Joint Bacterial Traces in the Gut and Oral Cavity of Obesity Patients Provide Evidence for Saliva as a Rich Microbial Biomarker Source
Abstract
1. Introduction
2. Materials and Methods
3. Results
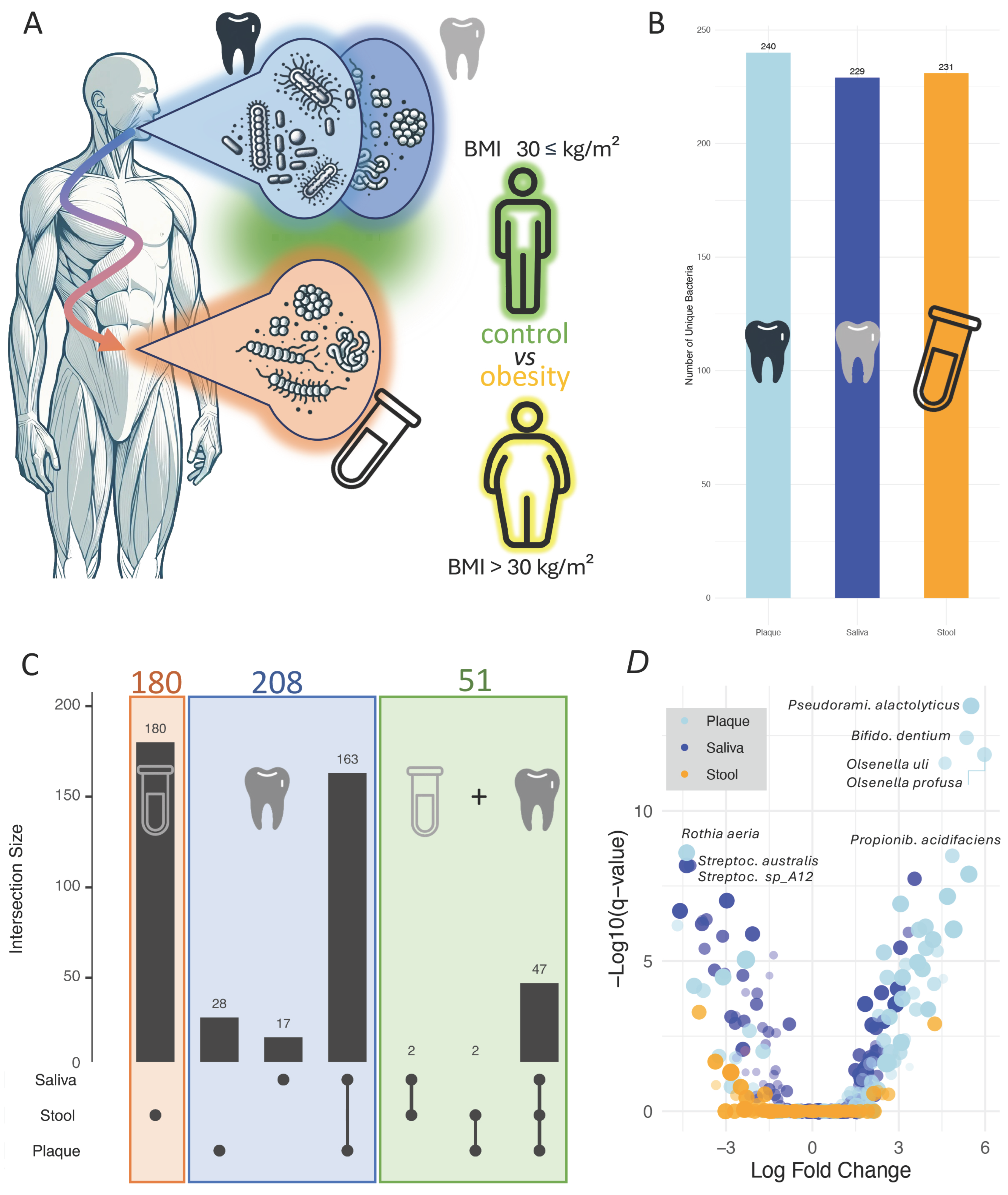
3.1. Microbiome-Disease Associations Vary Across Specimen Types with Inter-Site Overlap
3.2. Oral Samples Display Significantly Higher Effect Sizes as Compared to Stool Samples
3.3. Cross-Site Consistency and Divergence of Obesity-Associated Microbial Signatures
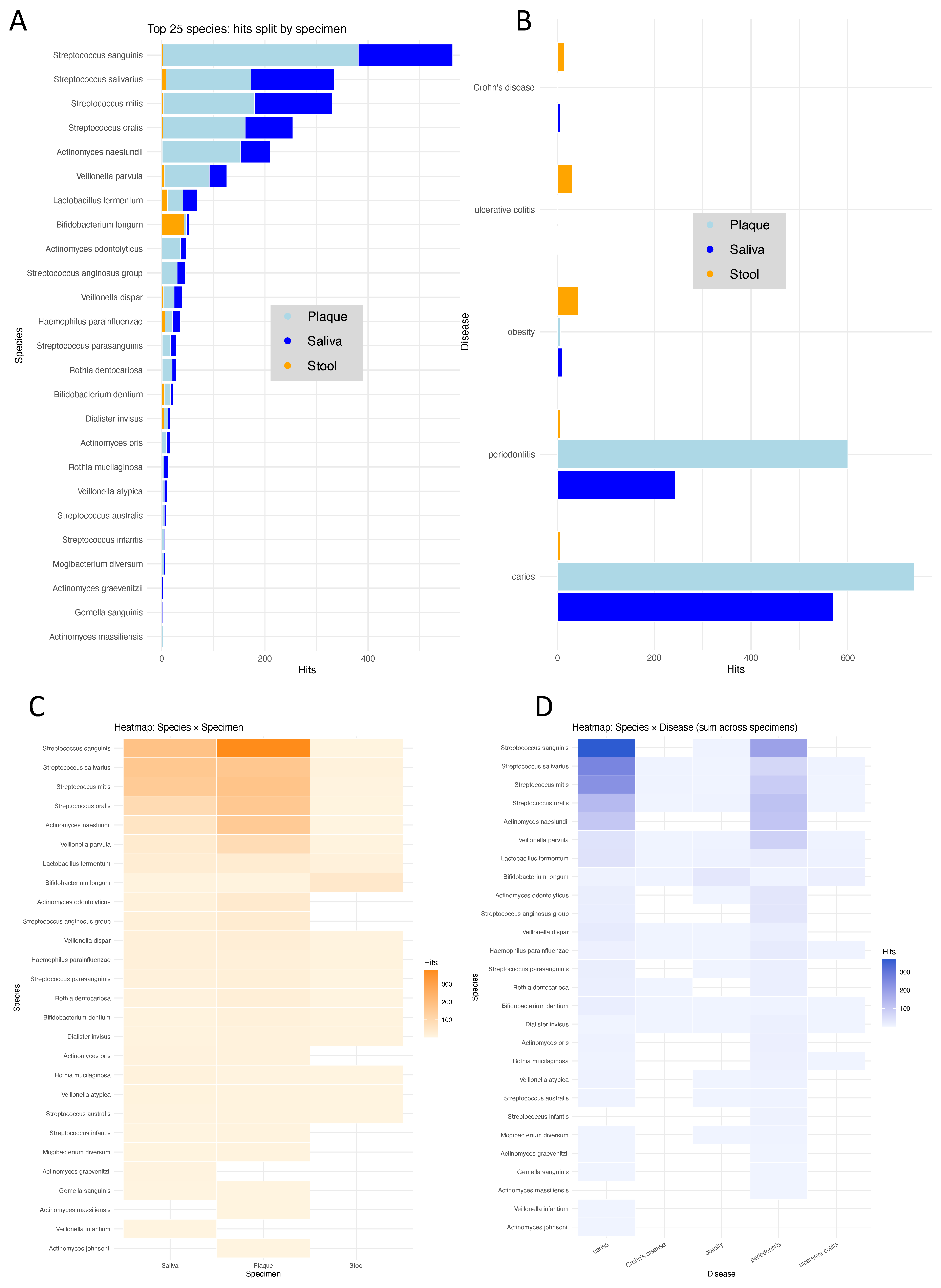
3.4. Literature Mining Supports Relevance of Identified Species Across Diseases and Sites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Forcina, G.; Di Filippo, P.; De Biasio, D.; Cesaro, F.G.; Frattolillo, V.; Massa, A.; De Cesare, M.; Marzuillo, P.; Miraglia Del Giudice, E.; Di Sessa, A. Targeting the Gut Microbiota in Pediatric Obesity: A Paradigm Shift in Prevention and Treatment? A Comprehensive Review. Nutrients 2025, 17, 2942. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Van Treuren, W.; Fischer, C.R.; Merrill, B.D.; DeFelice, B.C.; Sanchez, J.M.; Higginbottom, S.K.; Guthrie, L.; Fall, L.A.; Dodd, D.; et al. A metabolomics pipeline for the mechanistic interrogation of the gut microbiome. Nature 2021, 595, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Cuevas-Sierra, A.; de la O, V.; Salvado, R.; Barreiros-Mota, I.; Castela, I.; Camelo, A.; Brandao, I.; Santo, C.E.; Faria, A.; et al. Gut Microbiota Shifts After a Weight Loss Program in Adults with Obesity: The WLM3P Study. Nutrients 2025, 17, 2360. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Zhao, S.; Jang, C.; Liu, J.; Uehara, K.; Gilbert, M.; Izzo, L.; Zeng, X.; Trefely, S.; Fernandez, S.; Carrer, A.; et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 2020, 579, 586–591. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Selvaraju, V.; Chen, J.; Ayine, P.; Yang, L.; Babu, J.R.; Geetha, T.; Taneja, V. Ethnic variability associating gut and oral microbiome with obesity in children. Gut Microbes 2021, 13, 1882926. [Google Scholar] [CrossRef]
- Akagbosu, C.O.; McCauley, K.E.; Namasivayam, S.; Romero-Soto, H.N.; O’Brien, W.; Bacorn, M.; Bohrnsen, E.; Schwarz, B.; Mistry, S.; Burns, A.S.; et al. Gut microbiome shifts in adolescents after sleeve gastrectomy with increased oral-associated taxa and pro-inflammatory potential. Gut Microbes 2025, 17, 2467833. [Google Scholar] [CrossRef]
- Stagaman, K.; Kmiecik, M.J.; Wetzel, M.; Aslibekyan, S.; Sonmez, T.F.; Fontanillas, P.; Auton, A.; Babalola, E.; Bell, R.K.; Bielenberg, J.; et al. Oral and gut microbiome profiles in people with early idiopathic Parkinson’s disease. Commun. Med. 2024, 4, 209. [Google Scholar] [CrossRef]
- Brown, E.L.; Essigmann, H.T.; Hoffman, K.L.; Palm, N.W.; Gunter, S.M.; Sederstrom, J.M.; Petrosino, J.F.; Jun, G.; Aguilar, D.; Perkison, W.B.; et al. Impact of Diabetes on the Gut and Salivary IgA Microbiomes. Infect. Immun. 2020, 88, e00301-20. [Google Scholar] [CrossRef]
- Arbildo-Vega, H.I.; Cruzado-Oliva, F.H.; Infantes-Ruiz, E.D.; Coronel-Zubiate, F.T.; Becerra-Atoche, E.G.; Terrones-Campos, W.; Herrera-Plasencia, P.M.; Seminario-Trelles, O.A.; Ortega-Gallegos, R.E. An Umbrella Review of the Association Between Periodontal Disease and Diabetes Mellitus. Healthcare 2024, 12, 2311. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Devkota, S.; Ghosh, T.S. Gut microbiome: A biomedical revolution. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Xu, Z.; Yeoh, Y.K.; Tun, H.M.; Fei, N.; Zhang, J.; Morrison, M.; Kamm, M.A.; Yu, J.; Chan, F.K.L.; Ng, S.C. Variation in the metagenomic analysis of fecal microbiome composition calls for a standardized operating approach. Microbiol. Spectr. 2024, 12, e01516-24. [Google Scholar] [CrossRef]
- Rehner, J.; Schmartz, G.P.; Groeger, L.; Dastbaz, J.; Ludwig, N.; Hannig, M.; Rupf, S.; Seitz, B.; Flockerzi, E.; Berger, T.; et al. Systematic Cross-biospecimen Evaluation of DNA Extraction Kits for Long- and Short-read Multi-metagenomic Sequencing Studies. Genom. Proteom. Bioinform. 2022, 20, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Schmartz, G.P.; Rehner, J.; Gund, M.P.; Keller, V.; Molano, L.G.; Rupf, S.; Hannig, M.; Berger, T.; Flockerzi, E.; Seitz, B.; et al. Decoding the diagnostic and therapeutic potential of microbiota using pan-body pan-disease microbiomics. Nat. Commun. 2024, 15, 8261. [Google Scholar] [CrossRef]
- Beghini, F.; McIver, L.J.; Blanco-Miguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 2021, 10, e65088. [Google Scholar] [CrossRef] [PubMed]
- Katz, K.S.; Shutov, O.; Lapoint, R.; Kimelman, M.; Brister, J.R.; O’Sullivan, C. STAT: A fast, scalable, MinHash-based k-mer tool to assess Sequence Read Archive next-generation sequence submissions. Genome Biol. 2021, 22, 270. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Kunath, B.J.; De Rudder, C.; Laczny, C.C.; Letellier, E.; Wilmes, P. The oral–gut microbiome axis in health and disease. Nat. Rev. Microbiol. 2024, 22, 791–805. [Google Scholar] [CrossRef]
- Chén, O.Y.; Bodelet, J.S.; Saraiva, R.G.; Phan, H.; Di, J.; Nagels, G.; Schwantje, T.; Cao, H.; Gou, J.; Reinen, J.M.; et al. The roles, challenges, and merits of the p value. Patterns 2023, 4, 100878. [Google Scholar] [CrossRef]
- Maher, J.M.; Markey, J.C.; Ebert-May, D. The Other Half of the Story: Effect Size Analysis in Quantitative Research. CBE—Life Sci. Educ. 2013, 12, 345–351. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Xin, S.; Wang, Y.; Xu, J. Insight into the Relationship between Oral Microbiota and the Inflammatory Bowel Disease. Microorganisms 2022, 10, 1868. [Google Scholar] [CrossRef]
- Wang, Z.; Kaplan, R.C.; Burk, R.D.; Qi, Q. The Oral Microbiota, Microbial Metabolites, and Immuno-Inflammatory Mechanisms in Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 12337. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, L.; Zhang, G.; Dong, J.; Ren, Z.; Li, Z. The pathogenesis of gut microbiota in hepatic encephalopathy by the gut-liver-brain axis. Biosci. Rep. 2023, 43, BSR20222524. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, P.; Shi, L.; Gao, N.; Li, Y.; Shu, C.; Xu, Y.; Yu, Y.; He, J.; Guo, D.; et al. Bifidobacterium longum promotes postoperative liver function recovery in patients with hepatocellular carcinoma. Cell Host Microbe 2024, 32, 131–144.e136. [Google Scholar] [CrossRef]
- Li, Y.L.; Chen, B.Y.; Feng, Z.H.; Zhou, L.J.; Liu, T.; Lin, W.Z.; Zhu, H.; Xu, S.; Bai, X.B.; Meng, X.Q.; et al. Roles of oral and gut microbiota in acute myocardial infarction. J. Adv. Res. 2024, 74, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Rehner, J.; Schmartz, G.P.; Kramer, T.; Keller, V.; Keller, A.; Becker, S.L. The Effect of a Planetary Health Diet on the Human Gut Microbiome: A Descriptive Analysis. Nutrients 2023, 15, 1924. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Xu, X.; Zhang, J.; Wang, X.; Han, T.; Zhang, Y.; Pan, S.; Ming, Z.; Li, R.; Lou, F.; et al. Timing of unsaturated fat intake improves insulin sensitivity via the gut microbiota-bile acid axis: A randomized controlled trial. Nat. Commun. 2025, 16, 4211. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, R.; Wu, J.; Tan, Z.; Jiao, J. Dietary selection of distinct gastrointestinal microorganisms drives fiber utilization dynamics in goats. Microbiome 2025, 13, 118. [Google Scholar] [CrossRef]
- Häcker, D.; Siebert, K.; Smith, B.J.; Köhler, N.; Riva, A.; Mahapatra, A.; Heimes, H.; Nie, J.; Metwaly, A.; Hölz, H.; et al. Exclusive enteral nutrition initiates individual protective microbiome changes to induce remission in pediatric Crohn’s disease. Cell Host Microbe 2024, 32, 2019–2034.e2018. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Jiang, M.; Lang, T.; Wan, L.; Dai, J. 5-aminosalicylic acid alleviates colitis and protects intestinal barrier function by modulating gut microbiota in mice. Naunyn Schmiedeberg’s Arch. Pharmacol. 2025, 398, 3681–3695. [Google Scholar] [CrossRef]
- Ning, S.; Zhang, Z.; Zhou, C.; Wang, B.; Liu, Z.; Feng, B. Cross-talk between macrophages and gut microbiota in inflammatory bowel disease: A dynamic interplay influencing pathogenesis and therapy. Front. Med. 2024, 11, 1457218. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Paglia, L. Childhood obesity, sugar, and Early Childhood Caries: The sweet trap. Eur. J. Paediatr. Dent. 2024, 25, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Wong, H.M.; Huang, F.; Peng, S. Altered salivary profiles in adolescents with dental caries and central obesity: A cross-sectional study. J. Dent. 2025, 161, 105979. [Google Scholar] [CrossRef]
- Yu, S.T.; Hua, J.Y.; Zeng, Y.H.; Yu, S.Y.; Zhang, Z.Y.; Xiao, W.S.; Li, D.L. Multimorbidity patterns of dental caries and obesity/overweight among adults: A systematic review and meta-analysis. BMC Oral Health 2025, 25, 1037. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehner, J.; Gund, M.; Becker, S.L.; Hannig, M.; Rupf, S.; Schattenberg, J.M.; Keller, A.; the IMAGINE Consortium; Molano, L.-A.G.; Keller, V. Joint Bacterial Traces in the Gut and Oral Cavity of Obesity Patients Provide Evidence for Saliva as a Rich Microbial Biomarker Source. Nutrients 2025, 17, 3527. https://doi.org/10.3390/nu17223527
Rehner J, Gund M, Becker SL, Hannig M, Rupf S, Schattenberg JM, Keller A, the IMAGINE Consortium, Molano L-AG, Keller V. Joint Bacterial Traces in the Gut and Oral Cavity of Obesity Patients Provide Evidence for Saliva as a Rich Microbial Biomarker Source. Nutrients. 2025; 17(22):3527. https://doi.org/10.3390/nu17223527
Chicago/Turabian StyleRehner, Jacqueline, Madline Gund, Sören L. Becker, Matthias Hannig, Stefan Rupf, Jörn M. Schattenberg, Andreas Keller, the IMAGINE Consortium, Leidy-Alejandra G. Molano, and Verena Keller. 2025. "Joint Bacterial Traces in the Gut and Oral Cavity of Obesity Patients Provide Evidence for Saliva as a Rich Microbial Biomarker Source" Nutrients 17, no. 22: 3527. https://doi.org/10.3390/nu17223527
APA StyleRehner, J., Gund, M., Becker, S. L., Hannig, M., Rupf, S., Schattenberg, J. M., Keller, A., the IMAGINE Consortium, Molano, L.-A. G., & Keller, V. (2025). Joint Bacterial Traces in the Gut and Oral Cavity of Obesity Patients Provide Evidence for Saliva as a Rich Microbial Biomarker Source. Nutrients, 17(22), 3527. https://doi.org/10.3390/nu17223527







