Patterns of Dietary Fatty Acids and Fat Spreads in Relation to Blood Pressure, Lipids and Insulin Resistance in Young Adults: A Repeat Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Demographic and Clinical Data
2.3. Outcome Data
2.4. Exposure Data
2.5. Clustering of Dietary Fatty Acids and Fat Spreads
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Dietary Fat and Fat Spread Clusters
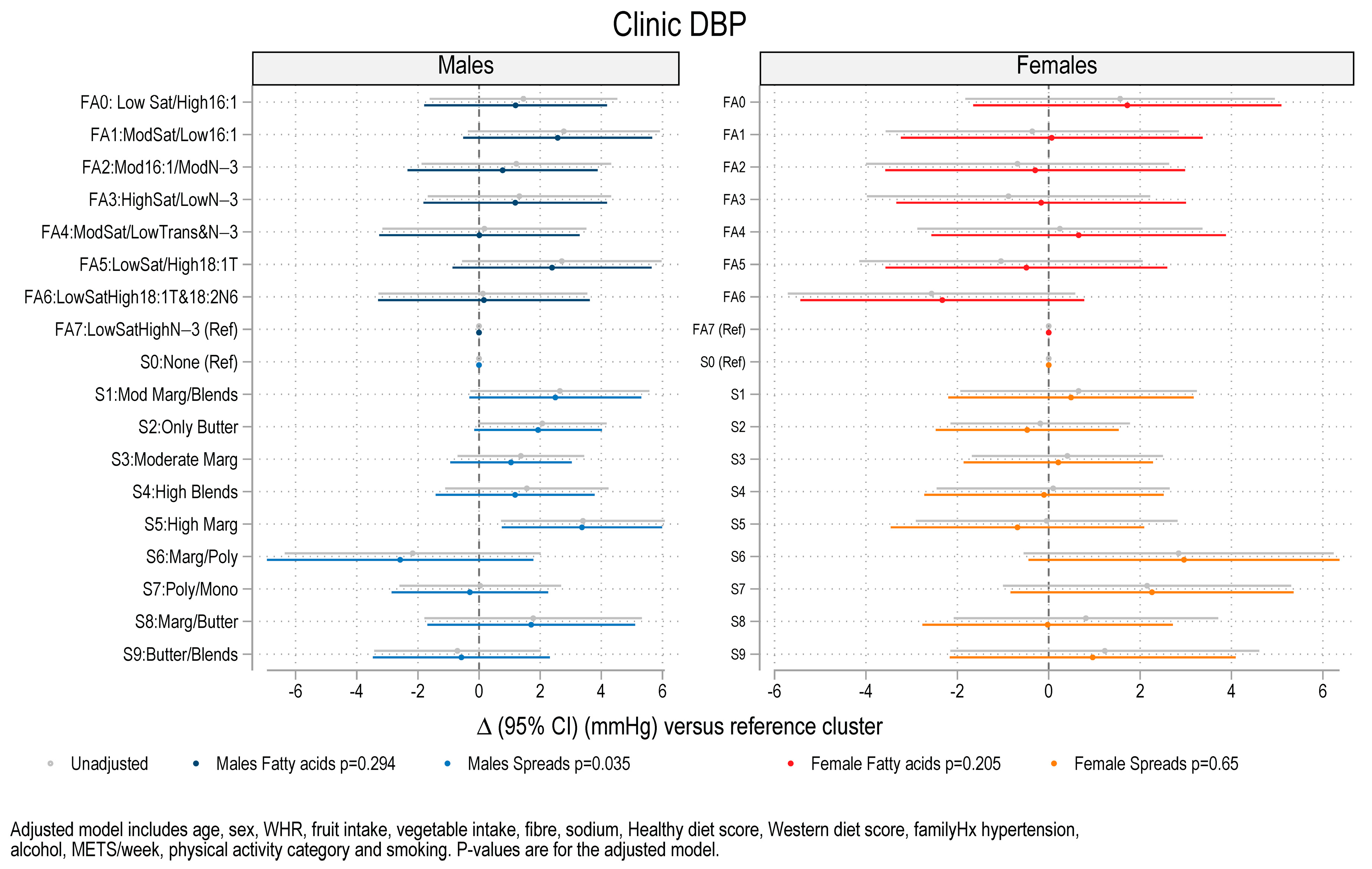
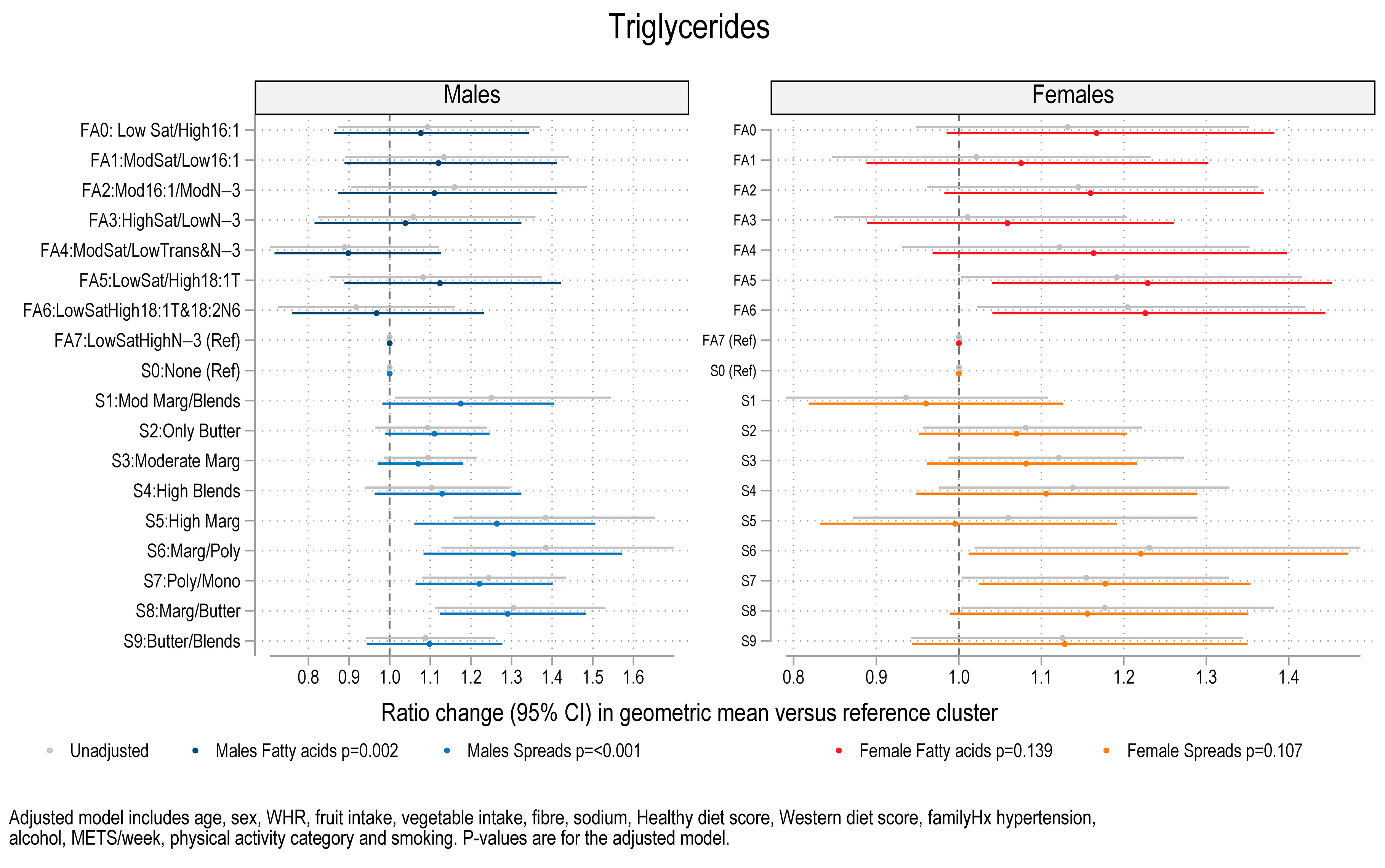
3.3. Association Between Fat Spread Clusters, Fatty Acid Clusters, and Blood Pressure, Lipids and HOMA-IR
4. Discussion
Implications for Clinical Practice and Directions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, J.A.; Fong, J.; Bernert, J.T. Serum Fatty Acids and Blood Pressure. Hypertension 1996, 27, 303–307. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.A.; Bremner, A.P.; Beilin, L.J.; Ambrosini, G.L.; Mori, T.A.; Huang, R.C.; Oddy, W.H. Polyunsaturated fatty acid intake and blood pressure in adolescents. J. Hum. Hypertens. 2012, 26, 178–187. [Google Scholar] [CrossRef]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 53. [Google Scholar] [CrossRef]
- Garsetti, M.; Balentine, D.A.; Zock, P.L.; Blom, W.A.; Wanders, A.J. Fat composition of vegetable oil spreads and margarines in the USA in 2013: A national marketplace analysis. Int. J. Food Sci. Nutr. 2016, 67, 372–382. [Google Scholar] [CrossRef]
- Nagpal, T.; Sahu, J.K.; Khare, S.K.; Bashir, K.; Jan, K. Trans fatty acids in food: A review on dietary intake, health impact, regulations and alternatives. J. Food Sci. 2021, 86, 5159–5174. [Google Scholar] [CrossRef]
- Vafeiadou, K.; Weech, M.; Altowaijri, H.; Todd, S.; Yaqoob, P.; Jackson, K.G.; Lovegrove, J.A. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study12. Am. J. Clin. Nutr. 2015, 102, 40–48. [Google Scholar] [CrossRef]
- Silva, T.J.; Barrera-Arellano, D.; Ribeiro, A.P.B. Margarines: Historical approach, technological aspects, nutritional profile, and global trends. Food Res. Int. 2021, 147, 110486. [Google Scholar] [CrossRef]
- Ras, R.T.; Fuchs, D.; Koppenol, W.P.; Garczarek, U.; Greyling, A.; Keicher, C.; Verhoeven, C.; Bouzamondo, H.; Wagner, F.; Trautwein, E.A. The effect of a low-fat spread with added plant sterols on vascular function markers: Results of the Investigating Vascular Function Effects of Plant Sterols (INVEST) study. Am. J. Clin. Nutr. 2015, 101, 733–741. [Google Scholar] [CrossRef]
- O’Sullivan, T.A.; Ambrosini, G.; Beilin, L.J.; Mori, T.A.; Oddy, W.H. Dietary intake and food sources of fatty acids in Australian adolescents. Nutrition 2011, 27, 153–159. [Google Scholar] [CrossRef]
- Ervin, R.B.; Wright, J.D.; Wang, C.Y.; Kennedy-Stephenson, J. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Adv. Data 2004, 1–6. [Google Scholar]
- Goldstein, B.A.; Navar, A.M.; Carter, R.E. Moving beyond regression techniques in cardiovascular risk prediction: Applying machine learning to address analytic challenges. Eur. Heart J. 2016, 38, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Cartus, A.R.; Kirkpatrick, S.I.; Himes, K.P.; Kennedy, E.H.; Simhan, H.N.; Grobman, W.A.; Duffy, J.Y.; Silver, R.M.; Parry, S. Machine learning as a strategy to account for dietary synergy: An illustration based on dietary intake and adverse pregnancy outcomes. Am. J. Clin. Nutr. 2020, 111, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Berisha, V.; Krantsevich, C.; Hahn, P.R.; Hahn, S.; Dasarathy, G.; Turaga, P.; Liss, J. Digital medicine and the curse of dimensionality. npj Digit. Med. 2021, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Bonassi, S. Prospects and Pitfalls of Machine Learning in Nutritional Epidemiology. Nutrients 2022, 14, 1705. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 37. [Google Scholar] [CrossRef]
- Woodman, R.J.; Baghdadi, L.R.; Shanahan, E.M.; de Silva, I.; Hodgson, J.M.; Mangoni, A.A. Diets high in n-3 fatty acids are associated with lower arterial stiffness in patients with rheumatoid arthritis: A latent profile analysis. Br. J. Nutr. 2019, 121, 182–194. [Google Scholar] [CrossRef]
- Moeller, S.M.; Reedy, J.; Millen, A.E.; Dixon, L.B.; Newby, P.K.; Tucker, K.L.; Krebs-Smith, S.M.; Guenther, P.M. Dietary Patterns: Challenges and Opportunities in Dietary Patterns Research: An Experimental Biology Workshop, April 1, 2006. J. Am. Diet. Assoc. 2007, 107, 1233–1239. [Google Scholar] [CrossRef]
- Solans, M.; Coenders, G.; Marcos-Gragera, R.; Castelló, A.; Gràcia-Lavedan, E.; Benavente, Y.; Moreno, V.; Pérez-Gómez, B.; Amiano, P.; Fernández-Villa, T.; et al. Compositional analysis of dietary patterns. Stat. Methods Med. Res. 2018, 28, 2834–2847. [Google Scholar] [CrossRef]
- Kirk, D.; Kok, E.; Tufano, M.; Tekinerdogan, B.; Feskens, E.J.M.; Camps, G. Machine Learning in Nutrition Research. Adv. Nutr. 2022, 13, 2573–2589. [Google Scholar] [CrossRef]
- Kanter, I.; Yaari, G.; Kalisky, T. Applications of Community Detection Algorithms to Large Biological Datasets. Methods Mol. Biol. 2021, 2243, 59–80. [Google Scholar] [CrossRef]
- Levine, J.H.; Simonds, E.F.; Bendall, S.C.; Davis, K.L.; Amir el, A.D.; Tadmor, M.D.; Litvin, O.; Fienberg, H.G.; Jager, A.; Zunder, E.R.; et al. Data-Driven Phenotypic Dissection of AML Reveals Progenitor-like Cells that Correlate with Prognosis. Cell 2015, 162, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Straker, L.; Mountain, J.; Jacques, A.; White, S.; Smith, A.; Landau, L.; Stanley, F.; Newnham, J.; Pennell, C.; Eastwood, P. Cohort Profile: The Western Australian Pregnancy Cohort (Raine) Study–Generation 2. Int. J. Epidemiol. 2017, 46, 1384–1385j. [Google Scholar] [CrossRef] [PubMed]
- Groves, T. Enhancing the quality and transparency of health research. BMJ 2008, 337, a718. [Google Scholar] [CrossRef] [PubMed]
- Appannah, G.; Murray, K.; Trapp, G.; Dymock, M.; Oddy, W.H.; Ambrosini, G.L. Dietary pattern trajectories across adolescence and early adulthood and their associations with childhood and parental factors. Am. J. Clin. Nutr. 2021, 113, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Baghurst, K.I.; Record, S.J. A computerised dietary analysis system for use with diet diaries or food frequency questionnaires. Community Health Stud. 1984, 8, 11–18. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; Oddy, W.H.; Robinson, M.; O’Sullivan, T.A.; Hands, B.P.; de Klerk, N.H.; Silburn, S.R.; Zubrick, S.R.; Kendall, G.E.; Stanley, F.J.; et al. Adolescent dietary patterns are associated with lifestyle and family psycho-social factors—CORRIGENDUM. Public Health Nutr. 2016, 19, 765. [Google Scholar] [CrossRef]
- Thøgersen-Ntoumani, C.; Gucciardi, D.F.; McVeigh, J.A.; O’Sullivan, T.A.; Dontje, M.; Stamatakis, E.; Eastwood, P.R.; Straker, L. Health behaviour profiles in young Australian adults in relation to physical and mental health: The Raine Study. Health Promot. J. Aust. 2023, 35, 1010–1021. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Warnick, G.R.; Albers, J.J. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J. Lipid Res. 1978, 19, 65–76. [Google Scholar] [CrossRef]
- Hodge, A.; Patterson, A.J.; Brown, W.J.; Ireland, P.; Giles, G. The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public Health 2000, 24, 576–583. [Google Scholar] [CrossRef]
- Hebden, L.; Kostan, E.; O’Leary, F.; Hodge, A.; Allman-Farinelli, M. Validity and reproducibility of a food frequency questionnaire as a measure of recent dietary intake in young adults. PLoS ONE 2013, 8, e75156. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A. Trivial Names of Fatty Acids—Parts 1 & 2. Available online: https://lipidlibrary.aocs.org/resource-material/trivial-names-of-fatty-acids-part-1 (accessed on 17 July 2024).
- Panchal, B.; Bhandari, B. Butter and Dairy Fat Spreads. In Dairy Fat Products and Functionality: Fundamental Science and Technology; Truong, T., Lopez, C., Bhandari, B., Prakash, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 509–532. [Google Scholar] [CrossRef]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, K.; Lawrence, M.A.; Russell, C.; McNaughton, S.A. Evidence Use in the Development of the Australian Dietary Guidelines: A Qualitative Study. Nutrients 2021, 13, 3748. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.M. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements edited by JJ Otten, JP Hellwig, and LD Meyers, 2006. The National Academies Press, Washington, DC. Am. J. Clin. Nutr. 2007, 85, 924. [Google Scholar] [CrossRef]
- Luo, D.; Cheng, Y.; Zhang, H.; Ba, M.; Chen, P.; Li, H.; Chen, K.; Sha, W.; Zhang, C.; Chen, H. Association between high blood pressure and long term cardiovascular events in young adults: Systematic review and meta-analysis. BMJ 2020, 370, m3222. [Google Scholar] [CrossRef]
- Matheson, B.A.; Walker, K.Z.; Taylor, D.M.; Peterkin, R.; Lugg, D.; O’dea, K. Effect on serum lipids of monounsaturated oil and margarine in the diet of an Antarctic Expedition. Am. J. Clin. Nutr. 1996, 63, 933–938. [Google Scholar] [CrossRef]
- Sweazea, K.L. Compounding evidence implicating Western diets in the development of metabolic syndrome. Acta Physiol. 2014, 211, 471–473. [Google Scholar] [CrossRef][Green Version]
- Spence, M.; McKinley, M.C.; Hunter, S.J. Session 4: CVD, diabetes and cancer: Diet, insulin resistance and diabetes: The right (pro)portions. Proc. Nutr. Soc. 2010, 69, 61–69. [Google Scholar] [CrossRef]
- Chew, G.T.; Gan, S.K.; Watts, G.F. Revisiting the metabolic syndrome. Med. J. Aust. 2006, 185, 445–449. [Google Scholar] [CrossRef]
- Stehouwer, C.D.; Henry, R.M.; Ferreira, I. Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease. Diabetologia 2008, 51, 527–539. [Google Scholar] [CrossRef]
- Agbaje, A.O. Arterial stiffness precedes hypertension and metabolic risks in youth: A review. J. Hypertens. 2022, 40, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, M.; Rawal, S.; Fernandez, M.L.; Huedo-Medina, T.B.; Duffy, V.B. Taste phenotype associates with cardiovascular disease risk factors via diet quality in multivariate modeling. Physiol. Behav. 2018, 194, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Katan, M.B. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N. Engl. J. Med. 1990, 323, 439–445. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organisation Validation Programme for Trans Fat Elimination 2023. Available online: https://www.who.int/teams/nutrition-and-food-safety/replace-trans-fat/validation-programme-for-trans-fat-elimination (accessed on 15 January 2025).
- Huang, L.; Federico, E.; Jones, A.; Wu, J.H.Y. Presence of trans fatty acids containing ingredients in pre-packaged foods in Australia in 2018. Aust. N. Z. J. Public Health 2020, 44, 419–420. [Google Scholar] [CrossRef]
- Clifton, P.M.; Keogh, J.B.; Noakes, M. Trans Fatty Acids in Adipose Tissue and the Food Supply Are Associated with Myocardial Infarction. J. Nutr. 2004, 134, 874–879. [Google Scholar] [CrossRef][Green Version]
- Dhaka, V.; Gulia, N.; Ahlawat, K.S.; Khatkar, B.S. Trans fats-sources, health risks and alternative approach—A review. J. Food Sci. Technol. 2011, 48, 534–541. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.M.; Bouzas, C.; Pastor, Ó.; Ugarriza, L.; Llompart, I.; Cevallos-Ibarra, K.; Sureda, A.; Tur, J.A. Plasma Fatty Acid Composition, Oxidative and Inflammatory Status, and Adherence to the Mediterranean Diet of Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants 2023, 12, 1554. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Benčič, A.; Knüppel, S.; Boeing, H.; Hoffmann, G. Effects of oils and solid fats on blood lipids: A systematic review and network meta-analysis. J. Lipid Res. 2018, 59, 1771–1782. [Google Scholar] [CrossRef]
- Chamberlain, J.; Johnson, E.L.; Leal, S.; Rhinehart, A.S.; Shubrook, J.H.; Peterson, L. Cardiovascular Disease and Risk Management: Review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann. Intern. Med. 2018, 168, 640–650. [Google Scholar] [CrossRef]
- Sosa-Holwerda, A.; Park, O.H.; Albracht-Schulte, K.; Niraula, S.; Thompson, L.; Oldewage-Theron, W. The Role of Artificial Intelligence in Nutrition Research: A Scoping Review. Nutrients 2024, 16, 2066. [Google Scholar] [CrossRef]
- Sun, X.; Yin, Y.; Yang, Q.; Huo, T. Artificial intelligence in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. J. Med. Res. 2023, 28, 242. [Google Scholar] [CrossRef] [PubMed]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Fruchterman, T.M.J.; Reingold, E.M. Graph drawing by force-directed placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Csárdi, G.; Nepusz, T. The igraph software package for complex network research. InterJournal 2006, Complex Systems, 1695. Available online: https://igraph.org (accessed on 15 January 2025).
- Blondel, V.D.; Guillaume, J.-L.; Lambiotte, R.; Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, 2008, P10008. [Google Scholar] [CrossRef]



| All (N = 785) (100%) | Males N = 409 (52.1%) | Females N = 376 (47.9%) | p-Value 3 | |
|---|---|---|---|---|
| Age, years At the visit aged 20 At the visit aged 22 | 19.95 ± 0.42 22.11 ± 0.59 | 19.99 ± 0.43 22.15 ± 0.62 | 19.90 ± 0.41 22.06 ± 0.57 | 0.005 0.041 |
| Body mass index (kg/m2) At the visit aged 20 At the visit aged 22 | 24.2 ± 4.7 25.0 ± 5.0 | 24.2 ± 4.1 25.2 ± 4.4 | 24.25 ± 5.3 24.9 ± 5.55 | 0.960 0.296 |
| Family history 5 of high blood pressure, n (%) | 244 (31.1) | 129 (31.5) | 115 (30.6) | 0.773 |
| Systolic blood pressure 1, mmHg At the visit aged 20 At the visit aged 22 | 117.2 ± 12.2 118.9 ± 11.3 | 122.2 ± 11.6 123.4 ± 10.6 | 111.7 ± 10.3 113.9 ± 9.8 | <0.001 <0.001 |
| Diastolic blood pressure 1, mmHg At the visit aged 20 At the visit aged 22 | 65.5 ± 7.3 67.0 ± 7.3 | 65.1 ± 7.5 66.8 ± 7.4 | 65.9 ± 7.1 67.3 ± 7.1 | 0.149 0.328 |
| Stage 1 hypertension 6 [34] At the visit aged 20 At the visit aged 22 | 103 (13.1) 103 (13.1) | 74 (18.1) 80 (19.6) | 29 (7.7) 23 (6.1) | <0.001 <0.001 |
| Stage 2 hypertension 6 [34] At the visit aged 20 At the visit aged 22 | 27 (3.44) 31 (3.95) | 25 (6.1) 26 (6.4) | 2 (0.5) 5 (1.3) | <0.001 <0.001 |
| Triglycerides (mmol/L) At the visit aged 20 At the visit aged 22 | 1.04 ± 0.48 1.06 ± 0.45 | 1.08 ± 0.63 1.04 ± 0.48 | 1.13 ± 0.53 1.06 ± 0.45 | 0.332 0.053 |
| Cholesterol (mmol/L) At the visit aged 20 At the visit aged 22 | 4.33 ± 0.79 4.62 ± 0.80 | 4.16 ± 0.74 4.54 ± 0.79 | 4.52 ± 0.80 4.71 ± 0.81 | <0.001 0.003 |
| Non-HDL cholesterol At the visit aged 20 At the visit aged 22 | 3.00 ± 0.74 3.26 ± 0.77 | 2.93 ± 0.74 3.30 ± 0.80 | 3.07 ± 0.73 3.22 ± 0.74 | 0.010 0.154 |
| HDL cholesterol (mmol/L) At the visit aged 20 At the visit aged 22 | 1.33 ± 0.32 1.36 ± 0.34 | 1.23 ± 0.24 1.24 ± 0.24 | 1.45 ± 0.35 1.49 ± 0.39 | <0.001 <0.001 |
| Fruit consumption (age 17), n (%) 0: Rarely or never 1: 1–2 times a month 2: 1–2 times per week 3: 3–5 times per week 4: 6 + times per week Missing | 13 (1.7) 33 (4.2) 104 (13.25) 234 (29.8) 252 (32.1) 149 (19.0) | 9 (2.7) 21 (6.4) 59 (18.0) 119 (36.3) 120 (36.6) 81 (19.8) | 4 (1.3) 12 (3.9) 45 (14.6) 115 (37.3) 132 (42.9) 68 (18.1) | 0.247 |
| Fruit consumption category (age 17), Median (IQR) Mean (SD) | 3 (3–4) 3.07 ± 0.97 | 3 (2–4) 2.98 ± 1.03 | 3 (3–4) 3.17 ± 0.91 | 0.025 0.014 |
| Vegetable consumption (age 17), n (%) 0: Rarely or never 1: 1–2 times a month 2: 1–2 times per week 3: 3–5 times per week 4: 6 + times per week Missing | 1 (0.1) 11 (1.4) 60 (7.6) 212 (27.0) 405 (45.0) 172 (18.85) | 1 (0.2) 7 (1.7) 38 (9.3) 114 (27.9) 168 (41.1) 81 (19.8) | 0 (0.0) 4 (1.1) 22 (5.85) 98 (26.1) 185 (49.2) 67 (17.8) | 0.153 |
| Vegetable consumption cat (age 17) Median (IQR) Mean (SD) | 4 (3–4) 3.42 ± 0.75 | 4 (3–4) 3.34 ± 0.79 | 4 (3–4) 3.50 ± 0.69 | 0.012 0.008 |
| Weekly alcohol consumption (age 20) 2 Median (IQR) grams per day | 8.6 (0–21.4) | 10 (0–27.1) | 5.7 (0–15.7) | 0.132 |
| Alcohol categories (age 20) 4 Less than 1 standard drink 1 to 3 standard drinks More than 3 standard drinks | 604 (89.5) 47 (7.0) 24 (3.6) | 293 (87.7) 26 (7.8) 15 (4.5) | 311 (91.2) 21 (6.2) 9 (2.6) | 0.287 |
| Smoking status (age 22) No Yes | 618 (85.7) 103 (14.3) | 311 (83.8) 60 (16.2) | 307 (87.7) 43 (12.3) | 0.136 |
| Sodium intake (mg/day) (age 17) | 2977 ± 1214 | 3441 ± 1245 | 2530 ± 999 | <0.001 |
| CSIRO diet factor analysis scores (age 17) Western diet score Healthy diet score | −0.06 ± 0.82 0.07 ± 0.90 | 0.26 ± 0.83 0.01 ± 0.92 | −0.36 ± 0.68 0.13 ± 0.89 | <0.001 0.163 |
| Males | Females | |
|---|---|---|
| FA0: Low saturated/High 16:1 | - | - |
| FA1: Moderate saturated/Low 16:1 | - | Lower HDL (−12.4%) |
| FA2: Moderate 16:1/Moderate N-3 | - | Lower HDL (−10.1%) |
| FA3: High saturated/Low N-3 | - | Lower HDL (−14.0%) |
| FA4: Moderate saturated/Low Trans and N-3 | - | Lower HDL (−15.0%) |
| FA5: Low saturated/High 18:1T | - | Lower HDL (−16.0%) |
| FA6: Low saturated/High 18:1T and 18:2N-6 | - | - |
| FA7: Low saturated/High N-3 | Reference | Reference |
| S0: No spreads (Reference) | Reference | Reference |
| S1: Moderate margarine/blends | - | - |
| S2: Butter alone | Higher SBP (+4.26 mmHg) | - |
| S3: Moderate margarine | - | - |
| S4: High blends | - | - |
| S5: High margarine | Higher SBP (+6.61 mmHg) Higher DBP (+3.4 mmHg) Higher Trig (+26.4%) | - |
| S6: Margarine/polyunsaturated | Higher Trig (+30.5%) | - |
| S7: Polyunsaturated/Monounsaturated | Higher Trig (+22.1%) | - |
| S8: Margarine/Butter | Higher Trig (+29.1%) | - |
| S9: Butter/Blend | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodman, R.; Mangoni, A.A.; Cohen-Woods, S.; Mori, T.A.; Beilin, L.; Murphy, K.; Hodgson, J. Patterns of Dietary Fatty Acids and Fat Spreads in Relation to Blood Pressure, Lipids and Insulin Resistance in Young Adults: A Repeat Cross-Sectional Study. Nutrients 2025, 17, 869. https://doi.org/10.3390/nu17050869
Woodman R, Mangoni AA, Cohen-Woods S, Mori TA, Beilin L, Murphy K, Hodgson J. Patterns of Dietary Fatty Acids and Fat Spreads in Relation to Blood Pressure, Lipids and Insulin Resistance in Young Adults: A Repeat Cross-Sectional Study. Nutrients. 2025; 17(5):869. https://doi.org/10.3390/nu17050869
Chicago/Turabian StyleWoodman, Richard, Arduino A. Mangoni, Sarah Cohen-Woods, Trevor A. Mori, Lawrence Beilin, Karen Murphy, and Jonathan Hodgson. 2025. "Patterns of Dietary Fatty Acids and Fat Spreads in Relation to Blood Pressure, Lipids and Insulin Resistance in Young Adults: A Repeat Cross-Sectional Study" Nutrients 17, no. 5: 869. https://doi.org/10.3390/nu17050869
APA StyleWoodman, R., Mangoni, A. A., Cohen-Woods, S., Mori, T. A., Beilin, L., Murphy, K., & Hodgson, J. (2025). Patterns of Dietary Fatty Acids and Fat Spreads in Relation to Blood Pressure, Lipids and Insulin Resistance in Young Adults: A Repeat Cross-Sectional Study. Nutrients, 17(5), 869. https://doi.org/10.3390/nu17050869








