Body Composition and Its Outcomes and Management in Multiple Sclerosis: Narrative Review
Abstract
1. Introduction
2. Concepts and Measurement of Body Composition
2.1. Adiposity (Fat Mass)
2.2. Bone Mineral Density (Surrogate for Bone Mass)
2.3. Lean Soft Tissue (Surrogate for Muscle Mass)
3. Methods
3.1. Quality Assessment of Narrative Reviews
3.2. Rationale and Objective
3.3. Literature Search and Study Selection
4. Results
4.1. Body Composition in Multiple Sclerosis Versus Controls
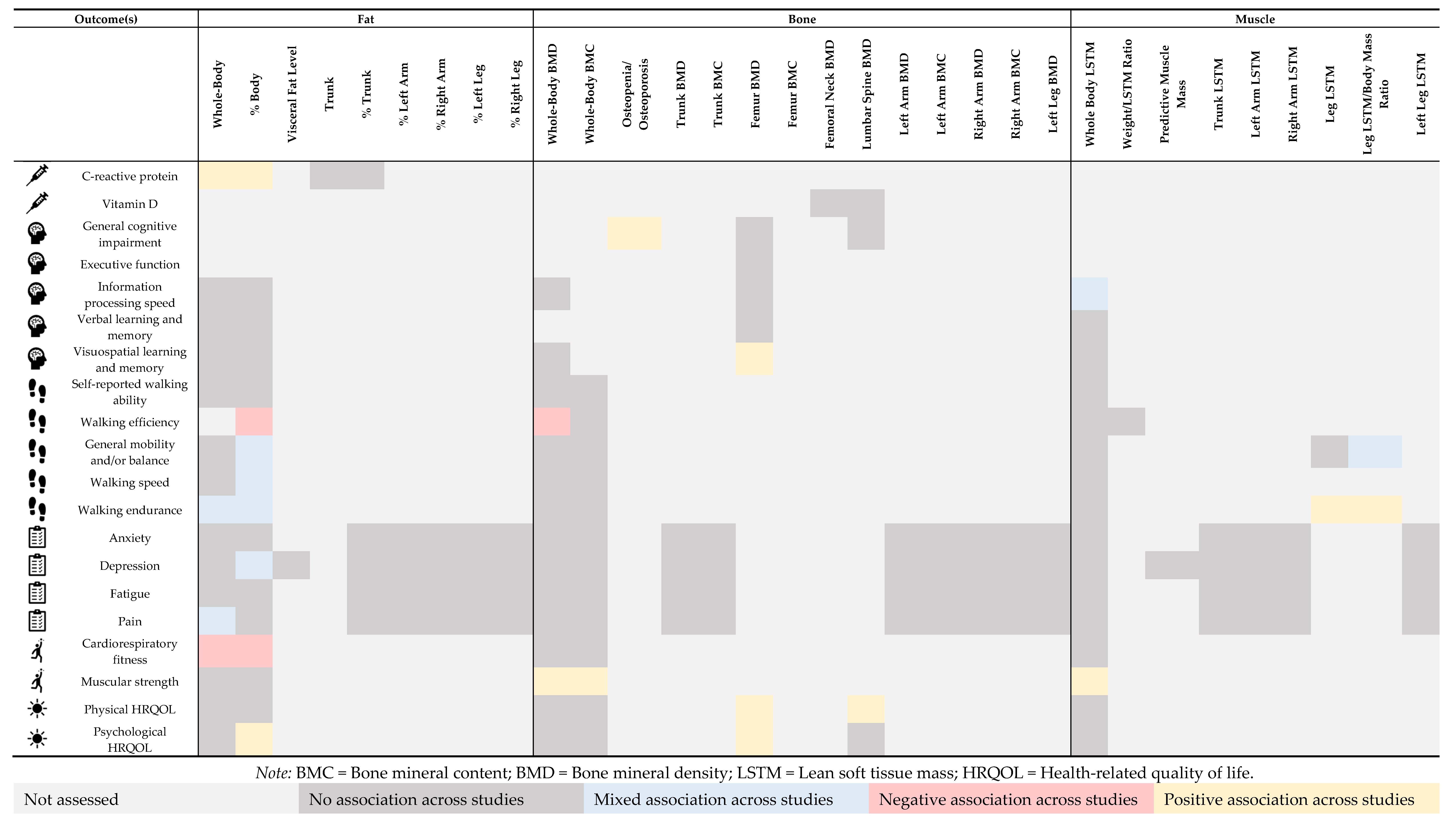
4.2. Body Composition via DEXA and Disease- and Health-Related Outcomes in Multiple Sclerosis
4.2.1. Biomarkers
4.2.2. Cognition
4.2.3. Mobility
4.2.4. Symptoms
4.2.5. Fitness
4.2.6. Quality of Life
4.3. Interventions and Management of Body Composition in Multiple Sclerosis
4.3.1. Physical Activity Interventions
4.3.2. Diet Interventions
4.3.3. Combined Physical Activity and Diet-Based Intervention
5. Limitations
6. Future Research Directions
7. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- International Classification of Diseases, Eleventh Revision (ICD-11), World Health Organization (WHO) 2019/2021. Attribution-NoDerivatives 3.0 IGO. Available online: https://icd.who.int/browse11 (accessed on 12 November 2024).
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.-C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes; National Health Statistics Reports, Number 158; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. [Google Scholar]
- Emmerich, S.D.; Fryar, C.D.; Stierman, B.; Ogden, C.L. Obesity and Severe Obesity Prevalence in Adults: United States, August 2021–August 2023; NCHS Data Brief, No 508; National Center for Health Statistics: Hyattsville, MD, USA, 2024. [Google Scholar] [CrossRef]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Johnson, C.L. Prevalence and trends in obesity among U.S. adults, 1999–2000. JAMA 2002, 288, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Marrie, R.A.; Fisk, J.D.; Fitzgerald, K.; Kowalec, K.; Maxwell, C.; Rotstein, D.; Salter, A.; Tremlett, H. Etiology, effects and management of comorbidities in multiple sclerosis: Recent advances. Front. Immunol. 2023, 14, 1197195. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779, Correction in JAMA 2021, 325, 2211. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Genes, T.M. Obesity and Multiple Sclerosis-A Multifaceted Association. J. Clin. Med. 2021, 10, 2689. [Google Scholar] [CrossRef]
- Nourbakhsh, B.; Mowry, E.M. Multiple Sclerosis Risk Factors and Pathogenesis. Continuum 2019, 25, 596–610. [Google Scholar] [CrossRef]
- Lutfullin, I.; Eveslage, M.; Bittner, S.; Antony, G.; Flaskamp, M.; Luessi, F.; Salmen, A.; Gisevius, B.; Klotz, L.; Korsukewitz, C.; et al. Association of obesity with disease outcome in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2023, 94, 57–61. [Google Scholar] [CrossRef]
- Galioto, R.; Berenholz, O.; Wang, Z.; Conway, D.S.; Planchon, S.M.; Rao, S.M. Does obesity exacerbate brain lesion volume and atrophy in patients with multiple sclerosis? Mult. Scler. Relat. Disord. 2020, 46, 102502. [Google Scholar] [CrossRef]
- Chu, D.T.; Rosso, M.; Gonzalez, C.T.; Saxena, S.; Healy, B.C.; Weiner, H.L.; Chitnis, T. Obesity is associated with the Optic Neuritis severity in Male patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 51, 102910. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.W.; Mostert, J.; Repovic, P.; Bowen, J.D.; Strijbis, E.; Uitdehaag, B.; Cutter, G. Smoking, obesity, and disability worsening in PPMS: An analysis of the INFORMS original trial dataset. J. Neurol. 2022, 269, 1663–1669. [Google Scholar] [CrossRef]
- Papetti, L.; Panella, E.; Monte, G.; Ferilli, M.A.N.; Tarantino, S.; Checchi, M.P.; Valeriani, M. Pediatric Onset Multiple Sclerosis and Obesity: Defining the Silhouette of Disease Features in Overweight Patients. Nutrients 2023, 15, 4880. [Google Scholar] [CrossRef] [PubMed]
- Duren, D.L.; Sherwood, R.J.; Czerwinski, S.A.; Lee, M.; Choh, A.C.; Siervogel, R.M.; Chumlea, W.C. Body composition methods: Comparisons and interpretation. J. Diabetes Sci. Technol. 2008, 2, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Lemos, T.; Gallagher, D. Current body composition measurement techniques. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 310–314. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Leinhard, O.D. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Kuriyan, R. Body composition techniques. Indian J. Med. Res. 2018, 148, 648–658. [Google Scholar] [CrossRef]
- Pray, R.; Riskin, S. The History and Faults of the Body Mass Index and Where to Look Next: A Literature Review. Cureus 2023, 15, e48230. [Google Scholar] [CrossRef]
- Wu, Y.; Li, D.; Vermund, S.H. Advantages and Limitations of the Body Mass Index (BMI) to Assess Adult Obesity. Int. J. Environ. Res. Public Health 2024, 21, 757. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.L.; Korinek, J.; Allison, T.G.; A Batsis, J.; Sert-Kuniyoshi, F.H.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef]
- Razak, F.; Anand, S.S.; Shannon, H.; Vuksan, V.; Davis, B.; Jacobs, R.; Teo, K.K.; McQueen, M.; Yusuf, S. Defining obesity cut points in a multiethnic population. Circulation 2007, 115, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; DeJonge, S.R.; Flores, V.A.; Jeng, B.; Motl, R.W. Systematic review and meta-analysis of sedentary behavior in persons with multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 80, 105124. [Google Scholar] [CrossRef]
- Kinnett-Hopkins, D.; Adamson, B.; Rougeau, K.; Motl, R.W. People with MS are less physically active than healthy controls but as active as those with other chronic diseases: An updated meta-analysis. Mult. Scler. Relat. Disord. 2017, 13, 38–43. [Google Scholar] [CrossRef]
- Bisson, E.J.; Finlayson, M.L.; Ekuma, O.; Leslie, W.D.; Marrie, R.A. Multiple sclerosis is associated with low bone mineral density and osteoporosis. Neurol. Clin. Pract. 2019, 9, 391–399. [Google Scholar] [CrossRef]
- Gunsalus, K.T.W.; Mixon, J.K.; House, E.M. Medical Nutrition Education for Health, Not Harm: BMI, Weight Stigma, Eating Disorders, and Social Determinants of Health. Med. Sci. Educ. 2024, 34, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Voruganti, V.S. Precision Nutrition: Recent Advances in Obesity. Physiology 2023, 38, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Acosta, A. Precision Medicine and Obesity. Gastroenterol. Clin. N. Am. 2021, 50, 127–139. [Google Scholar] [CrossRef]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Shephard, R.J.; Bouchard, C. Principal components of fitness: Relationship to physical activity and lifestyle. Can. J. Appl. Physiol. 1994, 19, 200–214. [Google Scholar] [CrossRef]
- Klaus, S. Adipose tissue as a regulator of energy balance. Curr. Drug Targets 2004, 5, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef]
- Johnston, E.K.; Abbott, R.D. Adipose Tissue Paracrine-, Autocrine-, and Matrix-Dependent Signaling during the Development and Progression of Obesity. Cells 2023, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef]
- Stults-Kolehmainen, M.A.; Stanforth, P.R.; Bartholomew, J.B.; Lu, T.; Abolt, C.J.; Sinha, R. DXA estimates of fat in abdominal, trunk and hip regions varies by ethnicity in men. Nutr. Diabetes 2013, 3, e64. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Ng, B.K.; Sommer, M.J.; Heymsfield, S.B. Body composition by DXA. Bone 2017, 104, 101–105. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Guo, Q.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.; Luo, X. Endocrine role of bone in the regulation of energy metabolism. Bone Res. 2021, 9, 25. [Google Scholar] [CrossRef]
- Haseltine, K.N.; Chukir, T.; Smith, P.J.; Jacob, J.T.; Bilezikian, J.P.; Farooki, A. Bone Mineral Density: Clinical Relevance and Quantitative Assessment. J. Nucl. Med. 2021, 62, 446–454. [Google Scholar] [CrossRef]
- Morgan, S.L.; Prater, G.L. Quality in dual-energy X-ray absorptiometry scans. Bone 2017, 104, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Hew-Butler, T.; Jurczyszyn, H.; Sabourin, J.; VanSumeren, M.; Smith-Hale, V. Too Tall for the DXA Scan? Contributions of the Feet and Head to Overall Body Composition. J. Clin. Densitom. 2022, 25, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Spiro, A.J.; Hoang, T.D.; Shakir, M.K.M. Artifacts Affecting Dual-Energy X-Ray Absorptiometry Measurements. AACE Clin. Case Rep. 2019, 5, e263–e266. [Google Scholar] [CrossRef]
- Brooks, S.V.; Guzman, S.D.; Ruiz, L.P. Skeletal muscle structure, physiology, and function. Handb. Clin. Neurol. 2023, 195, 3–16. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, A.; Tarantini, F.; Di Bari, M. Skeletal muscle: An endocrine organ. Clin. Cases Miner. Bone Metab. 2013, 10, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Di Ludovico, A.; La Bella, S.; Ciarelli, F.; Chiarelli, F.; Breda, L.; Mohn, A. Skeletal muscle as a pro- and anti-inflammatory tissue: Insights from children to adults and ultrasound findings. J. Ultrasound 2024, 27, 769–779. [Google Scholar] [CrossRef]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Chaves, L.G.C.M.; Gonçalves, T.J.M.; Bitencourt, A.G.V.; Rstom, R.A.; Pereira, T.R.; Velludo, S.F. Assessment of body composition by whole-body densitometry: What radiologists should know. Radiol. Bras. 2022, 55, 305–311. [Google Scholar] [CrossRef]
- Cheung, C.L.; Lee, G.K.; Au, P.C.; Li, G.H.-Y.; Chan, M.; Li, H.-L.; Cheung, B.M.-Y.; Wong, I.C.-K.; Lee, V.H.-F.; Mok, J.; et al. Systematic review and meta-analysis of lean mass and mortality: Rationale and study description. Osteoporos. Sarcopenia 2021, 7 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef]
- Fink, A.; Kosecoff, J.; Chassin, M.; Brook, R.H. Consensus methods: Characteristics and guidelines for use. Am. J. Public Health 1984, 74, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Tsouris, Z.; Aslanidou, P.; Aloizou, A.-M.; Sokratous, M.; Provatas, A.; Siokas, V.; Deretzi, G.; Hadjigeorgiou, G.M. Body mass index in patients with Multiple Sclerosis: A meta-analysis. Neurol. Res. 2019, 41, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Pilutti, L.; Silveira, S.L.; Herring, M.; Jeng, B.; Edwards, T.; Cederberg, K.L.J.; Fournier, K.; Motl, R.W. Multiple Sclerosis is Associated with Worse Body Composition Across Compartments: Results from a Systematic Review and Meta-Analysis. Manuscript Submitted for Publication.
- Baynard, T.; Hilgenkamp, T.I.M.; Schroeder, E.C.; Motl, R.W.; Fernhall, B. Measures of adiposity differentially correlate with C-reactive protein among persons with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 25, 1–4. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Gallagher, E.; Baier, M.; Green, L.; Feichter, J.; Patrick, K.; Miller, C.; Wrest, K.; Ramanathan, M. Risk of bone loss in men with multiple sclerosis. Mult. Scler. 2004, 10, 170–175. [Google Scholar] [CrossRef]
- Triantafyllou, N.; Lambrinoudaki, I.; Thoda, P.; Andreadou, E.; Kararizou, E.; Alexandrou, A.; Limouris, G.; Antoniou, A.; Tsivgoulis, G. Lack of association between vitamin D levels and bone mineral density in patients with multiple sclerosis. J. Neurol. Sci. 2012, 313, 137–141. [Google Scholar] [CrossRef]
- Kirbas, A.; Kirbas, S.; Anlar, O.; Turkyilmaz, A.K.; Cure, M.C.; Efe, H. Investigation of the relationship between vitamin D and bone mineral density in newly diagnosed multiple sclerosis. Acta Neurol. Belg. 2013, 113, 43–47. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, J.; Chiaravalloti, N.D.; Sandroff, B.M. Treatment and management of cognitive dysfunction in patients with multiple sclerosis. Nat. Rev. Neurol. 2020, 16, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Preziosa, P.; Barkhof, F.; Ciccarelli, O.; Cossarizza, A.; De Stefano, N.; Gasperini, C.; Geraldes, R.; Granziera, C.; Haider, L.; et al. The ageing central nervous system in multiple sclerosis: The imaging perspective. Brain 2024, 147, 3665–3680. [Google Scholar] [CrossRef]
- Sandroff, B.M.; Hubbard, E.A.; Pilutti, L.A.; Motl, R.W. No association between body composition and cognition in ambulatory persons with multiple sclerosis: A brief report. J. Rehabil. Res. Dev. 2015, 52, 301–308. [Google Scholar] [CrossRef]
- Batista, S.; Teter, B.; Sequeira, K.; Josyula, S.; Hoogs, M.; Ramanathan, M.; Benedict, R.H.; Weinstock-Guttman, B. Cognitive impairment is associated with reduced bone mass in multiple sclerosis. Mult. Scler. 2012, 18, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Pilutti, L.A.; Motl, R.W. Body composition and disability in people with multiple sclerosis: A dual-energy x-ray absorptiometry study. Mult. Scler. Relat. Disord. 2019, 29, 41–47. [Google Scholar] [CrossRef]
- Anand, S.S.; Friedrich, M.G.; Lee, D.S.; Awadalla, P.; Després, J.P.; Desai, D.; de Souza, R.J.; Dummer, T.; Parraga, G.; Larose, E.; et al. Evaluation of Adiposity and Cognitive Function in Adults. JAMA Netw. Open 2022, 5, e2146324. [Google Scholar] [CrossRef]
- Xie, C.; Wang, C.; Luo, H. Increased risk of osteoporosis in patients with cognitive impairment: A systematic review and meta-analysis. BMC Geriatr. 2023, 23, 797. [Google Scholar] [CrossRef]
- Tessier, A.J.; Wing, S.S.; Rahme, E.; Morais, J.A.; Chevalier, S. Association of Low Muscle Mass With Cognitive Function During a 3-Year Follow-up Among Adults Aged 65 to 86 Years in the Canadian Longitudinal Study on Aging. JAMA Netw. Open 2022, 5, e2219926. [Google Scholar] [CrossRef]
- Baird, J.F.; Sandroff, B.M.; Motl, R.W. Therapies for mobility disability in persons with multiple sclerosis. Expert. Rev. Neurother. 2018, 18, 493–502. [Google Scholar] [CrossRef]
- Rooney, S.; McWilliam, G.; Wood, L.; Moffat, F.; Paul, L. Oxygen Cost of Walking in People With Multiple Sclerosis and Its Association With Fatigue: A Systematic Review and Meta-analysis. Int. J. MS Care 2022, 24, 74–80. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.G.; Justice, J.N.; Freitas, E.C.; Kershaw, E.E.; Sparks, L.M. Adipose Tissue Quality in Aging: How Structural and Functional Aspects of Adipose Tissue Impact Skeletal Muscle Quality. Nutrients 2019, 11, 2553. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Ashraf, A.; Zeynali, N.; Ebrahimi, B.; AJehu, D. Balance and functional mobility predict low bone mineral density among postmenopausal women undergoing recent menopause with osteoporosis, osteopenia, and normal bone mineral density: A cross-sectional study. Geriatr. Nurs. 2021, 42, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.F.; Naumova, E.N.; Carabello, R.J.; Phillips, E.M.; Fielding, R.A. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J. Nutr. Health Aging 2008, 12, 493–498. [Google Scholar] [CrossRef]
- Cozart, J.S.; Bruce, A.S.; Shook, R.P.; Befort, C.; Siengsukon, C.; Simon, S.; Lynch, S.; Mahmoud, R.; Drees, B.; Posson, P.; et al. Body metrics are associated with clinical, free-living, and self-report measures of mobility in a cohort of adults with obesity and multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 79, 105010. [Google Scholar] [CrossRef]
- Ward, C.L.; Suh, Y.; Lane, A.D.; Yan, H.; Ranadive, S.M.; Fernhall, B.; Motl, R.W.; Evans, E.M. Body composition and physical function in women with multiple sclerosis. J. Rehabil. Res. Dev. 2013, 50, 1139–1147. [Google Scholar] [CrossRef]
- Jeng, B.; Huynh, T.L.T.; Feasel, C.D.; Motl, R.W. Oxygen cost of walking and its relationship with body composition in multiple sclerosis. Int. J. Obes. 2023, 47, 138–143. [Google Scholar] [CrossRef]
- Jeng, B.; Motl, R.W. No association between body composition and walking outcomes in multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 68, 104242. [Google Scholar] [CrossRef]
- Nameni, G.; Jazayeri, S.; Salehi, M.; Esrafili, A.; Hajebi, A.; Motevalian, S.A. Association between visceral adiposity and generalized anxiety disorder (GAD). BMC Psychol. 2024, 12, 49. [Google Scholar] [CrossRef]
- Lv, X.; Cai, J.; Li, X.; Wang, X.; Ma, H.; Heianza, Y.; Qi, L.; Zhou, T. Body composition, lifestyle, and depression: A prospective study in the UK biobank. BMC Public Health 2024, 24, 393. [Google Scholar] [CrossRef]
- van Baar, H.; Bours, M.J.L.; Beijer, S.; van Zutphen, M.; van Duijnhoven, F.J.B.; Kok, D.E.; Wesselink, E.; de Wilt, J.H.W.; Kampman, E.; Winkels, R.M. Body composition and its association with fatigue in the first 2 years after colorectal cancer diagnosis. J. Cancer Surviv. 2021, 15, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.P.; Arnold, J.B.; Evans, A.M.; Yaxley, A.; Damarell, R.A.; Shanahan, E.M. The association between body fat and musculoskeletal pain: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2018, 19, 233. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, E.; Jasińska, E.; Głuszek-Osuch, M.; Suliga, E. Depressive Symptoms in Multiple Sclerosis: Links to Body Composition, Physical Activity, and Functional Ability. Med. Sci. Monit. 2024, 30, e943977. [Google Scholar] [CrossRef]
- Silveira, S.L.; Pilutti, L.A.; Motl, R.W. No evidence of associations among body composition and symptoms in persons with multiple sclerosis. Rehabil. Psychol. 2020, 65, 80–86. [Google Scholar] [CrossRef]
- Klaren, R.E.; Sandroff, B.M.; Fernhall, B.; Motl, R.W. Comprehensive Profile of Cardiopulmonary Exercise Testing in Ambulatory Persons with Multiple Sclerosis. Sports Med. 2016, 46, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.; Dalgas, U.; Wens, I.; Hvid, L.G. Muscle strength and power in persons with multiple sclerosis—A systematic review and meta-analysis. J. Neurol. Sci. 2017, 376, 225–241. [Google Scholar] [CrossRef]
- Heileson, J.L.; Papadakis, Z.; Ismaeel, A.; Richardson, K.A.; Torres, R.; Funderburk, L.; Gallucci, A.; Koutakis, P.; Forsse, J.S. The Benefits of Utilizing Total Body Composition as a Predictor of Cardiorespiratory Fitness Based on Age: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 5758. [Google Scholar] [CrossRef]
- Wen, Z.; Gu, J.; Chen, R.; Wang, Q.; Ding, N.; Meng, L.; Wang, X.; Liu, H.; Sheng, Z.; Zheng, H. Handgrip Strength and Muscle Quality: Results from the National Health and Nutrition Examination Survey Database. J. Clin. Med. 2023, 12, 3184. [Google Scholar] [CrossRef]
- O’Mahony, J.; Salter, A.; Ciftci-Kavaklioglu, B.; Fox, R.J.; Cutter, G.R.; Marrie, R.A. Physical and Mental Health-Related Quality of Life Trajectories Among People with Multiple Sclerosis. Neurology 2022, 99, e1538–e1548. [Google Scholar] [CrossRef]
- Sehanovic, A.; Kunic, S.; Ibrahimagic, O.C.; Smajlovic, D.; Tupkovic, E.; Mehicevic, A.; Zoletic, E. Contributing Factors to the Quality of Life in Multiple Sclerosis. Med. Arch. 2020, 74, 368–373. [Google Scholar] [CrossRef]
- Mikkola, T.M.; Kautiainen, H.; von Bonsdorff, M.B.; Salonen, M.K.; Wasenius, N.; Kajantie, E.; Eriksson, J.G. Body composition and changes in health-related quality of life in older age: A 10-year follow-up of the Helsinki Birth Cohort Study. Qual. Life Res. 2020, 29, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Nipp, R.D.; Fuchs, G.; El-Jawahri, A.; Mario, J.; Troschel, F.M.; Greer, J.A.; Gallagher, E.R.; Jackson, V.A.; Kambadakone, A.; Hong, T.S.; et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. Oncologist 2018, 23, 97–104. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, D.; Yadav, G.; Mishra, S.R.; Gupta, A.K.; Jauhari, S.; Roy, M.S. Comparison of Quality of Life and Bone Mass Density among Postmenopausal Women: A Cross-sectional Study. J. Midlife Health 2020, 11, 224–230. [Google Scholar] [CrossRef]
- Ayatollahi, A.; Mohajeri-Tehrani, M.R.; Nafissi, S. Factors affecting bone mineral density in multiple sclerosis patients. Iran. J. Neurol. 2013, 12, 19–22. [Google Scholar] [PubMed]
- Motl, R.W.; Sandroff, B.M. Benefits of Exercise Training in Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2015, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.-A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Multiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Lin, Z.; Shi, G.; Liao, X.; Huang, J.; Yu, M.; Liu, W.; Luo, X.; Zhan, H.; Cai, X. Correlation between sedentary activity, physical activity and bone mineral density and fat in America: National Health and Nutrition Examination Survey, 2011–2018. Sci Rep. 2023, 13, 10054. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Learmonth, Y.C.; PHerring, M.; Russell, D.I.; A Pilutti, L.; Day, S.; Marck, C.H.; Chan, B.; Metse, A.P.; Motl, R.W. Safety of exercise training in multiple sclerosis: An updated systematic review and meta-analysis. Mult. Scler. 2023, 29, 1604–1631. [Google Scholar] [CrossRef]
- Youssef, H.; Gönül, M.N.; Sobeeh, M.G.; Akar, K.; Feys, P.; Cuypers, K.; Vural, A. Is High-Intensity Interval Training More Effective Than Moderate Continuous Training in Rehabilitation of Multiple Sclerosis: A Comprehensive Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2024, 105, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Keytsman, C.; Van Noten, P.; Spaas, J.; Nieste, I.; Van Asch, P.; Eijnde, B.O. Periodized home-based training: A new strategy to improve high intensity exercise therapy adherence in mildly affected patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 28, 91–97. [Google Scholar] [CrossRef]
- Keytsman, C.; Van Noten, P.; Verboven, K.; Van Asch, P.; Eijnde, B.O. Periodized versus classic exercise therapy in Multiple Sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2021, 49, 102782. [Google Scholar] [CrossRef] [PubMed]
- Wens, I.; Dalgas, U.; Vandenabeele, F.; Grevendonk, L.; Verboven, K.; Hansen, D.; Eijnde, B.O. High Intensity Exercise in Multiple Sclerosis: Effects on Muscle Contractile Characteristics and Exercise Capacity, a Randomised Controlled Trial. PLoS ONE 2015, 10, e0133697. [Google Scholar] [CrossRef]
- Nietse, I.; Spaas, J.; Franssen, W.; Asch, P.V.; Savelberg, H.H.C.M.; Eijnde, B.O. The effect of a structured running exercise intervention on non-exercise physical activity and sedentary behaviour in persons with mild Multiple Sclerosis and healthy controls. J. Act. Sedentary Sleep Behav. 2023, 2, 1–12. [Google Scholar] [CrossRef]
- Kalb, R.; Brown, T.R.; Coote, S.; Costello, K.; Dalgas, U.; Garmon, E.; Giesser, B.; Halper, J.; Karpatkin, H.; Keller, J.; et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult. Scler. 2020, 26, 1459–1469. [Google Scholar] [CrossRef]
- Pilutti, L.A.; Dlugonski, D.; Sandroff, B.M.; Klaren, R.E.; Motl, R.W. Internet-delivered lifestyle physical activity intervention improves body composition in multiple sclerosis: Preliminary evidence from a randomized controlled trial. Arch. Phys. Med. Rehabil. 2014, 95, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Marques, K.A.P.; Trindade, C.B.B.; Almeida, M.C.V.; Bento-Torres, N.V.O. Pilates for rehabilitation in patients with multiple sclerosis: A systematic review of effects on cognition, health-related physical fitness, general symptoms and quality of life. J. Bodyw. Mov. Ther. 2020, 24, 26–36. [Google Scholar] [CrossRef]
- Duff, W.R.D.; Andrushko, J.W.; Renshaw, D.W.; Chilibeck, P.D.; Farthing, J.P.; Danielson, J.; Evans, C.D. Impact of Pilates Exercise in Multiple Sclerosis: A Randomized Controlled Trial. Int. J. MS Care 2018, 20, 92–100. [Google Scholar] [CrossRef]
- Swan, W.I.; Vivanti, A.; Hakel-Smith, N.A.; Hotson, B.; Orrevall, Y.; Trostler, N.; Howarter, K.B.; Papoutsakis, C. Nutrition Care Process and Model Update: Toward Realizing People-Centered Care and Outcomes Management. J. Acad. Nutr. Diet. 2017, 117, 2003–2014. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Vizthum, D.; Henry-Barron, B.; Schweitzer, A.; Cassard, S.D.; Kossoff, E.; Hartman, A.L.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 23, 33–39. [Google Scholar] [CrossRef]
- Ghezzi, L.; Tosti, V.; Shi, L.; Cantoni, C.; Mikesell, R.; Lancia, S.; Zhou, Y.; Obert, K.; Dula, C.; Sen, M.K.; et al. Randomised controlled trial of intermittent calorie restriction in people with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2024, 96, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Wingo, B.C.; Rinker, J.R., 2nd; Green, K.; Peterson, C.M. Feasibility and acceptability of time-restricted eating in a group of adults with multiple sclerosis. Front. Neurol. 2023, 13, 1087126. [Google Scholar] [CrossRef] [PubMed]
- Wingo, B.C.; Rinker, J.R.; Goss, A.M.; Green, K.; Wicks, V.; Cutter, G.R.; Motl, R.W. Feasibility of improving dietary quality using a telehealth lifestyle intervention for adults with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 46, 102504. [Google Scholar] [CrossRef]
- Koppold, D.A.; Breinlinger, C.; Hanslian, E.; Kessler, C.; Cramer, H.; Khokhar, A.R.; Peterson, C.M.; Tinsley, G.; Vernieri, C.; Bloomer, R.J.; et al. International consensus on fasting terminology. Cell Metab. 2024, 36, 1779–1794.e4. [Google Scholar] [CrossRef]
- Aamir, A.B.; Kumari, R.; Latif, R.; Ahmad, S.; Rafique, N.; Salem, A.M.; Alasoom, L.I.; Alsunni, A.; Alabdulhadi, A.S.; Chander, S. Effects of intermittent fasting and caloric restriction on inflammatory biomarkers in individuals with obesity/overweight: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2025, 26, e13838. [Google Scholar] [CrossRef]
- Stringer, E.J.; Cloke, R.W.G.; Van der Meer, L.; Murphy, R.A.; Macpherson, N.A.; Lum, J.J. The Clinical Impact of Time-restricted Eating on Cancer: A Systematic Review. In Nutrition Reviews; Oxford University Press: Oxford, UK, 2024. [Google Scholar] [CrossRef]
- Lin, X.; Wang, S.; Gao, Y. The effects of intermittent fasting for patients with multiple sclerosis (MS): A systematic review. Front. Nutr. 2024, 10, 1328426. [Google Scholar] [CrossRef]
- Rigby, R.R.; Mitchell, L.J.; Hamilton, K.; Williams, L.T. The Use of Behavior Change Theories in Dietetics Practice in Primary Health Care: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2020, 120, 1172–1197. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Abraham, C.; Whittington, C.; McAteer, J.; Gupta, S. Effective techniques in healthy eating and physical activity interventions: A meta-regression. Health Psychol. 2009, 28, 690–701. [Google Scholar] [CrossRef]
- Bruce, J.M.; Cozart, J.S.; Shook, R.P.; Befort, C.; Siengsukon, C.F.; Simon, S.; Lynch, S.G.; Mahmoud, R.; Drees, B.; Posson, P.; et al. Modifying diet and exercise in multiple sclerosis (MoDEMS): A randomized controlled trial for behavioral weight loss in adults with multiple sclerosis and obesity. Mult. Scler. 2023, 29, 1860–1871. [Google Scholar] [CrossRef]
- McLeroy, K.R.; Bibeau, D.; Steckler, A.; Glanz, K. An ecological perspective on health promotion programs. Health Educ. Q. 1988, 15, 351–377. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kidwell-Chandler, A.; Jackson, J.; Jeng, B.; Silveira, S.L.; Pilutti, L.A.; Hibbing, P.R.; Motl, R.W. Body Composition and Its Outcomes and Management in Multiple Sclerosis: Narrative Review. Nutrients 2025, 17, 1021. https://doi.org/10.3390/nu17061021
Kidwell-Chandler A, Jackson J, Jeng B, Silveira SL, Pilutti LA, Hibbing PR, Motl RW. Body Composition and Its Outcomes and Management in Multiple Sclerosis: Narrative Review. Nutrients. 2025; 17(6):1021. https://doi.org/10.3390/nu17061021
Chicago/Turabian StyleKidwell-Chandler, Ariel, Justin Jackson, Brenda Jeng, Stephanie L. Silveira, Lara A. Pilutti, Paul R. Hibbing, and Robert W. Motl. 2025. "Body Composition and Its Outcomes and Management in Multiple Sclerosis: Narrative Review" Nutrients 17, no. 6: 1021. https://doi.org/10.3390/nu17061021
APA StyleKidwell-Chandler, A., Jackson, J., Jeng, B., Silveira, S. L., Pilutti, L. A., Hibbing, P. R., & Motl, R. W. (2025). Body Composition and Its Outcomes and Management in Multiple Sclerosis: Narrative Review. Nutrients, 17(6), 1021. https://doi.org/10.3390/nu17061021







