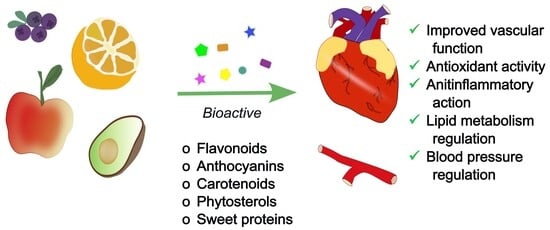
Natural Sweetness and Bioactivity: The Cardiovascular Promise of Fruits
Abstract
1. Introduction
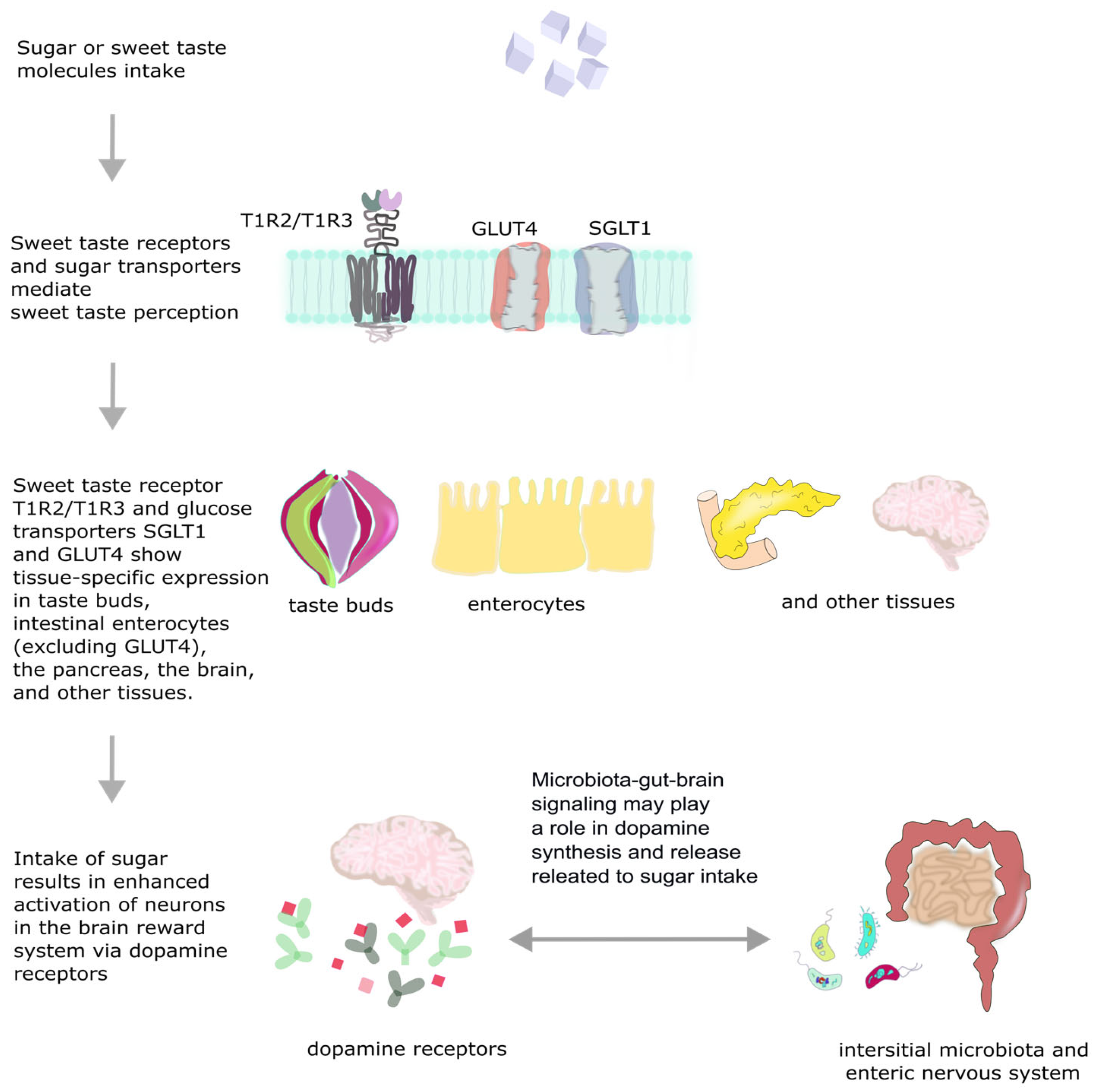
2. Biology of Sweet Taste Perception
2.1. Sweet Taste Receptors
2.2. The Natural Drive for Sweetness
2.2.1. Sugar Intake Regulation
2.2.2. Reward Signaling System
3. Refined Sugars in the Diet and Cardiovascular Health Consequences
3.1. Insulin Resistance and Hyperinsulinemia
3.2. Dyslipidemia
3.3. Oxidative Stress and Inflammation
3.4. Hypertension and Hyperuricemia
3.5. Sweet Taste and Obesity
4. Fruits as a Remedy for Cardiovascular Health
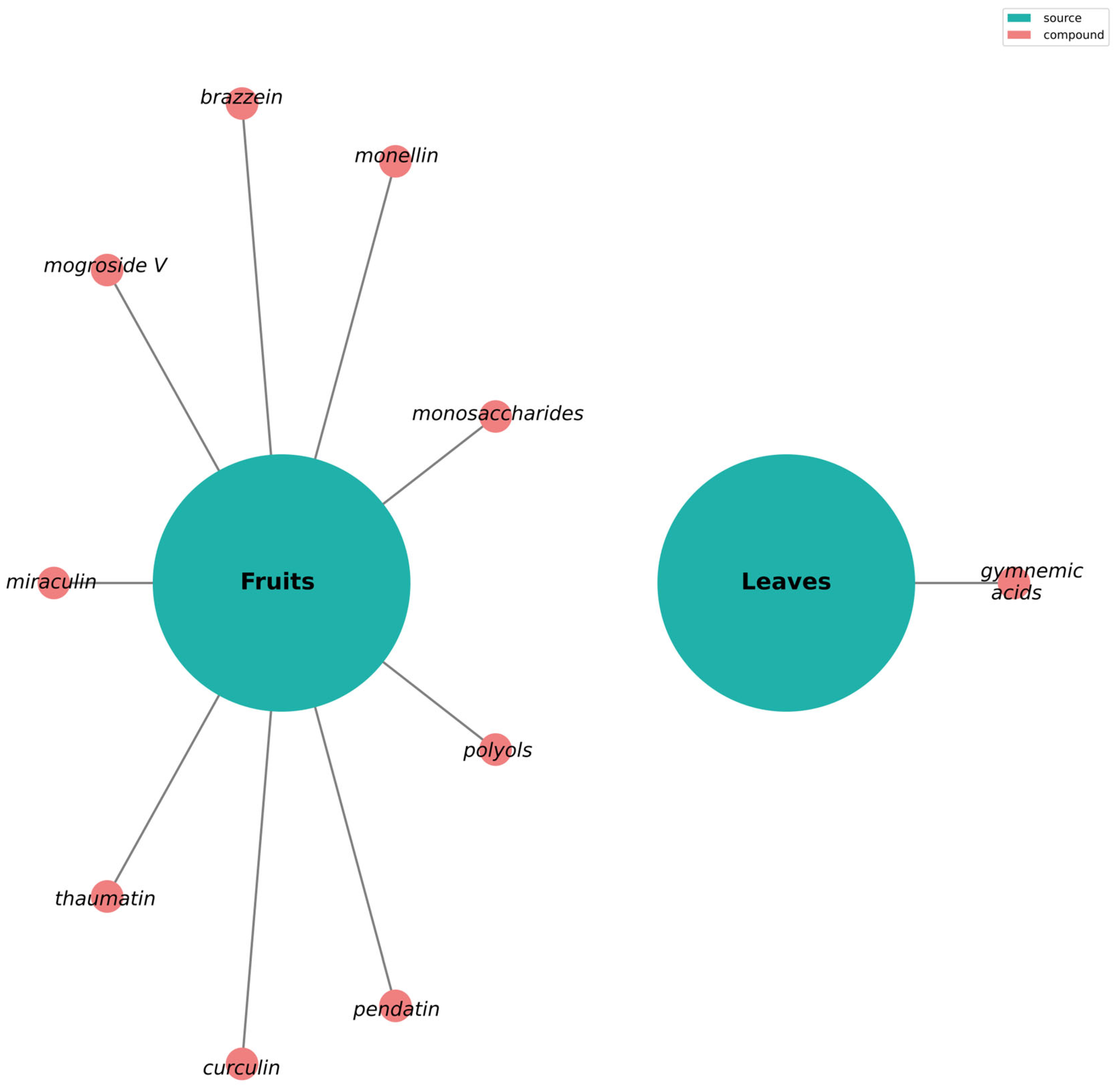
4.1. Plants as a Natural Source of Sweetness
4.1.1. Sweet Proteins
4.1.2. Glycosides and Sweetness Perception
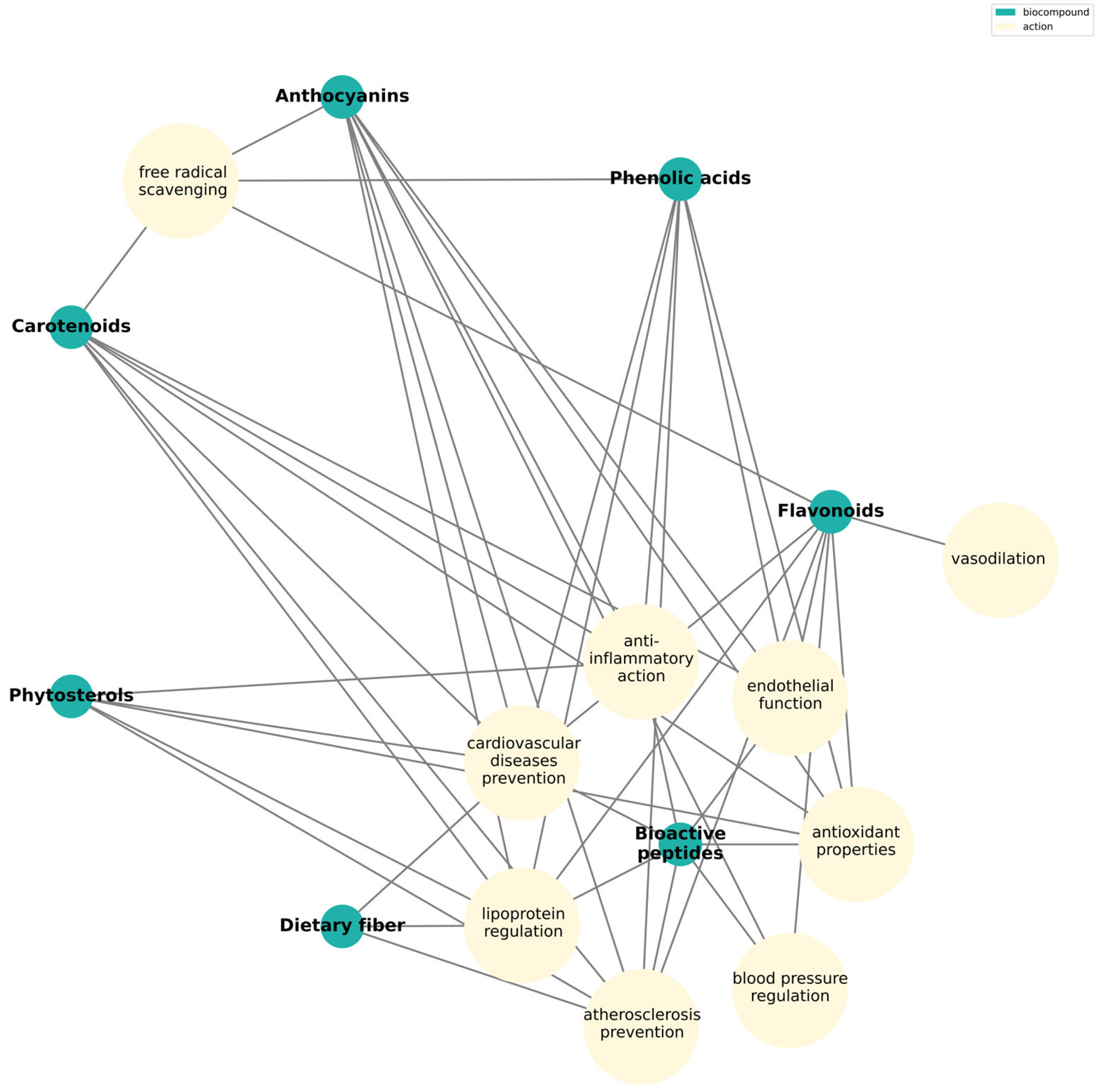
4.2. Fruits as a Source of Variety of Bioactive Compounds
4.2.1. Flavonoids
4.2.2. Phenolic Acids
4.2.3. Anthocyanins
4.2.4. Carotenoids
4.2.5. Phytosterols
4.2.6. Dietary Fiber
4.2.7. Bioactive Peptides
5. Fruit-Based Diets in Cardiovascular Prevention
5.1. The Role of Specific Fruits in the Prevention of Cardiovascular Diseases
5.2. Recommendations Regarding Fruit Intake
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tan, S.C.W.; Zheng, B.-B.; Tang, M.-L.; Chu, H.; Zhao, Y.-T.; Weng, C. Global Burden of Cardiovascular Diseases and Its Risk Factors, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. QJM Int. J. Med. 2025, 118, 411–422. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the Future of Cardiovascular Disease in the United States. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.E.H.; Timpel, P.; Harst, L.; Greaves, C.J.; Ali, M.K.; Lambert, J.; Weber, M.B.; Almedawar, M.M.; Morawietz, H. Blood Sugar Regulation for Cardiovascular Health Promotion and Disease Prevention: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1829–1844. [Google Scholar] [CrossRef] [PubMed]
- Monnard, C.R.; Grasser, E.K. Perspective: Cardiovascular Responses to Sugar-Sweetened Beverages in Humans: A Narrative Review with Potential Hemodynamic Mechanisms. Adv. Nutr. 2018, 9, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, K.M.; Kemps, E.; White, M.J.; Bartlett, S.E. The Impact of Free Sugar on Human Health—A Narrative Review. Nutrients 2023, 15, 889. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. The Role of Sugar-Sweetened Beverages in the Global Epidemics of Obesity and Chronic Diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Blanco-Gandia, M.C.; Montagud-Romero, S.; Rodríguez-Arias, M. Binge Eating and Psychostimulant Addiction. World J. Psychiatry 2021, 11, 517–529. [Google Scholar] [CrossRef]
- Schamarek, I.; Richter, F.C.; Finlayson, G.; Tönjes, A.; Stumvoll, M.; Blüher, M.; Rohde-Zimmermann, K. Association of Salty and Sweet Taste Recognition with Food Reward and Subjective Control of Eating Behavior. Nutrients 2024, 16, 2661. [Google Scholar] [CrossRef]
- Greenberg, D.; St Peter, J.V. Sugars and Sweet Taste: Addictive or Rewarding? Int. J. Environ. Res. Public Health 2021, 18, 9791. [Google Scholar] [CrossRef]
- Appleton, K.M. Liking for Sweet Taste, Sweet Food Intakes, and Sugar Intakes. Nutrients 2024, 16, 3672. [Google Scholar] [CrossRef]
- Calabro, R.; Kemps, E.; Prichard, I. Socio-Cognitive Determinants of Sugar-Sweetened Beverage Consumption Among Young People: A Systematic Review and Meta-Analysis. Appetite 2023, 180, 106334. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Smithers, L.G.; Harford, J.; Merlin, T.; Braunack-Mayer, A. Determinants of Knowledge and Attitudes About Sugar and the Association of Knowledge and Attitudes with Sugar Intake Among Adults: A Systematic Review. Appetite 2018, 126, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D. In Search for an Alternative to Sugar to Reduce Obesity. Int. J. Vitam. Nutr. Res. 2019, 89, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.T.M.; Threapleton, D.E.; Day, A.J.; Williamson, G.; Cade, J.E.; Burley, V.J. Fruit Intake and Cardiovascular Disease Mortality in the UK Women’s Cohort Study. Eur. J. Epidemiol. 2015, 30, 1035–1048. [Google Scholar] [CrossRef]
- Scheffers, F.R.; Boer, J.M.; Wijga, A.H.; van der Schouw, Y.T.; Smit, H.A.; Verschuren, W.M. Substitution of Pure Fruit Juice for Fruit and Sugar-Sweetened Beverages and Cardiometabolic Risk in European Prospective Investigation into Cancer and Nutrition (EPIC)-NL: A Prospective Cohort Study. Public Health Nutr. 2022, 25, 1504–1514. [Google Scholar] [CrossRef]
- Bae, J.H.; Kang, H. Longitudinal Analysis of Sweet Taste Preference Through Genetic and Phenotypic Data Integration. Foods 2024, 13, 3370. [Google Scholar] [CrossRef]
- Kimmeswenger, I.; Lieder, B. Novel Perspective on the Plasticity of Taste Perception: Is Food- and Exercise-Induced Inflammation Associated with Sweet Taste Sensitivity and Preference? J. Agric. Food Chem. 2024, 72, 15122–15127. [Google Scholar] [CrossRef]
- World Health Organization. Use of Non-Sugar Sweeteners: WHO Guideline; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-007361-6. [Google Scholar]
- Prasad, K.; Dhar, I. Oxidative Stress as a Mechanism of Added Sugar-Induced Cardiovascular Disease. Int. J. Angiol. 2014, 23, 217–226. [Google Scholar] [CrossRef]
- Gutierrez, R.; Fonseca, E.; Simon, S.A. The Neuroscience of Sugars in Taste, Gut-Reward, Feeding Circuits, and Obesity. Cell. Mol. Life Sci. 2020, 77, 3469–3502. [Google Scholar] [CrossRef]
- Yoshida, R.; Ninomiya, Y. Mechanisms and Functions of Sweet Reception in Oral and Extraoral Organs. Int. J. Mol. Sci. 2024, 25, 7398. [Google Scholar] [CrossRef]
- Tan, H.-E.; Sisti, A.C.; Jin, H.; Vignovich, M.; Villavicencio, M.; Tsang, K.S.; Goffer, Y.; Zuker, C.S. The Gut–Brain Axis Mediates Sugar Preference. Nature 2020, 580, 511–516. [Google Scholar] [CrossRef]
- Witek, K.; Wydra, K.; Filip, M. A High-Sugar Diet Consumption, Metabolism and Health Impacts with a Focus on the Development of Substance Use Disorder: A Narrative Review. Nutrients 2022, 14, 2940. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Bohórquez, D.V. The Neural Basis of Sugar Preference. Nat. Rev. Neurosci. 2022, 23, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Van Opstal, A.M.; Hafkemeijer, A.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Mulder, T.P.J.; Pijl, H.; Rombouts, S.A.R.B.; van der Grond, J. Brain Activity and Connectivity Changes in Response to Nutritive Natural Sugars, Non-Nutritive Natural Sugar Replacements and Artificial Sweeteners. Nutr. Neurosci. 2021, 24, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Morville, T.; Madsen, K.H.; Siebner, H.R.; Hulme, O.J. Reward Signalling in Brainstem Nuclei Under Fluctuating Blood Glucose. PLoS ONE 2021, 16, e0243899. [Google Scholar] [CrossRef]
- Klenowski, P.M.; Zhao-Shea, R.; Freels, T.G.; Molas, S.; Zinter, M.; M’Angale, P.; Xiao, C.; Martinez-Núñez, L.; Thomson, T.; Tapper, A.R. A Neuronal Coping Mechanism Linking Stress-Induced Anxiety to Motivation for Reward. Sci. Adv. 2023, 9, eadh9620. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; Rossi, V.; Santero, S.; Bianchi, A.; Zuccotti, G. Ultra-Processed Food, Reward System and Childhood Obesity. Children 2023, 10, 804. [Google Scholar] [CrossRef]
- Bijoch, Ł.; Klos, J.; Pawłowska, M.; Wiśniewska, J.; Legutko, D.; Szachowicz, U.; Kaczmarek, L.; Beroun, A. Whole-Brain Tracking of Cocaine and Sugar Rewards Processing. Transl. Psychiatry 2023, 13, 20. [Google Scholar] [CrossRef]
- Wiss, D.A.; Avena, N.; Rada, P. Sugar Addiction: From Evolution to Revolution. Front. Psychiatry 2018, 9, 545. [Google Scholar] [CrossRef]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Short-Term Exposure to a Diet High in Fat and Sugar, or Liquid Sugar, Selectively Impairs Hippocampal-Dependent Memory, with Differential Impacts on Inflammation. Behav. Brain Res. 2016, 306, 1–7. [Google Scholar] [CrossRef]
- Giona, L.; Musillo, C.; De Cristofaro, G.; Ristow, M.; Zarse, K.; Siems, K.; Tait, S.; Cirulli, F.; Berry, A. Western Diet-Induced Cognitive and Metabolic Dysfunctions in Aged Mice Are Prevented by Rosmarinic Acid in a Sex-Dependent Fashion. Clin. Nutr. 2024, 43, 2236–2248. [Google Scholar] [CrossRef]
- Li, J.-M.; Yu, R.; Zhang, L.-P.; Wen, S.-Y.; Wang, S.-J.; Zhang, X.-Y.; Xu, Q.; Kong, L.-D. Dietary Fructose-Induced Gut Dysbiosis Promotes Mouse Hippocampal Neuroinflammation: A Benefit of Short-Chain Fatty Acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef]
- Edwin Thanarajah, S.; DiFeliceantonio, A.G.; Albus, K.; Kuzmanovic, B.; Rigoux, L.; Iglesias, S.; Hanßen, R.; Schlamann, M.; Cornely, O.A.; Brüning, J.C.; et al. Habitual Daily Intake of a Sweet and Fatty Snack Modulates Reward Processing in Humans. Cell Metab. 2023, 35, 571–584.e6. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, B.P. Neuroscience: Dissecting Appetite. Nature 2014, 508, S64–S65. [Google Scholar] [CrossRef] [PubMed]
- Mandelblat-Cerf, Y.; Ramesh, R.N.; Burgess, C.R.; Patella, P.; Yang, Z.; Lowell, B.B.; Andermann, M.L. Arcuate Hypothalamic AgRP and Putative POMC Neurons Show Opposite Changes in Spiking across Multiple Timescales. eLife 2015, 4, e07122. [Google Scholar] [CrossRef]
- Lavielle, P.; Gómez-Díaz, R.A.; Valdez, A.L.; Wacher, N.H. Food Addiction Behavior in Patients with Newly-Diagnosed Type 2 Diabetes. Gac. Médica Méx. 2023, 159, 12113. [Google Scholar] [CrossRef]
- Cattaneo, C.; Mambrini, S.P.; Gilardini, L.; Scacchi, M.; Castelnuovo, G.; Pagliarini, E.; Bertoli, S. The Phenomenon of Abnormal Eating and Taste Perception: What’s the Link in Subjects with Obesity and Eating Disorders? Food Qual. Prefer. 2023, 104, 104744. [Google Scholar] [CrossRef]
- Spetter, M.S.; Smeets, P.A.M.; de Graaf, C.; Viergever, M.A. Representation of Sweet and Salty Taste Intensity in the Brain. Chem. Senses 2010, 35, 831–840. [Google Scholar] [CrossRef]
- Lustig, R.H. Ultraprocessed Food: Addictive, Toxic, and Ready for Regulation. Nutrients 2020, 12, 3401. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-154902-8. [Google Scholar]
- Feingold, K.R. The Effect of Diet on Cardiovascular Disease and Lipid and Lipoprotein Levels. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Stanhope, K.L. Sugar Consumption, Metabolic Disease and Obesity: The State of the Controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; OKeefe, J.H. Added Sugars Drive Coronary Heart Disease via Insulin Resistance and Hyperinsulinaemia: A New Paradigm. Open Heart 2017, 4, e000729. [Google Scholar] [CrossRef]
- Bhargava, S.; de la Puente-Secades, S.; Schurgers, L.; Jankowski, J. Lipids and Lipoproteins in Cardiovascular Diseases: A Classification. Trends Endocrinol. Metab. TEM 2022, 33, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Soppert, J.; Lehrke, M.; Marx, N.; Jankowski, J.; Noels, H. Lipoproteins and Lipids in Cardiovascular Disease: From Mechanistic Insights to Therapeutic Targeting. Adv. Drug Deliv. Rev. 2020, 159, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Huang, Y.; Reilly, K.H.; Li, S.; Zheng, R.; Barrio-Lopez, M.T.; Martinez-Gonzalez, M.A.; Zhou, D. Sugar-Sweetened Beverages and Risk of Hypertension and CVD: A Dose-Response Meta-Analysis. Br. J. Nutr. 2015, 113, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primer 2018, 4, 18014. [Google Scholar] [CrossRef]
- Herman, M.A.; Birnbaum, M.J. Molecular Aspects of Fructose Metabolism and Metabolic Disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef]
- Gherghina, M.-E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress—Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Ribeiro, G.; Oliveira-Maia, A.J. Sweet Taste and Obesity. Eur. J. Intern. Med. 2021, 92, 3–10. [Google Scholar] [CrossRef]
- Sartor, F.; Donaldson, L.F.; Markland, D.A.; Loveday, H.; Jackson, M.J.; Kubis, H.-P. Taste Perception and Implicit Attitude toward Sweet Related to Body Mass Index and Soft Drink Supplementation. Appetite 2011, 57, 237–246. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Mennella, J.A. Habituation to the Pleasure Elicited by Sweetness in Lean and Obese Women. Appetite 2012, 58, 800–805. [Google Scholar] [CrossRef]
- Trius-Soler, M.; Santillán-Alarcón, D.A.; Martínez-Huélamo, M.; Lamuela-Raventós, R.M.; Moreno, J.J. Effect of Physiological Factors, Pathologies, and Acquired Habits on the Sweet Taste Threshold: A Systematic Review and Meta-Analysis. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3755–3773. [Google Scholar] [CrossRef]
- Kotackova, L.; Marecek, R.; Mouraviev, A.; Tang, A.; Brazdil, M.; Cierny, M.; Paus, T.; Pausova, Z.; Mareckova, K. Bariatric Surgery and Its Impact on Depressive Symptoms, Cognition, Brain and Inflammation. Front. Endocrinol. 2023, 14, 1171244. [Google Scholar] [CrossRef]
- Alshwaiyat, N.M.; Ahmad, A.; Al-Jamal, H.A.N. Effect of Diet-Induced Weight Loss on Iron Status and Its Markers Among Young Women with Overweight/Obesity and Iron Deficiency Anemia: A Randomized Controlled Trial. Front. Nutr. 2023, 10, 1155947. [Google Scholar] [CrossRef]
- May, C.E.; Rosander, J.; Gottfried, J.; Dennis, E.; Dus, M. Dietary Sugar Inhibits Satiation by Decreasing the Central Processing of Sweet Taste. eLife 2020, 9, e54530. [Google Scholar] [CrossRef] [PubMed]
- Romaní-Pérez, M.; Líebana-García, R.; Flor-Duro, A.; Bonillo-Jiménez, D.; Bullich-Vilarrubias, C.; Olivares, M.; Sanz, Y. Obesity and the Gut Microbiota: Implications of Neuroendocrine and Immune Signaling. FEBS J. 2025, 292, 1397–1420. [Google Scholar] [CrossRef] [PubMed]
- Swartz, T.D.; Duca, F.A.; de Wouters, T.; Sakar, Y.; Covasa, M. Up-Regulation of Intestinal Type 1 Taste Receptor 3 and Sodium Glucose Luminal Transporter-1 Expression and Increased Sucrose Intake in Mice Lacking Gut Microbiota. Br. J. Nutr. 2012, 107, 621–630. [Google Scholar] [CrossRef]
- Bernard, A.; Ancel, D.; Neyrinck, A.M.; Dastugue, A.; Bindels, L.B.; Delzenne, N.M.; Besnard, P. A Preventive Prebiotic Supplementation Improves the Sweet Taste Perception in Diet-Induced Obese Mice. Nutrients 2019, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Miras, A.D.; le Roux, C.W. Mechanisms Underlying Weight Loss after Bariatric Surgery. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 575–584. [Google Scholar] [CrossRef]
- Dragomir, N.; Grigore, D.-M.; Pogurschi, E.N. Beyond Sugar: A Holistic Review of Sweeteners and Their Role in Modern Nutrition. Foods 2025, 14, 3182. [Google Scholar] [CrossRef]
- Zuraini, N.Z.A.; Sekar, M.; Wu, Y.S.; Gan, S.H.; Bonam, S.R.; Mat Rani, N.N.I.; Begum, M.Y.; Lum, P.T.; Subramaniyan, V.; Fuloria, N.K.; et al. Promising Nutritional Fruits Against Cardiovascular Diseases: An Overview of Experimental Evidence and Understanding Their Mechanisms of Action. Vasc. Health Risk Manag. 2021, 17, 739–769. [Google Scholar] [CrossRef]
- Du, M.; Zhu, Y.; Nan, H.; Zhou, Y.; Pan, X. Regulation of Sugar Metabolism in Fruits. Sci. Hortic. 2024, 326, 112712. [Google Scholar] [CrossRef]
- Wee, M.; Tan, V.; Forde, C. A Comparison of Psychophysical Dose-Response Behaviour Across 16 Sweeteners. Nutrients 2018, 10, 1632. [Google Scholar] [CrossRef]
- Novik, T.S.; Koveshnikova, E.I.; Kotlobay, A.A.; Sycheva, L.P.; Kurochkina, K.G.; Averina, O.A.; Belopolskaya, M.V.; Sergiev, P.V.; Dontsova, O.A.; Lazarev, V.N.; et al. Sweet-Tasting Natural Proteins Brazzein and Monellin: Safe Sugar Substitutes for the Food Industry. Foods 2023, 12, 4065. [Google Scholar] [CrossRef]
- Gnanavel, M.; Serva Peddha, M. Identification of Novel Sweet Protein for Nutritional Applications. Bioinformation 2011, 7, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, S.S. Structure-Dependent Activity of Plant-Derived Sweeteners. Molecules 2020, 25, 1946. [Google Scholar] [CrossRef] [PubMed]
- Peteliuk, V.; Rybchuk, L.; Bayliak, M.; Storey, K.B.; Lushchak, O. Natural Sweetener Stevia Rebaudiana: Functionalities, Health Benefits and Potential Risks. EXCLI J. 2021, 20, 1412–1430. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.; Mohamed Elfadil, O.; Patel, S.; Ghanem, O.M.; Hurt, R.T.; Mundi, M.S. Rediscovering Sweetness: The Evolution and Impact of Non-Nutritive and Natural Sweeteners. Curr. Nutr. Rep. 2025, 14, 54. [Google Scholar] [CrossRef]
- Freeman, E.L.; Ward, R.; Murphy, M.M.; Wang, T.; Ryder, J. Comprehensive Safety Assessment of Serendipity Berry Sweet Protein Produced from Komagataella Phaffii. Regul. Toxicol. Pharmacol. 2024, 147, 105562. [Google Scholar] [CrossRef]
- Saraiva, A.; Carrascosa, C.; Ramos, F.; Raheem, D.; Pedreiro, S.; Vega, A.; Raposo, A. Brazzein and Monellin: Chemical Analysis, Food Industry Applications, Safety and Quality Control, Nutritional Profile and Health Impacts. Foods 2023, 12, 1943. [Google Scholar] [CrossRef]
- Cancelliere, R.; Leone, S.; Gatto, C.; Mazzoli, A.; Ercole, C.; Iossa, S.; Liverini, G.; Picone, D.; Crescenzo, R. Metabolic Effects of the Sweet Protein MNEI as a Sweetener in Drinking Water. A Pilot Study of a High Fat Dietary Regimen in a Rodent Model. Nutrients 2019, 11, 2643. [Google Scholar] [CrossRef]
- Chung, J.-H.; Kong, J.-N.; Choi, H.-E.; Kong, K.-H. Antioxidant, Anti-Inflammatory, and Anti-Allergic Activities of the Sweet-Tasting Protein Brazzein. Food Chem. 2018, 267, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ji, L.; Xu, S.; Zhang, Y.; Iqbal, H.M.N.; Cheng, H. Bioprospecting and Biotechnological Insights into Sweet-Tasting Proteins by Microbial Hosts—A Review. Bioengineered 2022, 13, 9816–9829. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Mercado, A.I.; López-Plaza, B.; Plaza-Diaz, J.; Arcos-Castellanos, L.; Ruiz-Ojeda, F.J.; Brandimonte-Hernández, M.; Feliú-Batlle, J.; Hummel, T.; Palma-Milla, S.; Gil, Á. Miraculin Can Contribute to a Reduction in Inflammatory Biomarkers and Cachexia in Malnourished Patients with Cancer and Taste Disorders. Pharmaceuticals 2025, 18, 622. [Google Scholar] [CrossRef] [PubMed]
- Shivani; Thakur, B.K.; Mallikarjun, C.P.; Mahajan, M.; Kapoor, P.; Malhotra, J.; Dhiman, R.; Kumar, D.; Pal, P.K.; Kumar, S. Introduction, Adaptation and Characterization of Monk Fruit (Siraitia grosvenorii): A Non-Caloric New Natural Sweetener. Sci. Rep. 2021, 11, 6205. [Google Scholar] [CrossRef]
- Xiao, R.; Liao, W.; Luo, G.; Qin, Z.; Han, S.; Lin, Y. Modulation of Gut Microbiota Composition and Short-Chain Fatty Acid Synthesis by Mogroside V in an In Vitro Incubation System. ACS Omega 2021, 6, 25486–25496. [Google Scholar] [CrossRef]
- Qin, T.; Li, Y.; Wu, Y.; Meng, F.; Lin, G.; Xia, X. Mogroside Alleviates Diabetes Mellitus and Modulates Intestinal Microflora in Type 2 Diabetic Mice. Biol. Pharm. Bull. 2024, 47, 1043–1053. [Google Scholar] [CrossRef]
- Wang, S.; Cui, K.; Liu, J.; Hu, J.; Yan, K.; Xiao, P.; Lu, Y.; Yang, X.; Liang, X. Mogroside-Rich Extract from Siraitia grosvenorii Fruits Ameliorates High-Fat Diet-Induced Obesity Associated with the Modulation of Gut Microbiota in Mice. Front. Nutr. 2022, 9, 870394. [Google Scholar] [CrossRef]
- Turner, S.; Diako, C.; Kruger, R.; Wong, M.; Wood, W.; Rutherfurd-Markwick, K.; Ali, A. Consuming Gymnema sylvestre Reduces the Desire for High-Sugar Sweet Foods. Nutrients 2020, 12, 1046. [Google Scholar] [CrossRef]
- Jeytawan, N.; Yadoung, S.; Jeeno, P.; Yana, P.; Sutan, K.; Naksen, W.; Wongkaew, M.; Sommano, S.R.; Hongsibsong, S. Antioxidant and Phytochemical Potential of and Phytochemicals in Gymnema inodorum (Lour.) Decne in Northern Thailand. Plants 2022, 11, 3498. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and Pharmacological Properties of Gymnema sylvestre: An Important Medicinal Plant. BioMed Res. Int. 2014, 2014, 830285. [Google Scholar] [CrossRef]
- Kanetkar, P.; Singhal, R.; Kamat, M. Gymnema sylvestre: A Memoir. J. Clin. Biochem. Nutr. 2007, 41, 77–81. [Google Scholar] [CrossRef]
- Weerasinghe, D.M.K.P.; Brough, L.; Everett, D.W.; Rashidinejad, A. Gymnema lactiferum: A Review of Its Traditional Applications, Phytochemical Constituents, and Biological Properties. Food Sci. Nutr. 2024, 12, 8742–8754. [Google Scholar] [CrossRef] [PubMed]
- Saiki, P.; Kawano, Y.; Ogi, T.; Klungsupya, P.; Muangman, T.; Phantanaprates, W.; Kongchinda, P.; Pinnak, N.; Miyazaki, K. Purified Gymnemic Acids from Gymnema inodorum Tea Inhibit 3T3-L1 Cell Differentiation into Adipocytes. Nutrients 2020, 12, 2851. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Diako, C.; Kruger, R.; Wong, M.; Wood, W.; Rutherfurd-Markwick, K.; Stice, E.; Ali, A. The Effect of a 14-Day Gymnema sylvestre Intervention to Reduce Sugar Cravings in Adults. Nutrients 2022, 14, 5287. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Yokum, S. Effects of Gymnemic Acids Lozenge on Reward Region Response to Receipt and Anticipated Receipt of High-Sugar Food. Physiol. Behav. 2018, 194, 568–576. [Google Scholar] [CrossRef]
- Jangam, A.; Tirunavalli, S.K.; Adimoolam, B.M.; Kasireddy, B.; Patnaik, S.S.; Erukkambattu, J.; Thota, J.R.; Andugulapati, S.B.; Addlagatta, A. Anti-Inflammatory and Antioxidant Activities of Gymnema sylvestre Extract Rescue Acute Respiratory Distress Syndrome in Rats via Modulating the NF-κB/MAPK Pathway. Inflammopharmacology 2023, 31, 823–844. [Google Scholar] [CrossRef]
- Rachh, P.R.; Rachh, M.R.; Ghadiya, N.R.; Modi, D.C.; Modi, K.P.; Patel, N.M.; Rupareliya, M.T. Antihyperlipidemic Activity of Gymenma Sylvestre R. Br. Leaf Extract on Rats Fed with High Cholesterol Diet. Int. J. Pharmacol. 2010, 6, 138–141. [Google Scholar] [CrossRef]
- World Health Organization. Prevention of Cardiovascular Disease: Guidelines for Assessment and Management of Total Cardiovascular Risk; World Health Organization: Geneva, Switzerland, 2007; ISBN 978-92-4-154717-8. [Google Scholar]
- Alissa, E.M.; Ferns, G.A. Dietary Fruits and Vegetables and Cardiovascular Diseases Risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef]
- Billowria, K.; Ali, R.; Rangra, N.K.; Kumar, R.; Chawla, P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Crit. Rev. Anal. Chem. 2024, 54, 1002–1016. [Google Scholar] [CrossRef]
- Li, C.; Dai, T.; Chen, J.; Chen, M.; Liang, R.; Liu, C.; Du, L.; McClements, D.J. Modification of Flavonoids: Methods and Influences on Biological Activities. Crit. Rev. Food Sci. Nutr. 2023, 63, 10637–10658. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and Their Role in Oxidative Stress, Inflammation, and Human Diseases. Chem.-Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef]
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent Research on Flavonoids and Their Biomedical Applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar] [CrossRef]
- Hejazi, J.; Ghanavati, M.; Hejazi, E.; Poustchi, H.; Sepanlou, S.G.; Khoshnia, M.; Gharavi, A.; Sohrabpour, A.A.; Sotoudeh, M.; Dawsey, S.M.; et al. Habitual Dietary Intake of Flavonoids and All-Cause and Cause-Specific Mortality: Golestan Cohort Study. Nutr. J. 2020, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.P.; Dalgaard, F.; Kyrø, C.; Murray, K.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; Gislason, G.; Scalbert, A.; Cassidy, A.; et al. Flavonoid Intake Is Associated with Lower Mortality in the Danish Diet Cancer and Health Cohort. Nat. Commun. 2019, 10, 3651. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid Intake and Risk of CVD: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Sullivan, T.R.; Fakler, P.; Frank, O.R.; Stocks, N.P. Effect of Cocoa on Blood Pressure. Cochrane Database Syst. Rev. 2017, 4, CD008893. [Google Scholar] [CrossRef]
- García-Pérez, P.; Gallego, P.P. Plant Phenolics as Dietary Antioxidants: Insights on Their Biosynthesis, Sources, Health-Promoting Effects, Sustainable Production, and Effects on Lipid Oxidation. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 405–426. ISBN 978-3-030-87222-9. [Google Scholar]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. ISBN 978-0-12-814774-0. [Google Scholar]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.-J.; Tomas, M.; et al. Functional Implications of Bound Phenolic Compounds and Phenolics-Food Interaction: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Kukavica, B. Phenolic Compounds as Antidiabetic, Anti-Inflammatory, and Anticancer Agents and Improvement of Their Bioavailability by Liposomes. Cell Biochem. Funct. 2021, 39, 926–944. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as Anti-Inflammatory Agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Leifert, W.R.; Abeywardena, M.Y. Cardioprotective Actions of Grape Polyphenols. Nutr. Res. 2008, 28, 729–737. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Escobedo-Avellaneda, Z.; Welti-Chanes, J. Phenolic Compounds in Mesoamerican Fruits-Characterization, Health Potential and Processing with Innovative Technologies. Int. J. Mol. Sci. 2020, 21, 8357. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, X.; Lin, X.; Mostafa, S.; Zou, H.; Wang, L.; Jin, B. Plant Anthocyanins: Classification, Biosynthesis, Regulation, Bioactivity, and Health Benefits. Plant Physiol. Biochem. PPB 2024, 217, 109268. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Activity of Anthocyanins and Anthocyanidins: A Critical Review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Song, L.; Yang, Z.; Qiu, M.; Wang, J.; Shi, S. Anthocyanins: Promising Natural Products with Diverse Pharmacological Activities. Molecules 2021, 26, 3807. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Huang, Y.; Evans, P.C.; Little, P.J.; Tian, X.; Weng, J.; Xu, S. Anthocyanins in Vascular Health and Disease: Mechanisms of Action and Therapeutic Potential. J. Cardiovasc. Pharmacol. 2024, 84, 289–302. [Google Scholar] [CrossRef]
- Carvalho, F.; Lahlou, R.A.; Silva, L.R. Phenolic Compounds from Cherries and Berries for Chronic Disease Management and Cardiovascular Risk Reduction. Nutrients 2024, 16, 1597. [Google Scholar] [CrossRef]
- Xin, M.; Xu, A.; Tian, J.; Wang, L.; He, Y.; Jiang, H.; Yang, B.; Li, B.; Sun, Y. Anthocyanins as Natural Bioactives with Anti-Hypertensive and Atherosclerotic Potential: Health Benefits and Recent Advances. Phytomedicine 2024, 132, 155889. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits-A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Młynarska, E.; Hajdys, J.; Czarnik, W.; Fularski, P.; Leszto, K.; Majchrowicz, G.; Lisińska, W.; Rysz, J.; Franczyk, B. The Role of Antioxidants in the Therapy of Cardiovascular Diseases-A Literature Review. Nutrients 2024, 16, 2587. [Google Scholar] [CrossRef] [PubMed]
- Sumalla-Cano, S.; Eguren-García, I.; Lasarte-García, Á.; Prola, T.A.; Martínez-Díaz, R.; Elío, I. Carotenoids Intake and Cardiovascular Prevention: A Systematic Review. Nutrients 2024, 16, 3859. [Google Scholar] [CrossRef]
- Terao, J. Revisiting Carotenoids as Dietary Antioxidants for Human Health and Disease Prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Q.; Zhu, J.; Miao, L.-J.; Lv, S.-A.; Shen, G.-Y.; Wu, J. Associations of Serum Carotenoids with Cardiovascular Disease Mortality among Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease. Hepatol. Res. 2025, 55, 1111–1127. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- El Omari, N.; Bakrim, S.; Khalid, A.; Abdalla, A.N.; Iesa, M.A.M.; El Kadri, K.; Tang, S.Y.; Goh, B.H.; Bouyahya, A. Unveiling the Molecular Mechanisms: Dietary Phytosterols as Guardians against Cardiovascular Diseases. Nat. Prod. Bioprospecting 2024, 14, 27. [Google Scholar] [CrossRef]
- Arshad, M.T.; Ali, M.K.M.; Maqsood, S.; Ikram, A.; Hossain, M.S.; Aljameel, A.I.; Al-Farga, A.; Gnedeka, K.T. Dietary Phytochemicals in Cardiovascular Disease Prevention and Management: A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70872. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A. Functional Foods for Cholesterol Management: A Review of the Mechanisms, Efficacy, and a Novel Cholesterol-Lowering Capacity Index. Nutrients 2025, 17, 2648. [Google Scholar] [CrossRef]
- Qiao, W.; Feng, H.; Zhang, Y.-F.; Zhang, Z.; Yang, J.; Wu, M.; Xie, J.; Huang, J.; Zhou, T.; Zhang, Y. Protective Association between Dietary Phytosterol Intake and Cardiovascular Health: An Analysis of the UK Biobank Cohort. Food Funct. 2025, 16, 1157–1168. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Cao, S.; Liu, C.; Han, Y.; Cheng, J.; Zhang, S.; Zhao, J.; Shi, Y. Let Food Be Your Medicine—Dietary Fiber. Food Funct. 2024, 15, 7733–7756. [Google Scholar] [CrossRef]
- Deehan, E.C.; Mocanu, V.; Madsen, K.L. Effects of Dietary Fibre on Metabolic Health and Obesity. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 301–318. [Google Scholar] [CrossRef]
- Arayici, M.E.; Basbinar, Y.; Ellidokuz, H. High and Low Dietary Fiber Consumption and Cancer Risk: A Comprehensive Umbrella Review with Meta-Meta-Analysis Involving Meta-Analyses of Observational Epidemiological Studies. Crit. Rev. Food Sci. Nutr. 2025, 65, 1617–1630. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Yang, Q.; Guo, J.; Zhou, S.; Zhong, T.; Xiao, Y.; Yu, X.; Feng, K.; Peng, Y.; et al. The Impact of Dietary Fiber on Cardiovascular Diseases: A Scoping Review. Nutrients 2025, 17, 444. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Gianfredi, V.; Solmi, M.; Barbagallo, M.; Dominguez, L.J.; Mandalà, C.; Di Palermo, C.; Carruba, L.; Solimando, L.; Stubbs, B.; et al. The Impact of Dietary Fiber Consumption on Human Health: An Umbrella Review of Evidence from 17,155,277 Individuals. Clin. Nutr. 2025, 51, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, M.E.; Zurbau, A.; Glenn, A.J.; Oguntala, J.O.; Josse, R.G.; Malik, V.S.; Chiavaroli, L.; Liu, S.; Kendall, C.W.C.; Jenkins, D.J.A.; et al. The Portfolio Dietary Pattern and Risk of Cardiovascular Disease Mortality during 1988-2019 in US Adults: A Prospective Cohort Study. BMC Med. 2025, 23, 287. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vázquez, A. Bioactive Peptides: A Review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Nong, N.T.P.; Hsu, J.-L. Characteristics of Food Protein-Derived Antidiabetic Bioactive Peptides: A Literature Update. Int. J. Mol. Sci. 2021, 22, 9508. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.-B.; Shim, J.-H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides from Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Erdmann, K.; Cheung, B.W.Y.; Schröder, H. The Possible Roles of Food-Derived Bioactive Peptides in Reducing the Risk of Cardiovascular Disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar] [CrossRef]
- Sosalagere, C.; Adesegun Kehinde, B.; Sharma, P. Isolation and Functionalities of Bioactive Peptides from Fruits and Vegetables: A Reviews. Food Chem. 2022, 366, 130494. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bollati, C.; d’Adduzio, L.; Fanzaga, M.; Cruz-Chamorro, I.; Arnoldi, A.; Sirtori, C.R.; Lammi, C. Food-Derived Peptides with Hypocholesterolemic Activity: Production, Transepithelial Transport and Cellular Mechanisms. Trends Food Sci. Technol. 2024, 143, 104279. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.-Y.; Li, H.-B. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Li, L.; Bennett, D.; Guo, Y.; Key, T.J.; Bian, Z.; Sherliker, P.; Gao, H.; Chen, Y.; Yang, L.; et al. Fresh Fruit Consumption and Major Cardiovascular Disease in China. N. Engl. J. Med. 2016, 374, 1332–1343. [Google Scholar] [CrossRef]
- Chan, H.-T.; Yiu, K.-H.; Wong, C.-Y.; Li, S.-W.; Tam, S.; Tse, H.-F. Increased Dietary Fruit Intake Was Associated with Lower Burden of Carotid Atherosclerosis in Chinese Patients with Type 2 Diabetes Mellitus. Diabet. Med. J. 2013, 30, 100–108. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Blekkenhorst, L.C.; Considine, M.J.; Maghzal, G.; Stocker, R.; Woodman, R.J.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Flavonoid-Rich Apple Improves Endothelial Function in Individuals at Risk for Cardiovascular Disease: A Randomized Controlled Clinical Trial. Mol. Nutr. Food Res. 2018, 62, 1700674. [Google Scholar] [CrossRef]
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Jensen, R.I.; Németh-Balogh, M.; Paulovicsová, B.; Bergström, A.; Wilcks, A.; Licht, T.R.; et al. Intake of Whole Apples or Clear Apple Juice Has Contrasting Effects on Plasma Lipids in Healthy Volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef]
- Serra, A.T.; Rocha, J.; Sepodes, B.; Matias, A.A.; Feliciano, R.P.; de Carvalho, A.; Bronze, M.R.; Duarte, C.M.M.; Figueira, M.E. Evaluation of Cardiovascular Protective Effect of Different Apple Varieties—Correlation of Response with Composition. Food Chem. 2012, 135, 2378–2386. [Google Scholar] [CrossRef]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Calderón-Pérez, L.; Companys, J.; Pla-Pagà, L.; Ludwig, I.A.; Romero, M.P.; Solà, R. The Effects and Associations of Whole-Apple Intake on Diverse Cardiovascular Risk Factors. A Narrative Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Donoso, W.; Sandoval, N.; Reyes, M.; Gonzalez, P.; Gajardo, M.; Morales, E.; Neira, A.; Razmilic, I.; Yuri, J.A.; et al. Apple Peel Supplemented Diet Reduces Parameters of Metabolic Syndrome and Atherogenic Progression in ApoE-/- Mice. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 918384. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.F.; Chaves, M.A.; Borges, C.D.; Mendonça, C.R.B. Avocado: Characteristics, Health Benefits and Uses. Ciênc. Rural 2016, 46, 747–754. [Google Scholar] [CrossRef]
- Mahmassani, H.A.; Avendano, E.E.; Raman, G.; Johnson, E.J. Avocado Consumption and Risk Factors for Heart Disease: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 107, 523–536. [Google Scholar] [CrossRef]
- Villa-Rodríguez, J.A.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Effect of Maturity Stage on the Content of Fatty Acids and Antioxidant Activity of ‘Hass’ Avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Burton-Freeman, B. Avocado Fruit on Postprandial Markers of Cardio-Metabolic Risk: A Randomized Controlled Dose Response Trial in Overweight and Obese Men and Women. Nutrients 2018, 10, E1287. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, D.G.; Flores-García, M.; Silva-Platas, C.; Rizzo, S.; Torre-Amione, G.; De la Peña-Diaz, A.; Hernández-Brenes, C.; García-Rivas, G. Isolation and Chemical Identification of Lipid Derivatives from Avocado (Persea americana) Pulp with Antiplatelet and Antithrombotic Activities. Food Funct. 2015, 6, 193–203. [Google Scholar] [CrossRef]
- Carvajal-Zarrabal, O.; Nolasco-Hipolito, C.; Aguilar-Uscanga, M.G.; Melo-Santiesteban, G.; Hayward-Jones, P.M.; Barradas-Dermitz, D.M. Avocado Oil Supplementation Modifies Cardiovascular Risk Profile Markers in a Rat Model of Sucrose-Induced Metabolic Changes. Dis. Markers 2014, 2014, 386425. [Google Scholar] [CrossRef]
- Gouegni, E.F.; Abubakar, H. Phytochemical, Toxicological, Biochemical and Haematological Studies on Avocado (Persea americana) in Experimental Animals. Niger. Food J. 2013, 31, 64–69. [Google Scholar] [CrossRef]
- Luzak, B.; Kosiorek, A.; Syska, K.; Rozalski, M.; Bijak, M.; Podsedek, A.; Balcerczak, E.; Watala, C.; Golanski, J. Does Grape Seed Extract Potentiate the Inhibition of Platelet Reactivity in the Presence of Endothelial Cells? Adv. Med. Sci. 2014, 59, 178–182. [Google Scholar] [CrossRef]
- Shanmuganayagam, D.; Beahm, M.R.; Kuhns, M.A.; Krueger, C.G.; Reed, J.D.; Folts, J.D. Differential Effects of Grape (Vitis vinifera) Skin Polyphenolics on Human Platelet Aggregation and Low-Density Lipoprotein Oxidation. J. Agric. Food Chem. 2012, 60, 5787–5794. [Google Scholar] [CrossRef]
- Al-Jarallah, A.; Igdoura, F.; Zhang, Y.; Tenedero, C.B.; White, E.J.; MacDonald, M.E.; Igdoura, S.A.; Trigatti, B.L. The Effect of Pomegranate Extract on Coronary Artery Atherosclerosis in SR-BI/APOE Double Knockout Mice. Atherosclerosis 2013, 228, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yan, C.; Frost, B.; Wang, X.; Hou, C.; Zeng, M.; Gao, H.; Kang, Y.; Liu, J. Pomegranate Extract Decreases Oxidative Stress and Alleviates Mitochondrial Impairment by Activating AMPK-Nrf2 in Hypothalamic Paraventricular Nucleus of Spontaneously Hypertensive Rats. Sci. Rep. 2016, 6, 34246. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Waghulde, H.; Kasture, S. Effect of Pomegranate Juice on Angiotensin II-Induced Hypertension in Diabetic Wistar Rats. Phytother. Res. PTR 2010, 24 (Suppl. 2), S196–S203. [Google Scholar] [CrossRef] [PubMed]
- Haghighian, M.K.; Rafraf, M.; Moghaddam, A.; Hemmati, S.; Jafarabadi, M.A.; Gargari, B.P. Pomegranate (Punica granatum L.) Peel Hydro Alcoholic Extract Ameliorates Cardiovascular Risk Factors in Obese Women with Dyslipidemia: A Double Blind, Randomized, Placebo Controlled Pilot Study. Eur. J. Integr. Med. 2016, 8, 676–682. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Ishisaka, A.; Mawatari, K.; Vidal-Diez, A.; Spencer, J.P.E.; Terao, J. Blueberry Intervention Improves Vascular Reactivity and Lowers Blood Pressure in High-Fat-, High-Cholesterol-Fed Rats. Br. J. Nutr. 2013, 109, 1746–1754. [Google Scholar] [CrossRef]
- Castello, F.; Fernández-Pachón, M.-S.; Cerrillo, I.; Escudero-López, B.; Ortega, Á.; Rosi, A.; Bresciani, L.; Del Rio, D.; Mena, P. Absorption, Metabolism, and Excretion of Orange Juice (Poly)Phenols in Humans: The Effect of a Controlled Alcoholic Fermentation. Arch. Biochem. Biophys. 2020, 695, 108627. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G. Effect of Pineapple, Orange and Watermelon Juices on Phosphodiesterase, Monoamine Oxidase and Angiotensin-I Converting Enzyme Activities in Rat Heart and Brain Homogenates. Orient. Pharm. Exp. Med. 2017, 17, 269–276. [Google Scholar] [CrossRef]
- Duttaroy, A.K.; Jørgensen, A. Effects of Kiwi Fruit Consumption on Platelet Aggregation and Plasma Lipids in Healthy Human Volunteers. Platelets 2004, 15, 287–292. [Google Scholar] [CrossRef]
- Sims, I.M.; Monro, J.A. Fiber: Composition, Structures, and Functional Properties. Adv. Food Nutr. Res. 2013, 68, 81–99. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries Improve Endothelial Function, but Not Blood Pressure, in Adults with Metabolic Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and Time Dependence of Blueberry Flavonoid-Induced Improvements in Vascular Function: A Randomized, Controlled, Double-Blind, Crossover Intervention Study with Mechanistic Insights into Biological Activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Arráez-Román, D.; Segura-Carretero, A. Antiplatelet Activity of Natural Bioactive Extracts from Mango (Mangifera indica L.) and Its By-Products. Antioxidants 2019, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Laurindo, L.F.; Machado, N.M.; Duarte, L.G.; Guiguer, E.L.; Araujo, A.C.; Dias, J.A.; Lamas, C.B.; Nunes, Y.C.; Bechara, M.D.; et al. Mangifera indica L., By-Products, and Mangiferin on Cardio-Metabolic and Other Health Conditions: A Systematic Review. Life 2023, 13, 2270. [Google Scholar] [CrossRef]
- Castro, R.J.; Pedroza, K.; Hong, M.Y. The Effects of Mango Consumption on Vascular Health and Immune Function. Metab. Open 2023, 20, 100260. [Google Scholar] [CrossRef]
- Lucas, E.A.; Li, W.; Peterson, S.K.; Brown, A.; Kuvibidila, S.; Perkins-Veazie, P.; Clarke, S.L.; Smith, B.J. Mango Modulates Body Fat and Plasma Glucose and Lipids in Mice Fed a High-Fat Diet. Br. J. Nutr. 2011, 106, 1495–1505. [Google Scholar] [CrossRef]
- Razavi, S.-M.; Gholamin, S.; Eskandari, A.; Mohsenian, N.; Ghorbanihaghjo, A.; Delazar, A.; Rashtchizadeh, N.; Keshtkar-Jahromi, M.; Argani, H. Red Grape Seed Extract Improves Lipid Profiles and Decreases Oxidized Low-Density Lipoprotein in Patients with Mild Hyperlipidemia. J. Med. Food 2013, 16, 255–258. [Google Scholar] [CrossRef]
- Borde, P.; Mohan, M.; Kasture, S. Effect of Myricetin on Deoxycorticosterone Acetate (DOCA)-Salt-Hypertensive Rats. Nat. Prod. Res. 2011, 25, 1549–1559. [Google Scholar] [CrossRef]
- Freedman, J.E.; Parker, C.; Li, L.; Perlman, J.A.; Frei, B.; Ivanov, V.; Deak, L.R.; Iafrati, M.D.; Folts, J.D. Select Flavonoids and Whole Juice from Purple Grapes Inhibit Platelet Function and Enhance Nitric Oxide Release. Circulation 2001, 103, 2792–2798. [Google Scholar] [CrossRef]
- Quintieri, A.M.; Baldino, N.; Filice, E.; Seta, L.; Vitetti, A.; Tota, B.; De Cindio, B.; Cerra, M.C.; Angelone, T. Malvidin, a Red Wine Polyphenol, Modulates Mammalian Myocardial and Coronary Performance and Protects the Heart against Ischemia/Reperfusion Injury. J. Nutr. Biochem. 2013, 24, 1221–1231. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Betts, N.M.; Nguyen, A.; Newman, E.D.; Fu, D.; Lyons, T.J. Freeze-Dried Strawberries Lower Serum Cholesterol and Lipid Peroxidation in Adults with Abdominal Adiposity and Elevated Serum Lipids. J. Nutr. 2014, 144, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Koutsos, A.; Riccadonna, S.; Ulaszewska, M.M.; Franceschi, P.; Trošt, K.; Galvin, A.; Braune, T.; Fava, F.; Perenzoni, D.; Mattivi, F.; et al. Two Apples a Day Lower Serum Cholesterol and Improve Cardiometabolic Biomarkers in Mildly Hypercholesterolemic Adults: A Randomized, Controlled, Crossover Trial. Am. J. Clin. Nutr. 2020, 111, 307–318. [Google Scholar] [CrossRef]
- Soleti, R.; Trenteseaux, C.; Fizanne, L.; Coué, M.; Hilairet, G.; Kasbi-Chadli, F.; Mallegol, P.; Chaigneau, J.; Boursier, J.; Krempf, M.; et al. Apple Supplementation Improves Hemodynamic Parameter and Attenuates Atherosclerosis in High-Fat Diet-Fed Apolipoprotein E-Knockout Mice. Biomedicines 2020, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Liddle, D.M.; Lin, X.; Cox, L.C.; Ward, E.M.; Ansari, R.; Wright, A.J.; Robinson, L.E. Daily Apple Consumption Reduces Plasma and Peripheral Blood Mononuclear Cell-Secreted Inflammatory Biomarkers in Adults with Overweight and Obesity: A 6-Week Randomized, Controlled, Parallel-Arm Trial. Am. J. Clin. Nutr. 2021, 114, 752–763. [Google Scholar] [CrossRef]
- Olas, B. The Pulp, Peel, Seed, and Food Products of Persea americana as Sources of Bioactive Phytochemicals with Cardioprotective Properties: A Review. Int. J. Mol. Sci. 2024, 25, 13622. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, W.; Li, J.; Du, L.; Lv, O.; Zhao, S.; Li, J. Beneficial Effects of Pomegranate on Lipid Metabolism in Metabolic Disorders. Mol. Nutr. Food Res. 2019, 63, e1800773. [Google Scholar] [CrossRef]
- Asgary, S.; Keshvari, M. Effects of Citrus Sinensis Juice on Blood Pressure. ARYA Atheroscler. 2013, 9, 98–101. [Google Scholar]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef]
- Iwasawa, H.; Morita, E.; Yui, S.; Yamazaki, M. Anti-Oxidant Effects of Kiwi Fruit in Vitro and in Vivo. Biol. Pharm. Bull. 2011, 34, 128–134. [Google Scholar] [CrossRef]
- Karlsen, A.; Svendsen, M.; Seljeflot, I.; Laake, P.; Duttaroy, A.K.; Drevon, C.A.; Arnesen, H.; Tonstad, S.; Blomhoff, R. Kiwifruit Decreases Blood Pressure and Whole-Blood Platelet Aggregation in Male Smokers. J. Hum. Hypertens. 2013, 27, 126–130. [Google Scholar] [CrossRef]
- Stonehouse, W.; Gammon, C.S.; Beck, K.L.; Conlon, C.A.; von Hurst, P.R.; Kruger, R. Kiwifruit: Our Daily Prescription for Health. Can. J. Physiol. Pharmacol. 2013, 91, 442–447. [Google Scholar] [CrossRef]
- American Heart Association. The American Heart Association Diet and Lifestyle Recommendations. 13 June 2024. Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/aha-diet-and-lifestyle-recommendations (accessed on 22 August 2025).
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, ACT, Australia, 2013.
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Government Publishing Office: Washington, DC, USA, 2020.
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- European Commission. Food-Based Dietary Guidelines Recommendations for Fruit and Vegetables. Health Promotion Knowledge Gateway. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/food-based-dietary-guidelines-europe-table-3_en (accessed on 22 August 2025).
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality-a Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A Comparative Risk Assessment of Burden of Disease and Injury Attributable to 67 Risk Factors and Risk Factor Clusters in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Adults Meeting Fruit and Vegetable Intake Recommendations—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Devirgiliis, C.; Guberti, E.; Mistura, L.; Raffo, A. Effect of Fruit and Vegetable Consumption on Human Health: An Update of the Literature. Foods 2024, 13, 3149. [Google Scholar] [CrossRef]
- Bui, T.V.; Blizzard, C.L.; Luong, K.N.; Truong, N.L.V.; Tran, B.Q.; Otahal, P.; Srikanth, V.; Nelson, M.R.; Au, T.B.; Ha, S.T.; et al. Fruit and Vegetable Consumption in Vietnam, and the Use of a “standard Serving” Size to Measure Intake. Br. J. Nutr. 2016, 116, 149–157. [Google Scholar] [CrossRef]
- Kanungsukkasem, U.; Ng, N.; Van Minh, H.; Razzaque, A.; Ashraf, A.; Juvekar, S.; Masud Ahmed, S.; Huu Bich, T. Fruit and Vegetable Consumption in Rural Adults Population in INDEPTH HDSS Sites in Asia. Glob. Health Action 2009, 2, 1988. [Google Scholar] [CrossRef]
- Nomura, M.; Yamaguchi, M.; Inada, Y.; Nishi, N. Current Dietary Intake of the Japanese Population in Reference to the Planetary Health Diet-Preliminary Assessment. Front. Nutr. 2023, 10, 1116105. [Google Scholar] [CrossRef]
- World Health Organization. Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2014; Available online: https://www.who.int/tools/elena/bbc/fruit-vegetables-ncds (accessed on 22 August 2025).
- Renzella, J.; Townsend, N.; Jewell, J.; Breda, J.; Roberts, N.; Rayner, M.; Wickramasinghe, K. What National and Subnational Interventions and Policies Based on Mediterranean and Nordic Diets are Recommended or Implemented in the WHO European Region, and Is There Evidence of Effectiveness in Reducing Noncommunicable Diseases? WHO Health Evidence Network Synthesis Reports; WHO Regional Office for Europe: Copenhagen, Denmark, 2018; ISBN 978-92-890-5301-3. [Google Scholar]

| Fruit | Compounds | CV Activity | References |
|---|---|---|---|
| Blueberry (Vaccinium corymbosum) | Anthocyanins; Flavonoids; Phenolic acids; Vitamin C, B complex, E, A; Carotenoids. | ↑ Antioxidant capacity; ↓ Inflammation; Improved endothelial function—flow-mediated dilation. | Stull et al. [167]; Rodrigues-Mateos et al. [168]; Zuraini et al. [63] |
| Mango (Mangifera indica) | Vitamin C; Carotenoids; Polyphenols (maginferin); Flavonoids; Anthocyanins; Tannins; Phenolic acids; Coumarin. | Antioxidant; Anitinflammatory; Antiplatlet activity; Hypodipidemic: ↓ triglycerides, ↓ LDL ↓ TC, ↑ HDL; Enhanced endothelial function. | Alañón et al. [169]; Minniti et al. [170]; Castro et al. [171]; Lucas et al. [172]; Zuraini et al. [63] |
| Grape (Vitis vinifera) | Flavonoids (PGPFs); Anthocyanins (malvidin); Polyphenols (resveratrol). | Hypolipidemic ↓LDL, ↓TC; ↑ Antioxidant capacity; Improves blood pressure inhibiting platelet aggregation; Improved endothelial function. | Shanmuganayagam et al. [157]; Razavi et al. [173]; Borde et al. [174]; Freedman et al. [175]; Quintieri et al. [176]; Zuraini et al. [63]. |
| Strawberry (Fragaria × ananassa) | Flavonoids; Anthocyanins; Phenolic acids; high content Vitamin C; rich Folate source. | ↑ Antioxidant capacity; ↓ Inflammation; ↓ TC. | Miller et al. [177]; Basu et al. [178]. |
| Apple (Malus domestica) | Flavonoids; Phenolic acids (chlorogenic acid and caffeic acid); Fiber (pectins); Phytosterols; Vitamin C; β-carotene | Hypolipidemic ↓ decreases LDL ↓TC; ↓Blood pressure; Antioxidant capacity; Antinflammatory; Enhances endothelial function. | Koutsos et al. [179]; Soleti et al. [180]; Liddle et al. [181]; Zuraini et al. [63]. |
| Avocado (Persea americana) | Flavonoids; Phenolic acids; Carotenoids; Phytosterols; Fiber; Vitamin E; Monounsaturated Fatty Acids; Fatty alcohols | Antithrombotic; Antiplatelets; Antinflammatory; Hypolipidemic ↓ decreases LDL ↓ TC; Enhances endothelial function. | Park et al. [152]; Rodriguez-Sanchez et al. [153]; Olas et al. [182]; Zuraini et al. [63] |
| Pomegranate (Punica granatum L.) | Polyphenols; Flavonoids; Alkaloids; Vitamins; Sterols; Unsaturated fatty acids | Lipid Metabolism Regulation; ↓ Blood pressure; Protection of endothelial function; ↓ Oxidative stress; ↓ Inflammation | Haghighian et al. [161]; Sun et al. [159]; Hou C. et al. [183]; Zuraini et al. [63]. |
| Orange (Citrus sinensis) | Vitamin C; Polyphenols (hesperidin, narirutin, naringin); Folate; Potassium | Lipid Metabolism Regulation; ↓ Blood pressure; ↓ Oxidative stress; ↓ Inflammation | Asgary et al. [184]; Miles et al. [185]; Zuraini et al. [63]. |
| Kiwi (Actinidia deliciosa, Actinidia chinensis) | Vitamin C, K, E Folate; Polyphenols; Carotenoids; Potassium; Fiber | Lipid Metabolism Regulation; Antiplatelets; Antithrombotic; ↓ Blood pressure; | Duttaroy et al. [165]; Iwasawa et al. [186]; Karlsen et al. [187]; Stonehouse et al. [188]; Zuraini et al. [63]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fałczyńska, A.; Miller-Kasprzak, E.; Rosiejka, D.; Michałowska, J.; Błażejewska, W.; Bogdańska, A.; Bogdański, P. Natural Sweetness and Bioactivity: The Cardiovascular Promise of Fruits. Nutrients 2025, 17, 3417. https://doi.org/10.3390/nu17213417
Fałczyńska A, Miller-Kasprzak E, Rosiejka D, Michałowska J, Błażejewska W, Bogdańska A, Bogdański P. Natural Sweetness and Bioactivity: The Cardiovascular Promise of Fruits. Nutrients. 2025; 17(21):3417. https://doi.org/10.3390/nu17213417
Chicago/Turabian StyleFałczyńska, Aleksandra, Ewa Miller-Kasprzak, Dawid Rosiejka, Joanna Michałowska, Wiktoria Błażejewska, Adela Bogdańska, and Paweł Bogdański. 2025. "Natural Sweetness and Bioactivity: The Cardiovascular Promise of Fruits" Nutrients 17, no. 21: 3417. https://doi.org/10.3390/nu17213417
APA StyleFałczyńska, A., Miller-Kasprzak, E., Rosiejka, D., Michałowska, J., Błażejewska, W., Bogdańska, A., & Bogdański, P. (2025). Natural Sweetness and Bioactivity: The Cardiovascular Promise of Fruits. Nutrients, 17(21), 3417. https://doi.org/10.3390/nu17213417