Gnetin C in Cancer and Other Diseases: What Do We Know So Far?
Abstract
1. Introduction
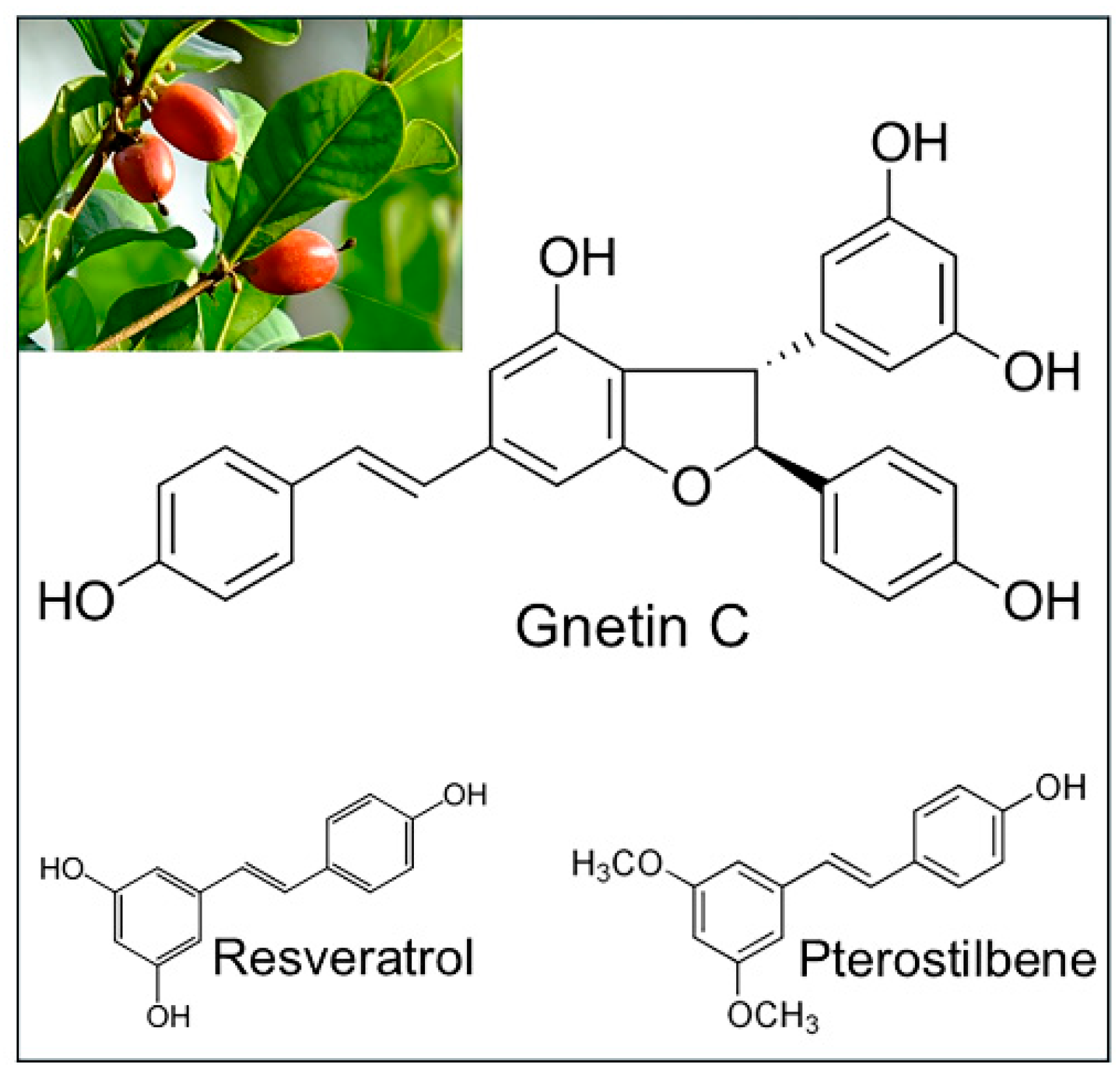
2. Gnetin C, a Resveratrol Dimer
3. Gnetin C and Cancer
4. Gnetin C and Prostate Cancer
5. Gnetin C and Other Effects
6. Gnetin C and Human Clinical Trials
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid beta |
| Aβ42 | Amyloid beta 42 |
| ACC1 | Acetyl coenzyme A carboxylase 1 |
| AKT | V-akt murine thymoma viral oncogene (protein kinase B) |
| ALT | Alanine aminotransferase |
| AML | Acute myeloid leukemia |
| AML-MT | Acute myeloid leukemia xenograft |
| APN | Adiponectin |
| AR | Androgen receptor |
| AR-FL | Androgen receptor-full length |
| AR-V7 | Androgen receptor-variant 7 |
| AsPC1 | Human pancreatic cancer cells |
| AT1 | Angiotensin II type 1 receptor |
| BACE1 | Beta-site amyloid precursor protein cleaving enzyme 1 |
| Bak | Homolog of Bax |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell leukemia/lymphoma 2 |
| B16-F10 | Mouse melanoma cells |
| BFGF | Basic fibroblast growth factor |
| bw | Body weight |
| CaP8 | Mouse prostate cancer cells |
| chREBP | Carbohydrate response element binding protein |
| CC3 | Cleaved caspase 3 |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CCL5 | Chemokine (C-C motif) ligand 5 |
| CD31 | Cluster of differentiation 31 |
| CML | Chronic myeloid leukemia |
| Col1a1 | Collagen type I alpha 1 chain |
| COS-1 | Monkey kidney cells |
| Cre | Cre recombinase |
| CRP | C-reactive protein |
| CRPC | Castrate-resistant prostate cancer |
| CTL | Cytotoxic T lymphocytes |
| DGAT1 | Diacylglycerol acyltransferase 1 |
| DGAT2 | Diacylglycerol acyltransferase 2 |
| DU145 | Human prostate cancer cells |
| eNOS | Endothelial nitric oxide synthase |
| Enz | Enzalutamide |
| ERK1/2 | Mitogen-activated protein kinase 1 and 2 |
| ETS2 | ETS Proto-oncogene 2, transcription factor |
| GnC | Gnetin C |
| HDAC | Histone deacetylase |
| HDL-C | High-density lipoprotein cholesterol |
| HEK-293T | Human kidney cells |
| HFCD | High-fat choline deficient |
| HIF-1α | Hypoxia-inducible factor 1- alpha |
| HL60 | Human leukemia cells |
| HT-29 | Human colon cancer cells |
| HUVEC | Human umbilical vein endothelial cells |
| IFN-β | Interferon beta |
| IFN-γ | Interferon gamma |
| IL-1β | Interleukin-1 beta |
| IL-2 | Interleukin 2 |
| IL-6 | Interleukin 6 |
| i.p | Intraperitoneal |
| K562 | Human CML cells |
| KH88 | Human CML cells |
| Ki67 | Cellular protein marker of proliferation |
| LDH | Lactate dehydrogenase |
| LDL-C | Low-density lipoprotein-cholesterol |
| LDLR | Low-density lipoprotein receptor |
| LNCaP | Human prostate cancer cells |
| Luc | Luciferase |
| MAO-A | Monoamine oxidase A |
| MAO-B | Monoamine oxidase B |
| MCF7 | Human breast cancer cells |
| miR-22 | MicroRNA-22 |
| miR-34a | MicroRNA-34a |
| MMP-14 | Matrix metallopeptidase 14 |
| MRT | Mean residence time |
| MSE | Melinjo seed extract |
| MTA1 | Metastasis-associated protein 1 |
| MTP | Microsomal triglyceride transfer protein |
| mTOR | Mammalian target of rapamycin |
| MV4 | Human AML cells |
| NAFLD | Nonalcoholic fatty liver disease |
| NEFA | Nonesterified fatty acids |
| NF- κβ | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | Natural killer cells |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NuRD | Nucleosome remodeling deacetylation complex |
| Oun1 | Human CML cells |
| p21 | Cyclin-dependent kinase inhibitor 1A |
| p4EBP1 | Eukaryotic translation initiation factor 4E-binding protein 1 |
| p53 | Tumor protein p53 |
| PAI1 | Plasminogen activator inhibitor-1 |
| p-AKT | Phosphorylated AKT |
| Pan-02 | Mouse pancreatic cancer cells |
| PANC-1 | Human pancreatic cancer cells |
| PC3 | Human prostate cancer cells |
| PC3M | Human prostate cancer cells |
| PGC-1a | peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
| PIN | Prostatic intraepithelial neoplasia |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| pS6K | p70-S6 Kinase |
| PSA | Prostate specific antigen |
| PTEN | Phosphatase and tensin homolog |
| PWPE1 | normal prostate epithelial cells |
| R26 | Rosa26 |
| Res | Resveratrol |
| ROS | Reactive oxygen species |
| 22Rv1 | Human prostate cancer cells |
| SA-β-gal | Senescence-associated beta-galactosidase |
| SAHA | Suberoylanilide hydroxamic acid |
| shMTA1 | Short-hairpin MTA1 |
| Sirt1 | Sirtuin 1 |
| SOD1 | Superoxide dismutase 1 |
| STAT1 | Signal transducer and activator of transcription 1 |
| STZ | Streptozotocin |
| Tg | Triglyceride |
| TGFβ | Transforming growth factor beta |
| THP1 | Human leukemia cells |
| TLR3 | Toll-like receptor 3 |
| U937 | Human lymphoma cells |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VCaP | Human prostate cancer cells |
| VEGF | Vascular epidermal growth factor |
| WT | Wild type |
References
- Ahmadi, R.; Ebrahimzadeh, M.A. Resveratrol—A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Li, S.; Lin, C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Li, S. Resveratrol, pterostilbene, and dementia. Biofactors 2018, 44, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, K.H.; Yokoyama, W. Chemistry of Pterostilbene and Its Metabolic Effects. J. Agric. Food Chem. 2020, 68, 12836–12841. [Google Scholar] [CrossRef]
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216. [Google Scholar] [CrossRef]
- Miksits, M.; Wlcek, K.; Svoboda, M.; Kunert, O.; Haslinger, E.; Thalhammer, T.; Szekeres, T.; Jager, W. Antitumor activity of resveratrol and its sulfated metabolites against human breast cancer cells. Planta Med. 2009, 75, 1227–1230. [Google Scholar] [CrossRef]
- Hoshino, J.; Park, E.J.; Kondratyuk, T.P.; Marler, L.; Pezzuto, J.M.; van Breemen, R.B.; Mo, S.; Li, Y.; Cushman, M. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J. Med. Chem. 2010, 53, 5033–5043. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Ito, T. Resveratrol oligomer structure in Dipterocarpaceaeous plants. J. Nat. Med. 2020, 74, 619–637. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Q.; Li, P.; Wang, Z.; Liu, S.; He, C.; Zhang, C.; Xiao, P. Update on Phytochemistry and Pharmacology of Naturally Occurring Resveratrol Oligomers. Molecules 2017, 22, 2050. [Google Scholar] [CrossRef]
- Kang, J.E.; Yoo, N.; Jeon, B.J.; Kim, B.S.; Chung, E.H. Resveratrol Oligomers, Plant-Produced Natural Products With Anti-virulence and Plant Immune-Priming Roles. Front. Plant Sci. 2022, 13, 885625. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Yi, F.; Liu, Q.; Chen, N.; He, X.; He, C.; Xiao, P. Resveratrol oligomers from Paeonia suffruticosa protect mice against cognitive dysfunction by regulating cholinergic, antioxidant and anti-inflammatory pathways. J. Ethnopharmacol. 2020, 260, 112983. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Gromek, S.; Niesen, D.; Seeram, N.P.; Henry, G.E. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. J. Agric. Food Chem. 2011, 59, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.Q.; Di, J.M.; Luo, Y.; Cheng, K.J.; Wei, X.; Shi, Z. Resveratrol oligomers for the prevention and treatment of cancers. Oxid. Med. Cell. Longev. 2014, 2014, 765832. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Tokunaga, Y.; Sakan, F. Stilbenoids isolated from the seeds of Melinjo (Gnetum gnemon L.) and their biological activity. J. Agric. Food Chem. 2009, 57, 2544–2549. [Google Scholar] [CrossRef]
- Ota, H.; Akishita, M.; Tani, H.; Tatefuji, T.; Ogawa, S.; Iijima, K.; Eto, M.; Shirasawa, T.; Ouchi, Y. trans-Resveratrol in Gnetum gnemon protects against oxidative-stress-induced endothelial senescence. J. Nat. Prod. 2013, 76, 1242–1247. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Kunimasa, K.; Yamori, Y.; Mori, M.; Mori, H.; Nakamura, K.; Miller, G.; Manne, U.; Tiwari, A.K.; Narayanan, B. Antitumor activity of melinjo (Gnetum gnemon L.) seed extract in human and murine tumor models in vitro and in a colon-26 tumor-bearing mouse model in vivo. Cancer Med. 2015, 4, 1767–1780. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Elbadry, M.I.; Taniwaki, M.; Harada, K.; Trung, L.Q.; Nakagawa, N.; Takami, A.; Ishiyama, K.; Yamauchi, T.; Takenaka, K.; et al. The simultaneous inhibition of the mTOR and MAPK pathways with Gnetin-C induces apoptosis in acute myeloid leukemia. Cancer Lett. 2017, 400, 127–136. [Google Scholar] [CrossRef]
- Kunimasa, K.; Ohta, T.; Tani, H.; Kato, E.; Eguchi, R.; Kaji, K.; Ikeda, K.; Mori, H.; Mori, M.; Tatefuji, T.; et al. Resveratrol derivative-rich melinjo (Gnetum gnemon L.) seed extract suppresses multiple angiogenesis-related endothelial cell functions and tumor angiogenesis. Mol. Nutr. Food Res. 2011, 55, 1730–1734. [Google Scholar] [CrossRef]
- Tatefuji, T.; Yanagihara, M.; Fukushima, S.; Hashimoto, K. Safety assessment of melinjo (Gnetum gnemon L.) seed extract: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2014, 67, 230–235. [Google Scholar] [CrossRef]
- Konno, H.; Kanai, Y.; Katagiri, M.; Watanabe, T.; Mori, A.; Ikuta, T.; Tani, H.; Fukushima, S.; Tatefuji, T.; Shirasawa, T. Melinjo (Gnetum gnemon L.) Seed Extract Decreases Serum Uric Acid Levels in Nonobese Japanese Males: A Randomized Controlled Study. Evid. Based Complement. Alternat Med. 2013, 2013, 589169. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Hikami, S.; Iizuna, S.; Yoshimatsu, M.; Asama, T.; Ota, H.; Kimura, Y.; Tatefuji, T.; Hashimoto, K.; Higaki, K. Pharmacokinetics and safety of resveratrol derivatives in humans after oral administration of melinjo (Gnetum gnemon L.) seed extract powder. J. Agric. Food Chem. 2014, 62, 1999–2007. [Google Scholar] [CrossRef]
- Espinoza, J.L.; An, D.T.; Trung, L.Q.; Yamada, K.; Nakao, S.; Takami, A. Stilbene derivatives from melinjo extract have antioxidant and immune modulatory effects in healthy individuals. Integr. Mol. Med. 2015, 2, 405–413. [Google Scholar]
- Nakagami, Y.; Suzuki, S.; Espinoza, J.L.; Vu Quang, L.; Enomoto, M.; Takasugi, S.; Nakamura, A.; Nakayama, T.; Tani, H.; Hanamura, I.; et al. Immunomodulatory and Metabolic Changes after Gnetin-C Supplementation in Humans. Nutrients 2019, 11, 1403. [Google Scholar] [CrossRef]
- Oniki, K.; Kawakami, T.; Nakashima, A.; Miyata, K.; Watanabe, T.; Fujikawa, H.; Nakashima, R.; Nasu, A.; Eto, Y.; Takahashi, N.; et al. Melinjo seed extract increases adiponectin multimerization in physiological and pathological conditions. Sci. Rep. 2020, 10, 4313. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Inaoka, P.T. Gnetin-C and other resveratrol oligomers with cancer chemopreventive potential. Ann. N. Y. Acad. Sci. 2017, 1403, 5–14. [Google Scholar] [CrossRef]
- Yanagihara, M.; Yoshimatsu, M.; Inoue, A.; Kanno, T.; Tatefuji, T.; Hashimoto, K. Inhibitory effect of gnetin C, a resveratrol dimer from melinjo (Gnetum gnemon), on tyrosinase activity and melanin biosynthesis. Biol. Pharm. Bull. 2012, 35, 993–996. [Google Scholar] [CrossRef]
- Kato, H.; Samizo, M.; Kawabata, R.; Takano, F.; Ohta, T. Stilbenoids from the melinjo (Gnetum gnemon L.) fruit modulate cytokine production in murine Peyer’s patch cells ex vivo. Planta Med. 2011, 77, 1027–1034. [Google Scholar] [CrossRef]
- Iliya, I.; Akao, Y.; Matsumoto, K.; Nakagawa, Y.; Zulfiqar, A.; Ito, T.; Oyama, M.; Murata, H.; Tanaka, T.; Nozawa, Y.; et al. Growth inhibition of stilbenoids in Welwitschiaceae and Gnetaceae through induction of apoptosis in human leukemia HL60 cells. Biol. Pharm. Bull. 2006, 29, 1490–1492. [Google Scholar] [CrossRef]
- Ferrer, P.; Asensi, M.; Segarra, R.; Ortega, A.; Benlloch, M.; Obrador, E.; Varea, M.T.; Asensio, G.; Jorda, L.; Estrela, J.M. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia 2005, 7, 37–47. [Google Scholar] [CrossRef]
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Wlodarek, D.; Gromadzka-Ostrowska, J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients 2021, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.C.; Tsai, Y.C.; Lin, J.N.; Fan, L.L.; Pan, M.H.; Ho, C.T.; Wu, J.Y.; Way, T.D. Activation of AMPK by pterostilbene suppresses lipogenesis and cell-cycle progression in p53 positive and negative human prostate cancer cells. J. Agric. Food Chem. 2012, 60, 6399–6407. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Atarashi, K.; Murata, Y.; Yamaji, R.; Nakano, Y.; Inui, H. Inhibitory mechanisms of the transcriptional activity of androgen receptor by resveratrol: Implication of DNA binding and acetylation of the receptor. J. Steroid Biochem. Mol. Biol. 2011, 123, 65–70. [Google Scholar] [CrossRef]
- Mitchell, S.H.; Zhu, W.; Young, C.Y. Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Cancer Res. 1999, 59, 5892–5895. [Google Scholar] [PubMed]
- Harada, N.; Murata, Y.; Yamaji, R.; Miura, T.; Inui, H.; Nakano, Y. Resveratrol down-regulates the androgen receptor at the post-translational level in prostate cancer cells. J. Nutr. Sci. Vitaminol. 2007, 53, 556–560. [Google Scholar] [CrossRef]
- Wang, Y.; Romigh, T.; He, X.; Orloff, M.S.; Silverman, R.H.; Heston, W.D.; Eng, C. Resveratrol regulates the PTEN/AKT pathway through androgen receptor-dependent and -independent mechanisms in prostate cancer cell lines. Hum. Mol. Genet. 2010, 19, 4319–4329. [Google Scholar] [CrossRef]
- Streicher, W.; Luedeke, M.; Azoitei, A.; Zengerling, F.; Herweg, A.; Genze, F.; Schrader, M.G.; Schrader, A.J.; Cronauer, M.V. Stilbene induced inhibition of androgen receptor dimerization: Implications for AR and ARDeltaLBD-signalling in human prostate cancer cells. PLoS ONE 2014, 9, e98566. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kumar, A.; Butt, N.A.; Zhang, L.; Williams, R.; Rimando, A.M.; Biswas, P.K.; Levenson, A.S. Molecular insight into the differential anti-androgenic activity of resveratrol and its natural analogs: In silico approach to understand biological actions. Mol. Biosyst. 2016, 12, 1702–1709. [Google Scholar] [CrossRef]
- Nikhil, K.; Sharan, S.; Chakraborty, A.; Roy, P. Pterostilbene-isothiocyanate conjugate suppresses growth of prostate cancer cells irrespective of androgen receptor status. PLoS ONE 2014, 9, e93335. [Google Scholar] [CrossRef]
- Lundqvist, J.; Tringali, C.; Oskarsson, A. Resveratrol, piceatannol and analogs inhibit activation of both wild-type and T877A mutant androgen receptor. J. Steroid Biochem. Mol. Biol. 2017, 174, 161–168. [Google Scholar] [CrossRef]
- Dhar, S.; Kumar, A.; Zhang, L.; Rimando, A.M.; Lage, J.M.; Lewin, J.R.; Atfi, A.; Zhang, X.; Levenson, A.S. Dietary pterostilbene is a novel MTA1-targeted chemopreventive and therapeutic agent in prostate cancer. Oncotarget 2016, 7, 18469–18484. [Google Scholar] [CrossRef]
- Kai, L.; Levenson, A.S. Combination of resveratrol and antiandrogen flutamide has synergistic effect on androgen receptor inhibition in prostate cancer cells. Anticancer. Res. 2011, 31, 3323–3330. [Google Scholar] [PubMed]
- Kumar, A.; Lin, S.-Y.; Dhar, S.; Rimando, A.M.; Levenson, A.S. Stilbenes inhibit androgen receptor expression in 22Rv1 castrate-resistant prostate cancer cells. J. Med. Act. Plants 2014, 3, 1–8. [Google Scholar]
- Campanelli, G.; Deabel, R.A.; Puaar, A.; Devarakonda, L.S.; Parupathi, P.; Zhang, J.; Waxner, N.; Yang, C.; Kumar, A.; Levenson, A.S. Molecular Efficacy of Gnetin C as Dual-Targeted Therapy for Castrate-Resistant Prostate Cancer. Mol. Nutr. Food Res. 2023, 67, e2300479. [Google Scholar] [CrossRef]
- Ma, J.; Li, C.; Qian, H.; Zhang, Y. MTA1: A Vital Modulator in Prostate Cancer. Curr. Protein Pept. Sci. 2022, 23, 456–464. [Google Scholar]
- Sen, N.; Gui, B.; Kumar, R. Role of MTA1 in cancer progression and metastasis. Cancer Metastasis Rev. 2014, 33, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Samuel, S.K.; Levenson, A.S. Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. Int. J. Cancer 2010, 126, 1538–1548. [Google Scholar] [CrossRef]
- Li, K.; Dias, S.J.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Lewin, J.R.; Levenson, A.S. Pterostilbene acts through metastasis-associated protein 1 to inhibit tumor growth, progression and metastasis in prostate cancer. PLoS ONE 2013, 8, e57542. [Google Scholar] [CrossRef]
- Butt, N.A.; Kumar, A.; Dhar, S.; Rimando, A.M.; Akhtar, I.; Hancock, J.C.; Lage, J.M.; Pound, C.R.; Lewin, J.R.; Gomez, C.R.; et al. Targeting MTA1/HIF-1alpha signaling by pterostilbene in combination with histone deacetylase inhibitor attenuates prostate cancer progression. Cancer Med. 2017, 6, 2673–2685. [Google Scholar] [CrossRef]
- Hemani, R.; Patel, I.; Inamdar, N.; Campanelli, G.; Donovan, V.; Kumar, A.; Levenson, A.S. Dietary Pterostilbene for MTA1-Targeted Interception in High-Risk Premalignant Prostate Cancer. Cancer Prev. Res. 2022, 15, 87–100. [Google Scholar] [CrossRef]
- Kumar, A.; Dholakia, K.; Sikorska, G.; Martinez, L.A.; Levenson, A.S. MTA1-Dependent Anticancer Activity of Gnetin C in Prostate Cancer. Nutrients 2019, 11, 2096. [Google Scholar] [CrossRef]
- Gadkari, K.; Kolhatkar, U.; Hemani, R.; Campanelli, G.; Cai, Q.; Kumar, A.; Levenson, A.S. Therapeutic Potential of Gnetin C in Prostate Cancer: A Pre-Clinical Study. Nutrients 2020, 12, 3631–3642. [Google Scholar] [CrossRef]
- Campanelli, G.; Francois, E.; Parupathi, P.; Devarakonda, L.S.; Yang, C.; Kumar, A.; Levenson, A.S. The Therapeutic Efficacy and Mechanism of Action of Gnetin C, a Natural Compound from the Melinjo Plant, in a Preclinical Mouse Model of Advanced Prostate Cancer. Cancers 2024, 16, 1344. [Google Scholar] [CrossRef]
- Parupathi, P.; Campanelli, G.; Deabel, R.A.; Puaar, A.; Devarakonda, L.S.; Kumar, A.; Levenson, A.S. Gnetin C Intercepts MTA1-Associated Neoplastic Progression in Prostate Cancer. Cancers 2022, 14, 6038. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Lim, J.; Lee, J.; Hur, S.; Kim, S.S.; Lim, S.; Hyun, C.G.; Kim, Y.S.; Park, D. Involvement of nuclear factor-kappaB in the inhibition of pro-inflammatory mediators by pinosylvin. Planta Med. 2006, 72, 801–806. [Google Scholar] [CrossRef]
- Clarke, J.O.; Mullin, G.E. A review of complementary and alternative approaches to immunomodulation. Nutr. Clin. Pract. 2008, 23, 49–62. [Google Scholar] [CrossRef]
- Pradhan, R.; Chatterjee, S.; Hembram, K.C.; Sethy, C.; Mandal, M.; Kundu, C.N. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J. Nutr. Biochem. 2021, 92, 108624. [Google Scholar] [CrossRef]
- Vo, N.T.; Madlener, S.; Bago-Horvath, Z.; Herbacek, I.; Stark, N.; Gridling, M.; Probst, P.; Giessrigl, B.; Bauer, S.; Vonach, C.; et al. Pro- and anticarcinogenic mechanisms of piceatannol are activated dose dependently in MCF-7 breast cancer cells. Carcinogenesis 2010, 31, 2074–2081. [Google Scholar] [CrossRef]
- Sauter, E.R.; Mohammed, A. Natural Products for Cancer Prevention and Interception: Preclinical and Clinical Studies and Funding Opportunities. Pharmaceuticals 2024, 17, 136. [Google Scholar] [CrossRef]
- Narisawa, T.; Fukaura, Y.; Hasebe, M.; Nomura, S.; Oshima, S.; Inakuma, T. Prevention of N-methylnitrosourea-induced colon carcinogenesis in rats by oxygenated carotenoid capsanthin and capsanthin-rich paprika juice. Proc. Soc. Exp. Biol. Med. 2000, 224, 116–122. [Google Scholar]
- Kresty, L.A.; Morse, M.A.; Morgan, C.; Carlton, P.S.; Lu, J.; Gupta, A.; Blackwood, M.; Stoner, G.D. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001, 61, 6112–6119. [Google Scholar]
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Kloypan, C.; Jeenapongsa, R.; Sri-in, P.; Chanta, S.; Dokpuang, D.; Tip-pyang, S.; Surapinit, N. Stilbenoids from Gnetum macrostachyum attenuate human platelet aggregation and adhesion. Phytother. Res. 2012, 26, 1564–1568. [Google Scholar] [CrossRef]
- Seino, S.; Kimoto, T.; Yoshida, H.; Tanji, K.; Matsumiya, T.; Hayakari, R.; Seya, K.; Kawaguchi, S.; Tsuruga, K.; Tanaka, H.; et al. Gnetin C, a resveratrol dimer, reduces amyloid-beta 1-42 (Abeta42) production and ameliorates Abeta42-lowered cell viability in cultured SH-SY5Y human neuroblastoma cells. Biomed. Res. 2018, 39, 105–115. [Google Scholar] [CrossRef]
- Yoshida, H.; Imaizumi, T.; Matsumiya, T.; Seya, K.; Kawaguchi, S.; Tanaka, H. Gnetin C suppresses double-stranded RNA-induced C-C motif chemokine ligand 2 (CCL2) and CCL5 production by inhibiting Toll-like receptor 3 signaling pathway. Biomed. Res. 2018, 39, 231–240. [Google Scholar] [CrossRef]
- Kabir, T.; Yoshiba, H.; Agista, A.Z.; Sultana, H.; Ohsaki, Y.; Yeh, C.L.; Hirakawa, R.; Tani, H.; Ikuta, T.; Nochi, T.; et al. Protective Effects of Gnetin C from Melinjo Seed Extract against High-Fat Diet-Induced Hepatic Steatosis and Liver Fibrosis in NAFLD Mice Model. Nutrients 2023, 15, 3888. [Google Scholar] [CrossRef]
- Ikeda, E.; Tanaka, D.; Glogauer, M.; Tenenbaum, H.C.; Ikeda, Y. Healing effects of monomer and dimer resveratrol in a mouse periodontitis model. BMC Oral Health 2022, 22, 460. [Google Scholar] [CrossRef]
- Ikeda, E.; Ikeda, Y.; Wang, Y.; Fine, N.; Sheikh, Z.; Viniegra, A.; Barzilay, O.; Ganss, B.; Tenenbaum, H.C.; Glogauer, M. Resveratrol derivative-rich melinjo seed extract induces healing in a murine model of established periodontitis. J. Periodontol. 2018, 89, 586–595. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Franchini, M.; Favaloro, E.J.; Targher, G. Moderate red wine consumption and cardiovascular disease risk: Beyond the “French paradox”. Semin. Thromb. Hemost. 2010, 36, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Y.; Xie, X. Resveratrol inhibiting TGF/ERK signaling pathway can improve atherosclerosis: Backgrounds, mechanisms and effects. Biomed. Pharmacother. 2022, 155, 113775. [Google Scholar] [CrossRef]
- Riviere, C.; Papastamoulis, Y.; Fortin, P.Y.; Delchier, N.; Andriamanarivo, S.; Waffo-Teguo, P.; Kapche, G.D.; Amira-Guebalia, H.; Delaunay, J.C.; Merillon, J.M.; et al. New stilbene dimers against amyloid fibril formation. Bioorg Med. Chem. Lett. 2010, 20, 3441–3443. [Google Scholar] [CrossRef]
- Buffeteau, T.; Cavagnat, D.; Bisson, J.; Marchal, A.; Kapche, G.D.; Battistini, I.; Da Costa, G.; Badoc, A.; Monti, J.P.; Merillon, J.M.; et al. Unambiguous Determination of the Absolute Configuration of Dimeric Stilbene Glucosides from the Rhizomes of Gnetum africanum. J. Nat. Prod. 2014, 77, 1981–1985. [Google Scholar] [CrossRef]
- Zhang, Z.; Hamada, H.; Gerk, P.M. Selectivity of Dietary Phenolics for Inhibition of Human Monoamine Oxidases A and B. Biomed. Res. Int. 2019, 2019, 8361858. [Google Scholar] [CrossRef]
- Herraiz, T.; Flores, A.; Fernandez, L. Analysis of monoamine oxidase (MAO) enzymatic activity by high-performance liquid chromatography-diode array detection combined with an assay of oxidation with a peroxidase and its application to MAO inhibitors from foods and plants. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1073, 136–144. [Google Scholar] [CrossRef]
- Freyssin, A.; Page, G.; Fauconneau, B.; Rioux Bilan, A. Natural stilbenes effects in animal models of Alzheimer’s disease. Neural Regen. Res. 2020, 15, 843–849. [Google Scholar]
- Wicinski, M.; Domanowska, A.; Wodkiewicz, E.; Malinowski, B. Neuroprotective Properties of Resveratrol and Its Derivatives-Influence on Potential Mechanisms Leading to the Development of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2749. [Google Scholar] [CrossRef]
- Richard, T.; Pawlus, A.D.; Iglesias, M.L.; Pedrot, E.; Waffo-Teguo, P.; Merillon, J.M.; Monti, J.P. Neuroprotective properties of resveratrol and derivatives. Ann. N. Y. Acad. Sci. 2011, 1215, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, T.; Saito, S.; Tani, H.; Tatefuji, T.; Hashimoto, K. Resveratrol derivative-rich melinjo (Gnetum gnemon L.) seed extract improves obesity and survival of C57BL/6 mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2015, 79, 2044–2049. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J.; Bhaumik, D. Two faces of p53: Aging and tumor suppression. Nucleic Acids Res. 2007, 35, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Shibuya, S.; Ozawa, Y.; Izuo, N.; Shimizu, T. Resveratrol Derivative-Rich Melinjo Seed Extract Attenuates Skin Atrophy in Sod1-Deficient Mice. Oxid. Med. Cell. Longev. 2015, 2015, 391075. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Larrosa, M.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Fjeldborg, K.; Ornstrup, M.J.; Kjaer, T.N.; Nohr, M.K.; Pedersen, S.B. Resveratrol and inflammation: Challenges in translating pre-clinical findings to improved patient outcomes. Biochim. Biophys. Acta 2015, 1852, 1124–1136. [Google Scholar] [CrossRef]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef]
| Compound | Model | Dose | Cell/Animal | Mechanism of Action | Ref |
|---|---|---|---|---|---|
| Gnetin C | In vitro | 0–100 µM | Acute myelogenous leukemia (AML) cells: MV4, THP1, U937, HL60 Chronic myeloid leukemia (CML) cells: K562, Oun1, KH88 | Inhibition of ERK 1/2 and AKT/mTOR pathways; cell cycle arrest | [18] |
| In vivo | 5 mg/kg/day, 5 weeks | AML-MT xenograft mice | Inhibition of leukemia; antitumor effects (blood, spleen, bone marrow); extended survival of mice | ||
| Gnetin C MSE Resveratrol Gnemoside A, C, D | In vitro | 0.5–10 µM 40 µg/mL 5 µM | Human umbilical vein endothelial cells (HUVECs) | Inhibition of tube formation stimulated with VEGF and BFGF; reduction of cell viability and migration; ERK1/2 inactivation | [19] |
| In vivo | 5% MSE | Mouse dorsal air sac assay | Inhibition of tumor angiogenesis | ||
| Gnetin C Resveratrol MSE | In vitro | 0–100 µM 0–400 µg/mL | LNCaP, PC3, Murine CaP8 prostate cancer cells; MCF7 breast cancer cells; HT-29, colon-26 colon cancer cells; PANC-1, AsPC1, Pan-02 pancreatic cancer cells; PWPE1 and HEK-293T cells | Inhibitory effects on cancer cells without affecting normal cells; induction of apoptosis via caspase 3/7-dependent mechanisms | [17] |
| MSE | In vivo | 50 and 100 mg/kg/day, oral | Colon-26 tumor-bearing mouse model | Inhibition of tumor growth, angiogenesis and liver metastasis | |
| Gnetin C Resveratrol | In vitro | 2–16 µM | Murine melanoma B16 cells | Inhibitory activity against tyrosinase and melanin biosynthesis | [27] |
| Gnetin C Melinjo fruit extract | Ex vivo | 50% extract at 100 mg/kg/day | Cultured murine Peyer’s patch cells | Enhanced T-cell-dependent immune responses, IL2↑, IFNγ↑ | [28] |
| Compound | Model | Dose | Mechanism of Action | Ref | ||
|---|---|---|---|---|---|---|
| Mechanistic | In vitro | Gnetin C Resveratrol | DU145, PC3M, PC3M-shMTA1, DU145-shMTA1 | 0–50 µM | MTA1-mediated inhibitory effects on cell viability, colony formation, migration, induction of apoptosis; MTA1↓ (protein and RNA), ETS2↓ | [51] |
| Gnetin C Resveratrol Pterostilbene | DU145, PC3M | 0–100 µM | Cytotoxicity, reduction of clonogenic survival and motility | [52] | ||
| Gnetin C Enzalutamide combination | 22Rv1, VCaP | 0–100 µM/ 0–50 µM | Inhibition of cell viability, clonogenic survival and migration, synergism at certain doses; MTA1↓, AR-FL↓, AR-V7↓, PSA↓ | [44] | ||
| Gnetin C Pterostilbene | 22Rv1 | 25 µM | MTA1↓, pAkt/Akt↓, PTEN↑ | [54] | ||
| Gnetin C | PC3M, PC3M-shMTA1 | 25, 50 µM | MTA1↓, Cyclin D1↓, pAkt/Akt↓, p-mTOR/pS6K/p4EBP1↓ | [53] | ||
| Preclinical | Xenografts | Gnetin C Resveratrol Pterostilbene | PC3M-Luc | 25 and 50 mg/kg/day, i.p. | Tumor growth reduction, inhibition of angiogenesis and induction of apoptosis; MTA1↓, CyclinD1↓, Notch2↓ | [52] |
| Gnetin C Enzalutamide combination | 22Rv1-Luc | GnC 40 mg/kg/day + Enz 7 or 10 mg/kg/day, i.p. | Inhibition of tumor growth and angiogenesis, induction of apoptosis; MTA1↓, AR-FL↓, AR-V7↓ | [44] | ||
| Transgenic mice | Gnetin C Pterostilbene | R26MTA1; Pten+/f; Cre+ | GnC-Diet 35 and 70 mg/kg diet, or Pter-Diet 70 mg/kg diet | Reduction of cell proliferation, angiogenesis and inflammation; MTA1↓, pAkt/Akt↓, PTEN↑, IL2↓ in serum | [54] | |
| Gnetin C | R26MTA1; Ptenf/f, Cre+ | 7 mg/kg bw, i.p., 12 weeks | Inhibition of cell cycle progression, proliferation and angiogenesis, induction of apoptosis; MTA1↓, Cyclin D1↓, p-mTOR/pS6K/p4EBP1↓, IL2↓ in serum | [53] |
| Compound | Model | Dose | Cell/Animal | Mechanism of Action | Ref. | |
|---|---|---|---|---|---|---|
| Cardioprotective | Gnetin C Resveratrol Other stilbenes | Ex vivo | 500 µM | Human platelet-collagen adhesion assay | Res inhibits arachidonic acid- and thrombin-induced platelet aggregation; Gnetin C more potently inhibits platelet–collagen adhesion compared to Res | [63] |
| Gnetin C MSE Resveratrol Grape extract | In vitro | 0.05 nM angiotensin II + GnC 30 µM, MSE 300 µg/mL or Res 30 µM | Radioligand binding assay in transfected HEK-293 cells transfected COS-1 cells | GnC and MSE: inhibit ATII-type 1 receptor binding; GnC, MSE, grape extract, Res: mild agonists at PPARα and PPARγ | [21] | |
| Neuroprotective | Gnetin C Resveratrol ε-viniferin | In vitro | 0–20 µM | SH-SY5Y cells | Gnetin C more potently Aβ42↓ secretion, BACE1↓, Aβ oligomers↓, Aβ monomers↑, MMP-14↑, mitigated Aβ42-induced cytotoxicity | [64] |
| Gnetin C | In vitro | 0–10 µM | U373MG and SH-SY5Y cells: poly-IC-induced TLR3-mediated inflammation | IFN-β↓, STAT1 phosphorylation↓, CCL2↓, CCL5↓ | [65] | |
| Metabolic | Gnetin C MSE Resveratrol | In vivo | HFCD diet supplemented with 0.5% MSE, 12 wk | HFCD diet-induced NAFLD mouse model | MSE: body weight↓, liver weight↓, Tg↓, NEFAs↓, ALT↓, liver steatosis↓, hepatic fibrosis↓ | [66] |
| In vivo | HFCD diet supplemented with GnC or Resv 150 mg/kgbw/day, 12 wk | HFCD diet-induced NAFLD mouse model | GnC and Res: body weight↓, liver weight↓, IL-1b↓, adiponectin↑, liver steatosis↓, hepatic fibrosis↓, collagen deposition↓, COL1A1↓, TGFβ1↓; GnC: glucose↓, insulin sensitivity↑, lipids↓, ACC1↓, chREBP↓, DGAT1↓, DGAT2↓, MTP↓, LDLR↓, PPARα↓, PGC-1α↓, SIRT1↓ | |||
| Gnetin C MSE | In vivo | 2% MSE-supplemented diet, 21 days | Streptozotocin-induced diabetic mice (model for endothelial senescence) | SA-β-gal-positive cells↓, aortic SIRT1↑; Plasma Gnetin C component 6-fold higher than resveratrol | [16] | |
| Resveratrol Gnemonosides A, D | In vitro | 100 µmol/L | HUVEC H2O2-induced endothelial senescence | Only resveratrol component able to SA-β-gal-positive cells↓, SIRT1↑, eNOS↑, PAI1↓ | ||
| Gnetin C Resveratrol Gnetin L Gnemonosides A, C, D | In vitro | Constituents extracted from dried melinjo endosperm and purified | DPPH radical scavenging activity | Gnetin C ED50: 10.7 µM Resveratrol ED50: 13.2 µM | [15] | |
| Pancreatic digestive enzymes | Gnetin C has greater lipase and α-amylase inhibition than resveratrol | |||||
| Food | Moderate antimicrobial activity | |||||
| Anti-inflammatory—Anti-aging | Gnetin C Resveratrol | In vitro | 2–8 µM | Murine B16 cells | GnC and Res: Similar inhibitory potency of tyrosine activity and melanin biosynthesis; neither is cytotoxic | [27] |
| In vitro | 2–16 µM | Cell-free inhibition of tyrosinase enzyme | GnC has less direct inhibition of tyrosine activity | |||
| Gnetin C Resveratrol | In vivo | 10 mg/kg i.p. daily for 7–8 days | Ligature-induced periodontitis mouse model | GnC and Res: 8-OHdG↓; GnC: bone healing↑ and IL-1β↓ | [67,68] | |
| In vivo | As above | Nrf2−/− transgenic mice | Neither treatment able to induce bone healing |
| Trial Type | Treatment | Population | Number of Participants and Duration | Markers | Outcome | Ref |
|---|---|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled | Gnetin C 150 mg/day, orally | Healthy Japanese subjects | N = 12 Days: 14 | No change in CRP, Tg, 8-OHdG or pentosidine; LDL-C↓, HDL-C↓, adiponectin↓, NK cells↑ | Safety; Cardioprotective effect | [24] |
| Randomized, double-blind, placebo-controlled | MSE 750 mg daily, orally | Nonobese Japanese males, 35–70 yrs old | N = 30 Weeks: 8 | Serum uric acid↓, LDL-C no change, HDL-C↑ | Cardioprotective | [21] |
| No placebo | Single-dose study: Res 6.80 mg/day; MSE 1000 mg/day, orally | Healthy volunteers | N = 10 (6 men, 4 women) 23–34 yrs old Days: 28 | Safety; Pharmacokinetics | [22] | |
| Placebo-controlled | Repeated doses MSE 1000 mg, 2000 mg or 5000 mg | Healthy volunteers | N = 44 (22 men, 22 women) 32–49 yrs old Days: 14 and 28 | Blood pressure, pulse, body mass index; biochemical parameters in blood, urine | ||
| No placebo | MSE tablets (38.5% MSE powder equivalent to 262 mg Gnetin C): 20 tabs/day | Healthy volunteers | N = 5 (3 males, 2 females) 34–46 yrs old Days: 28 | Blood, circulating: Immune cells Surface immune receptors Treg cells CTL (GZMB) NK (NKG2D receptor) Inflammatory cytokines IFNγ; TNFα 8-OHdG↓ | Safety Antioxidant effects Effects on circulating lymphocytes Chemopreventive potential | [23] |
| Randomized, double-blind, placebo-controlled | MSE 150 mg or 300 mg daily vs. placebo | Healthy young volunteers | N = 42 Days:14 | HMW/total APN↑ LDL-C↓, ALT↓ | Anti-inflammatory Insulin sensitivity Cardioprotective | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanelli, G.; Levenson, A.S. Gnetin C in Cancer and Other Diseases: What Do We Know So Far? Nutrients 2025, 17, 863. https://doi.org/10.3390/nu17050863
Campanelli G, Levenson AS. Gnetin C in Cancer and Other Diseases: What Do We Know So Far? Nutrients. 2025; 17(5):863. https://doi.org/10.3390/nu17050863
Chicago/Turabian StyleCampanelli, Gisella, and Anait S. Levenson. 2025. "Gnetin C in Cancer and Other Diseases: What Do We Know So Far?" Nutrients 17, no. 5: 863. https://doi.org/10.3390/nu17050863
APA StyleCampanelli, G., & Levenson, A. S. (2025). Gnetin C in Cancer and Other Diseases: What Do We Know So Far? Nutrients, 17(5), 863. https://doi.org/10.3390/nu17050863








