Feasibility and Tolerability of Daily Microgreen Consumption in Healthy Middle-Aged/Older Adults: A Randomized, Open-Label, Controlled Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Participant Recruitment
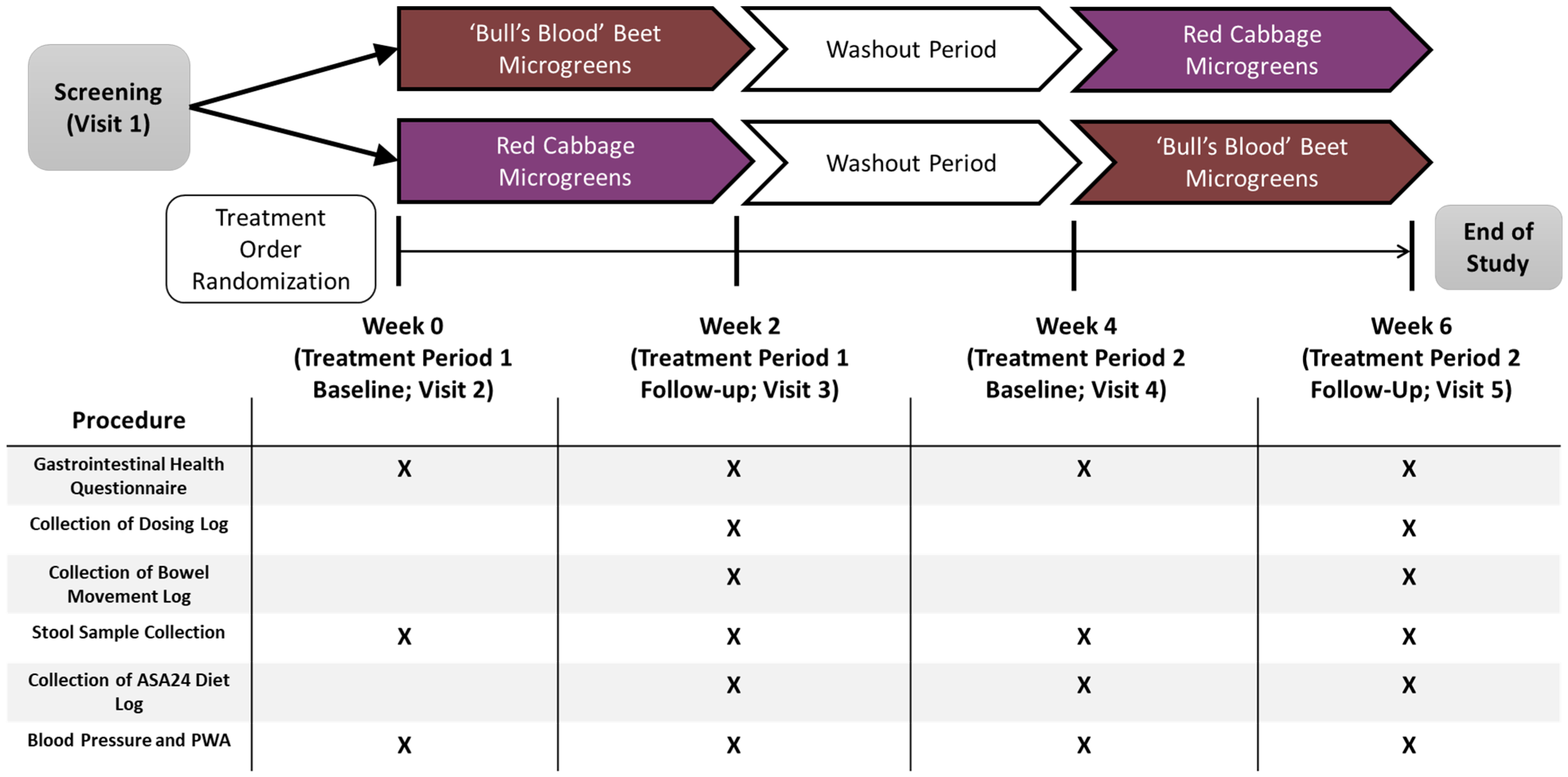
2.3. Study Design, Randomization, and Intervention
2.4. Microgreens Production
2.5. Outcome Measures
2.5.1. Compliance and Feasibility
2.5.2. Gastrointestinal Tolerability
2.5.3. Fecal Collection and Gut Microbiota Analysis
2.5.4. Blood Collection and Biomarker Analysis
2.5.5. Dietary Intake, Energy Expenditure, and Anthropometric Assessment
2.5.6. Hemodynamics
2.5.7. Food Neophobia, Leafy Green Vegetable Consumption and Intention to Eat Microgreens
2.6. Statistical Analysis
3. Results
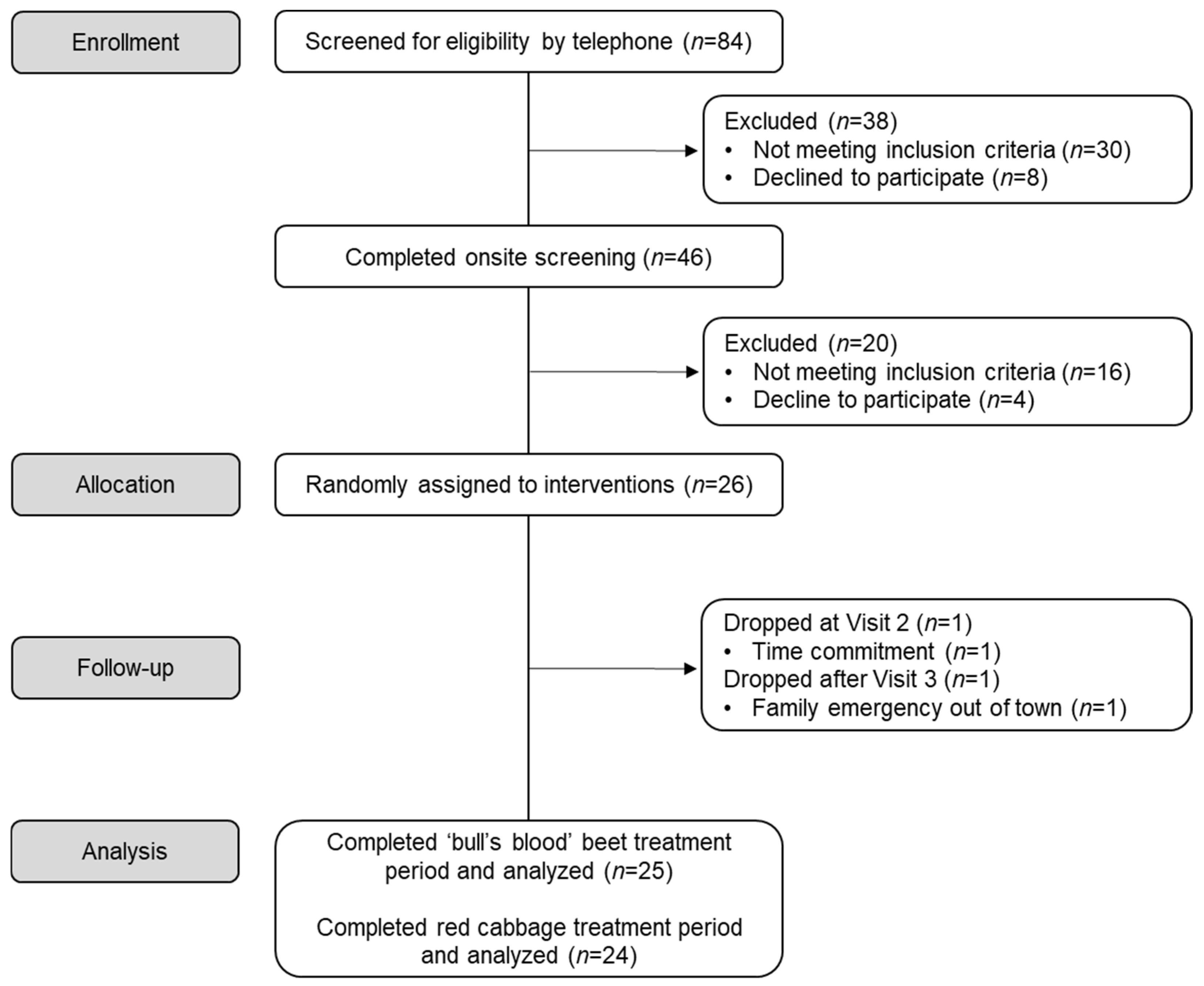
3.1. Participant Retention and Baseline Characteristics
3.2. Compliance and Feasibility
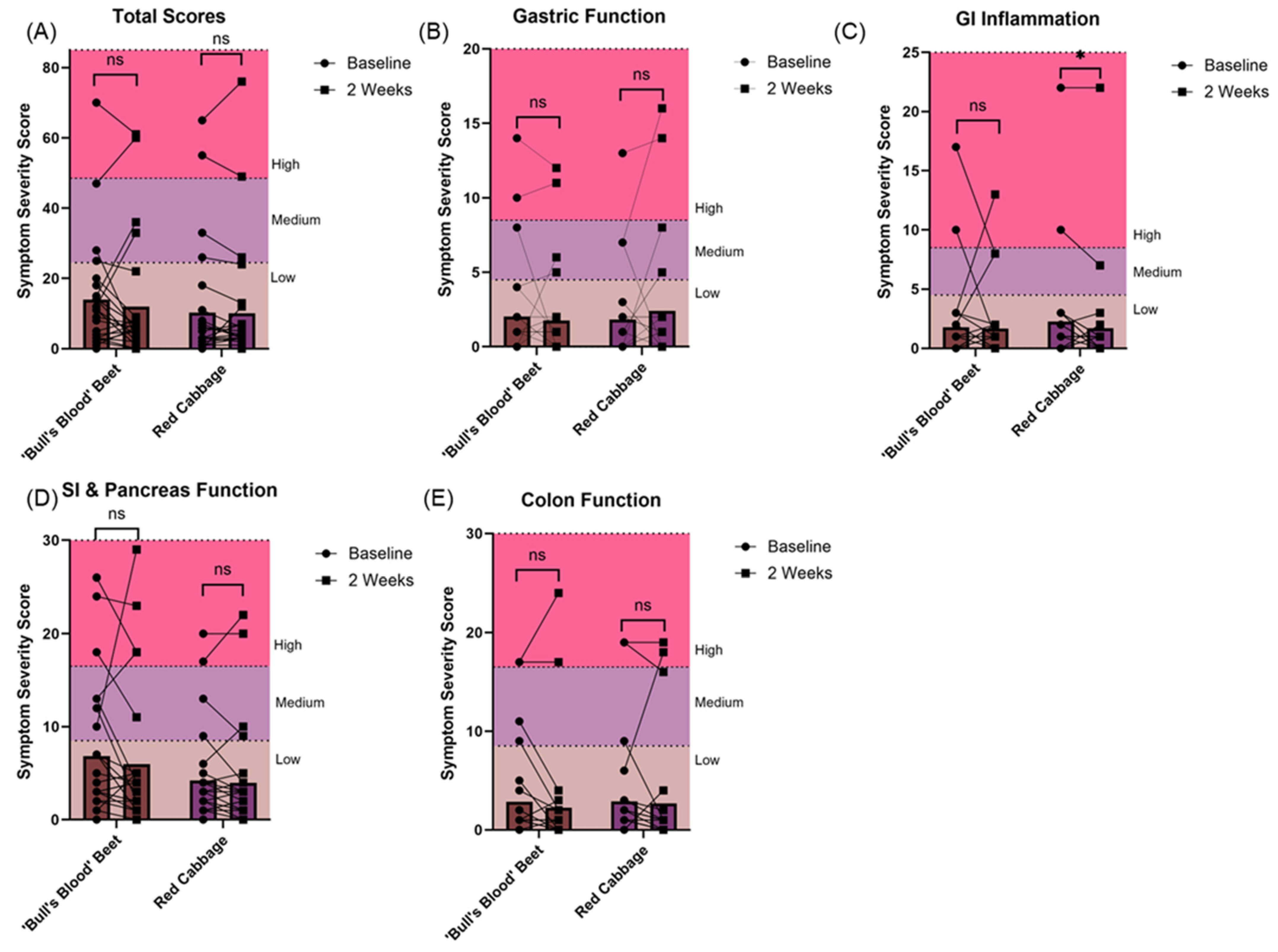
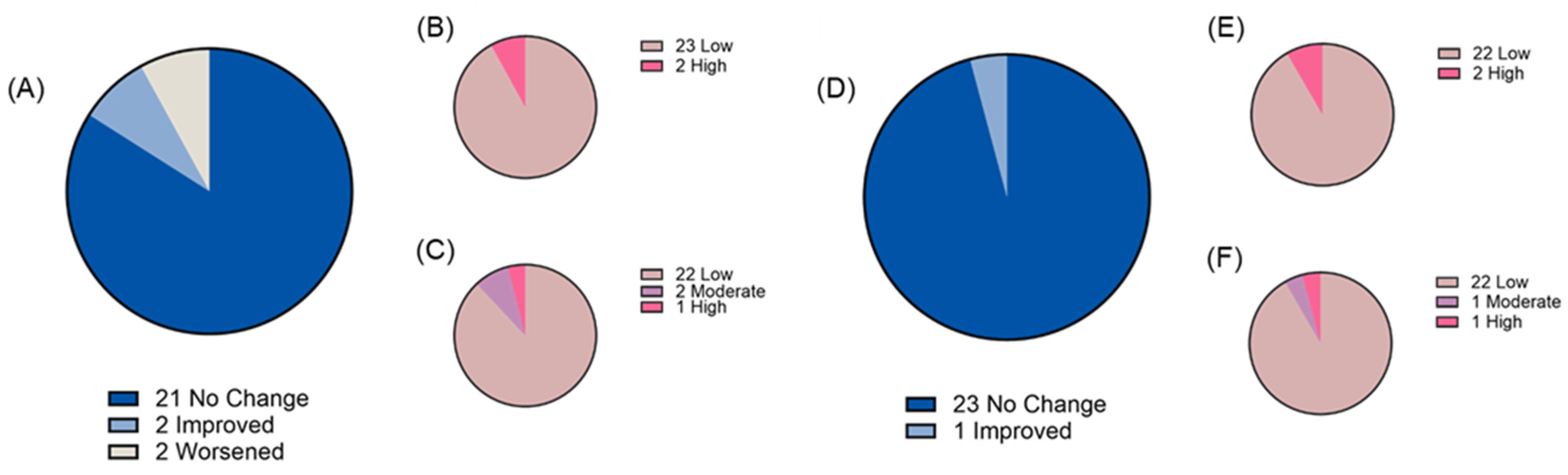
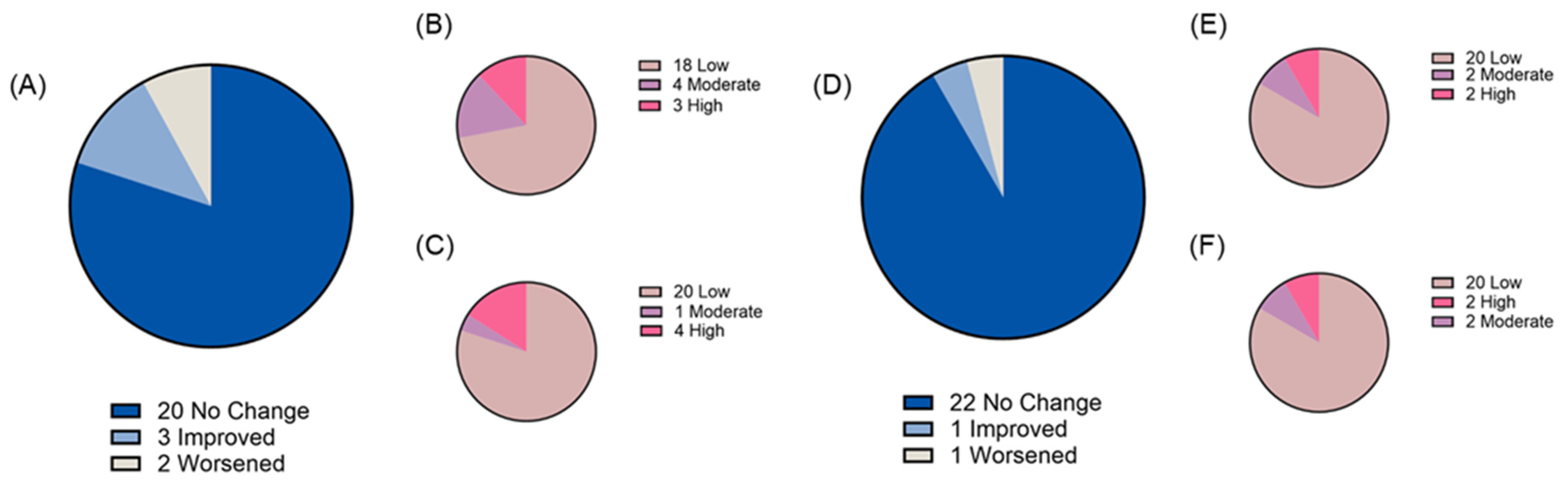
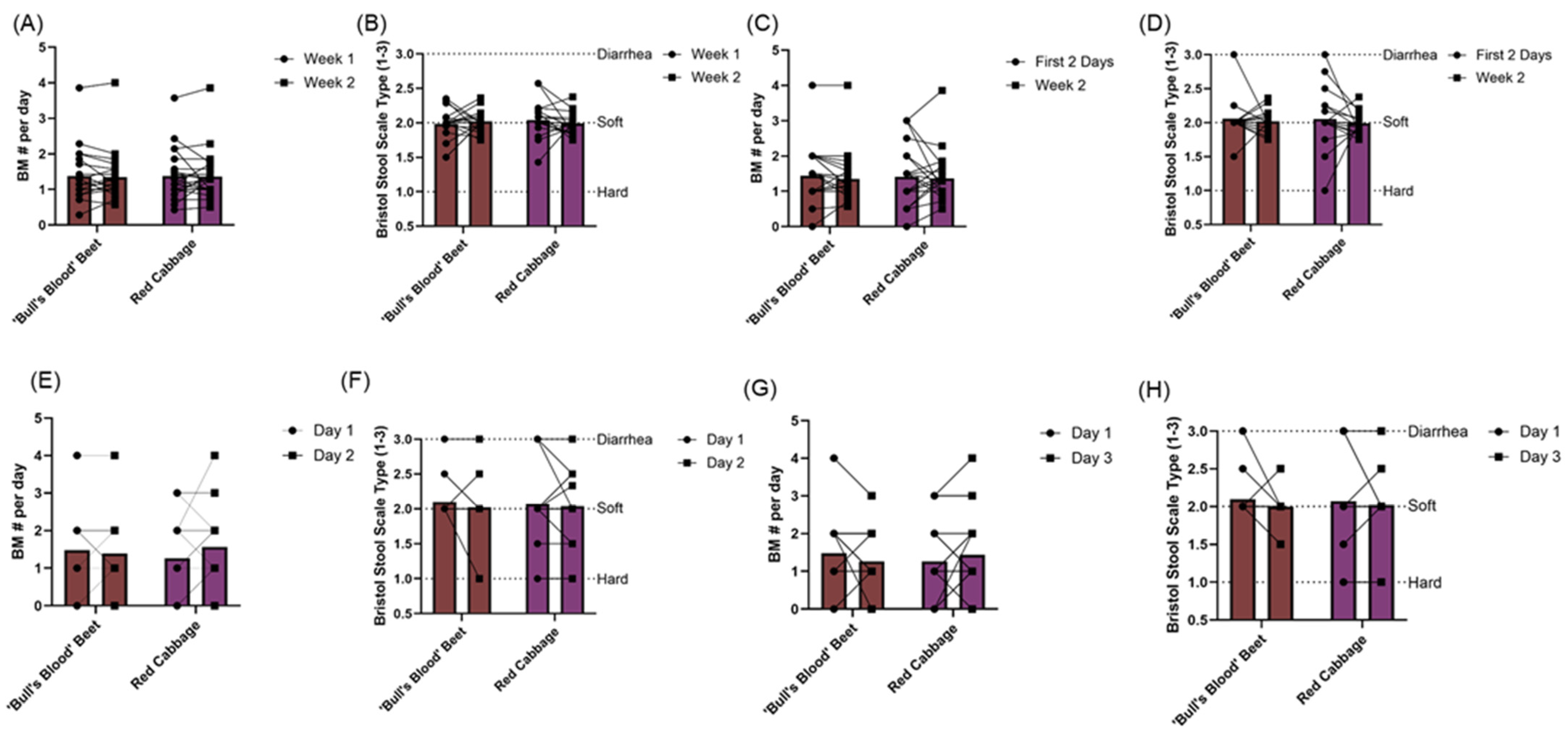
3.3. Gastrointestinal Tolerability
3.4. Gut Microbiota
3.5. Anthropometrics, Energy Expenditure, and Dietary Intake
3.6. Hemodynamics
3.7. Baseline Dietary Habits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional quality and health benefits of microgreens, a crop of modern agriculture. J. Future Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Xiao, Z.; Yu, L.; Pham, Q.; Sun, J.; Chen, P.; Yokoyama, W.; Yu, L.L.; Luo, Y.S.; et al. Red Cabbage Microgreens Lower Circulating Low-Density Lipoprotein (LDL), Liver Cholesterol, and Inflammatory Cytokines in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2016, 64, 9161–9171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pham, Q.; Wang, Y.; Huang, H.; Jiang, X.; Li, R.W.; Yu, L.; Luo, Y.; Wang, J.; Wang, T.T.Y. Red cabbage microgreen modulation of gut microbiota is associated with attenuation of diet-induced obesity risk factors in a mouse model. Food Funct. 2023, 14, 6654–6664. [Google Scholar] [CrossRef]
- Michell, K.A.; Isweiri, H.; Newman, S.E.; Bunning, M.; Bellows, L.L.; Dinges, M.M.; Grabos, L.E.; Rao, S.; Foster, M.T.; Heuberger, A.L.; et al. Microgreens: Consumer sensory perception and acceptance of an emerging functional food crop. J. Food Sci. 2020, 85, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Prenni, J.; Heuberger, A.; Isweiri, H.; Chaparro, J.; Newman, S.; Uchanski, M.; Omerigic, H.; Mitchell, K.; Bunning, M.; et al. Comprehensive Evaluation of Metabolites and Minerals in 6 Microgreen Species and the Influence of Maturity. Curr. Dev. Nutr. 2021, 5, nzaa180. [Google Scholar] [CrossRef]
- Gindin, M.; Febvre, H.P.; Rao, S.; Wallace, T.C.; Weir, T.L. Bacteriophage for Gastrointestinal Health (PHAGE) Study: Evaluating the Safety and Tolerability of Supplemental Bacteriophage Consumption. J. Am. Coll. Nutr. 2019, 38, 68–75. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P.; Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 7. [Google Scholar] [CrossRef]
- Janssen, S.; McDonald, D.; Gonzalez, A.; Navas-Molina, J.A.; Jiang, L.; Xu, Z.Z.; Winker, K.; Kado, D.M.; Orwoll, E.; Manary, M.; et al. Phylogenetic Placement of Exact Amplicon Sequences Improves Associations with Clinical Information. mSystems 2018, 3, e00021-18. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 8. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Robeson, M.; O'Rourke, D.; Kaehler, B.; Ziemski, M.; Dillon, M.; Foster, J.; Bokulich, N. RESCRIPt: Reproducible sequence taxonomy reference database management-PubMed. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J.; Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The Automated Self-Administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef]
- Kirkpatrick, S.I.; Thompson, F.E.; Subar, A.F.; Douglass, D.; Zimmerman, T.P.; Kahle, L.L.; George, S.M.; Potischman, N. Validity of the National Cancer Institute's Automated Self-Administered 24-hour Recall (ASA24): Results of a feeding study. FASEB J. 2013, 27, 230–234. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Pannucci, T.E.; Lerman, J.L.; Herrick, K.A.; Zimmer, M.; Mathieu, K.M.; Stoody, E.E.; Reedy, J. Healthy Eating Index-2020: Review and Update Process to Reflect the Dietary Guidelines for Americans, 2020–2025. J. Acad. Nutr. Diet. 2023, 123, 1280–1288. [Google Scholar] [CrossRef]
- National Cancer Institute. Healthy Eating Index SAS Code. Available online: https://epi.grants.cancer.gov/hei/sas-code.html (accessed on 11 July 2024).
- Sallis, J.F.; Haskell, W.L.; Wood, P.D.; Fortmann, S.P.; Rogers, T.; Blair, S.N.; Paffenbarger, R.S. Physical activity assessment methodology in the Five-City Project. Am. J. Epidemiol. 1985, 121, 91–106. [Google Scholar] [CrossRef]
- Hayden-Wade, H.A.; Coleman, K.J.; Sallis, J.F.; Armstrong, C. Validation of the telephone and in-person interview versions of the 7-day PAR. Med. Sci. Sports Exerc. 2003, 35, 801–809. [Google Scholar] [CrossRef]
- Woolf, E.K.; Terwoord, J.D.; Litwin, N.S.; Vazquez, A.R.; Lee, S.Y.; Ghanem, N.; Michell, K.A.; Smith, B.T.; Grabos, L.E.; Ketelhut, N.B.; et al. Daily blueberry consumption for 12 weeks improves endothelial function in postmenopausal women with above-normal blood pressure through reductions in oxidative stress: A randomized controlled trial. Food Funct. 2023, 14, 2621–2641. [Google Scholar] [CrossRef]
- Litwin, N.; Van Ark, H.; Hartley, S.; Michell, K.; Vazquez, A.; Fischer, E.; Melby, C.; Weir, T.; Wei, Y.; Rao, S.; et al. Impact of Red Beetroot Juice on Vascular Endothelial Function and Cardiometabolic Responses to a High-Fat Meal in Middle-Aged/Older Adults with Overweight and Obesity: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Curr. Dev. Nutr. 2019, 3, nzz113. [Google Scholar] [CrossRef] [PubMed]
- Pliner, P.; Hobden, K. Development of a scale to measure the trait of food neophobia in humans. Appetite 1992, 19, 105–120. [Google Scholar] [CrossRef] [PubMed]
- NCI. Usual Dietary Intakes: NHANES Food Frequency Questionnaire (FFQ). Available online: https://epi.grants.cancer.gov/diet/usualintakes/ffq.html (accessed on 30 April 2019).
- Di Gioia, F.; Hong, J.C.; Pisani, C.; Petropoulos, S.A.; Bai, J.; Rosskopf, E.N. Frontiers | Yield performance, mineral profile, and nitrate content in a selection of seventeen microgreen species. Front. Plant Sci. 2023, 14, 1220691. [Google Scholar] [CrossRef]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological effects of red beetroot and betalains: A review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef]
- Bryan, N.S. Nitric oxide deficiency is a primary driver of hypertension. Biochem. Pharmacol. 2022, 206, 115325. [Google Scholar] [CrossRef]
- Rocchetti, G.; Tomas, M.; Zhang, L.; Zengin, G.; Lucini, L.; Capanoglu, E. Red beet (Beta vulgaris) and amaranth (Amaranthus sp.) microgreens: Effect of storage and in vitro gastrointestinal digestion on the untargeted metabolomic profile. Food Chem. 2020, 332, 127415. [Google Scholar] [CrossRef]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef]
- Ma, L.; Liu, G.; Zong, G.; Sampson, L.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.E.; Rexrode, K.M.; Sun, Q. Intake of glucosinolates and risk of coronary heart disease in three l|CLEP. Clin. Epidemiol. 2018, 10, 749–762. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Bouranis, J.A.; Beaver, L.M.; Wong, C.P.; Choi, J.; Hamer, S.; Davis, E.W.; Brown, K.S.; Jiang, D.; Sharpton, T.J.; Stevens, J.F.; et al. Sulforaphane and Sulforaphane-Nitrile Metabolism in Humans Following Broccoli Sprout Consumption: Inter-individual Variation, Association with Gut Microbiome Composition, and Differential Bioactivity. Mol. Nutr. Food Res. 2024, 68, e2300286. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-gut microbiota-health axis: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 7563–7588. [Google Scholar] [CrossRef] [PubMed]
- Bouranis, J.A.; Beaver, L.M.; Ho, E. Frontiers|Metabolic Fate of Dietary Glucosinolates and Their Metabolites: A Role for the Microbiome. Front. Nutr. 2021, 8, 748433. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R.; Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 7415. [Google Scholar] [CrossRef]
- Bouranis, J.A.; Wong, C.P.; Beaver, L.M.; Uesugi, S.L.; Papenhausen, E.M.; Choi, J.; Davis, E.W.; Da Silva, A.N.; Kalengamaliro, N.; Chaudhary, R.; et al. Sulforaphane Bioavailability in Healthy Subjects Fed a Single Serving of Fresh Broccoli Microgreens. Foods 2023, 12, 3784. [Google Scholar] [CrossRef] [PubMed]
- Metabolomic insight into the profile, in vitro bioaccessibility and bioactive properties of polyphenols and glucosinolates from four Brassicaceae microgreens. Food Res. Int. 2021, 140, 110039. [CrossRef]
- Orouji, N.; Asl, S.K.; Taghipour, Z.; Habtemariam, S.; Nabavi, S.M.; Rahimi, R.; Orouji, N.; Asl, S.K.; Taghipour, Z.; Habtemariam, S.; et al. Glucosinolates in cancer prevention and treatment: Experimental and clinical evidence. Med. Oncol. 2023, 40, 12. [Google Scholar] [CrossRef]
- Kamal, R.M.; Razis, A.F.A.; Sukri, N.S.M.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases-PubMed. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]
- Ellis, L.R.; Boesch, C.; Dye, L. Effects of Anthocyanins on Cognition and Vascular Function: A Systematic Review. Mol. Nutr. Food Res. 2024, 68, e2300502. [Google Scholar] [CrossRef]
- Podsędek, A.; Frąszczak, B.; Sosnowska, D.; Kajszczak, D.; Szymczak, K.; Bonikowski, R.; Podsędek, A.; Frąszczak, B.; Sosnowska, D.; Kajszczak, D.; et al. LED Light Quality Affected Bioactive Compounds, Antioxidant Potential, and Nutritional Value of Red and White Cabbage Microgreens. Appl. Sci. 2023, 13, 5435. [Google Scholar] [CrossRef]
- Acharya, J.; Gautam, S.; Neupane, P.; Niroula, A. Pigments, ascorbic acid, and total polyphenols content and antioxidant capacities of beet (Beta vulgaris) microgreens during growth. Int. J. Food Prop. 2021, 24, 1175–1186. [Google Scholar] [CrossRef]
- Hobbs, D.; Goulding, M.; Nquyen, A.; Malaver, T.; Walker, C.; George, T.; Methven, L.; Lovegrove, J. Acute Ingestion of Beetroot Bread Increases Endothelium-Independent Vasodilation and Lowers Diastolic Blood Pressure in Healthy Men: A Randomized Controlled Trial. J. Nutr. 2013, 143, 1399–1405. [Google Scholar] [CrossRef]



| Completed (n = 24) | |
|---|---|
| Age, years | 57 ± 1 |
| Years postmenopausal (women only) | 8 ± 1 |
| Sex, M:F (n) | 9:15 |
| BMI, kg/m2 | 23.7 ± 0.5 |
| Waist-to-hip ratio | 0.84 ± 0.02 |
| Total cholesterol, mg/dL | 200 ± 4 |
| HDL, mg/dL | 71 ± 3 |
| LDL, mg/dL | 110 ± 4 |
| Triglycerides, mg/dL | 88 ± 6 |
| HDL:LDL ratio | 0.67 ± 0.04 |
| Hemoglobin A1c, % | 5.3 ± 0.1 |
| SBP, mmHg | 110 ± 3 |
| DBP, mmHg | 69 ± 2 |
| Days Compliant | Days Non-Compliant | Percent Compliance | |
|---|---|---|---|
| Overall | 13.4 ± 0.2 | 0.6 ± 0.2 | 95.6 ± 1.5 |
| ‘Bull’s Blood’ Beet | 13.6 ± 0.2 | 0.4 ± 0.2 | 96.9 ± 1.2 |
| Red Cabbage | 13.2 ± 0.2 | 0.8 ± 0.2 | 94.4 ± 1.6 |
| ‘Bull’s Blood’ Beet | Red Cabbage | |||
|---|---|---|---|---|
| Baseline | 2 Weeks | Baseline | 2 Weeks | |
| Body weight, kg | 65.4 ± 1.8 | 65.6 ± 1.8 | 66.2 ± 2.0 | 65.9 ± 2.0 |
| BMI, kg/m2 | 23.6 ± 0.5 | 23.7 ± 0.5 | 23.8 ± 0.5 | 23.7 ± 0.5 |
| WC, cm | 81 ± 2 | 81 ± 2 | 80 ± 2 | 82 ± 2 |
| HC, cm | 97 ± 1 | 97 ± 1 | 97 ± 1 | 98 ± 1 |
| WC:HC | 0.84 ± 0.01 | 0.83 ± 0.01 | 0.83 ± 0.02 | 0.84 ± 0.02 |
| ‘Bull’s Blood’ Beet | Red Cabbage | |||
|---|---|---|---|---|
| Baseline | 2 Weeks | Baseline | 2 Weeks | |
| Average daily energy expenditure, Kcal | 2536 ± 137 | 2318 ± 79 * | 2430 ± 68 | 2399 ± 76 |
| Average daily METs | 38.3 ± 1.2 | 35.2 ± 0.4 * | 36.9 ± 0.6 | 36.5 ± 0.7 |
| ‘Bull’s Blood’ Beet | Red Cabbage | Washout | |
|---|---|---|---|
| Score Range: 0–5 | |||
| Total Vegetables | 4.7 ± 0.2 a# | 4.9 ± 0.1 a | 4.4 ± 0.4 a |
| Greens and Beans | 3.6 ± 0.4 a | 3.1 ± 0.4 a | 3.1 ± 0.7 a |
| Total Fruit | 3.2 ± 0.4 a | 3.7 ± 0.3 a | 3.4 ± 0.7 a |
| Whole Fruit | 3.8 ± 0.4 a | 4.2 ± 0.3 a | 3.7 ± 0.6 a |
| Total Protein | 4.2 ± 0.3 a | 4.6 ± 0.2 a | 4.2 ± 0.4 a |
| Seafood and Plant Protein | 3.9 ± 0.4 a | 4.5 ± 0.2 a | 3.7 ± 0.6 a |
| Score Range: 0–10 | |||
| Whole Grain | 3.8 ± 0.6 a | 5.7 ± 0.7 a | 4.5 ± 1.1 a |
| Total Dairy | 5.4 ± 0.6 a | 4.7 ± 0.5 a | 5.6 ± 1.0 a |
| Fatty Acids | 7.6 ± 0.5 a | 7.9 ± 0.5 a | 6.2 ± 0.9 a |
| Sodium | 2.9 ± 0.7 a | 4.8 ± 0.7 b | 4.4 ± 0.9 ab |
| Refined Grain | 7.7 ± 0.7 a | 8.4 ± 0.5 a | 7.4 ± 1.1 a |
| Saturated Fat | 7.6 ± 0.4 a | 6.8 ± 0.5 a | 5.2 ± 1.0 b |
| Added Sugar | 7.8 ± 0.4 a#% | 8.7 ± 0.3 a | 9.1 ± 0.4 a |
| Total | 66.2 ± 2.2 a | 72.0 ± 2.0 a# | 64.9 ± 3.4 a |
| ‘Bull’s Blood’ Beet | Red Cabbage | |||
|---|---|---|---|---|
| Baseline | 2 Weeks | Baseline | 2 Weeks | |
| Brachial SBP, mmHg | 118 ± 2 | 117 ± 2 | 118 ± 2 | 118 ± 3 |
| Brachial DBP, mmHg | 71 ± 2 | 70 ± 2 | 73 ± 1 | 72 ± 2 |
| Aortic SBP, mmHg | 107 ± 2 | 106 ± 2 | 109 ± 2 | 109 ± 3 |
| Aortic DBP, mmHg | 72 ± 2 | 71 ± 2 | 73 ± 1 | 72 ± 2 |
| HR, bpm | 56 ± 2 | 56 ± 2 | 55 ± 2 | 57 ± 2 |
| MAP, mmHg | 84 ± 2 | 83 ± 2 | 86 ± 2 | 85 ± 2 |
| AP, mmHg | 12 ± 3 | 9 ± 1 | 9 ± 1 | 13 ± 3 |
| AIx, % | 24 ± 2 | 25 ± 3 | 25 ± 3 | 28 ± 3 |
| AIx@75, % | 16 ± 3 | 17 ± 3 | 16 ± 3 | 20 ± 4 |
| Completed (n = 24) | |
|---|---|
| Food neophobia score | 21.3 ± 1.3 |
| How often did you eat raw greens (such as spinach, turnip, collard, mustard, chard, or kale)? | 6.2 ± 0.5 |
| How often did you eat lettuce salads (with or without other vegetables? | 7.1 ± 0.4 |
| How often were the lettuce salads you ate made with dark green leaves? | 7.2 ± 0.3 |
| How familiar are you with microgreens? | 2.6 ± 0.2 |
| How frequently have you consumed microgreens? | 2.1 ± 0.2 |
| How likely are you to purchase microgreens in the future? | 4.7 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Michell, K.A.; Butler, M.M.; Smith, B.T.; Woolf, E.K.; Holmes, S.C.; Grabos, L.E.; Vazquez, A.R.; Isweiri, H.; Bunning, M.; et al. Feasibility and Tolerability of Daily Microgreen Consumption in Healthy Middle-Aged/Older Adults: A Randomized, Open-Label, Controlled Crossover Trial. Nutrients 2025, 17, 467. https://doi.org/10.3390/nu17030467
Lee SY, Michell KA, Butler MM, Smith BT, Woolf EK, Holmes SC, Grabos LE, Vazquez AR, Isweiri H, Bunning M, et al. Feasibility and Tolerability of Daily Microgreen Consumption in Healthy Middle-Aged/Older Adults: A Randomized, Open-Label, Controlled Crossover Trial. Nutrients. 2025; 17(3):467. https://doi.org/10.3390/nu17030467
Chicago/Turabian StyleLee, Sylvia Y., Kiri A. Michell, Michelle M. Butler, Brayden T. Smith, Emily K. Woolf, Sydney C. Holmes, Lauren E. Grabos, Allegra R. Vazquez, Hanan Isweiri, Marisa Bunning, and et al. 2025. "Feasibility and Tolerability of Daily Microgreen Consumption in Healthy Middle-Aged/Older Adults: A Randomized, Open-Label, Controlled Crossover Trial" Nutrients 17, no. 3: 467. https://doi.org/10.3390/nu17030467
APA StyleLee, S. Y., Michell, K. A., Butler, M. M., Smith, B. T., Woolf, E. K., Holmes, S. C., Grabos, L. E., Vazquez, A. R., Isweiri, H., Bunning, M., Uchanski, M. E., Rao, S., Newman, S. E., Weir, T. L., & Johnson, S. A. (2025). Feasibility and Tolerability of Daily Microgreen Consumption in Healthy Middle-Aged/Older Adults: A Randomized, Open-Label, Controlled Crossover Trial. Nutrients, 17(3), 467. https://doi.org/10.3390/nu17030467








