A Preliminary Analysis of the Influence of Elderberry Juice Consumption on Thyroid Metabolism in Mice and Humans Fed High-Fat Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Clinical Trial and Rodent Study Design
2.2. Human Serum T4, TSH, Tg Quantification
2.3. Mouse Serum T4 Quantification
2.4. Statistical Analysis
3. Results
3.1. Human Thyroid Hormone Levels
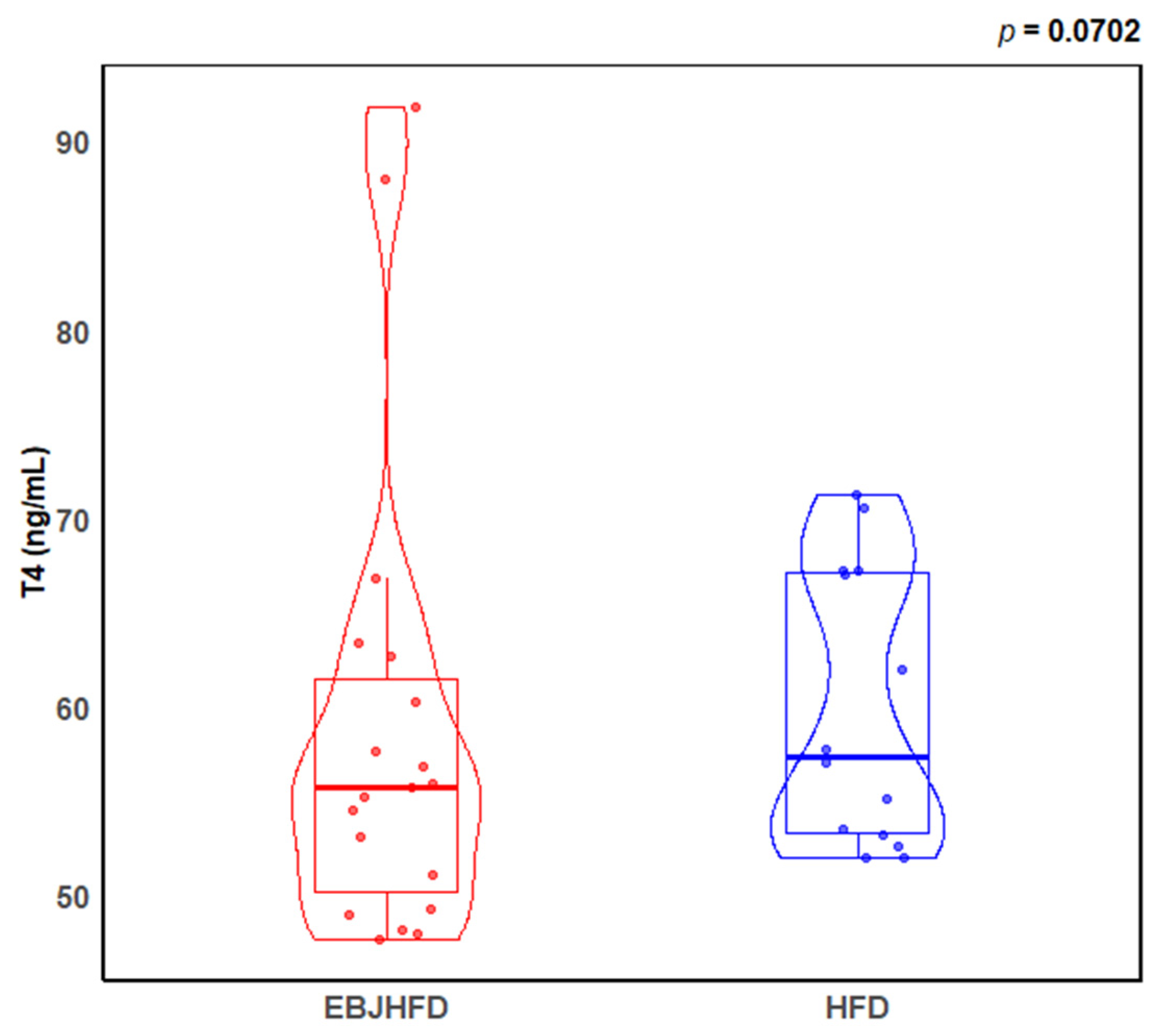
3.2. Mouse Thyroid Hormone Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minj, J.; Riordan, J.; Teets, C.; Fernholz-Hartman, H.; Tanggono, A.; Lee, Y.; Chauvin, T.; Carbonero, F.; Solverson, P. Diet-Induced Rodent Obesity Is Prevented and the Fecal Microbiome Is Improved with Elderberry. J. Agric. Food Chem. 2024, 72, 12555–12565. [Google Scholar] [CrossRef] [PubMed]
- Teets, C.; Ghanem, N.; Ma, G.; Minj, J.; Perkins-Veazie, P.; Johnson, S.A.; Etter, A.J.; Carbonero, F.G.; Solverson, P.M. A One-Week Elderberry Juice Intervention Augments the Fecal Microbiota and Suggests Improvement in Glucose Tolerance and Fat Oxidation in a Randomized Controlled Trial. Nutrients 2024, 16, 3555. [Google Scholar] [CrossRef] [PubMed]
- Rust, B.M.; Riordan, J.O.; Carbonero, F.G.; Solverson, P.M. One-Week Elderberry Juice Treatment Increases Carbohydrate Oxidation after a Meal Tolerance Test and Is Well Tolerated in Adults: A Randomized Controlled Pilot Study. Nutrients 2023, 15, 2072. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.F.; Musich, M.; Costa, A.N.; Gonzales, J.; Gonzales, H.; Ferguson, B.J.; Kille, B.; Thomas, A.L.; Wei, X.; Liu, P.; et al. Feasibility and Preliminary Efficacy of American Elderberry Juice for Improving Cognition and Inflammation in Patients with Mild Cognitive Impairment. Int. J. Mol. Sci. 2024, 25, 4352. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, X.Q.; Wu, D.T.; Li, H.B.; Feng, Y.B.; Zou, L.; Gan, R.Y. Elderberry (Sambucus nigra L.): Bioactive Compounds, Health Functions, and Applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef]
- Xia, S.F.; Duan, X.M.; Hao, L.Y.; Li, L.T.; Cheng, X.R.; Xie, Z.X.; Qiao, Y.; Li, L.R.; Tang, X.; Shi, Y.H.; et al. Role of thyroid hormone homeostasis in obesity-prone and obesity-resistant mice fed a high-fat diet. Metabolism 2015, 64, 566–579. [Google Scholar] [CrossRef]
- Wu, J.; Jia, C.; Wang, Q.; Li, X. Association between vitamin C intake and thyroid function among U.S. adults: A population-based study. Front. Endocrinol. 2024, 15, 1462251. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; García-Almagro, F.J.; Avilés-Plaza, F.; Parra, S.; Yáñez-Gascón, M.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef]
- Rosli, H.; Shahar, S.; Rajab, N.F.; Che Din, N.; Haron, H. The effects of polyphenols-rich tropical fruit juice on cognitive function and metabolomics profile—A randomized controlled trial in middle-aged women. Nutr. Neurosci. 2022, 25, 1577–1593. [Google Scholar] [CrossRef]
- Florio, P.; Folli, C.; Cianci, M.; Del Rio, D.; Zanotti, G.; Berni, R. Transthyretin Binding Heterogeneity and Anti-amyloidogenic Activity of Natural Polyphenols and Their Metabolites. J. Biol. Chem. 2015, 290, 29769–29780. [Google Scholar] [CrossRef]
- Johnson, M.C.; Dela Libera Tres, M.; Thomas, A.L.; Rottinghaus, G.E.; Greenlief, C.M. Discriminant Analyses of the Polyphenol Content of American Elderberry Juice from Multiple Environments Provide Genotype Fingerprint. J. Agric. Food Chem. 2017, 65, 4044–4050. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, D.L.; Galgani, J.E.; Johannsen, N.M.; Zhang, Z.; Covington, J.D.; Ravussin, E. Effect of short-term thyroxine administration on energy metabolism and mitochondrial efficiency in humans. PLoS ONE 2012, 7, e40837. [Google Scholar] [CrossRef]
- De Sales, N.F.; Silva da Costa, L.; Carneiro, T.I.; Minuzzo, D.A.; Oliveira, F.L.; Cabral, L.M.; Torres, A.G.; El-Bacha, T. Anthocyanin-rich grape pomace extract (Vitis vinifera L.) from wine industry affects mitochondrial bioenergetics and glucose metabolism in human hepatocarcinoma HepG2 cells. Molecules 2018, 23, 611. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Skates, E.; Overall, J.; DeZego, K.; Wilson, M.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Berries containing anthocyanins with enhanced methylation profiles are more effective at ameliorating high fat diet-induced metabolic damage. Food Chem. Toxicol. 2018, 111, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P. Anthocyanin Bioactivity in Obesity and Diabetes: The Essential Role of Glucose Transporters in the Gut and Periphery. Cells 2020, 9, 2515. [Google Scholar] [CrossRef]
- Solverson, P.; Albaugh, G.P.; Debelo, H.A.; Ferruzzi, M.G.; Baer, D.J.; Novotny, J.A. Mixed Berry Juice and Cellulose Fiber Have Differential Effects on Peripheral Blood Mononuclear Cell Respiration in Overweight Adults. Nutrients 2023, 15, 1709. [Google Scholar] [CrossRef]
- Shulhai, A.M.; Rotondo, R.; Petraroli, M.; Patianna, V.; Predieri, B.; Iughetti, L.; Esposito, S.; Street, M.E. The Role of Nutrition on Thyroid Function. Nutrients 2024, 16, 2496. [Google Scholar] [CrossRef]
- Wu, J.; Jia, C.; Zhang, Z.; Hou, Z.; Cui, Y. The relationship between dietary total flavonoids and thyroid function in U.S.adults, NHANES 2007–2010. PLoS ONE 2024, 19, e0303169. [Google Scholar] [CrossRef] [PubMed]
- Welch-White, V.; Dawkins, N.; Graham, T.; Pace, R. The impact of high fat diets on physiological changes in euthyroid and thyroid altered rats. Lipids Health Dis. 2013, 12, 100. [Google Scholar] [CrossRef]
- Araujo, R.L.; Andrade, B.M.; Padrón, A.S.; Gaidhu, M.P.; Perry, R.L.; Carvalho, D.P.; Ceddia, R.B. High-fat diet increases thyrotropin and oxygen consumption without altering circulating 3,5,3′-triiodothyronine (T3) and thyroxine in rats: The role of iodothyronine deiodinases, reverse T3 production, and whole-body fat oxidation. Endocrinology 2010, 151, 3460–3469. [Google Scholar] [CrossRef]
- Reinehr, T. Obesity and thyroid function. Mol. Cell Endocrinol. 2010, 316, 165–171. [Google Scholar] [CrossRef]
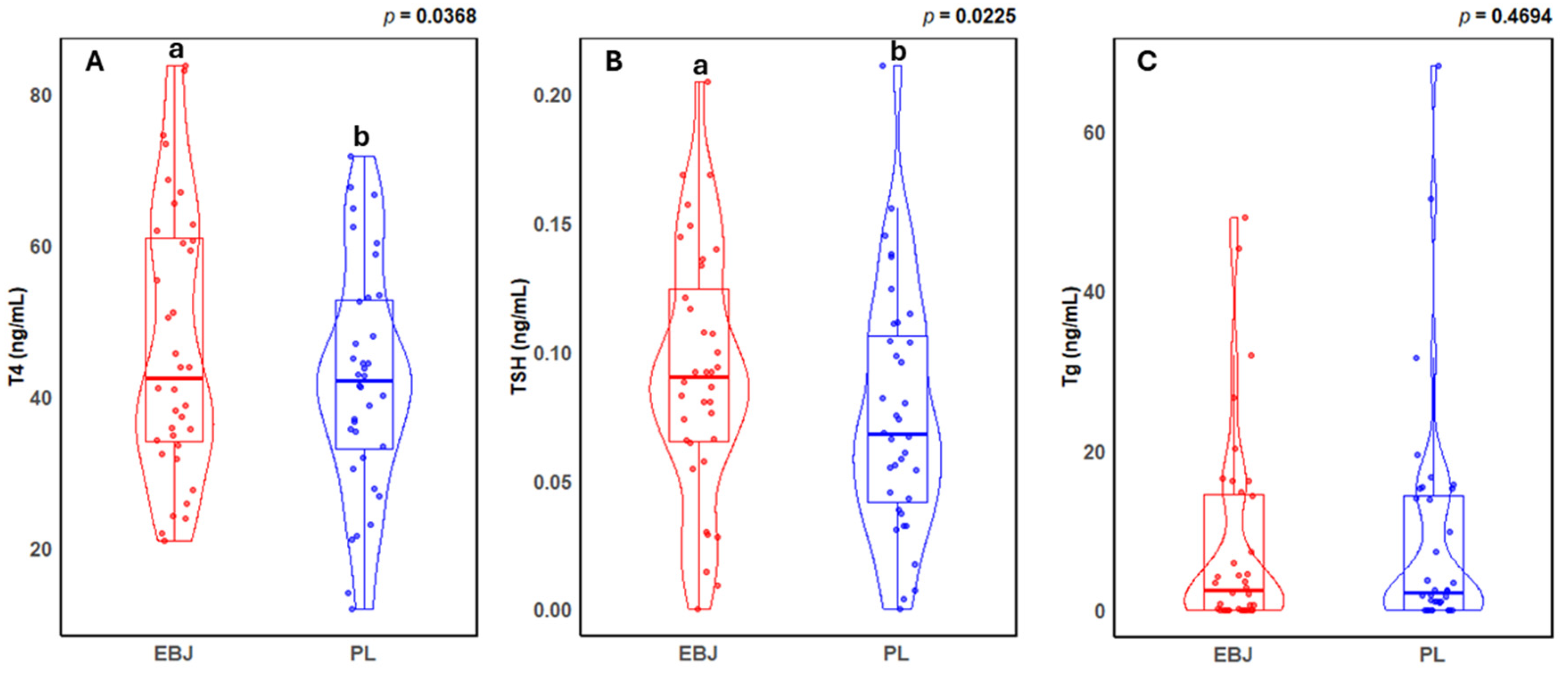
| EBJ | PL | p | |
|---|---|---|---|
| T4 (ng/mL) | 47.0 ± 2.97 | 42.19 ± 2.51 | 0.037 |
| TSH (ng/mL) | 0.092 ± 0.008 | 0.076 ± 0.008 | 0.023 |
| Tg (ng/mL) | 11.72 ± 2.76 a | 12.23 ± 3.18 b | 0.469 |
| EBJHFD | HFD | p | |
|---|---|---|---|
| T4 (ng/mL) | 55.0 ± 1.42 a | 59.9 ± 1.96 b | 0.070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarrett, C.L.; Teets, C.; Carbonero, F.G.; Etter, A.J.; Solverson, P.M. A Preliminary Analysis of the Influence of Elderberry Juice Consumption on Thyroid Metabolism in Mice and Humans Fed High-Fat Diets. Nutrients 2025, 17, 612. https://doi.org/10.3390/nu17040612
Jarrett CL, Teets C, Carbonero FG, Etter AJ, Solverson PM. A Preliminary Analysis of the Influence of Elderberry Juice Consumption on Thyroid Metabolism in Mice and Humans Fed High-Fat Diets. Nutrients. 2025; 17(4):612. https://doi.org/10.3390/nu17040612
Chicago/Turabian StyleJarrett, Catherine L., Christy Teets, Franck G. Carbonero, Andrea J. Etter, and Patrick M. Solverson. 2025. "A Preliminary Analysis of the Influence of Elderberry Juice Consumption on Thyroid Metabolism in Mice and Humans Fed High-Fat Diets" Nutrients 17, no. 4: 612. https://doi.org/10.3390/nu17040612
APA StyleJarrett, C. L., Teets, C., Carbonero, F. G., Etter, A. J., & Solverson, P. M. (2025). A Preliminary Analysis of the Influence of Elderberry Juice Consumption on Thyroid Metabolism in Mice and Humans Fed High-Fat Diets. Nutrients, 17(4), 612. https://doi.org/10.3390/nu17040612









