High Rates of Abnormal Glucose Metabolism Detected by 75 g Oral Glucose Tolerance Test in Major Psychiatric Patients with Normal HbA1c and Fasting Glucose Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement
2.3. Classification of Glucose Metabolism Using 75 g OGTT
2.4. Statistical Analysis
3. Results
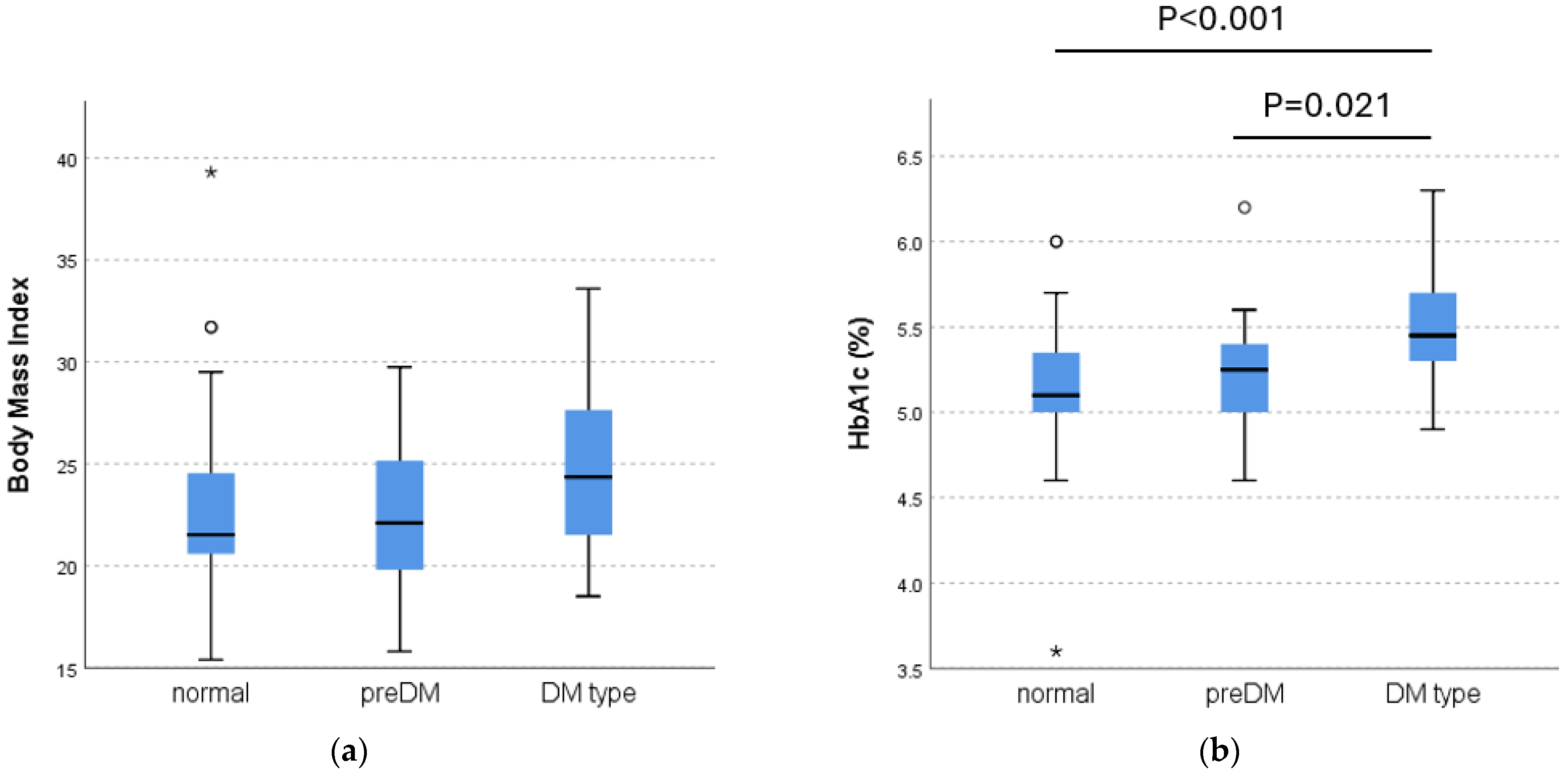
3.1. Comparison of Characteristics
3.2. Medication
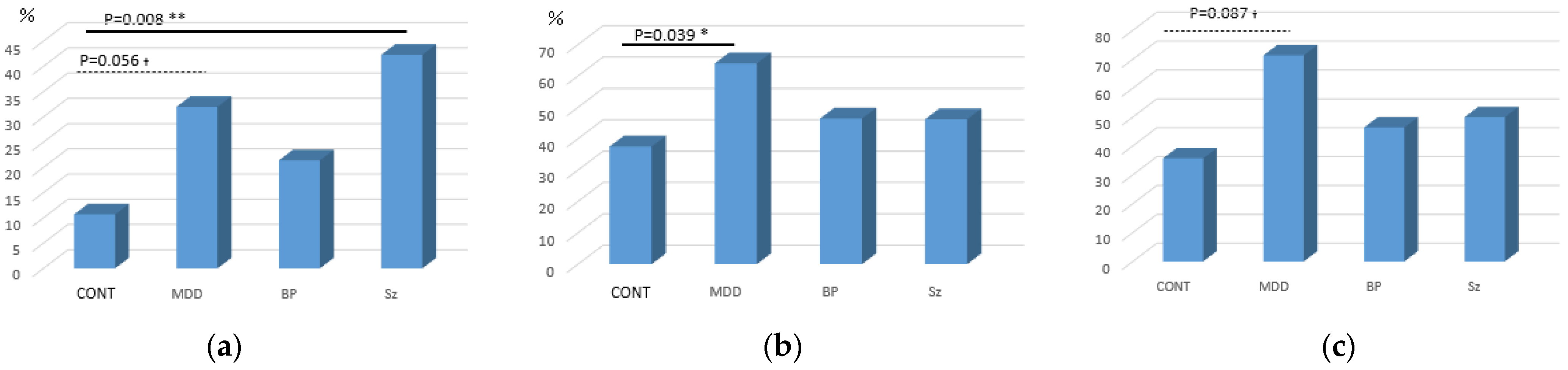
3.3. Oral Glucose Tolerance Test
4. Discussion
4.1. Schizophrenia
4.2. Major Depressive Disorder
4.3. Bipolar Disorder
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| BP | bipolar disorder |
| CRE | creatinine clearance |
| DM | diabetes mellitus |
| DSM-IV | Diagnostic and Statistical Manual of Mental Disorders, 4th edition |
| γ-GT | γ-glutamyltransferase |
| GLP-1 | glucagon-like peptide-1 |
| HbA1c | hemoglobin A1c |
| MDD | major depressive disorder |
| OGTT | oral glucose tolerance test |
| Sz | schizophrenia |
| T-Cho | total cholesterol |
| TG | triglycerides |
| UN | urea nitrogen |
References
- Mai, Q.; Holman, C.D.; Sanfilippo, F.M.; Emery, J.D.; Preen, D.B. Mental illness related disparities in diabetes prevalence, quality of care and outcomes: A population-based longitudinal study. BMC Med. 2011, 9, 118. [Google Scholar] [CrossRef]
- Nuevo, R.; Chatterji, S.; Fraguas, D.; Verdes, E.; Naidoo, N.; Arango, C.; Ayuso-Mateos, J.L. Increased risk of diabetes mellitus among persons with psychotic symptoms: Results from the WHO World Health Survey. J. Clin. Psychiatry 2011, 72, 1592–1599. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit. Pathw. Cardiol. 2005, 4, 198–203. [Google Scholar]
- McIntyre, R.S.; Woldeyohannes, H.O.; Soczynska, J.K.; Miranda, A.; Lachowski, A.; Liauw, S.S.; Grossman, T.; Lourenco, M.T.; Kim, B.; Alsuwaidan, M.T.; et al. The rate of metabolic syndrome in euthymic Canadian individuals with bipolar I/II disorder. Adv. Ther. 2010, 27, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.; D’Ambrosio, V.; Rosso, G.; Bogetto, F.; Maina, G. Age-specific prevalence of metabolic syndrome in Italian patients with bipolar disorder. Psychiatry Clin. Neurosci. 2011, 65, 47–54. [Google Scholar] [CrossRef] [PubMed]
- McElroy, S.L.; Keck, P.E., Jr. Metabolic syndrome in bipolar: A review with a focus on bipolar depression. J. Clin. Psychiatry 2014, 75, 46–61. [Google Scholar] [CrossRef]
- Braceland, R.J.; Meduna, L.J.; Vaichulis, J.A. Delayed action of insulin in schizophrenia. Am. J. Psychiatry 1945, 102, 108–110. [Google Scholar] [CrossRef]
- Freeman, H. Resistance to insulin in mentally disturbed soldiers. Arch. Neurol. Psychiatry 1946, 56, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Langfeldt, G. The insulin torelance test in mental disorders. Acta Psychiatr. Scand. 1952, 80, 189–200. [Google Scholar]
- Mukherjee, S.; Schnur, D.B.; Reddy, R. Family history of type 2 diabetes in schizophrenic patients. Lancet 1989, 1, 495. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Birtwistle, J.; Roe, I.; Thompson, C. The unhealthy lifestyle of people with schizophrenia. Pscychol. Med. 1999, 29, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.; Weiden, P.; Delahanty, J.; Goldberg, R.; Postrado, L.; Lucksted, A.; Lehman, A. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr. Bull. 2000, 26, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Thakore, J.H.; Mann, J.N.; Vlahos, I.; Martin, A.; Reznek, R. Increased visceral fat distribution in drug-naïve and drug-free patients with schizophrenia. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 137–141. [Google Scholar] [CrossRef]
- Lindekilde, N.; Scheuer, S.H.; Rutters, F.; Knudsen, L.; Lasgaard, M.; Rubin, K.H.; Henriksen, J.E.; Kivimäki, M.; Andersen, G.S.; Pouwer, F. Prevalence of type 2 diabetes in psychiatric disorders: An umbrella review with meta-analysis of 245 observational studies from 32 systematic reviews. Diabetologia 2022, 65, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hirai, M.; Suzuki, S.; Awata, S.; Oka, Y. Neuropathy is associated with depression independently of health-related quality of life in Japanese patients with diabetes. Psychiatry Clin. Neurosci. 2009, 63, 65–72. [Google Scholar] [CrossRef]
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997, 20, 1183–1197. [Google Scholar] [CrossRef]
- Kaur, G.; Lakshmi, P.V.M.; Rastogi, A.; Bhansali, A.; Jain, S.; Teerawattananon, Y.; Bano, H.; Prinja, S. Diagnostic accuracy of tests for type 2 diabetes and prediabetes: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0242415. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Stolk, R.P.; Grobbee, D.E.; Gispen-de Wied, C.C. Hyperglycemia and diabetes in patients with schizophrenia or schizoaffective disorders. Diabetes Care 2006, 29, 786–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33, quiz 34–57. [Google Scholar] [PubMed]
- Otsubo, T.; Tanaka, K.; Koda, R.; Shinoda, J.; Sano, N.; Tanaka, S.; Aoyama, H.; Mimura, M.; Kamijima, K. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin. Neurosci. 2005, 59, 517–526. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Inagaki, A. Psychotropic dose equivalence in Japan. Psychiatry Clin. Neurosci. 2015, 69, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus; Seino, Y.; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes. Investig. 2010, 1, 212–228. [Google Scholar] [CrossRef]
- Al-Zoairy, R.; Ress, C.; Tschoner, A.; Kaser, S.; Ebenbichler, C. The effects of psychotropic drugs on the regulation of glucose metabolism. Curr. Diabetes Rev. 2013, 9, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.; Rico-Villademoros, F.; García-Rizo, C.; Rojo, R.; Gómez-Huelgas, R. Real-world data on the adverse metabolic effects of second-generation antipsychotics and their potential determinants in adult patients: A systematic review of population-based studies. Adv. Ther. 2021, 38, 2491–2512. [Google Scholar] [CrossRef]
- Mamakou, V.; Thanopoulou, A.; Gonidakis, F.; Tentolouris, N.; Kontaxakis, V. Schizophrenia and type 2 diabetes mellitus. Psychiatriki 2018, 29, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.I.; Shuldiner, A.R. Rethinking the genetic basis for comorbidity of schizophrenia and type 2 diabetes. Schizophr Res. 2010, 123, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Zhang, Q.; Wang, L.; Ma, X.; Li, R.; Ping, J.; Zhu, J.; Tian, H.; Jiang, D. Insulin resistance/diabetes and schizophrenia: Potential shared genetic factors and implications for better management of patients with schizophrenia. CNS Drugs 2024, 38, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Ito, H.; Kobayashi, M.; Mayahara, K.; Matsumoto, Y.; Hirakawa, J. Prevalence of diabetes and antipsychotic prescription patterns in patients with schizophrenia: A nationwide retrospective cohort study. Schizophr. Res. 2010, 19, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sugai, T.; Suzuki, Y.; Yamazaki, M.; Shimoda, K.; Mori, T.; Ozeki, Y.; Matsuda, H.; Sugawara, N.; Yasui-Furukori, N.; Minami, Y.; et al. High prevalence of obesity, hypertension, hyperlipidemia, and diabetes mellitus in Japanese outpatients with schizophrenia: A nationwide survey. PLoS ONE 2016, 11, e0166429. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Suzuki, Y.; Fukui, N.; Sugai, T.; Watanabe, J.; Tsuneyama, N.; Saito, M.; Someya, T. The prevalence of glucose intolerance in Japanese schizophrenic patients with a normal fasting glucose level. J. Clin. Psychopharmacol. 2013, 33, 525–527. [Google Scholar] [CrossRef] [PubMed]
- de Silva, V.A.; Suraweera, C.; Ratnatunga, S.S.; Dayabandara, M.; Wanniarachchi, N.; Hanwella, R. Metformin in prevention and treatment of antipsychotic induced weight gain: A systematic review and meta-analysis. BMC Psychiatry 2016, 16, 341. [Google Scholar] [CrossRef]
- Trott, M.; Arnautovska, U.; Siskind, D. GLP-1 receptor agonists and weight loss in schizophrenia—Past, present, and future. Curr. Opin. Psychiatry 2024, 37, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Huang, J.; Zhao, Y.; Wang, W.; Tian, X.; Hei, G.; Kang, D.; Gao, Y.; Liu, F.; Zhao, J.; et al. Metformin improves cognitive impairment in patients with schizophrenia: Associated with enhanced functional connectivity of dorsolateral prefrontal cortex. Transl. Psychiatry 2023, 13, 315. [Google Scholar] [CrossRef]
- Flintoff, J.; Kesby, J.P.; Siskind, D.; Burne, T.H. Treating cognitive impairment in schizophrenia with GLP-1RAs: An overview of their therapeutic potential. Expert Opin. Investig. Drugs 2021, 30, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Kalocsányiová, E.; McCrone, P.; Elliott, H.; Milligan, W.; Gkaintatzi, E. Non-pharmacological interventions for type 2 diabetes in people living with severe mental illness: Results of a systematic review and meta-analysis. Int. J. Environ. Res Public Health 2024, 21, 423. [Google Scholar] [CrossRef] [PubMed]
- Dauwan, M.; Begemann, M.J.; Heringa, S.M.; Sommer, I.E. Exercise Improves Clinical Symptoms, Quality of Life, Global Functioning, and Depression in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr. Bull. 2016, 42, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Stubbs, B.; Rosenbaum, S.; Vancampfort, D.; Malchow, B.; Schuch, F.; Elliott, R.; Nuechterlein, K.H.; Yung, A.R. Aerobic Exercise Improves Cognitive Functioning in People with Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2017, 43, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Hidese, S.; Matsuo, J.; Ishida, I.; Hiraishi, M.; Teraishi, T.; Ota, M.; Hattori, K.; Kunugi, H. Relationship of handgrip strength and body mass index with cognitive function in patients with schizophrenia. Front. Psychiatry 2018, 9, 156. [Google Scholar] [CrossRef]
- Kawakami, N.; Takatsuka, N.; Shimizu, H.; Ishibashi, H. Depressive symptoms and occurrence of type 2 diabetes among Japanese men. Diabetes Care 1999, 22, 1071–1076. [Google Scholar] [CrossRef]
- Hidese, S.; Asano, S.; Saito, K.; Sasayama, D.; Kunugi, H. Association of depression with body mass index classification, metabolic disease, and lifestyle: A web-based survey involving 11,876 Japanese people. J. Psychiatr. Res. 2018, 102, 23–28. [Google Scholar] [CrossRef]
- Ali, S.; Stone, M.A.; Peters, J.L.; Davies, M.J.; Khunti, K. The prevalence of co-morbid depression in adults with Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2006, 23, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, H.; Ida, I.; Owashi, T.; Kimura, M.; Inoue, Y.; Nakagawa, S.; Yabana, T.; Urushibara, T.; Kanai, R.; Aihara, M.; et al. Assessment of the dexamethasone/CRH test as a state-dependent marker for hypothalamic-pituitary-adrenal (HPA) axis abnormalities in major depressive episode: A Multicenter Study. Neuropsychopharmacology 2006, 31, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Koga, N.; Komatsu, Y.; Shinozaki, R.; Ishida, I.; Shimizu, Y.; Ishimaru, S.; Kunugi, H. Simultaneous monitoring of activity and heart rate variability in depressed patients: A pilot study using a wearable monitor for 3 consecutive days. Neuropsychopharmacol. Rep. 2022, 42, 457–467. [Google Scholar] [CrossRef]
- Sgoifo, A.; Carnevali, L.; de los Angeles Pico Alfonso, M.; Amore, M. Autonomic dysfunction and heart rate variability in depression. Stress 2015, 18, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Steckler, T.; Holsboer, F.; Reul, J.M. Glucocorticoids and depression. Baillieres Best Pract. Res. Clin. Endocrinol. Metab. 1999, 13, 597–614. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P. Visceral fat accumulation: The missing link between psychosocial factors and cardiovascular disease? J. Int. Med. 1991, 230, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, R.; Fuller Tyszkiewicz, M.D. Factors contributing to depressive mood states in everyday life: A systematic review. J. Affect. Disord. 2016, 200, 103–110. [Google Scholar] [CrossRef]
- Pyykkönen, A.J.; Räikkönen, K.; Tuomi, T.; Eriksson, J.G.; Groop, L.; Isomaa, B. Stressful life events and the metabolic syndrome: The prevalence, prediction and prevention of diabetes (PPP)-Botnia Study. Diabet. Care 2010, 33, 378–384. [Google Scholar] [CrossRef]
- Serretti, A.; Mandelli, L. Antidepressants and body weight: A comprehensive review and meta-analysis. J. Clin. Psychiatry 2010, 71, 1259–1272. [Google Scholar] [CrossRef]
- Hennings, J.M.; Schaaf, L.; Fulda, S. Glucose metabolism and antidepressant medication. Curr. Pham. Des. 2012, 18, 5900–5919. [Google Scholar] [CrossRef]
- Kunugi, H. Depression and lifestyle: Focusing on nutrition, exercise, and their possible relevance to molecular mechanisms. Psychiatry Clin. Neurosci. 2023, 77, 420–433. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Powell, A.M.; Kaidanovich-Beilin, O.; Soczynska, J.K.; Alsuwaidan, M.; Woldeyohannes, H.O.; Kim, A.S.; Gallaugher, L.A. The neuroprotective effects of GLP-1: Possible treatments for cognitive deficits in individuals with mood disorders. Behav. Brain Res. 2013, 237, 164–171. [Google Scholar] [CrossRef]
- Nibber, A.; Singh, H.; Burnet, P.; Lennox, B.; Minichino, A. Investigating the pro-cognitive and anti-depressant efficacy of metformin: A systematic review and meta-analysis of randomised controlled trials. J. Affect. Disord. 2022, 310, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Possidente, C.; Fanelli, G.; Serretti, A.; Fabbri, C. Clinical insights into the cross-link between mood disorders and type 2 diabetes: A review of longitudinal studies and Mendelian randomisation analyses. Neurosci. Biobehav. Rev. 2023, 152, 105298. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Mi, J.; Jiang, Q.M.; Xu, J.M.; Tang, Y.Y.; Tian, G.; Wang, B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2014, 41, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.; Garcia, L.; Abbas, A.; Strain, T.; Schuch, F.B.; Golubic, R.; Kelly, P.; Khan, S.; Utukuri, M.; Laird, Y.; et al. Association between physical activity and risk of depression: A systematic review and meta-analysis. JAMA Psychiatry 2022, 79, 550–559. [Google Scholar] [CrossRef]
- Kvam, S.; Kleppe, C.L.; Nordhus, I.H.; Hovland, A. Exercise as a treatment for depression: A meta-analysis. J. Affect. Disord. 2016, 202, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Noetel, M.; Sanders, T.; Gallardo-Gómez, D.; Taylor, P.; Del Pozo Cruz, B.; van den Hoek, D.; Smith, J.J.; Mahoney, J.; Spathis, J.; Moresi, M.; et al. Effect of exercise for depression: Systematic review and network meta-analysis of randomised controlled trials. Br. Med. J. 2024, 384, e075847. [Google Scholar] [CrossRef]
- Mazloomi, S.N.; Talebi, S.; Mehrabani, S.; Bagheri, R.; Ghavami, A.; Zarpoosh, M.; Mohammadi, H.; Wong, A.; Nordvall, M.; Kermani, M.A.H.; et al. The association of ultra-processed food consumption with adult mental health disorders: A systematic review and dose-response meta-analysis of 260,385 participants. Nutr. Neurosci. 2023, 26, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Hojjati Kermani, M.A.; Bagheri, R.; Mohammadi, H.; Jayedi, A.; Lane, M.M.; Asbaghi, O.; Mehrabani, S.; Suzuki, K. Ultra-processed food consumption and adult diabetes risk: A systematic review and dose-response meta-analysis. Nutrients 2021, 13, 4410. [Google Scholar] [CrossRef]
- Kittel-Schneider, S.; Bury, D.; Leopold, K.; Haack, S.; Bauer, M.; Pfeiffer, S.; Sauer, C.; Pfennig, A.; Völzke, H.; Grabe, H.J.; et al. Prevalence of prediabetes and diabetes mellitus type II in bipolar disorder. Front. Psychiatry 2020, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Leopold, K.; Reif, A.; Haack, S.; Bauer, M.; Bury, D.; Löffler, A.; Kittel-Schneider, S.; Pfeiffer, S.; Sauer, C.; Schwarz, P.; et al. Type 2 diabetes and pre-diabetic abnormalities in patients with bipolar disorders. J. Affect. Disord. 2016, 189, 240–245. [Google Scholar] [CrossRef] [PubMed]
| Healthy Control | MDD | BP | Sz | |
|---|---|---|---|---|
| N = 107(M 50/F 57) | 28 (12/16) | 25 (14/11) | 28 (11/17) | 26 (13/13) |
| Age (years) | 41.6 ± 11.1 | 40.8 ± 12.0 | 41.0 ± 8.8 | 38.3 ± 9.3 |
| Education (years) | 15.8 ± 2.5 | 15.2 ± 2.6 | 15.7 ± 2.3 | 14.1 ± 3.0 |
| Height (cm) | 163.5 ± 8.9 | 165.6 ± 9.5 | 163.1 ± 8.5 | 166.1 ± 8.3 |
| Weight (kg) | 60.9 ± 11.7 | 62.2 ± 11.8 | 62.1 ± 14.5 | 68.4 ± 17.8 |
| Body mass index: BMI | 22.7 ± 3.2 | 22.5 ± 2.9 | 23.3 ± 4.5 | 24.7 ± 5.3 |
| Abdominal circumference (cm) | 80.9 ± 10.0 | 80.9 ± 8.7 | 82.0 ± 11.2 | 86.0 ± 14.5 |
| Fasting glucose (mg/dL) | 93.6 ± 8.3 | 95.3 ± 9.5 | 92.1 ± 8.5 | 94.6 ± 5.7 |
| HbA1c (NGSP: %) | 5.2 ± 0.5 | 5.3 ± 0.4 | 5.3 ± 0.4 | 5.3 ± 0.3 |
| AST (IU/L) | 22.5 ± 10.5 | 23.3 ± 15.4 | 22.2 ± 8.3 | 22.8 ± 11.8 |
| ALT (IU/L) | 18.6 ± 9.7 | 27.8 ± 37.6 | 24.7 ± 16.5 | 29.2 ± 24.1 |
| γGTP (IU/L) | 27.3 ± 28.3 | 33.4 ± 32.7 | 28.3 ± 26.1 | 31.6 ± 27.5 |
| TG (mg/dL) | 104.4 ± 99.5 | 116.6 ± 86.9 | 129.0 ± 89.0 | 144.0 ± 165.0 |
| T-Cho (mg/dL) | 204.4 ± 44.4 | 213.2 ± 29.4 | 199.8 ± 32.1 | 184.1 ± 36.9 |
| BUN | 12.1 ± 2.8 | 13.5 ± 3.6 | 11.1 ± 4.1 | 11.5 ± 4.3 |
| CRE | 0.67 ± 0.02 | 0.74 ± 0.03 | 0.70 ± 0.03 | 0.73 ± 0.03 |
| Antidepressant (mg) (imipramine equivalents) | 0.00 | 100.9 ± 102.8 | 41.0 ± 64.8 | 9.1 ± 31.1 |
| Antipsychotics (mg) (chlorpromazine equivalents) | 0.00 | 21.5 ± 55.4 | 84.1 ± 163.6 | 447.1 ± 511.3 |
| Psychiatric medication-free N | 28 | 7 | 11 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, S.; Aizawa, E.; Ishihara, N.; Hattori, K.; Segawa, K.; Kunugi, H. High Rates of Abnormal Glucose Metabolism Detected by 75 g Oral Glucose Tolerance Test in Major Psychiatric Patients with Normal HbA1c and Fasting Glucose Levels. Nutrients 2025, 17, 613. https://doi.org/10.3390/nu17040613
Yoshida S, Aizawa E, Ishihara N, Hattori K, Segawa K, Kunugi H. High Rates of Abnormal Glucose Metabolism Detected by 75 g Oral Glucose Tolerance Test in Major Psychiatric Patients with Normal HbA1c and Fasting Glucose Levels. Nutrients. 2025; 17(4):613. https://doi.org/10.3390/nu17040613
Chicago/Turabian StyleYoshida, Sumiko, Emiko Aizawa, Naoko Ishihara, Kotaro Hattori, Kazuhiko Segawa, and Hiroshi Kunugi. 2025. "High Rates of Abnormal Glucose Metabolism Detected by 75 g Oral Glucose Tolerance Test in Major Psychiatric Patients with Normal HbA1c and Fasting Glucose Levels" Nutrients 17, no. 4: 613. https://doi.org/10.3390/nu17040613
APA StyleYoshida, S., Aizawa, E., Ishihara, N., Hattori, K., Segawa, K., & Kunugi, H. (2025). High Rates of Abnormal Glucose Metabolism Detected by 75 g Oral Glucose Tolerance Test in Major Psychiatric Patients with Normal HbA1c and Fasting Glucose Levels. Nutrients, 17(4), 613. https://doi.org/10.3390/nu17040613





