A randomized controlled trial. Ann. Intern. Med. 2023, 176, 10–21. [Google Scholar] [CrossRef]
- Thomsen, M.N.; Skytte, M.J.; Samkani, A.; Carl, M.H.; Weber, P.; Astrup, A.; Chabanova, E.; Fenger, M.; Frystyk, J.; Hartmann, B. Dietary carbohydrate restriction augments weight loss-induced improvements in glycaemic control and liver fat in individuals with type 2 diabetes: A randomised controlled trial. Diabetologia 2022, 65, 506–517. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Xie, H.; Bai, H.; Lin, G.; Dong, Y.; Shi, D.; Wang, J.; Zhang, Q.; Zhang, Y. Impact of a low-carbohydrate and high-fiber diet on nonalcoholic fatty liver disease. Asia Pac. J. Clin. Nutr. 2020, 29, 483–490. [Google Scholar]
- Sylvetsky, A.C.; Edelstein, S.L.; Walford, G.; Boyko, E.J.; Horton, E.S.; Ibebuogu, U.N.; Knowler, W.C.; Montez, M.G.; Temprosa, M.; Hoskin, M.; et al. A high-carbohydrate, high-fiber, low-fat diet results in weight loss among adults at high risk of type 2 diabetes. J. Nutr. 2017, 147, 2060–2066. [Google Scholar] [CrossRef]
- De Natale, C.; Annuzzi, G.; Bozzetto, L.; Mazzarella, R.; Costabile, G.; Ciano, O.; Riccardi, G.; Rivellese, A.A. Effects of a plant-based high-carbohydrate/high-fiber diet versus high–monounsaturated fat/low-carbohydrate diet on postprandial lipids in type 2 diabetic patients. Diabetes Care 2009, 32, 2168–2173. [Google Scholar] [PubMed]
- Sievenpiper, J.L. Low-carbohydrate diets and cardiometabolic health: The importance of carbohydrate quality over quantity. Nutr. Rev. 2020, 78, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hartley, L.; May, M.D.; Loveman, E.; Colquitt, J.L.; Rees, K. Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2016, 1, CD011472. [Google Scholar]
- Chutkan, R.; Fahey, G.; Wright, W.L.; McRorie, J. Viscous versus nonviscous soluble fiber supplements: Mechanisms and evidence for fiber-specific health benefits. J. Am. Assoc. Nurse Pract. 2012, 24, 476–487. [Google Scholar]
- Ghavami, A.; Ziaei, R.; Talebi, S.; Barghchi, H.; Nattagh-Eshtivani, E.; Moradi, S.; Rahbarinejad, P.; Mohammadi, H.; Ghasemi-Tehrani, H.; Marx, W. Soluble fiber supplementation and serum lipid profile: A systematic review and dose-response meta-analysis of randomized controlled trials. Adv. Nutr. 2023, 14, 465–474. [Google Scholar]
- Brum, J.; Ramsey, D.; McRorie, J.; Bauer, B.; Kopecky, S.L. Meta-analysis of usefulness of psyllium fiber as adjuvant antilipid therapy to enhance cholesterol lowering efficacy of statins. Am. J. Cardiol. 2018, 122, 1169–1174. [Google Scholar]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar]
- Zhao, W.-T.; Luo, Y.; Zhang, Y.; Zhou, Y.; Zhao, T.-T. High protein diet is of benefit for patients with type 2 diabetes: An updated meta-analysis. Medicine 2018, 97, e13149. [Google Scholar]
- Papakonstantinou, E.; Triantafillidou, D.; Panagiotakos, D.; Koutsovasilis, A.; Saliaris, M.; Manolis, A.; Melidonis, A.; Zampelas, A. A high-protein low-fat diet is more effective in improving blood pressure and triglycerides in calorie-restricted obese individuals with newly diagnosed type 2 diabetes. Eur. J. Clin. Nutr. 2010, 64, 595–602. [Google Scholar]
- Paik, J.K.; Park, M.; Shin, J.E.; Jang, S.-Y.; Shin, J.-Y. Dietary protein to carbohydrate ratio and incidence of metabolic syndrome in Korean adults based on a long-term prospective community-based cohort. Nutrients 2020, 12, 3274. [Google Scholar] [CrossRef]
- Garg, A.; Grundy, S.M.; Koffler, M. Effect of high carbohydrate intake on hyperglycemia, islet function, and plasma lipoproteins in NIDDM. Diabetes Care 1992, 15, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Thomas, E.L.; Collins, A.L.; McCarthy, J.; Fitzpatrick, J.; Durighel, G.; Goldstone, A.P.; Bell, J.D. Estimation of abdominal fat compartments by bioelectrical impedance: The validity of the ViScan measurement system in comparison with MRI. Eur. J. Clin. Nutr. 2010, 64, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Komiya, H.; Mori, Y.; Ohta, T.; Kasahara, Y.; Ikeda, Y. Development of a new method for estimating visceral fat area with multi-frequency bioelectrical impedance. Tohoku J. Exp. Med. 2008, 214, 105–112. [Google Scholar] [CrossRef]
- World Health Organization. Waist Circumference and Waist-Hip Ratio. Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Ko, G.T.; So, W.-Y.; Tong, P.C.; Chan, W.-B.; Yang, X.; Ma, R.C.; Kong, A.P.; Ozaki, R.; Yeung, C.-Y.; Chow, C.-C. Effect of interactions between C peptide levels and insulin treatment on clinical outcomes among patients with type 2 diabetes mellitus. Cmaj 2009, 180, 919–926. [Google Scholar] [CrossRef]
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Lernmark, Å.; Metzger, B.E.; Nathan, D.M.; Kirkman, M.S. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin. Chem. 2023, 69, 808–868. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Fan, B.; Wu, H.; Shi, M.; Yang, A.; Lau, E.S.; Tam, C.H.; Mao, D.; Lim, C.K.; Kong, A.P.; Ma, R.C. Associations of the HOMA2-% B and HOMA2-IR with progression to diabetes and glycaemic deterioration in young and middle-aged Chinese. Diabetes/Metab. Res. Rev. 2022, 387, e3525. [Google Scholar] [CrossRef]
- Tschritter, O.; Fritsche, A.; Shirkavand, F.; Machicao, F.; Haring, H.; Stumvoll, M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003, 26, 1026–1033. [Google Scholar] [CrossRef]
- Boursier, J.; Zarski, J.P.; de Ledinghen, V.; Rousselet, M.C.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.-G.; Mi, Y.-Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.-H.; Cardoso, A.C. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar]
- De Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R. Baveno VII–renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar]
- Ji, Y.; Plourde, H.; Bouzo, V.; Kilgour, R.D.; Cohen, T.R. Validity and usability of a smartphone image-based dietary assessment app compared to 3-day food diaries in assessing dietary intake among Canadian adults: Randomized controlled trial. JMIR Mhealth Uhealth 2020, 8, e16953. [Google Scholar]
- Woo, J.; Ho, S.; Sham, A.; Sea, M.; Lam, K.; Lam, T.; Janus, E. Diet and glucose tolerance in a Chinese population. Eur. J. Clin. Nutr. 2003, 57, 523–530. [Google Scholar]
- Deriemaeker, P.; Aerenhouts, D.; Hebbelinck, M.; Clarys, P. Validation of a 3-day diet diary: Comparison with a 7-day diet diary and a FFQ: 1946: Board# 97 8: 30 AM–9: 30 AM. Med. Sci. Sports Exerc. 2006, 38, S328. [Google Scholar]
- The Government of the Hong Kong Special Administrative Region. Nutrient Information Inquiry. Available online: https://www.cfs.gov.hk/english/nutrient/fc-introduction.php (accessed on 1 December 2020).
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar]
- Ames, R.P. The effects of antihypertensive drugs on serum lipids and lipoproteins: I. Diuretics. Drugs 1986, 32, 260–278. [Google Scholar]
- Dierkes, J.; Luley, C.; Westphal, S. Effect of lipid-lowering and anti-hypertensive drugs on plasma homocysteine levels. Vasc. Health Risk Manag. 2007, 3, 99–108. [Google Scholar]
- Hashimoto, Y.; Tanaka, M.; Miki, A.; Kobayashi, Y.; Wada, S.; Kuwahata, M.; Kido, Y.; Yamazaki, M.; Fukui, M. Intake of carbohydrate to fiber ratio is a useful marker for metabolic syndrome in patients with type 2 diabetes: A cross-sectional study. Ann. Nutr. Metab. 2018, 72, 329–335. [Google Scholar]
- Fontanelli, M.d.M.; Micha, R.; Sales, C.H.; Liu, J.; Mozaffarian, D.; Fisberg, R.M. Application of the≤ 10: 1 carbohydrate to fiber ratio to identify healthy grain foods and its association with cardiometabolic risk factors. Eur. J. Nutr. 2020, 59, 3269–3279. [Google Scholar] [PubMed]
- Dong, Q.; Wang, L.; Hu, H.; Cui, L.; Lu, A.; Qian, C.; Wang, X.; Du, X. Greater protection of lower dietary carbohydrate to fiber ratio (CFR) against poor blood pressure control in patients with essential hypertension: A cross-sectional study. Nutrients 2022, 14, 4443. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, T. Association between dietary carbohydrate to fiber ratio and metabolic dysfunction associated fatty liver disease in adults: Evidence from the NHANES 2017–2020. J. Health Popul. Nutr. 2024, 43, 43. [Google Scholar]
- Chu, N.; Chan, T.Y.; Chu, Y.K.; Ling, J.; He, J.; Leung, K.; Ma, R.C.; Chan, J.C.; Chow, E. Higher dietary magnesium and potassium intake are associated with lower body fat in people with impaired glucose tolerance. Front. Nutr. 2023, 10, 1169705. [Google Scholar]
- Mosallanezhad, Z.; Jalali, M.; Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary sodium to potassium ratio is an independent predictor of cardiovascular events: A longitudinal follow-up study. BMC Public Health 2023, 23, 705. [Google Scholar]
- Jayedi, A.; Ghomashi, F.; Zargar, M.S.; Shab-Bidar, S. Dietary sodium, sodium-to-potassium ratio, and risk of stroke: A systematic review and nonlinear dose-response meta-analysis. Clin. Nutr. 2019, 38, 1092–1100. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Huang, L.-S. The insulin resistance syndrome: Impact on lipoprotein metabolism and atherothrombosis. J. Cardiovasc. Risk 2000, 7, 325–331. [Google Scholar] [CrossRef]
- Hernandez, P.; Passi, N.; Modarressi, T.; Kulkarni, V.; Soni, M.; Burke, F.; Bajaj, A.; Soffer, D. Clinical management of hypertriglyceridemia in the prevention of cardiovascular disease and pancreatitis. Curr. Atheroscler. Rep. 2021, 23, 1–13. [Google Scholar]
- Muzurović, E.; Mikhailidis, D.P.; Mantzoros, C. Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metabolism 2021, 119, 154770. [Google Scholar]
- Riccardi, G.; Costabile, G. Carbohydrate quality is key for a healthy and sustainable diet. Nat. Rev. Endocrinol. 2019, 15, 257–258. [Google Scholar]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Han, T.; Cheng, Y.; Tian, S.; Wang, L.; Liang, X.; Duan, W.; Na, L.; Sun, C. Changes in triglycerides and high-density lipoprotein cholesterol may precede peripheral insulin resistance, with 2-h insulin partially mediating this unidirectional relationship: A prospective cohort study. Cardiovasc. Diabetol. 2016, 15, 1–11. [Google Scholar]
- Jiang, Y.; Zhao, Y.; Milne, G.; Dai, Q.; Chen, Q.; Zhang, X.; Lan, Q.; Rothman, N.; Gao, Y.-T.; Cai, Q. Quality of dietary carbohydrate is more important than its quantity in lipid peroxidation. Am. J. Clin. Nutr. 2022, 116, 189–196. [Google Scholar] [PubMed]
- Lin, Y.-H.; Huang, Y.-Y.; Chen, H.-Y.; Hsieh, S.-H.; Sun, J.-H.; Chen, S.-T.; Lin, C.-H. Impact of carbohydrate on glucose variability in patients with type 1 diabetes assessed through professional continuous glucose monitoring: A retrospective study. Diabetes Ther. 2019, 10, 2289–2304. [Google Scholar]
- Zhang, L.; Zhang, Y.; Wang, X.; Dong, L.; Li, Q.; Ren, W.; Li, Y.; Bai, J.; Gong, Q.; Kuang, H. Safety and effectiveness of metformin plus lifestyle intervention compared with lifestyle intervention alone in preventing progression to diabetes in a Chinese population with impaired glucose regulation: A multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2023, 11, 567–577. [Google Scholar]
- Chu, N.; He, J.; Leung, K.H.; Chan, J.C.; Chow, E. Explaining the high rate of progression from prediabetes to type 2 diabetes in China. Lancet Diabetes Endocrinol. 2023, 11, 794. [Google Scholar]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Low-carbohydrate diets and all-cause mortality: A systematic review and meta-analysis of observational studies. PLoS ONE 2013, 8, e55030. [Google Scholar]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar]
- Jahromi, M.K.; Saber, N.; Norouzzadeh, M.; Daftari, G.; Pourhabibi-Zarandi, F.; Ahmadirad, H.; Farhadnejad, H.; Teymoori, F.; Salehi-Sahlabadi, A.; Mirmiran, P. Carbohydrate quality index and risk of non-alcoholic fatty liver disease in Iranian adults. BMC Endocr. Disord. 2024, 24, 195. [Google Scholar]
- McRae, M.P. Dietary fiber is beneficial for the prevention of cardiovascular disease: An umbrella review of meta-analyses. J. Chiropr. Med. 2017, 16, 289–299. [Google Scholar]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [PubMed]
- Zhu, Y.; Yang, H.; Zhang, Y.; Rao, S.; Mo, Y.; Zhang, H.; Liang, S.; Zhang, Z.; Yang, W. Dietary fiber intake and non-alcoholic fatty liver disease: The mediating role of obesity. Front. Public Health 2023, 10, 1038435. [Google Scholar]
- Zhao, H.; Yang, A.; Mao, L.; Quan, Y.; Cui, J.; Sun, Y. Association between dietary fiber intake and non-alcoholic fatty liver disease in adults. Front. Nutr. 2020, 7, 593735. [Google Scholar] [CrossRef]
- Kim, H.-K.; Nanba, T.; Ozaki, M.; Chijiki, H.; Takahashi, M.; Fukazawa, M.; Okubo, J.; Shibata, S. Effect of the intake of a snack containing dietary fiber on postprandial glucose levels. Foods 2020, 9, 1500. [Google Scholar] [CrossRef]
- Luk, A.O.; Zee, B.C.; Chong, M.; Ozaki, R.; Rausch, C.W.; Chan, M.H.; Ma, R.C.; Kong, A.P.; Chow, F.C.; Chan, J.C. A proof-of-concept study to evaluate the efficacy and safety of BTI320 on post-prandial hyperglycaemia in Chinese subjects with pre-diabetes. BMC Endocr. Disord. 2018, 18, 1–10. [Google Scholar]
- Jefferson, A.; Adolphus, K. The effects of intact cereal grain fibers, including wheat bran on the gut microbiota composition of healthy adults: A systematic review. Front. Nutr. 2019, 6, 33. [Google Scholar]
- Chu, N.H.; Chow, E.; Chan, J.C. The Therapeutic Potential of the Specific Intestinal Microbiome (SIM) Diet on Metabolic Diseases. Biology 2024, 13, 498. [Google Scholar] [CrossRef]
- Pérez-Montes de Oca, A.; Julián, M.T.; Ramos, A.; Puig-Domingo, M.; Alonso, N. Microbiota, fiber, and NAFLD: Is there any connection? Nutrients 2020, 12, 3100. [Google Scholar] [CrossRef]
- Berding, K.; Carbia, C.; Cryan, J.F. Going with the grain: Fiber, cognition, and the microbiota-gut-brain-axis. Exp. Biol. Med. 2021, 246, 796–811. [Google Scholar]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar]
- Martínez-Montoro, J.I.; Damas-Fuentes, M.; Fernández-García, J.C.; Tinahones, F.J. Role of the gut microbiome in beta cell and adipose tissue crosstalk: A review. Front. Endocrinol. 2022, 13, 869951. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary fibre modulates the gut microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a low-fat vegan diet on body weight, insulin sensitivity, postprandial metabolism, and intramyocellular and hepatocellular lipid levels in overweight adults: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef]
- Yanai, H.; Katsuyama, H.; Hamasaki, H.; Abe, S.; Tada, N.; Sako, A. Effects of carbohydrate and dietary fiber intake, glycemic index and glycemic load on HDL metabolism in Asian populations. J. Clin. Med. Res. 2014, 6, 321. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Krebs, M.; Krssak, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhäusl, W.; Roden, M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002, 51, 599–605. [Google Scholar] [CrossRef]
- Linn, T.; Santosa, B.; Grönemeyer, D.; Aygen, S.; Scholz, N.; Busch, M.; Bretzel, R. Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia 2000, 43, 1257–1265. [Google Scholar] [CrossRef]
- Burén, J.; Ericsson, M.; Damasceno, N.R.T.; Sjödin, A. A ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, normal-weight women: A randomized controlled feeding trial. Nutrients 2021, 13, 814. [Google Scholar] [CrossRef]
| Variable | Total Cohort (n = 177) | Men (n = 72) | Women (n = 105) | p-Value |
|---|---|---|---|---|
| Age, years | 60 (54–62) | 60 (57–62) | 60 (52–62) | 0.501 |
| Male, n (%) | 72 (41) | NA | NA | NA |
| Statins, n (%) | 66 (37.3) | 33 (45.8) | 33 (31.4) | 0.059 |
| Antihypertensive drugs, n (%) | 76 (42.9) | 38 (52.8) | 38 (36.2) | 0.032 |
| Weight, kg | 70.6 ± 12.9 | 78.1 ± 12.1 | 65.5 ± 10.8 | <0.0001 |
| Waist circumferences, cm | 93.5 ± 9.8 | 96.5 ± 9.7 | 91.5 ± 9.4 | 0.001 |
| Hip circumferences, cm | 99.8 ± 7.5 | 100.3 ± 7.4 | 99.5 ± 7.7 | 0.508 |
| BMI, kg/m2 | 26.7 ± 3.9 | 27.0 ± 3.7 | 26.5 ± 4.1 | 0.429 |
| Systolic blood pressure, mmHg | 133 ± 16.6 | 133 ± 16 | 133 ± 17 | 0.999 |
| Diastolic blood pressure, mmHg | 82.9 ± 10.4 | 86.3 ± 9.8 | 80.6 ± 10.1 | <0.0001 |
| Body fat, % | 31.8 ± 8.7 | 25.1 ± 5.3 | 36.4 ± 7.4 | <0.0001 |
| Fasting plasma glucose, mmol/L | 5.3 ± 0.5 | 5.4 ± 0.5 | 5.3 ± 0.5 | 0.646 |
| 1 h plasma glucose, mmol/L | 10.9 ± 1.6 | 11.1 ± 1.8 | 10.8 ± 1.5 | 0.269 |
| 2 h plasma glucose, mmol/L | 8.4 ± 1.4 | 8.2 ± 1.6 | 8.5 ± 1.2 | 0.106 |
| AUC-PG mmol/L.min−1 | 18.5 ± 1.9 | 18.5 ± 2.0 | 18.6 ± 1.8 | 0.875 |
| Fasting plasma CP, pmol/L | 563 (434–742) | 635 (478–796) | 520 (352–712) | 0.008 |
| 2 hr plasma CP, pmol/L | 2955 (2295–3757) | 3141 (2537–3809) | 2774 (2270–3668) | 0.130 |
| HOMA2-IR | 1.27 (0.94–1.67) | 1.37 (1.07–1.78) | 1.16 (0.80–1.54) | 0.009 |
| HOMA2-β (%) | 99.9 (77.0–125.5) | 111.8 (81.3–130.8) | 93.2 (73.7–117.8) | 0.057 |
| HOMA2-S (%) | 78.4 (59.0–104.1) | 72.8 (56.3–93.1) | 85.4 (63.9–118.7) | 0.015 |
| Lipid profiles | ||||
| Total cholesterol, mmol/L | 4.9 ± 1.0 | 4.7 ± 1.1 | 5.1 ± 0.9 | 0.026 |
| LDL, mmol/L | 3.0 (2.3–3.5) | 2.8 (2.1–3.5) | 3.0 (2.4–3.6) | 0.239 |
| HDL, mmol/L | 1.3 (1.1–1.6) | 1.1 (1.0–1.3) | 1.1 (1.3–1.7) | <0.0001 |
| Triglycerides, mmol/L | 1.2 (0.9–3.5) | 1.3 (0.9–1.5) | 1.1 (0.9–1.5) | 0.139 |
| Hepatic parameters | ||||
| Liver stiffness score (kPa) | 4.4 (3.9–5.3) | 4.7 (3.9–5.5) | 4.4 (3.8–5.2) | 0.316 |
| CAP score (dB/m) | 264 ± 54 | 267 ± 51 | 262 ± 56 | 0.645 |
| Physical Activities | ||||
| Vigorous, MET-min/week | 0 (0–0) | 0 (0–0) | 0 (0–240) | 0.369 |
| Moderate, MET-min/week | 0 (0–480) | 0 (0–720) | 120 (0–480) | 0.980 |
| Light, MET-min/week | 693 (330–1386) | 693 (297–1386) | 693 (347–1386) | 0.428 |
| Total physical activity MET-min/week | 1166 (484–2243) | 1188 (594–2772) | 1208 (495–2316) | 0.815 |
| Sedentary, min/day | 300 (180–480) | 300 (180–480) | 300 (180–420) | 0.429 |
| Variable | Total Cohort (n = 177) | Men (n = 72) | Women (n = 105) | p-Value |
|---|---|---|---|---|
| Dietary information | ||||
| Energy, kcal/day | 1885 (1553–2182) | 2110 (1809–2515) | 1757 (1454–2046) | <0.0001 |
| Carbohydrates, g/day | 201 (165–248) | 232 (183–274) | 190 (155–234) | <0.0001 |
| Protein, g/day | 87 (72–102) | 98 (85–115) | 81 (64–90) | <0.0001 |
| Protein/CHO | 0.42 (0.34–0.52) | 0.44 (0.36–0.54) | 0.42 (0.33–0.51) | 0.176 |
| Fat, g/day | 80 ± 25 | 89.6 ± 25 | 73.7 ± 23 | <0.0001 |
| Saturated fat, g/day | 19.4 (15.5–24.4) | 21.5 (18.1–27.0) | 12.3 (13.0–21.7) | <0.0001 |
| Fat/CHO | 0.37 (0.30–0.47) | 0.38 (0.30–0.47) | 0.36 (0.29–0.65) | 0.678 |
| Fibre, g/day | 11 (8–15) | 10 (8–15) | 12 (8–16) | 0.232 |
| Soluble fibre, g/day | 0.70 (0.36–1.26) | 0.59 (0.33–1.29) | 0.77 (0.42–1.22) | 0.232 |
| Fibre/CHO | 0.05 (0.04–0.08) | 0.05 (0.03–0.07) | 0.06 (0.04–0.08) | <0.0001 |
| Total sugar, g/day | 41 (29–58) | 47 (31–60) | 39 (27–56) | 0.080 |
| Minerals | ||||
| Potassium, mg | 2291 (1726–2959) | 2331 (1849–3014) | 2202 (1646–2955) | 0.092 |
| Sodium, mg | 3743 (3104–4528) | 3975 (3524–4999) | 3620 (2867–4248) | 0.008 |
| Sodium/potassium ratio | 1.72 (1.18–2.17) | 1.76 (1.27–2.17) | 1.68 (1.17–2.19) | 0.836 |
| Dependent Variable (fibre/CHO) | Standardised Beta Coefficient | 95% CI | Adjusted R2 | p Value |
|---|---|---|---|---|
| Systolic blood pressure (mmHg) | ||||
| Base model | −0.232 | [−188.74 to −41.15] | 0.092 | 0.002 |
| Model 1 | −0.245 | [−200.24 to −41.74] | 0.139 | 0.003 |
| Model 2 | −0.281 | [−227.5 to −56.35] | 0.157 | 0.001 |
| Model 3 | −0.277 | [−236.32 to −43.48] | 0.151 | 0.005 |
| Model 4 | −0.281 | [−231.94 to −51.83] | 0.145 | 0.002 |
| Triglycerides (mmol/L) | ||||
| Base model | −0.198 | [−9.908 to −1.299] | 0.054 | 0.011 |
| Model 1 | −0.176 | [−9.612 to −0.356] | 0.103 | 0.035 |
| Model 2 | −0.190 | [−10.929 to −0.531] | 0.119 | 0.031 |
| Model 3 | −0.270 | [−13.889 to −2.361] | 0.134 | 0.006 |
| Model 4 | −0.195 | [−11.332 to −0.410] | 0.110 | 0.035 |
| Fatty liver, CAP (dB/m) | ||||
| Base model | −0.249 | [−696.8 to −64.6] | 0.047 | 0.019 |
| Model 1 | −0.271 | [−782.0 to −45.9] | 0.073 | 0.028 |
| Model 2 | −0.252 | [−747.3 to −21.2] | 0.108 | 0.038 |
| Model 3 | −0.308 | [−866.9 to −74.3] | 0.109 | 0.020 |
| Model 4 | −0.259 | [−787.3 to −5.02] | 0.097 | 0.047 |
| Insulin resistance, HOMA2-IR | ||||
| Base model | −0.272 | [−7.286 to −2.225] | 0.150 | <0.0001 |
| Model 1 | −0.276 | [−7.581 to −2.044] | 0.160 | 0.001 |
| Model 2 | −0.263 | [−7.866 to −1.726] | 0.170 | 0.002 |
| Model 3 | −0.252 | [−8.024 to −1.148] | 0.165 | 0.009 |
| Model 4 | −0.237 | [−7.529 to −1.098] | 0.165 | 0.009 |
| Triglycerides (>1.7 mmol/L) | Unadjusted Model OR (95% CI), p-Value | Model 1 OR (95% CI), p-Value | Model 2 OR (95% CI), p-Value |
|---|---|---|---|
| 1st quartile (<0.038 fibre/CHO ratio) | Ref | Ref | Ref |
| 2nd quartile (0.038–0.055) fibre/CHO ratio) | 0.123 (0.014–1.046), p = 0.598 | 0.139 (0.016–1.218), p = 0.075 | 0.145 (0.016–1.273), p = 0.081 |
| 3rd quartile (0.055–0.076 fibre/CHO ratio) | 0.079 (0.010–0.648), p = 0.018 | 0.081 (0.010–0.667), p = 0.019 | 0.078 (0.009–0.645), p = 0.018 |
| 4th quartile (>0.076 fibre/CHO ratio) | 0.079 (0.010–0.648), p = 0.018 | 0.080 (0.010–0.659), p = 0.019 | 0.072 (0.009–0.603), p = 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, N.H.S.; Yu, Y.; He, J.; Li, C.R.H.; Pai, S.I.; Leung, K.H.T.; Ma, R.C.W.; Chan, J.C.N.; Chow, E. Carbohydrate Quality Is Independently Associated with Cardiometabolic Risk in Chinese Individuals with Impaired Glucose Tolerance. Nutrients 2025, 17, 1123. https://doi.org/10.3390/nu17071123
Chu NHS, Yu Y, He J, Li CRH, Pai SI, Leung KHT, Ma RCW, Chan JCN, Chow E. Carbohydrate Quality Is Independently Associated with Cardiometabolic Risk in Chinese Individuals with Impaired Glucose Tolerance. Nutrients. 2025; 17(7):1123. https://doi.org/10.3390/nu17071123
Chicago/Turabian StyleChu, Natural H. S., Yelia Yu, Jie He, Cynthia R. H. Li, Seong I. Pai, Kathy H. T. Leung, Ronald C. W. Ma, Juliana C. N. Chan, and Elaine Chow. 2025. "Carbohydrate Quality Is Independently Associated with Cardiometabolic Risk in Chinese Individuals with Impaired Glucose Tolerance" Nutrients 17, no. 7: 1123. https://doi.org/10.3390/nu17071123
APA StyleChu, N. H. S., Yu, Y., He, J., Li, C. R. H., Pai, S. I., Leung, K. H. T., Ma, R. C. W., Chan, J. C. N., & Chow, E. (2025). Carbohydrate Quality Is Independently Associated with Cardiometabolic Risk in Chinese Individuals with Impaired Glucose Tolerance. Nutrients, 17(7), 1123. https://doi.org/10.3390/nu17071123






