Association Between Dietary Tomato Intake and Blood Eosinophil Count in Middle-Aged and Older Japanese Individuals: A Population-Based Cross-Sectional Study †
Abstract
1. Introduction
2. Materials and Methods
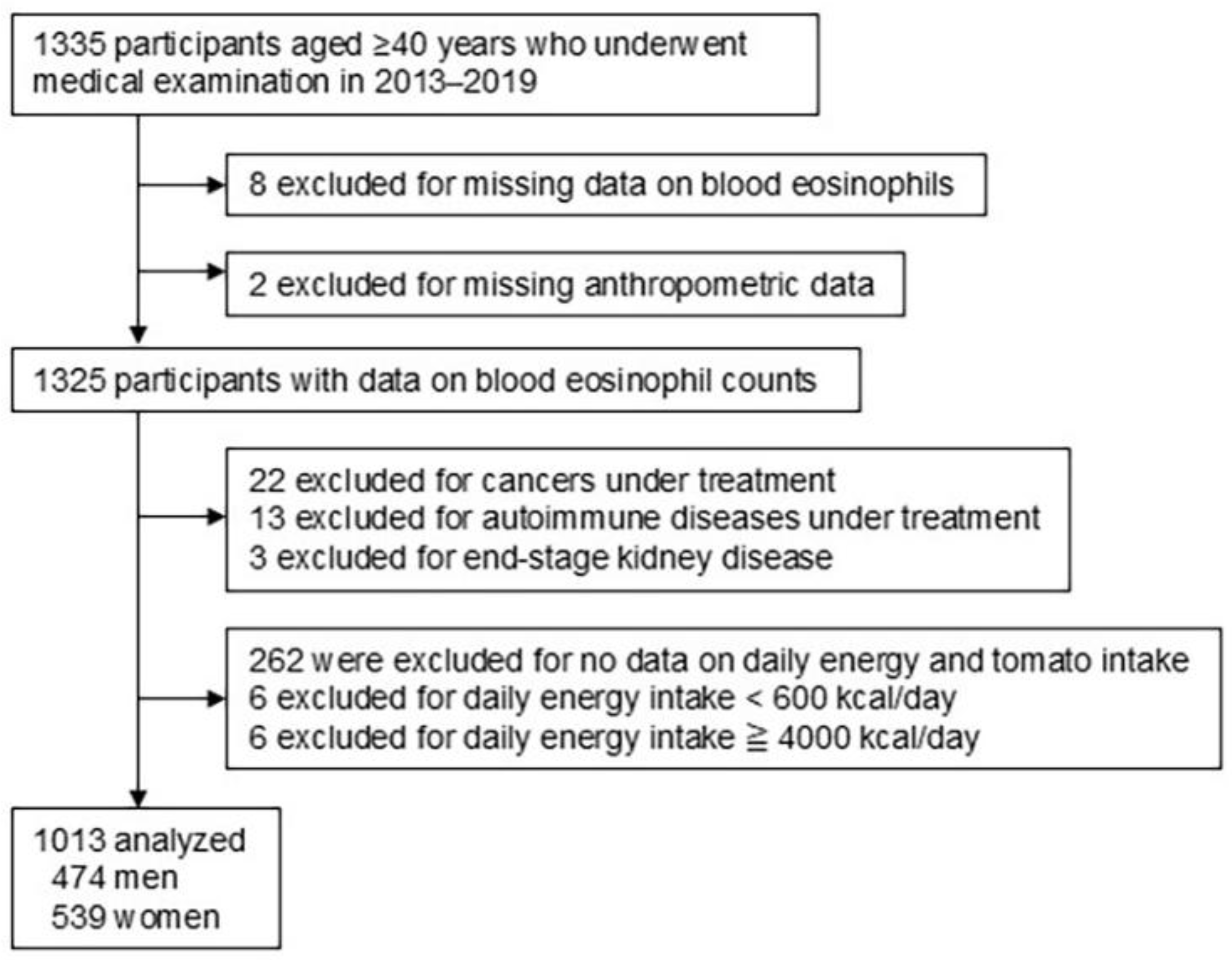
2.1. Study Design and Participants
2.2. Dietary Assessment of Tomato and Its Nutritional Components
2.3. Measurement of Blood Eosinophil Counts
2.4. Other Variables
2.5. Assessment of Genetic Variants
2.6. Calculation of Polygenic Risk Scores
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics According to Blood Eosinophil Counts
3.2. Association Between Dietary Tomato Intake and Blood Eosinophil Count
3.3. Association Between Intake of Major Vitamins Contained in Tomatoes and Blood Eosinophil Count
3.4. Subgroup Analysis by Participants’ Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, A.E.; Boersma, P. Diagnosed Allergic Conditions in Adults: United States, 2021. NCHS Data Brief No. 460; National Center for Health Statistics: Hyattsville, MD, USA, 2023; pp. 1–8. [Google Scholar]
- Ito, Y.; Kato, T.; Yoshida, K.; Takahashi, K.; Fukutomi, Y.; Nagao, M.; Fukuie, T. Prevalence of Allergic Diseases across All Ages in Japan: A Nationwide Cross-Sectional Study Employing Designated Allergic Disease Medical Hospital Network. JMA J. 2023, 6, 165–174. [Google Scholar] [CrossRef]
- Gigon, L.; Fettrelet, T.; Yousefi, S.; Simon, D.; Simon, H.U. Eosinophils from A to Z. Allergy Eur. J. Allergy Clin. Immunol. 2023, 78, 1810–1846. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F. Dupilumab for COPD with Blood Eosinophil Evidence of Type 2 Inflammation. N. Engl. J. Med. 2024, 390, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, F.; Asada, H. Current Perspective Regarding the Immunopathogenesis of Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (Dihs/Dress). Int. J. Mol. Sci. 2021, 22, 2147. [Google Scholar] [CrossRef] [PubMed]
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by Food: Impact on Gut Immunity and Immune Cell Function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599. [Google Scholar] [CrossRef]
- Acevedo, N.; Alhamwe, B.A.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial Effects and Potential Risks of Tomato Consumption for Human Health: An Overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and Lycopene and Multiple Health Outcomes: Umbrella Review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Dębińska, A.; Sozańska, B. Dietary Polyphenols—Natural Bioactive Compounds with Potential for Preventing and Treating Some Allergic Conditions. Nutrients 2023, 15, 4823. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Tsujiguchi, H.; Kambayashi, Y.; Hara, A.; Miyagi, S.; Yamada, Y.; Nakamura, H.; Shimizu, Y.; Hori, D.; Suzuki, F.; et al. Relationship between Vitamin Intake and Depressive Symptoms in Elderly Japanese Individuals: Differences with Gender and Body Mass Index. Nutrients 2017, 9, 1319. [Google Scholar] [CrossRef]
- Suzuki, K.; Tsujiguchi, H.; Miyagi, S.; Nguyen, T.T.T.; Hara, A.; Nakamura, H.; Shimizu, Y.; Hayashi, K.; Yamada, Y.; Nguyen, P.M.; et al. Association between Serum 25-Hydroxyvitamin d Concentrations and Chronic Pain: Effects of Drinking Habits. J. Pain Res. 2020, 13, 2987–2996. [Google Scholar] [CrossRef]
- Tsujiguchi, H.; Hara, A.; Miyagi, S.; Pham, K.O.; Suzuki, K.; Nguyen, T.T.T.; Ono, Y.; Kambayashi, Y.; Shimizu, Y.; Nakamura, H.; et al. Prospective Relationship between Autistic Traits and Nutrient Intakes among Japanese Children: Results of the Shika Study. Autism 2023, 27, 389–401. [Google Scholar] [CrossRef]
- Ogawa, A.; Tsujiguchi, H.; Nakamura, M.; Hayashi, K.; Hara, A.; Suzuki, K.; Miyagi, S.; Kannon, T.; Takazawa, C.; Zhao, J.; et al. Higher Intake of Vegetable Protein and Lower Intake of Animal Fats Reduce the Incidence of Diabetes in Non-Drinking Males: A Prospective Epidemiological Analysis of the Shika Study. Nutrients 2023, 15, 1040. [Google Scholar] [CrossRef]
- Hara, A.; Tsujiguchi, H.; Suzuki, K.; Nakamura, M.; Okada, M.; Zhao, J.; Takazawa, C.; Suzuki, F.; Kasahara, T.; Shimizu, Y.; et al. Distinct Associations between Dietary Omega-3 and Omega-6 Fatty Acids Intake with Chronic Kidney Disease in Adults with and without Diabetes: A Cross-Sectional Study. Nutrition 2023, 115, 112156. [Google Scholar] [CrossRef]
- Hara, A.; Sato, T.; Kress, S.; Suzuki, K.; Pham, K.O.; Tajima, A.; Schikowski, T.; Nakamura, H. Sex-Specific Associations between Air Pollutants and Asthma Prevalence in Japanese Adults: A Population-Based Study. Int. J. Environ. Health Res. 2025, 35, 310–318. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient Intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Yanagibori, R.; Amano, K. Self-Administered Diet History Questionnaire Developed for Health Education: A Relative Validation of the Test-Version by Comparison with 3-Day Diet Record in Women. J. Epidemiol. 1998, 8, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of Relative Validity of Food Group Intakes Estimated by Comprehensive and Brief-Type Self-Administered Diet History Questionnaires against 16 d Dietary Records in Japanese Adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Haneda, M.; Noda, M.; Origasa, H.; Noto, H.; Yabe, D.; Fujita, Y.; Goto, A.; Kondo, T.; Araki, E. Japanese Clinical Practice Guideline for Diabetes 2016. Diabetol. Int. 2018, 9, 1–45. [Google Scholar] [CrossRef]
- Kinoshita, M.; Yokote, K.; Arai, H.; Iida, M.; Ishigaki, Y.; Ishibashi, S.; Umemoto, S.; Egusa, G.; Ohmura, H.; Okamura, T.; et al. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J. Atheroscler. Thromb. 2018, 25, 846–984. [Google Scholar] [CrossRef]
- Tokumaru, T.; Toyama, T.; Hara, A.; Kitagawa, K.; Yamamura, Y.; Nakagawa, S.; Oshima, M.; Miyagawa, T.; Sato, K.; Ogura, H.; et al. Association between Unhealthy Dietary Habits and Proteinuria Onset in a Japanese General Population: A Retrospective Cohort Study. Nutrients 2020, 12, 2511. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- KDIGO. Chapter 1: Definition and Classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Hara, A.; Tsujiguchi, H.; Nguyen, T.T.T.; Kambayashi, Y.; Miyagi, S.; Yamada, Y.; Suzuki, K.; Shimizu, Y.; Nakamura, H. Relationship between Dietary N-6 Fatty Acid Intake and Hypertension: Effect of Glycated Hemoglobin Levels. Nutrients 2018, 10, 1825. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Kawai, Y.; Mimori, T.; Kojima, K.; Nariai, N.; Danjoh, I.; Saito, R.; Yasuda, J.; Yamamoto, M.; Nagasaki, M. Japonica Array: Improved Genotype Imputation by Designing a Population-Specific SNP Array with 1070 Japanese Individuals. J. Hum. Genet. 2015, 60, 581–587. [Google Scholar] [CrossRef]
- Browning, S.R.; Browning, B.L. Rapid and Accurate Haplotype Phasing and Missing-Data Inference for Whole-Genome Association Studies by Use of Localized Haplotype Clustering. Am. J. Hum. Genet. 2007, 81, 1084–1097. [Google Scholar] [CrossRef]
- Browning, B.L.; Browning, S.R. Genotype Imputation with Millions of Reference Samples. Am. J. Hum. Genet. 2016, 98, 116–126. [Google Scholar] [CrossRef]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Nomura, A.; Sato, T.; Tada, H.; Kannon, T.; Hosomichi, K.; Tsujiguchi, H.; Nakamura, H.; Takamura, M.; Tajima, A.; Kawashiri, M.-A. Polygenic Risk Scores for Low-Density Lipoprotein Cholesterol and Familial Hypercholesterolemia. J. Hum. Genet. 2021, 66, 1079–1087. [Google Scholar] [CrossRef]
- Vilhjálmsson, B.J.; Yang, J.; Finucane, H.K.; Gusev, A.; Lindström, S.; Ripke, S.; Genovese, G.; Loh, P.R.; Bhatia, G.; Do, R.; et al. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am. J. Hum. Genet. 2015, 97, 576–592. [Google Scholar] [CrossRef]
- Ishigaki, K.; Akiyama, M.; Kanai, M.; Takahashi, A.; Kawakami, E.; Sugishita, H.; Sakaue, S.; Matoba, N.; Low, S.K.; Okada, Y.; et al. Large-Scale Genome-Wide Association Study in a Japanese Population Identifies Novel Susceptibility Loci across Different Diseases. Nat. Genet. 2020, 52, 669–679. [Google Scholar] [CrossRef]
- Hartl, S.; Breyer, M.K.; Burghuber, O.C.; Ofenheimer, A.; Schrott, A.; Urban, M.H.; Agusti, A.; Studnicka, M.; Wouters, E.F.M.; Breyer-Kohansal, R. Blood Eosinophil Count in the General Population: Typical Values and Potential Confounders. Eur. Respir. J. 2020, 55, 1901874. [Google Scholar] [CrossRef]
- Wen, J.; Wang, C.; Xia, J.; Giri, M.; Guo, S. Relationship between Serum Iron and Blood Eosinophil Counts in Asthmatic Adults: Data from NHANES 2011–2018. Front. Immunol. 2023, 14, 1201160. [Google Scholar] [CrossRef] [PubMed]
- Vedel-Krogh, S.; Nielsen, S.F.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Blood Eosinophils and Exacerbations in Chronic Obstructive. Am. J. Respir. Crit. Care Med. 2016, 193, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Benson, V.S.; Hartl, S.; Barnes, N.; Galwey, N.; Van Dyke, M.K.; Kwon, N. Blood Eosinophil Counts in the General Population and Airways Disease: A Comprehensive Review and Meta-Analysis. Eur. Respir. J. 2022, 59, 2004590. [Google Scholar] [CrossRef] [PubMed]
- Sherrill, D.L.; Halonen, M.; Burrows, B. Relationships between Total Serum IgE, Atopy, and Smoking: A Twenty-Year Follow-up Analysis. J. Allergy Clin. Immunol. 1994, 94, 954–962. [Google Scholar] [CrossRef]
- Farzan, S.; Coyle, T.; Coscia, G.; Rebaza, A.; Santiago, M. Clinical Characteristics and Management Strategies for Adult Obese Asthma Patients. J. Asthma Allergy 2022, 15, 673–689. [Google Scholar] [CrossRef]
- Higuchi, T.; Omata, F.; Tsuchihashi, K.; Higashioka, K.; Koyamada, R.; Okada, S. Current Cigarette Smoking Is a Reversible Cause of Elevated White Blood Cell Count: Cross-Sectional and Longitudinal Studies. Prev. Med. Rep. 2016, 4, 417–422. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoshimura, M.; Yamaguchi, F.; Kouchi, T.; Tsuji, R.; Saito, M.; Obata, A.; Kikuchi, M. Anti-Allergic Activity of Naringenin Chalcone from a Tomato Skin Extract. Biosci. Biotechnol. Biochem. 2004, 68, 1706–1711. [Google Scholar] [CrossRef]
- Yoshimura, M.; Enomoto, T.; Dake, Y.; Okuno, Y.; Ikeda, H.; Cheng, L.; Obata, A. An Evaluation of the Clinical Efficacy of Tomato Extract for Perennial Allergic Rhinitis. Allergol. Int. 2007, 56, 225–230. [Google Scholar] [CrossRef]
- Bianchi, S.; Regolisti, G. Pivotal Clinical Trials, Meta-Analyses and Current Guidelines in the Treatment of Hyperkalemia. Nephrol. Dial. Transplant. 2019, 34, III51–III61. [Google Scholar] [CrossRef]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-Based Diets to Manage the Risks and Complications of Chronic Kidney Disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Sumida, K.; Biruete, A.; Kistler, B.M.; Khor, B.-H.; Ebrahim, Z.; Giannini, R.; Sussman-Dabach, E.J.; Avesani, C.M.; Chan, M.; Lambert, K.; et al. New Insights into Dietary Approaches to Potassium Management in Chronic Kidney Disease. J. Ren. Nutr. 2023, 33, S6–S12. [Google Scholar] [CrossRef]
- Aoki, A.; Hirahara, K.; Kiuchi, M.; Nakayama, T. Eosinophils: Cells Known for over 140 Years with Broad and New Functions. Allergol. Int. 2021, 70, 3–8. [Google Scholar] [CrossRef]

| Total (n = 1013) | Normal (n = 761) | High (n = 252) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Mean/n | SD/% | Mean/n | SD/% | Mean/n | SD/% | ||
| Men, n | 474 | 46.8 | 312 | 41.0 | 162 | 64.3 | <0.001 |
| Age, years | 62.5 | 11.2 | 63.0 | 11.1 | 60.81 | 11.4 | 0.007 |
| Asthma, n | 36 | 3.6 | 20 | 2.6 | 16 | 6.3 | 0.006 |
| Hypertension, n | 582 | 57.5 | 431 | 56.6 | 151 | 59.9 | 0.361 |
| Dyslipidemia, n | 358 | 35.3 | 262 | 34.4 | 96 | 38.1 | 0.291 |
| Diabetes, n | 137 | 13.5 | 94 | 12.4 | 43 | 17.1 | 0.058 |
| CVD, n | 60 | 5.9 | 47 | 6.2 | 13 | 5.2 | 0.553 |
| CKD, n | 211 | 20.8 | 156 | 20.5 | 55 | 21.8 | 0.653 |
| Current drinking, n | 485 | 47.9 | 344 | 45.2 | 141 | 56.0 | 0.003 |
| Current smoking, n | 196 | 19.3 | 117 | 15.4 | 79 | 31.3 | <0.001 |
| Physical activities, n | 572 | 56.5 | 434 | 57.0 | 138 | 54.8 | 0.529 |
| BMI, kg/m2 | 23.4 | 3.2 | 23.2 | 3.1 | 23.9 | 3.5 | 0.006 |
| White blood cell count, /μL | 5804 | 1659 | 5501 | 1519 | 6721 | 1726 | <0.001 |
| Eosinophil count, /μL | 155.0 | 126.7 | 98.3 | 48.3 | 326.3 | 135.9 | <0.001 |
| Eosinophil fraction, % | 2.68 | 2.05 | 1.87 | 0.98 | 5.13 | 2.48 | <0.001 |
| IgE, IU/mL | 219.1 | 573.3 | 169.5 | 319.8 | 362.7 | 980.0 | 0.005 |
| PRS_asthma | 0.267 | 0.090 | 0.261 | 0.089 | 0.283 | 0.092 | 0.019 |
| Energy intake, kcal/day | 1846.0 | 592.3 | 1828.8 | 592.5 | 1898.0 | 589.9 | 0.108 |
| Tomato intake, g/day | 18.4 | 25.3 | 19.8 | 27.0 | 14.1 | 18.8 | <0.001 |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Men, vs. women | 2.511 | 1.826–3.455 | <0.001 | 2.100 | 1.449–3.044 | <0.001 |
| Age, per +1 year | 0.983 | 0.970–0.996 | 0.011 | 0.986 | 0.973–1.000 | 0.051 |
| Energy intake, per +1 kcal/d | 1.000 | 1.000–1.000 | 0.815 | 1.000 | 1.000–1.000 | 0.716 |
| Tomato intake, per +10 g/d | 0.904 | 0.842–0.970 | 0.006 | 0.895 | 0.834–0.961 | 0.004 |
| BMI, per +1 kg/m2 | 1.051 | 1.003–1.100 | 0.037 | |||
| Drinking habit | 0.930 | 0.663–1.303 | 0.672 | |||
| Current smoking | 1.818 | 1.260–2.622 | 0.001 | |||
| Asthma | 3.238 | 1.590–6.595 | 0.001 | |||
| OR | 95% CI | p-Value | p-Value for Interaction | ||
|---|---|---|---|---|---|
| Sex | 0.915 | ||||
| Men | 0.904 | 0.825 | 1.000 | 0.042 | |
| Women | 0.877 | 0.776 | 0.990 | 0.034 | |
| Age | 0.995 | ||||
| <65 y | 0.877 | 0.784 | 0.970 | 0.013 | |
| ≥65 y | 0.923 | 0.834 | 1.030 | 0.148 | |
| Asthma | 0.571 | ||||
| No | 0.886 | 0.825 | 0.961 | 0.004 | |
| Yes | 0.942 | 0.722 | 1.219 | 0.650 | |
| BMI | 0.641 | ||||
| <25 kg/m2 | 0.886 | 0.809 | 0.970 | 0.011 | |
| ≥25 kg/m2 | 0.914 | 0.792 | 1.051 | 0.205 | |
| Hypertension | 0.662 | ||||
| No | 0.851 | 0.745 | 0.970 | 0.016 | |
| Yes | 0.923 | 0.842 | 1.020 | 0.111 | |
| Dyslipidemia | 0.897 | ||||
| No | 0.904 | 0.825 | 1.000 | 0.045 | |
| Yes | 0.868 | 0.768 | 0.980 | 0.025 | |
| Diabetes | 0.940 | ||||
| No | 0.904 | 0.834 | 0.990 | 0.022 | |
| Yes | 0.801 | 0.638 | 0.990 | 0.045 | |
| CKD | 0.019 | ||||
| No | 0.851 | 0.768 | 0.932 | <0.001 | |
| Yes | 1.083 | 0.942 | 1.231 | 0.270 | |
| CVD | 0.672 | ||||
| No | 0.886 | 0.817 | 0.961 | 0.004 | |
| Yes | 0.932 | 0.708 | 1.219 | 0.595 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, A.; Tsujiguchi, H.; Fukuchi, R.; Nakamura, M.; Camara, J.; Talica, M.; Zhao, J.; Takazawa, C.; Suzuki, F.; Ogawa, H.; et al. Association Between Dietary Tomato Intake and Blood Eosinophil Count in Middle-Aged and Older Japanese Individuals: A Population-Based Cross-Sectional Study. Nutrients 2025, 17, 3467. https://doi.org/10.3390/nu17213467
Hara A, Tsujiguchi H, Fukuchi R, Nakamura M, Camara J, Talica M, Zhao J, Takazawa C, Suzuki F, Ogawa H, et al. Association Between Dietary Tomato Intake and Blood Eosinophil Count in Middle-Aged and Older Japanese Individuals: A Population-Based Cross-Sectional Study. Nutrients. 2025; 17(21):3467. https://doi.org/10.3390/nu17213467
Chicago/Turabian StyleHara, Akinori, Hiromasa Tsujiguchi, Rio Fukuchi, Masaharu Nakamura, Jam Camara, Marama Talica, Jiaye Zhao, Chie Takazawa, Fumihiko Suzuki, Haruhiko Ogawa, and et al. 2025. "Association Between Dietary Tomato Intake and Blood Eosinophil Count in Middle-Aged and Older Japanese Individuals: A Population-Based Cross-Sectional Study" Nutrients 17, no. 21: 3467. https://doi.org/10.3390/nu17213467
APA StyleHara, A., Tsujiguchi, H., Fukuchi, R., Nakamura, M., Camara, J., Talica, M., Zhao, J., Takazawa, C., Suzuki, F., Ogawa, H., Kannon, T., Sato, T., Tajima, A., & Nakamura, H. (2025). Association Between Dietary Tomato Intake and Blood Eosinophil Count in Middle-Aged and Older Japanese Individuals: A Population-Based Cross-Sectional Study. Nutrients, 17(21), 3467. https://doi.org/10.3390/nu17213467








