Abstract
Background: China’s rapidly aging population has led to a growing burden of dementia, marked by cognitive decline and heavy social and economic costs. Dietary patterns have been identified as a critical means for prevention. Methods: This study drew on data from the China Longitudinal Health and Longevity Survey (CLHLS). Three logistic regression models were applied to examine the association between the Chinese version of the Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (cMIND) diet and dementia. To test the stability of the results, we conducted two sensitivity analyses. Restricted cubic spline (RCS) models were used to assess the potential for a nonlinear relationship. Subgroup and interaction analyses were conducted to explore heterogeneity across covariates and main effects. Propensity score matching (PSM) was performed as a secondary analysis to minimize the influence of confounding factors. Results: The study included 9142 participants, with a dementia prevalence of 10.7% among Chinese older adults. After adjusting for all covariates, each one-unit increase in the cMIND diet score was associated with an 11% lower prevalence of dementia (OR = 0.89; 95% CI: 0.84–0.93). After full adjustment, the RCS model confirmed a significant and linear dose–response association between adherence to the cMIND diet and dementia. Comparable associations were observed across most subgroups. Conclusions: Adherence to the cMIND diet was significantly associated with a lower prevalence of dementia in Chinese older adults, with evidence of a clear dose–response effect. These findings highlight the potential of the cMIND diet as a preventive strategy against dementia in this population.
1. Introduction
As the global population ages, dementia has become an urgent public health issue worldwide. Dementia is defined as a syndrome involving significant cognitive decline that disrupts independent daily activities []. Globally, dementia is the seventh leading cause of death and a major source of disability and dependency in older adults []. In 2019, approximately 57.4 million individuals were living with dementia, and this number is projected to increase to 152.8 million by 2050 due to population growth and population aging []. In 2021, the prevalence of dementia in China was 1194.2 per 100,000 people, with a mortality rate of 34.6 per 100,000. The prevalence among older Chinese adults is expected to further rise []. Beyond cognitive deterioration and poorer quality of life, dementia imposes substantial social and economic burdens on families and communities. Although overall global mortality rates have declined, dementia still increases the risk of death by approximately threefold, and this relative risk has remained unchanged across two cohorts more than two decades apart []. The burden of dementia in China also shows substantial regional variation, closely linked to economic development, geographic location, and exposure to risk factors []. Among modifiable risk factors, dietary patterns have been identified as a crucial determinant in dementia prevention [,].
In recent years, there has been extensive research on the impact of traditional regional diets (i.e., dietary patterns from various regions around the world) on the prevention of non-communicable diseases []. Traditional regional diets not only benefit physical health but also represent a sustainable lifestyle, aligning with cultural and environmental diversity. The association between diet and cognitive health has become a major area of research focus []. The “microbiome–gut–brain axis” offers a key theoretical framework for understanding diet–brain interactions []. This bidirectional system connects gut microbiota with the central nervous system through neural, endocrine, immune, and metabolic pathways []. Evidence shows that dietary patterns can modulate gut microbiota and its metabolites, thereby influencing brain health and cognitive function, partly via the vagus nerve [,,]. Diets rich in dietary fiber, polyphenols, and certain fatty acids have been shown to promote cognitive health in older adults []. The Mediterranean diet, which emphasizes fruits, vegetables, and olive oil, complemented by moderate alcohol intake [], has attracted considerable attention for its potential to slow neurodegenerative disease progression, largely due to its abundance of polyphenols, unsaturated fatty acids, and antioxidants [,]. The Dietary Approaches to Stop Hypertension (DASH) diet, originally developed to manage hypertension, emphasizes low sodium and high potassium, calcium, and magnesium intake. The DASH diet encourages the consumption of fruits, vegetables, low-fat dairy, and whole grains while restricting that of saturated fats and cholesterol []. A systematic review and meta-analysis showed that strict adherence to the Mediterranean diet lowered the risk of progression from mild cognitive impairment to dementia by 33% []. Moreover, long-term adherence to the DASH diet has also been linked to better preservation of cognitive function in older adults []. Morris et al. [] developed the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet, integrating the Mediterranean and DASH patterns to highlight foods with neuroprotective potential. The MIND diet emphasizes natural, plant-based foods while limiting animal-derived products and those high in saturated fat. A cohort study in Australia reported that the cognitive benefits of the MIND diet were broadly generalizable [].
However, the MIND dietary pattern emphasizes Western foods such as olive oil, deep-sea fish, and wine, which differ markedly from the traditional dietary habits of older Chinese adults []. To address this limitation, Huang et al. developed the Chinese version of the MIND (cMIND) diet based on the original MIND framework, adapting it to the characteristics of Chinese dietary patterns, and validated its applicability among older adults in China []. Its core components include whole grains, soy products, green tea, fresh fruits and vegetables, and low-salt cooking methods []. Several previous studies have examined the association between the cMIND diet and non-communicable diseases among older adults in China. For example, Adherence to the cMIND diet has been associated with the prevalence of hypertension, anxiety, and depression [,]. In addition, Huang X, Lin W, and their colleagues reported that moderate and high adherence to the cMIND diet was associated with a lower prevalence of cognitive impairment among Chinese older adults [,]. These findings provide further support for the potential benefits of the cMIND diet on cognitive function. However, given that dementia represents a more advanced stage along the continuum of cognitive decline, evidence regarding the association between the cMIND diet and dementia among Chinese older adults remains limited.
In conclusion, further studies are warranted to elucidate the association between the cMIND diet and dementia among older adults in China []. Therefore, this study explores the association between the cMIND diet and dementia in Chinese older adults, aiming to inform strategies for prevention and delay in this population.
2. Materials and Methods
2.1. Study Design and Population
The Chinese Longitudinal Healthy Longevity Survey (CLHLS) is a nationwide prospective cohort study of older adults in China. The survey aims to fill critical data and knowledge gaps in scientific research and policy analysis related to healthy aging in China []. From 1998 to 2018, eight survey waves were carried out in 23 provinces, municipalities, and autonomous regions. The study subjects of CLHLS were obtained through multi-stage stratified cluster sampling in 631 randomly selected cities and counties []. First, one eligible centenarian respondent was recruited in each selected city or county. Subsequently, one non-older adult, one octogenarian, and three older adults aged 65–79 years living in the same or nearby street, village, or town were matched to the centenarian. The age and sex of individuals aged 65–99 years were randomly assigned to ensure comparability with the randomly coded centenarians []. The survey collected comprehensive information on participants’ demographics, mental health, cognitive function, social participation, dietary behaviors, lifestyle habits, socioeconomic status, and family structure. All interviews and physical assessments were conducted one-on-one by trained interviewers to ensure data accuracy and reliability. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Peking University (protocol code: IRB00001052-13074 and date of approval 8 August 2022), and all participants provided informed consent before data collection. Further details on the CLHLS can be accessed at https://opendata.pku.edu.cn/dataverse/CHADS (accessed on 8 August 2022).
The determination of the study’s sample size was based on the formula used for calculating sample sizes in cross-sectional research studies: [n = (Z2α/2pq)/δ2] [], (1) n represents the sample size required for the study; (2) p denotes the prevalence rate of dementia in Chinese older adults; (3) q = (1 − p); (4) Zα/2 was set at 1.96 and α was set at 0.05 for a two-sided test; and (5) δ denotes the permissible error, calculated at 0.1 p. According to a previous study, the prevalence of dementia among older adults in China ranged from 2.6% to 13.5% []. Using the midpoint value of 8.1% for sample size estimation, the required minimum sample size for this study was calculated to be 4359 participants.
This analysis utilized cross-sectional data from the 2018 wave. Following the definition proposed by Livingston et al., older adults were defined as individuals aged 65 years and above []. The inclusion criteria for this study were as follows: (1) age ≥ 65 years; (2) completion of the 2018 assessments for the cMIND diet, dementia, and covariates; (3) voluntary participation in the study; and (4) ability to communicate normally with no family history of mental disorders. In this study, missing data were handled using case-wise deletion. The data cleaning process is illustrated in Figure 1. A total of 9142 eligible participants were included in the final analysis.
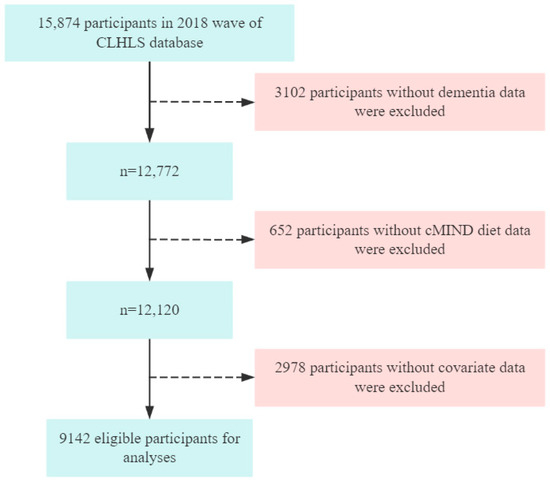
Figure 1.
Flow chart of participant selection.
2.2. Measurement of Independent Variables
To reflect Chinese dietary habits, Huang et al. adapted the MIND diet into a culturally appropriate version for older Chinese adults []. The applicability of the cMIND diet in this population has been confirmed in multiple studies [,]. Dietary intake frequency was assessed using a food frequency questionnaire (FFQ). The cMIND diet includes 12 components: Types of staple food, amount of staple food, fresh vegetables, fresh fruit, cooking oil, fish, food made from beans, nut, mushroom or algae, garlic, tea and white sugar or candy. Types of staple food, amount of staple food and cooking oil were scored 0 or 1, while the other components were scored 0, 0.5, or 1. The detailed criteria are shown in Supplementary Table S1. The total cMIND diet score ranges from 0 to 12, with higher scores indicating greater adherence to the cMIND diet. We categorized the total scores of the cMIND diet into tertiles as follows: Lower (0–4), Medium (4.5–5.5), High (6–12).
2.3. Measurement of Dependent Variables
Dementia was defined based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 and International Classification of Diseases 10th edition (ICD-10). Specifically, participants were classified as having dementia if they exhibited both cognitive impairment and functional impairment consistent with these diagnostic standards, or if they reported a physician’s diagnosis of dementia []. This definition has been validated by Liu et al. [], demonstrating reliability in the Chinese older population.
Functional impairment was assessed using the Activities of Daily Living (ADL) scale, which includes six domains: bathing, dressing, toileting, indoor activities, continence, and eating. The total ADL score ranges from 6 to 18, with a score ≥ 7 indicating functional impairment [].
Cognitive impairment was assessed with the Chinese version of the Mini-Mental State Examination (MMSE), covering orientation, registration, attention and calculation, recall, language, comprehension, and self-regulation abilities. The MMSE consists of 24 items, with a total score ranging from 0 to 30. A score of <18 indicating cognitive impairment [].
Self-reported doctor-diagnosed dementia was determined through a two-step question process. Participants were first asked, “Do you currently have dementia?” If the answer was “yes” the interviewer followed up with, “Was this diagnosed by a hospital or physician?” If participants also answered “yes”, they were defined as having self-reported doctor-diagnosed dementia [].
2.4. Measurement of Covariates
To control for potential confounding factors, we incorporated covariates encompassing participants’ demographic characteristics, lifestyle behaviors, and health conditions in the analyses. The demographic characteristics included age, gender, residence, marital status, economic status, education level and living arrangements. The lifestyle behaviors included smoking, drinking and exercise. The health conditions included hypertension, diabetes, heart disease, dyslipidemia and body mass index (BMI). More detailed information about these covariates can be found in Supplementary Table S2.
2.5. Statistical Analysis
The Kolmogorov–Smirnov test was applied to examine normality of continuous variables. Categorical variables are presented as numbers and percentages (n (%)). Continues variables are presented as median and interquartile range (M (Q1, Q3)). The chi-square test was used to evaluate group differences in categorical variables. Logistic regression models were employed to examine the association between the cMIND diet and dementia. A variance inflation factor of less than 5 was considered to indicate that there is no multicollinearity problem between the variables []. Three regression models were built: Model 1 was adjusted for age and gender; Model 2 was further adjusted for residence, marital status, education level, living arrangement, economic status, smoking, drinking and exercise based on Model 1; Model 3 further adjusted for hypertension, diabetes, heart disease, dyslipidemia and BMI based on Model 2. Two sensitivity analyses were carried out to test the robustness of the results: (1) missing covariate data were imputed using multiple chained equations, and (2) exclusion of participants with diabetes, hypertension, dyslipidemia, or heart disease. Using Model 3 with the continuous cMIND score, a restricted cubic spline (RCS) analysis was conducted to explore the potential non-linear association between the cMIND diet and dementia. Further subgroup analyses and interaction tests were conducted to examine the heterogeneity between covariates and primary variables, assessing potential effect modifications by these factors. To mitigate the potential for false positive errors arising from multiple comparisons, we adjusted the p values using the false discovery rate (FDR) method [].
Propensity score matching (PSM) was applied to minimize confounding by balancing covariates across groups with similar propensity scores. A secondary analysis was then conducted based on the matched sample.
All analyses were conducted with SPSS 26.0 and R 4.3.0, and a two-tailed p-value < 0.05 was considered statistically significant.
3. Results
3.1. Basic Characteristics of Study Participants
The prevalence of dementia among Chinese older adults in the study was 10.7%. The final analytic sample comprised 9142 participants, including 5058 females (55.3%) and 4084 males (44.7%), with 5380 individuals (58.9%) aged 80 years or older. Table 1 shows significant differences between dementia and non-dementia in terms of age, gender, marital status, economic status, education level, smoking, drinking, exercise, living arrangements, BMI, hypertension, diabetes, and dyslipidemia (p < 0.05).

Table 1.
Baseline characteristics of study population by dementia.
As presented in Table 2, participants were stratified into three groups based on cMIND diet tertiles (Lower, Medium and High), showing significant group differences in age, gender, residence, marital status, economic status, education level, smoking, drinking, exercise, living arrangements, BMI, hypertension, diabetes, heart disease and dyslipidemia (p < 0.05).

Table 2.
Baseline characteristics of study population by cMIND diet.
3.2. Association Between cMIND Diet and Dementia
Table 3 presents the results of the logistic regression models under different levels of covariate adjustment. After adjusting for gender, age, residence, marital status, education level, living arrangement, economic status, smoking, drinking, exercise, hypertension, diabetes, heart disease, dyslipidemia and BMI, each one-unit increase in the cMIND diet score was associated with an 11% lower prevalence of dementia (OR = 0.89; 95% CI: 0.84–0.93). Compared with participants in the lowest tertile of the cMIND diet score, those in the medium and high adherence groups had 18% (OR = 0.82; 95% CI: 0.69–0.97) and 24% (OR = 0.76; 95% CI: 0.61–0.94) lower odds of dementia, respectively.

Table 3.
Association between cMIND diet with dementia. (n = 9142).
The fully adjusted RCS model confirmed a significant association between cMIND diet and dementia (p for overall < 0.001), and a linear relationship was observed (non-linearity p = 0.192). Detailed results are presented in Figure 2.
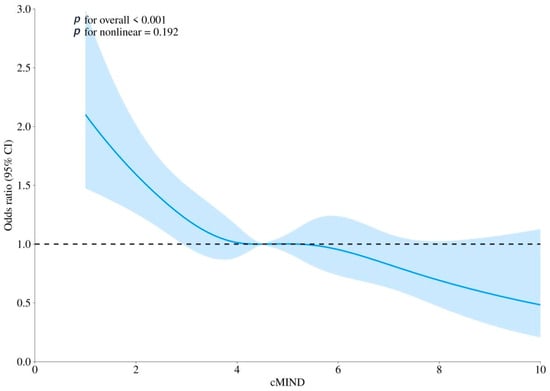
Figure 2.
The association between cMIND diet with dementia using restricted cubic spline (RCS) regression analysis. Note: Model was adjusted for gender, age, residence, marital status, education level, living arrangement, economic status, smoking, drinking, exercise, hypertension, diabetes, heart disease, dyslipidemia, and body mass index. CI: confidence interval; OR: odds ratio. The solid blue line represents the estimated odds ratio of the outcome across varying levels of adherence to the cMIND diet, with the blue shaded area indicating the 95% confidence interval. The horizontal black dashed line represents the reference value (OR = 1.0).
3.3. Sensitivity Analysis
Two sensitivity analyses were conducted to confirm robustness: (1) Missing values of covariates were imputed using multiple chained equations to reduce bias caused by direct deletion. Supplementary Table S3 shows that each one-unite increase in the cMIND diet score was associated with an 8.0% reduction in the odds of dementia in Model 3 (OR = 0.92; 95% CI: 0.88–0.97). Compared to the low adherence group, high adherence to the cMIND diet was significantly protective against dementia in older adults. (2) Participants with diabetes, hypertension, dyslipidemia, or heart disease were excluded. Supplementary Table S4 indicates that, in Model 3, the cMIND diet consistently showed protective effects against dementia when modeled as either a continuous or categorical variable.
3.4. Subgroup and Interaction Analysis
Subgroup analyses were performed across gender, age, residence, marital status, education level, living arrangement, economic status, smoking, drinking, exercise, hypertension, diabetes, heart disease, dyslipidemia and BMI to test robustness and population differences, with results shown in Figure 3.
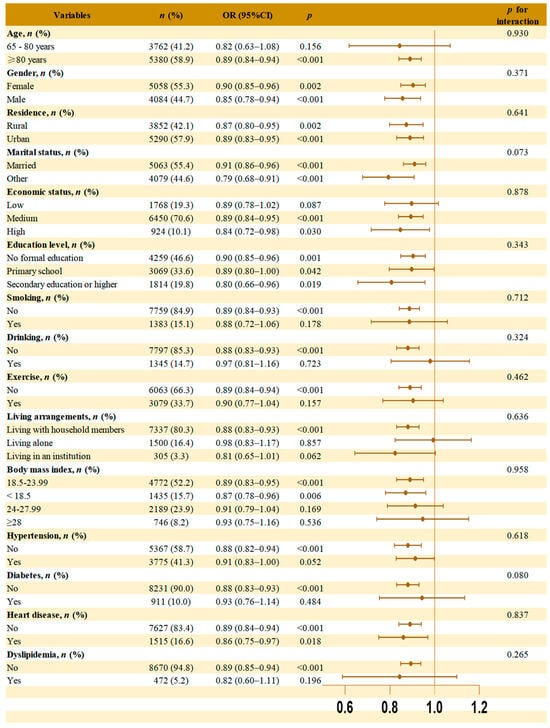
Figure 3.
Subgroup analyses and interaction analysis of the association between cMIND diet and dementia. Note: Model was adjusted for gender, age, residence, marital status, education level, living arrangement, economic status, smoking, drinking, exercise, hypertension, diabetes, heart disease, dyslipidemia, and body mass index. The brown dot in each subgroup represent the OR values. The horizontal line through the dot represents the 95% confidence interval (95% CI). The orange vertical line indicates the reference value (OR = 1.0).
The association between the cMIND diet and dementia remained statistically significant in several subgroups, including participants aged ≥80 years, with different gender, residence, marital status, education level, medium or high economic status, non-smoking, non-drinking, lack of exercise, living with household members, BMI ≤ 23.99, whether or not heart disease, and those without hypertension, diabetes, or dyslipidemia. No significant interaction was found between the subgroup variables and the association between the cMIND diet and dementia.
3.5. Propensity Score Matching
PSM (1:1 nearest neighbor matching algorithm and a caliper width of 0.05) was applied to match low and high adherence groups and reduce confounding effects. The probability density distributions before and after matching are shown in Supplementary Figure S1, and baseline characteristics of the matched sample are presented in Supplementary Table S5. All variables demonstrated standardized mean differences below 0.1, indicating acceptable balance between groups [].
The association analysis after matching is summarized in Table 4. In the fully adjusted Model 3, high adherence was associated with 25.0% lower odds of dementia versus low adherence (OR = 0.75; 95% CI: 0.58–0.97).

Table 4.
Association analysis between cMIND diet and dementia after propensity score matching (n = 3250).
4. Discussion
This study, using cross-sectional data from the 2018 CLHLS, investigated the association between the cMIND diet and dementia in 9142 Chinese older adults. After adjusting for multiple confounding factors, adherence to the cMIND diet was significantly associated with a lower risk of dementia. Specifically, compared with low adherence, moderate and high adherence were linked to 18.0% and 24.0% lower dementia risk, respectively. To test the stability of the results, we conducted two sensitivity analyses. RCS analysis demonstrated a significant linear relationship between cMIND diet and dementia. The association also remained significant across all subgroups, with no evidence of interaction. Finally, PSM was performed as a secondary analysis to minimize the influence of confounding factors.
4.1. The Prevalence and Pathogenesis of Diseases Among Older Adults in China
The prevalence of dementia among older adults in China is 10.7%, consistent with findings from Wang YQ [], Liu Y [], and others. Alzheimer’s disease (AD) is the most common form of dementia [], and its pathogenesis has been the most extensively studied. Current research can be broadly summarized into three major hypotheses. First, the amyloid cascade hypothesis. Proposed by Hardy and Allsop in 1991 [], this hypothesis suggests that the abnormal accumulation of β-amyloid (Aβ) plays a central role in AD development. Aβ, derived from the proteolytic cleavage of amyloid precursor protein (APP), progressively aggregates into neurotoxic plaques, leading to mitochondrial dysfunction, synaptic impairment, and neuronal death, thereby driving the onset and progression of AD []. Second, the hyperphosphorylated tau hypothesis. Tau, encoded by the MAPT gene [], becomes pathologically altered through abnormal hyperphosphorylation, resulting in conformational changes and loss of microtubule-stabilizing function [,]. This process disrupts neuronal structure and function. Higher levels of tau phosphorylation are associated with greater aggregation and more severe AD pathology []. Third, the neuroinflammation hypothesis. Pathological stimuli and neuronal injury can induce pro-inflammatory responses []. Pro-inflammatory cytokines promote the release of additional inflammatory mediators and reactive oxygen species (ROS), while also upregulating complement system activity, thereby amplifying neurotoxic effects. The synergistic interaction between these cytokines and reactive astrocytes may activate destructive neuronal signaling pathways, promoting the deposition of Aβ plaques and the hyperphosphorylation of tau proteins, leading to further neuronal damage.
4.2. Mechanistic Pathways Association the cMIND Diet to Dementia
The cMIND diet, as a comprehensive dietary pattern, is characterized by its emphasis on a balanced intake of multiple nutrients and their synergistic interactions with lifestyle factors []. Our findings align with prior studies and further support the potential role of healthy eating in dementia prevention. Polyphenols, vitamin E, and n-3 polyunsaturated fatty acids, which are abundant in berries, leafy greens, and olive oil—key components of the MIND diet—can inhibit excessive microglial activation, reduce pro-inflammatory cytokine release, neutralize free radicals, and repair oxidative neuronal damage. These combined mechanisms contribute to reduced Aβ deposition and tau protein phosphorylation [,]. Moreover, the low-sodium principle of the DASH diet, together with the flavonoids present in the MIND diet, enhances vascular endothelial function, regulates blood pressure, lowers the risk of cerebrovascular lesions, and safeguards cerebral blood flow and Aβ clearance. Collectively, these effects contribute to a reduced risk of dementia [].
4.3. The Association Between cMIND Diet and Dementia in Different Population Subgroups
This study systematically examined the associations between cMIND diet and dementia across different population subgroups, and consistent associations were observed. Specifically, in the subgroup aged ≥80 years, adherence to the cMIND diet was significantly associated with a lower prevalence of dementia. Among older adults, the brain typically accumulates pathological damage over decades. The cMIND diet’s rich anti-inflammatory components help promote a low-inflammation brain microenvironment, thereby slowing neurodegenerative processes and enhancing cognitive function []. Furthermore, this study found that rural residents benefited more from the cMIND diet than their urban counterparts. This suggests that preserving and promoting traditional dietary patterns may be crucial for maintaining neurological health in the context of rapid urbanization []. This study found that the protective effect of the cMIND diet against dementia was more pronounced among individuals with other marital statuses than among those who were married. Liu et al. found that individuals experiencing divorce, separation, widowhood, or remaining single exhibited a significantly higher risk of dementia compared with married individuals []. According to the marital resources model, marriage provides unique social, psychological, and economic resources that are difficult to obtain through other forms of relationships, thereby positively influencing health and longevity []. At the same time, socioeconomic disadvantage is a well-recognized risk factor for dementia []. Studies by French et al. and Hickey et al. have both found that individuals with lower income and education levels may face limitations in food choices and reduced health literacy [,]. Studies by Kivimäki et al. and Sanlier et al. have shown that individuals with lower socioeconomic status face a higher risk of dementia compared with those of higher status [,]. Furthermore, our findings indicate that living with household members was significantly associated with a lower prevalence of dementia, whereas no clear benefit was observed for those living alone. This observation underscores the synergistic role of social interaction in dietary interventions. Shared meals not only enhance adherence to healthy eating patterns but may also independently promote the development of cognitive reserve through social engagement. In the lifestyle dimension, this study found no significant protective effect of the cMIND diet against dementia among current smokers and drinkers. Tobacco and alcohol may diminish or even counteract the diet’s neuroprotective effects, consistent with the findings of Scarmeas et al. indicating that lifestyle and social support can modulate dietary outcomes []. Regarding health status, this study also found that adherence to the cMIND diet significantly lowered dementia risk among participants without hypertension or dyslipidemia. Compared with hypertensive individuals, those without hypertension exhibit healthier vascular function and maintain relatively stable cerebral blood flow. Additionally, the cMIND diet’s restriction of saturated and trans fats helps regulate lipid levels and slow the progression of atherosclerosis, thereby further improving cerebrovascular health []. No significant protective effect was observed among diabetic participants, whereas a risk reduction was evident in non-diabetic individuals. This finding aligns with the “metabolic–neurodegenerative axis” hypothesis, which suggests that chronic hyperglycemia and insulin resistance may undermine potential dietary benefits by promoting amyloid deposition, damaging the blood–brain barrier, and disrupting insulin signaling within the brain [].
4.4. Advantages and Limitations
The results of this study provide new perspectives for public health intervention strategies, particularly among older adults in China. The cMIND diet, as a non-pharmacological preventive approach, holds promise as an important tool for alleviating the burden of dementia. These findings offer scientific evidence for public health policymakers and support the implementation of cognitive function-focused dietary interventions, carrying significant public health value and practical implications. This study also has several limitations. First, this analysis was based on cross-sectional data, which limits the ability to establish a causal association between the cMIND diet and dementia and does not rule out the possibility of reverse causation. The findings of this study can only support an association between the cMIND diet and dementia. Future prospective cohort studies are needed to clarify the causality and its directionality. Second, although we have controlled for potential confounders as thoroughly as possible, the influence of other unmeasured factors, such as environmental exposures or genetic predispositions, cannot be entirely ruled out. Third, both the cMIND diet and dementia were assessed via self-report. Although data collectors received rigorous training, the possibility of social desirability and recall biases cannot be ruled out. Future studies should adopt objective measures whenever possible to minimize the impact of such potential biases. Finally, the database used in this study is specific to the Chinese population. Given the differences in diet and culture across regions globally, the applicability and generalizability of the cMIND diet may be limited. Future research should prioritize cross-cultural comparative studies to examine the effects of different dietary patterns in diverse populations. Such an approach would facilitate the development of more targeted dietary recommendations for dementia prevention on a global scale.
5. Conclusions
The findings suggest that greater adherence to the cMIND diet is associated with a lower prevalence of dementia among Chinese older adults, with a clear dose–response relationship. Further subgroup analyses confirm that this association persists across different demographic subgroups. The findings of this study highlight the potential of the cMIND diet in preventing dementia among older Chinese adults, indicating the need for larger cohort studies or randomized controlled trials to strengthen causal inference, and offering innovative strategies to reduce the dementia burden through localized dietary interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17223529/s1, Table S1: The value assignment of cMIND diet, Table S2: The value assignment of covariates, Table S3: Association between cMIND diet and dementia: A Multiple Imputation by Chained Equations Approach, Table S4: Association between cMIND diet and dementia, except hypertension, diabetes, heart disease and dyslipidemia, Table S5: Baseline characteristics before and after propensity score matching, Figure S1: Covariates matching effect.
Author Contributions
Y.Z. (Yu Zhang): conceptualization, methodology, formal analysis, writing—original draft, writing—review and editing. Y.L.: methodology, writing—original draft, writing—review and editing. Y.M.: visualization, writing—review and editing. Y.D.: visualization, writing—review and editing. Z.C.: formal analysis, writing—review and editing. Y.F.: formal analysis, writing—review and editing. Z.S.: writing—review and editing, supervision. L.Z.: writing—review and editing, supervision. Y.Z. (Yong Zhao): supervision, writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Special Fund of the Chinese Nutrition Society for the Balanced Diet Initiative—Research on the Development and Promotion of a Community-Based “Smart Breakfast” Science Popularization Model for Older Adults (Grant number: CNS-RDSF-2025-030).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Peking University (protocol code: IRB00001052-13074).
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
The original data presented in the study are openly available in http://opendata.pku.edu.cn/ (accessed on 8 August 2022).
Acknowledgments
We thank the CLHLS research and field team and every respondent in the study for their contributions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gale, S.A.; Acar, D.; Daffner, K.R. Dementia. Am. J. Med. 2018, 131, 1161–1169. [Google Scholar] [CrossRef]
- World Health Organization. Dementia. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/dementia (accessed on 17 June 2025).
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the global burden of disease study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J. Trends, inequalities, and cross-location similarities in global dementia burden and attributable risk factors across 204 countries and territories: A systematic analysis for the global burden of disease study 2021. Int. J. Surg. 2025, 111, 5298–5310. [Google Scholar] [CrossRef]
- Wetterberg, H.; Najar, J.; Rydén, L.; Ribbe, M.; Sterner, T.R.; Zettergren, A.; Guo, X.; Erhag, H.F.; Sacuiu, S.; Kern, S.; et al. Dementia remains the major predictor of death among octogenarians. A study of two population cohorts of 85-year-olds examined 22 years apart. Eur. J. Epidemiol. 2021, 36, 507–517. [Google Scholar] [CrossRef]
- Li, R.; Qi, J.; Yang, Y.; Wu, Y.; Yin, P.; Zhou, M.; Qian, Z.; LeBaige, M.H.; McMillin, S.E.; Guo, H.; et al. Disease burden and attributable risk factors of Alzheimer’s Disease and dementia in China from 1990 to 2019. J. Prev. Alzheimers Dis. 2022, 9, 306–314. [Google Scholar] [CrossRef]
- Shi, Y.; Lin, F.; Li, Y.; Wang, Y.; Chen, X.; Meng, F.; Ye, Q.; Cai, G. Association of pro-inflammatory diet with increased risk of all-cause dementia and Alzheimer’s dementia: A prospective study of 166,377 UK biobank participants. BMC Med. 2023, 21, 266. [Google Scholar] [CrossRef]
- Dorey, C.K.; Gierhart, D.; Fitch, K.A.; Crandell, I.; Craft, N.E. Low xanthophylls, retinol, lycopene, and tocopherols in grey and white matter of brains with Alzheimer’s disease. J. Alzheimers Dis. 2023, 94, 1–17. [Google Scholar] [CrossRef]
- Klonizakis, M.; Bugg, A.; Hunt, B.; Theodoridis, X.; Bogdanos, D.P.; Grammatikopoulou, M.G. Assessing the physiological effects of traditional regional diets targeting the prevention of cardiovascular disease: A systematic review of randomized controlled trials implementing mediterranean, New Nordic, Japanese, Atlantic, Persian and Mexican dietary interventions. Nutrients 2021, 13, 3034. [Google Scholar] [CrossRef] [PubMed]
- Ugartemendia, L.; Bravo, R.; Castaño, M.Y.; Cubero, J.; Zamoscik, V.; Kirsch, P.; Rodríguez, A.B.; Reuterd, M. Influence of diet on mood and social cognition: A pilot study. Food Funct. 2020, 11, 8320–8330. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, M.; Wang, P. The intricate interplay between dietary habits and cognitive function: Insights from the gut-brain axis. Front. Nutr. 2025, 12, 1539355. [Google Scholar] [CrossRef] [PubMed]
- Zyoud, S.H.; Smale, S.; Waring, W.S.; Sweileh, W.M.; Al-Jabi, S.W. Global research trends in microbiome-gut-brain axis during 2009–2018: A bibliometric and visualized study. BMC Gastroenterol. 2019, 19, 158. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, D.G.; Lavecchia, S.; Brennan, S.; Lawlor, B.A.; Kelly, M.E. The impact of the mediterranean diet on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Adv. Nutr. 2017, 8, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Fekete, M.; Varga, P.; Ungvari, Z.; Fekete, J.T.; Buda, A.; Szappanos, Á.; Lehoczki, A.; Mózes, N.; Grosso, G.; Godos, J.; et al. The role of the mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer’s disease: A meta-analysis. Geroscience 2025, 47, 3111–3130. [Google Scholar] [CrossRef] [PubMed]
- Martínez García, R.M.; Jiménez Ortega, A.I.; López Sobaler, A.M.; Ortega, R.M. Nutrition strategies that improve cognitive function. Nutr. Hosp. 2018, 35, 16–19. [Google Scholar]
- Wickman, B.E.; Enkhmaa, B.; Ridberg, R.; Romero, E.; Cadeiras, M.; Meyers, F.; Steinberg, F. Dietary management of heart failure: Dash diet and precision nutrition perspectives. Nutrients 2021, 13, 4424. [Google Scholar] [CrossRef]
- Gardener, H.; Caunca, M.R. Mediterranean diet in preventing neurodegenerative diseases. Curr. Nutr. Rep. 2018, 7, 10–20. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; Van De Rest, O. The mediterranean, dietary approaches to stop hypertension (dash), and mediterranean-dash intervention for neurodegenerative delay (mind) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease—A review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. Mind diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Hosking, D.E.; Eramudugolla, R.; Cherbuin, N.; Anstey, K.J. Mind not mediterranean diet related to 12-year incidence of cognitive impairment in an australian longitudinal cohort study. Alzheimers Dement. 2019, 15, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, I.; Tsiampalis, T.; Damigou, E.; Barkas, F.; Anastasiou, G.; Kravvariti, E.; Liberopoulos, E.; Sfikakis, P.P.; Chrysohoou, C.; Tsioufis, C.; et al. Long-term adherence to the mediterranean diet reduces 20-year diabetes incidence: The attica cohort study (2002–2022). Metabolites 2024, 14, 182. [Google Scholar] [CrossRef]
- Huang, X.; Aihemaitijiang, S.; Ye, C.; Halimulati, M.; Wang, R.; Zhang, Z. Development of the cMIND diet and its association with cognitive impairment in older Chinese people. J. Nutr. Health Aging 2022, 26, 760–770. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zeng, X.; Xian, X.; Chen, J.; Niu, T. Association between cMIND diet and hypertension among older adults in China: A nationwide survey. Aging Clin. Exp. Res. 2024, 36, 182. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Zhang, Y.; Zhou, X.; Shen, K.; Ji, X.; Zhu, J.; Wu, O.; Xian, X. Associations of cMIND diet with depressive and anxiety symptoms among old people in China: A nationwide study. Eur. J. Nutr. 2025, 64, 122. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhou, X.; Liu, X. Association of adherence to the Chinese version of the mind diet with reduced cognitive decline in older Chinese individuals: Analysis of the Chinese longitudinal healthy longevity survey. J. Nutr. Health Aging 2024, 28, 100024. [Google Scholar] [CrossRef]
- Thomas, A.; Ryan, C.P.; Caspi, A.; Liu, Z.; Moffitt, T.E.; Sugden, K.; Zhou, J.; Belsky, D.W.; Gu, Y. Diet, pace of biological aging, and risk of dementia in the framingham heart study. Ann. Neurol. 2024, 95, 1069–1079. [Google Scholar] [CrossRef]
- Zeng, Y. Towards deeper research and better policy for healthy aging—Using the unique data of Chinese longitudinal healthy longevity survey. China Econ. J. 2012, 5, 131–149. [Google Scholar] [CrossRef]
- Zhu, A.; Chen, H.; Shen, J.; Wang, X.; Li, Z.; Zhao, A.; Shi, X.; Yan, L.; Zeng, Y.; Yuan, C.; et al. Interaction between plant-based dietary pattern and air pollution on cognitive function: A prospective cohort analysis of Chinese older adults. Lancet Reg. Health West. Pac. 2022, 20, 100372. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Zhou, Q.; Dong, X.; Guo, Y.; Wang, P.; He, W.; Wang, R.; Wu, T.; Yao, Z.; et al. A network analysis of anxiety and depression symptoms in Chinese disabled elderly. J. Affect. Disord. 2023, 333, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti, P.; Wolf, L.E.; Segal, M.R.; McCulloch, C.E. Ethics and sample size. Am. J. Epidemiol. 2005, 161, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, X.; Zhang, Y.; Zeng, M.; Liu, Y.; Wu, Y.; Hu, W.; Lai, Y.; Liao, J. Geographical variation in dementia prevalence across China: A geospatial analysis. Lancet Reg. Health West. Pac. 2024, 47, 101117. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Wang, R.; Ye, C.; Huang, X.; Halimulati, M.; Sun, M.; Ma, Y.; Fan, R.; Zhang, Z. cMIND diet, indoor air pollution, and depression: A cohort study based on the CLHLS from 2011 to 2018. Nutrients 2023, 15, 1203. [Google Scholar] [CrossRef]
- Zeng, M.; Chen, Y.; Lobanov-Rostovsky, S.; Liu, Y.; Steptoe, A.; Brunner, E.J.; Liao, J. Adiposity and dementia among Chinese adults: Longitudinal study in the China health and retirement longitudinal study (CHARLS). Int. J. Obes. 2025, 49, 706–714. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Cai, J.; Huang, Y.; Chen, Y.; Venkatraman, T.M.; Lobanov-Rostovsky, S.; Bandosz, P.; Yang, Y.; Wu, Y.; et al. Is there a common latent cognitive construct for dementia estimation across two Chinese cohorts? Alzheimers Dement. 2022, 14, e12356. [Google Scholar] [CrossRef]
- Liu, N.; Cadilhac, D.A.; Kilkenny, M.F.; Liang, Y. Changes in the prevalence of chronic disability in China: Evidence from the China health and retirement longitudinal study. Public Health 2020, 185, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Du, J.; Song, Q.; Yang, J.; Wu, Y. A prediction model of cognitive impairment risk in elderly illiterate Chinese women. Front. Aging Neurosci. 2023, 15, 1148071. [Google Scholar] [CrossRef]
- Sun, Q.; Gao, T.; Han, S.; Mo, G.; Liu, H. Role of raising domestic animals in decreasing the risk of dementia occurrence in Chinese older adults: Evidence of a 10-year prospective cohort study. Geriatr. Nurs. 2022, 45, 174–179. [Google Scholar] [CrossRef]
- Xian, X.; Fan, X.; Wang, X.; Cao, S.; Wei, X.; Zhang, Y.; Shen, K. Association between indoor ventilation frequency and IADL disability among Chinese older adults. Indoor Air 2025, 2025, 7882633. [Google Scholar] [CrossRef]
- Xian, X.; Fu, Y.; Cao, S.; Niu, T. Association between mold exposure and depressive symptoms among Chinese older adults: Results from the Chinese longitudinal healthy longevity survey (CLHLS). J. Affect. Disord. 2025, 386, 119442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, X.-X.; Huang, Y.-P.; Wang, Y.-X.; Zou, H.; Xiong, L.; Liu, Z.-T.; Wen, Y.; Zhang, Z.-J. Prognosis prediction and comparison between pancreatic signet ring cell carcinoma and pancreatic duct adenocarcinoma: A retrospective observational study. Front. Endocrinol. 2023, 14, 1205594. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, R.; Liang, J.; Li, J.; Qian, S.; Li, J.; Xu, Y. Dementia in China (2015–2050) estimated using the 1% population sampling survey in 2015. Geriatr. Gerontol. Int. 2019, 19, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, T.; Chiu, M. From mild cognitive impairment to subjective cognitive decline: Conceptual and methodological evolution. Neuropsychiatr. Dis. Treat. 2017, 13, 491–498. [Google Scholar] [CrossRef]
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; et al. Abad directly links Aβ to mitochondrial toxicity in Alzheimer’s Disease. Science 2004, 304, 448–452. [Google Scholar] [CrossRef]
- Neve, R.L.; Harris, P.; Kosik, K.S.; Kurnit, D.M.; Donlon, T.A. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986, 387, 271–280. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef]
- Jara, C.; Aránguiz, A.; Cerpa, W.; Tapia-Rojas, C.; Quintanilla, R.A. Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol. 2018, 18, 279–294. [Google Scholar] [CrossRef]
- Mocanu, M.-M.; Nissen, A.; Eckermann, K.; Khlistunova, I.; Biernat, J.; Drexler, D.; Petrova, O.; Schönig, K.; Bujard, H.; Mandelkow, E.; et al. The potential for beta-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous tau in inducible mouse models of tauopathy. J. Neurosci. 2008, 28, 737–748. [Google Scholar] [CrossRef]
- Swaminathan, A.; Jicha, G.A. Nutrition and prevention of Alzheimer’s dementia. Front Aging Neurosci. 2014, 6, 282. [Google Scholar] [CrossRef]
- Wagner, M.; Agarwal, P.; Leurgans, S.E.; Bennett, D.A.; Schneider, J.A.; Capuano, A.W.; Grodstein, F. The association of mind diet with cognitive resilience to neuropathologies. Alzheimers Dement. 2023, 19, 3644–3653. [Google Scholar] [CrossRef]
- Agarwal, P.; Leurgans, S.E.; Agrawal, S.; Aggarwal, N.T.; Cherian, L.J.; James, B.D.; Dhana, K.; Barnes, L.L.; Bennett, D.A.; Schneider, J.A. Association of mediterranean-dash intervention for neurodegenerative delay and mediterranean diets with Alzheimer disease pathology. Neurology 2023, 100, e2259–e2268. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Zhang, Y.; Zheng, X.; Cepeda, C.; Wang, Y.; Duan, S.; Tong, X. Interactions of glial cells with neuronal synapses, from astrocytes to microglia and oligodendrocyte lineage cells. GLIA 2023, 71, 1383–1401. [Google Scholar] [CrossRef] [PubMed]
- Duplantier, S.C.; Gardner, C.D. A critical review of the study of neuroprotective diets to reduce cognitive decline. Nutrients 2021, 13, 2264. [Google Scholar] [CrossRef] [PubMed]
- Jain, U.; Liu, H.; Langa, K.M.; Farron, M.; Kabeto, M.; Lee, J. Widowhood and cognition among older women in India: New insights on widowhood duration and mediators. SSM Popul. Health 2022, 19, 101242. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Q.; Zhang, Q.; Li, S.; Zhao, J.; Chen, W.; Yang, J.; Xia, M.; Liu, Y. Association of adherence to the eat-lancet diet with risk of dementia according to social economic status: A prospective cohort in UK biobank. Geroscience 2025, 47, 3551–3564. [Google Scholar] [CrossRef] [PubMed]
- French, S.A.; Tangney, C.C.; Crane, M.M.; Wang, Y.; Appelhans, B.M. Nutrition quality of food purchases varies by household income: The shopper study. BMC Public Health 2019, 19, 231. [Google Scholar] [CrossRef]
- Hickey, K.T.; Creber, R.M.M.; Reading, M.; Sciacca, R.R.; Riga, T.C.; Frulla, A.P.; Casida, J.M. Low health literacy: Implications for managing cardiac patients in practice. Nurse Pract. 2018, 43, 49–55. [Google Scholar] [CrossRef]
- Kivimäki, M.; Batty, G.D.; Pentti, J.; Shipley, M.J.; Sipilä, P.; Nyberg, S.T.; Suominen, S.B.; Oksanen, T.; Stenholm, S.; Virtanen, M.; et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: A multi-cohort study. Lancet Public Health 2020, 5, e140–e149. [Google Scholar] [CrossRef] [PubMed]
- Sanlier, N.; Kocaay, F.; Kocabas, S.; Ayyildiz, P. The effect of sociodemographic and anthropometric variables on nutritional knowledge and nutrition literacy. Foods 2024, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Lopez, T. Peripheral inflammation and insulin resistance: Their impact on blood-brain barrier integrity and glia activation in Alzheimer’s disease. Int. J. Mol. Sci. 2025, 26, 4209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).