Watermelon Nutritional Composition with a Focus on L-Citrulline and Its Cardioprotective Health Effects—A Narrative Review
Abstract
1. Introduction
2. Watermelon and Its Nutritional Composition
3. L-Citrulline Metabolism and Transport
3.1. L-Citrulline Pharmacokinetics and Pharmacodynamics
3.2. L-Citrulline Regulatory Metabolism Roles
3.3. Nitric Oxide, the Final Cardiovascular Effector
4. Cardioprotective Effects of Watermelon Ingestion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baião, D.S.; da Silva, D.V.T.; Paschoalin, V.M.F. A Narrative Review on Dietary Strategies to Provide Nitric Oxide as a Non-Drug Cardiovascular Disease Therapy: Beetroot Formulations—A Smart Nutritional Intervention. Foods 2021, 10, 859. [Google Scholar] [CrossRef]
- Richardson, G.; Hicks, S.L.; O’Byrne, S.; Frost, M.T.; Moore, K.; Benjamin, N.; McKnight, G.M. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide 2002, 7, 24–29. [Google Scholar] [CrossRef]
- Hobbs, A.J.; Moncada, S. Antiplatelet properties of a novel, non-NO-based soluble guanylate cyclase activator. Vasc. Pharmacol. 2003, 40, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.A.; Steinert, J.R. Nitric oxide-mediated posttranslational modifications: Impacts at the synapse. Oxidative Med. Cell. Longev. 2016, 2016, 5681036. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Romaszko, E.; Szawara-Nowak, D.; Piskula, M.K. The impact of the matrix of red beet products and interindividual variability on betacyanins bioavailability in humans. Food Res. Int. 2018, 108, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A. Endothelial dysfunction in obesity: Role of inflammation. High Blood Press. Cardiovasc. Prev. 2016, 23, 83–85. [Google Scholar] [CrossRef]
- Morris, S.M., Jr. Arginine metabolism in vascular biology and disease. Vasc. Med. 2005, 10, S83–S87. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Karim, F.; Straznicky, N.E.; Fernandez, S.; Evans, R.G.; Head, G.A.; Kaye, D.M. Augmented endothelial-specific L-arginine transport prevents obesity-induced hypertension. Acta Physiol. 2014, 212, 39–48. [Google Scholar] [CrossRef]
- Vallance, P.; Chan, N. Endothelial function and nitric oxide: Clinical relevance. Heart 2001, 85, 342–350. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Grimble, G.K. Adverse gastrointestinal effects of arginine and related amino acids. J. Nutr. 2007, 137, 1693S–1701S. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in health and disease. Review on human studies. Clin. Nutr. 2017, 37, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA). Ministério da Agricultura, Pecuária e Abastecimento (MAPA). In Cultura da Melancia; Embrapa Hortaliças: Brasília, Brazil, 2014; pp. 1–303. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1026847/cultura-da-melancia (accessed on 14 August 2025).
- Zamuz, S.; Munekata, P.E.S.; Gullón, B.; Rocchetti, G.; Montesano, D.; Lorenzo, J.M. Citrullus lanatus as source of bioactive components: An up-to-date review. Trends Food Sci. Technol. 2021, 111, 208–222. [Google Scholar] [CrossRef]
- Naz, A.; Butt, M.S.; Sultan, M.T.; Qayyum, M.M.N.; Niaz, R.S. Watermelon lycopene and allied health claims. EXCLI J. 2014, 13, 650–660. Available online: https://www.excli.de/excli/article/view/729 (accessed on 5 September 2025).
- Jarret, R.L.; Levy, I.J. Oil and Fatty Acid Contents in Seed of Citrullus lanatus Schrad. J. Agric. Food Chem. 2012, 60, 5199–5204. [Google Scholar] [CrossRef]
- Mahla, H.R.; Rathore, S.S.; Venkatesan, K.; Sharma, R. Analysis of fatty acid methyl esters and oxidative stability of seed purpose watermelon (Citrullus lanatus) genotypes for edible oil. J. Food Sci. Technol. 2018, 55, 1552–1561. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.P.; Viegas, J.; Moldao-Martins, M.; Aguayo, E. Bioactive compounds from flesh and by-product of fresh-cut watermelon cultivars. J. Sci. Food Agric. 2011, 91, 805–812. [Google Scholar] [CrossRef]
- Egbuonu, A.C.C. Comparative Assessment of some Mineral, Amino Acid and Vitamin Compositions of Watermelon (Citrullus lanatus) Rind and Seed. Asian J. Biochem. 2015, 10, 230–236. Available online: https://scialert.net/abstract/?doi=ajb.2015.230.236 (accessed on 5 September 2025). [CrossRef]
- Abu-Hiamed, H.A. Chemical Composition, Flavonoids and $-sitosterol Contents of Pulp and Rind of Watermelon (Citrullus lanatus) Fruit. Pak. J. Nutr. 2017, 16, 502–507. Available online: https://scialert.net/abstract/?doi=pjn.2017.502.507 (accessed on 9 September 2025). [CrossRef]
- Dumitru, M.G.; Tutunea, D. Extraction and determination of physico-chemical properties of oil from watermelon seeds (Citrullus lanatus L.) to use in internal combustion engines. Rev. Chim. 2017, 68, 2676–2681. Available online: http://bch.ro/pdfRC/43%20DUMITRU%20MIHAELA%20%2011%2017.pdf (accessed on 11 September 2025). [CrossRef]
- Maoto, M.M.; Beswa, D.; Jideani, A.I.O. Watermelon as a potential fruit snack. Int. J. Food Prop. 2019, 22, 355–370. [Google Scholar] [CrossRef]
- Rezig, L.; Chouaibi, M.; Meddeb, W.; Msaada, K.; Hamdi, S. Chemical composition and bioactive compounds of Cucurbitaceae seeds: Potential sources for new trends of plant oils. Process Saf. Environ. Prot. 2019, 127, 73–81. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture (USDA). Seeds, Watermelon Seed Kernels, Dried (SR Legacy, 169407). 2019. Available online: https://fdc.nal.usda.gov/food-details/169407/nutrients (accessed on 11 September 2025).
- U.S. Department of Agriculture (USDA). Watermelon, Raw (SR Legacy, 167765). 2019. Available online: https://fdc.nal.usda.gov/food-details/167765/nutrients (accessed on 11 September 2025).
- Awotedu, O.L.; Ogunbamowo, P.O.; Awotedu, B.F.; Ariwoola, O.S. Comparative Nutritional Composition of Selected Medicinal Fruit Seeds. World News Nat. Sci. 2020, 29, 298–310. Available online: https://www.worldnewsnaturalsciences.com/wp-content/uploads/2020/01/WNOFNS-293-2020-298-310-1.pdf (accessed on 15 September 2025).
- Falade, O.S.; Otemuyiwa, I.O.; Adekunle, A.S.; Adewusi, S.A.; Oluwasefunmi, O. Nutrient composition of watermelon (Citrullis lanatus (Thunb.) Matsum.&Nakai) and egusi melon (Citrullus colocynthis (L.) Schrad.) seeds. Agric. Conspec. Sci. 2020, 85, 43–49. Available online: https://core.ac.uk/download/pdf/288493411.pdf (accessed on 15 September 2025).
- Omoniyi, S.A. Nutrient and Anti-nutritional Composition of Watermelon (Citrullus lanatus) Seed: A Review. Trends Sci. Technol. J. 2020, 5, 48–51. Available online: https://ftstjournal.com/uploads/docs/51%20Article%208.pdf (accessed on 17 September 2025).
- Yimer, Z.S.; Tehulie, N.S. Nutritional composition of different varieties of watermelon (Citrullus lanatus) fruit at gewane, Northeastern Ethiopia. J. Curr. Res. Food Sci. 2020, 1, 16–22. Available online: https://www.foodresearchjournal.com/article/20/2-1-4-110.pdf (accessed on 17 September 2025).
- Nadeem, M.; Ameer, K.; Siddique, F.; Navida, M. Watermelon nutrition profile, antioxidant activity, and processing. Korean J. Food Preserv. 2022, 29, 531–545. [Google Scholar] [CrossRef]
- Benmeziane, F.; Derradji. Composition, bioactive potential and food applications of watermelon (Citrullus lanatus) seeds—A review. Food Meas. 2023, 17, 5045–5061. [Google Scholar] [CrossRef]
- Kataria, D.; Kaur, J. From Waste to Wellness: Exploring the Nutritional Composition, Health Benefits and Utilization of Watermelon Rind. J. Food Chem. Nanotechnol. 2023, 9, S479–S482. [Google Scholar] [CrossRef]
- Tanvi, K.; Saxena, G. Comparative Investigation of the Proximate and Functional Properties of Watermelon (Citrullus lanatus) Rind and Custard Apple (Annona squamosa) Peel. J. Emerg. Technol. Innov. Res. 2023, 10, a40–a44. Available online: https://www.jetir.org/papers/JETIR2305006.pdf (accessed on 25 September 2025).
- Azman, W.M.F.W.; Shahar, A.; Azizam, S.A.; Rahim, A.A. Physical and Mechanical Properties of Watermelon Rind. Adv. Agric. Food Res. J. 2024, 5, 1–11. [Google Scholar] [CrossRef]
- Du, X.; Davila, M.; Ramirez, J.; Williams, C. Free Amino Acids and Volatile Aroma Compounds in Watermelon Rind, Flesh, and Three Rind-Flesh Juices. Molecules 2022, 27, 2536. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef]
- Collins, J.K.; Wu, G.; Perkins-Veazie, P.; Spears, K.; Claypool, P.L.; Baker, R.A.; Clevidence, B.A. Watermelon consumption increases plasma arginine concentrations in adults. Nutrition 2007, 23, 261–266. [Google Scholar] [CrossRef]
- Uyanga, V.A.; Amevor, F.K.; Liu, M.; Cui, Z.; Zhao, X.; Lin, H. Potential Implications of Citrulline and Quercetin on Gut Functioning of Monogastric Animals and Humans: A Comprehensive Review. Nutrients 2021, 13, 3782. [Google Scholar] [CrossRef]
- Joshi, V.; Joshi, M.; Silwal, D.; Noonan, K.; Rodriguez, S.; Penalosa, A. Systematized biosynthesis and catabolism regulate citrulline accumulation in watermelon. Phytochemistry 2019, 162, 129–140. [Google Scholar] [CrossRef]
- Ling, Z.N.; Jiang, Y.F.; Ru, J.N.; Lu, J.H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef]
- Tadmor, Y.; King, S.; Levi, A.; Davis, A.; Meir, A.; Wasserman, B.; Hirschberg, J.; Lewinsohn, E. Comparative fruit coloration in watermelon and tomato. Food Res. Int. 2005, 38, 837–841. [Google Scholar] [CrossRef]
- Fila, W.A.; Itam, E.H.; Johnson, J.T.; Odey, M.O.; Effiong, E.E.; Dasofunjo, K.; Ambo, E.D. Comparative proximate compositions of watermelon Citrullus lanatus, squash Cucurbita pepo’l and rambutan Nephelium lappaceum. Int. J. Sci. Technol. Res. 2013, 2, 81–88. Available online: http://www.journalofsciences-technology.org/archive/2013/jan_vol_2_no_1/55959134997818.pdf (accessed on 25 September 2025).
- Neglo, D.; Tettey, C.O.; Essuman, E.K.; Kortei, N.K.; Boakye, A.A.; Hunkpe, G.; Amarh, F.; Kwashie, P.; Devi, W.S. Comparative antioxidant and antimicrobial activities of the peels, rind, pulp and seeds of watermelon (Citrullus lanatus) fruit. Sci. Afr. 2021, 11, e00582. [Google Scholar] [CrossRef]
- Dhanani, T.; Dou, T.; Biradar, K.; Jifon, J.; Kurouski, D.; Patil, B.S. Raman Spectroscopy Detects Changes in Carotenoids on the Surface of Watermelon Fruits During Maturation. Front. Plant Sci. 2022, 13, 832522. [Google Scholar] [CrossRef]
- D’Eusanio, V. Assessment of Lycopene Levels in Dried Watermelon Pomace: A Sustainable Approach to Waste Reduction and Nutrient Valorization. Analytica 2024, 5, 311–321. [Google Scholar] [CrossRef]
- Sytar, O.; Smetanska, I. Special Issue Bioactive Compounds from Natural Sources (2020, 2021). Molecules 2022, 27, 1929. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]
- Sorokina, M.; McCaffrey, K.S.; Deaton, E.E.; Ma, G.; Ordovás, J.M.; Perkins-Veazie, P.M.; Steinbeck, C.; Levi, A.; Parnell, L.D. A Catalog of Natural Products Occurring in Watermelon—Citrullus lanatus. Front. Nutr. 2021, 8, 729822. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Manivannan, A.; Lee, E.S.; Han, K.; Lee, H.E.; Kim, D.S. Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules 2020, 25, 5258. [Google Scholar] [CrossRef] [PubMed]
- Meghwar, P.; Saeed, S.M.G.; Ullah, A.; Nikolakakis, E.; Panagopoulou, E.; Tsoupras, A.; Smaoui, S.; Khaneghah, A.M. Nutritional benefits of bioactive compounds from watermelon: A comprehensive review. Food Biosci. 2024, 61, 104609. [Google Scholar] [CrossRef]
- Zhao, W.; Lv, P.; Gu, H. Studies on carotenoids in watermelon flesh. Agric. Sci. 2013, 4, 13–20. Available online: https://api.semanticscholar.org/CorpusID:30750063 (accessed on 26 September 2025). [CrossRef]
- Edwards, A.J.; Vinyard, B.T.; Wiley, E.R.; Brown, E.D.; Collins, J.K.; Perkins-Veazie, P.; Baker, R.A.; Clevidence, B.A. Consumption of watermelon juice increases plasma concentrations of lycopene and beta-carotene in humans. J. Nutr. 2003, 133, 1043–1050. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef] [PubMed]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Timmers, C.D.; Francis, D.M.; Lesinski, G.B.; Clinton, S.K.; et al. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, crossover clinical trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Moia, V.M.; Portilho, F.L.; Pádua, T.A.; Corrêa, L.B.; Ricci-Junior, E.; Rosas, E.C.; Alencar, L.M.R.; Sinfronio, F.S.M.; Sampson, A.; Iram, S.H.; et al. Lycopene used as anti-inflammatory nanodrug for the treatment of rheumathoid arthritis: Animal assay, pharmacokinetics, ABC transporter and tissue deposition. Colloids Surf. B Biointerfaces 2020, 188, 110814. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Biddle, M.J.; Lennie, T.A.; Bricker, G.V.; Kopec, R.E.; Schwartz, S.J.; Moser, D.K. Lycopene dietary intervention: A pilot study in patients with heart failure. J. Cardiovasc. Nurs. 2015, 30, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Obermüller-Jevic, U.C.; Hellmis, E.; Koch, W.; Jacobi, G.; Biesalski, H.K. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J. Nutr. 2008, 138, 49–53. [Google Scholar] [CrossRef]
- Kim, J.Y.; Paik, J.K.; Kim, O.Y.; Park, H.W.; Lee, J.H.; Jang, Y.; Lee, J.H. Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis 2011, 215, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Fluhr, J.W.; Meinke, M.C.; Zastrow, L.; Sterry, W.; Lademann, J. Topical beta-carotene protects against infra-red-light-induced free radicals. Exp. Dermatol. 2011, 20, 125–129. [Google Scholar] [CrossRef]
- Marcelino, G.; Machate, D.J.; Freitas, K.D.C.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; Candido, C.J.; Guimarães, R.D.C.A. β-Carotene: Preventive Role for Type 2 Diabetes Mellitus and Obesity: A Review. Molecules 2020, 25, 5803. [Google Scholar] [CrossRef]
- Canas, J.A.; Lochrie, A.; McGowan, A.G.; Hossain, J.; Schettino, C.; Balagopal, P.B. Effects of Mixed Carotenoids on Adipokines and Abdominal Adiposity in Children: A Pilot Study. J. Clin. Endocrinol. Metab. 2017, 102, 1983–1990. [Google Scholar] [CrossRef]
- Asemi, Z.; Alizadeh, S.A.; Ahmad, K.; Goli, M.; Esmailzadeh, A. Effects of beta-carotene fortified symbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized crossover controlled clinical trial. Clin. Nutr. 2016, 35, 819–825. [Google Scholar] [CrossRef]
- Alcaino, J.; Baeza, M.; Cifuentes, V. Astaxanthin and related xanthophylls. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites, 1st ed.; Martín, S., García-Estrada, J.F., Zeilinger, C., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Kim, Y.C.; Choi, D.; Cha, A.; Lee, Y.-G.; Baek, N.-I.; Rimal, S.; Lee, S. Critical enzymes for biosynthesis of cucurbitacin derivatives in watermelon and their biological significance. Commun. Biol. 2020, 3, 444. [Google Scholar] [CrossRef]
- Kim, Y.C.; Choi, D.; Zhang, C.; Liu, H.-f.; Lee, S. Profiling cucurbitacins from diverse watermelons (Citrullus spp.). Hortic. Environ. Biotechnol. 2018, 59, 557–566. [Google Scholar] [CrossRef]
- Chen, X.; Bao, J.; Guo, J.; Ding, Q.; Lu, J.; Huang, M.; Wang, Y. Biological activities and potential molecular targets of cucurbitacins. Anti-Cancer Drugs 2012, 23, 777–787. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Seeram, N.P.; Nair, M.G. Anticancer and anti-inflammatory activities of Cucurbitacins from Cucurbita and reana. Cancer Lett. 2003, 189, 11–16. [Google Scholar] [CrossRef]
- Escandell, J.M.; Recio, M.C.; Manez, S.; Giner, R.M.; Cerda-Nicolas, M.; Gil-Benso, R.; Ríos, J.L. Dihydrocucurbitacin B inhibits delayed type hypersensitivity reactions by suppressing lymphocyte proliferation. J. Pharmacol. Exp. Ther. 2007, 322, 1261–1268. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Leskovar, D.I.; Colla, G.; Rouphael, Y. Watermelon and melon fruit quality: The genotypic and agro-environmental factors implicated. Sci. Hortic. 2018, 234, 393–408. [Google Scholar] [CrossRef]
- Yang, F.; Chen, E.; Dai, Y.; Xu, Y.; Liu, Y.; Bi, S. Elucidation of the interaction between fructose and key aroma compounds in watermelon juice via Raman spectroscopy and nuclear magnetic resonance. Food Res. Int. 2022, 159, 111613. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Bahri, S.; Zerrouk, N.; Aussel, C.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.C.; Cynober, L.; Sfar, S. Citrulline: From metabolism to therapeutic use. Nutrition 2013, 29, 479–484. [Google Scholar] [CrossRef]
- Aguayo, E.; Martínez-Sánchez, A.; Fernández-Lobato, B.; Alacid, F. L-Citrulline: A Non-Essential Amino Acid with Important Roles in Human Health. Appl. Sci. 2021, 11, 3293. [Google Scholar] [CrossRef]
- Breuillard, C.; Cynober, L.; Moinard, C. Citrulline and nitrogen homeostasis: An overview. Amino Acids 2015, 47, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.E.; Meininger, C.J.; Haynes, T.E.; Wu, G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed. Pharmacother. 2002, 56, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Benazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Morita, M.; Hayashi, T.; Kamimura, A. The effects on plasma L-arginine levels of combined oral L-citrulline and L-arginine supplementation in healthy males. Biosci. Biotechnol. Biochem. 2017, 81, 372–375. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Hsu, J.W.; Emrick, L.T.; Wong, L.J.; Craigen, W.J.; Jahoor, F.; Scaglia, F. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol. Genet. Metab. 2012, 105, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Shatanawi, A.; Momani, M.S.; Al-Aqtash, R.; Hamdan, M.H.; Gharaibeh, M.N. L-Citrulline Supplementation Increases Plasma Nitric Oxide Levels and Reduces Arginase Activity in Patients With Type 2 Diabetes. Front. Pharmacol. 2020, 22, 584669. [Google Scholar] [CrossRef]
- Shatanawi, A.; Momani, M.S. Plasma arginase activity is elevated in type 2 diabetic patients. Biomed. Res. 2017, 28, 4102–4106. Available online: https://www.alliedacademies.org/articles/plasma-arginase-activity-is-elevated-in-type-2-diabetic-patients.pdf (accessed on 2 October 2025).
- Douglass, M.S.; Kaplowitz, M.R.; Zhang, Y.; Fike, C.D. Impact of L-citrulline on nitric oxide signaling and arginase activity in hypoxic human pulmonary artery endothelial cells. Pulm. Circ. 2023, 13, e12221. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S. L-Citrulline. In Pharmacy Compounding Advisory Committee Meeting; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2017. Available online: https://fda.report/media/109619/FDA-Presentations-for-the-November-20-21--2017-Meeting-of-the-Pharmacy-Compounding-Advisory-Committee.pdf (accessed on 2 October 2025).
- Rhim, H.C.; Kim, S.J.; Park, J.; Jang, K.M. Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: A systematic review and meta-analysis. J. Sport Health Sci. 2020, 9, 553–561. [Google Scholar] [CrossRef]
- Kang, Y.; Dillon, K.N.; Martinez, M.A.; Maharaj, A.; Fischer, S.M.; Figueroa, A. L-Citrulline Supplementation Improves Arterial Blood Flow and Muscle Oxygenation during Handgrip Exercise in Hypertensive Postmenopausal Women. Nutrients 2024, 16, 1935. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. L-Citrulline supplementation improves O2 uptake kinetics and high intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. L-Citrulline supplementation: Impact on cardiometabolic health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef]
- Dawoud, H.; Malinski, T. Vitamin D3, L-Arginine, L-Citrulline, and antioxidant supplementation enhances nitric oxide bioavailability and reduces oxidative stress in the vascular endothelium-clinical implications for cardiovascular system. Pharmacogn. Res. 2020, 12, 17–23. Available online: https://phcogres.com/article/2020/12/1/104103prpr7919 (accessed on 3 October 2025).
- Abbaszadeh, F.; Azizi, S.; Mobasseri, M.; Ebrahimi-Mameghani, M. The effects of citrulline supplementation on meta-inflammation and insulin sensitivity in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Diabetol. Metab. Syndr. 2021, 13, 52. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.P.; Alacid, F.; Carrasco, M.; Martínez, I.; Aguayo, E. Watermelon Juice: Potential Functional Drink for Sore Muscle Relief in Athletes. J. Agric. Food Chem. 2013, 61, 7522–7528. [Google Scholar] [CrossRef]
- Barmore, W.; Azad, F.; Stone, W.L. Physiology, urea cycle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513323/ (accessed on 9 October 2025).
- Faure, C.; Raynaud-Simon, A.; Ferry, A.; Daugé, V.; Cynober, L.; Aussel, C.; Moinard, C. Leucine and citrulline modulate muscle function in malnourished aged rats. Amino Acids 2011, 42, 1425–1433. [Google Scholar] [CrossRef]
- Le Plénier, S.; Walrand, S.; Noirt, R.; Cynober, L.; Moinard, C. Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: A common activation pathway? Amino Acids 2012, 43, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yin, Y.; Tan, B.; Kong, X.; Wu, G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids 2011, 41, 1185–1193. [Google Scholar] [CrossRef]
- Baião, D.S.; Conte-Junior, C.A.; Silva, J.T.; Paschoalin, V.M.F.; Alvares, T.S. L-Arginine Supplementation and Nitric Oxide Production: No Additional Effect When Associated to Exercise. Food Nutr. Sci. 2013, 4, 779–784. [Google Scholar] [CrossRef]
- Morita, M.; Hayashi, T.; Ochiai, M.; Maeda, M.; Yamaguchi, T.; Ina, K.; Kuzuya, M. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem. Biophys. Res. Commun. 2014, 454, 53–57. [Google Scholar] [CrossRef]
- Morris, S.M. Regulation of arginine availability and its impact on NO synthesis. In Nitric Oxide: Biology & Pathobiology; Academic Press: Cambridge, UK, 2020; pp. 187–197. [Google Scholar] [CrossRef]
- Osowska, S.; Moinard, C.; Neveux, N.; Loi, C.; Cynober, L. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut 2004, 53, 1781–1786. [Google Scholar] [CrossRef]
- da Silva, D.V.T.; Baião, D.D.S.; Almeida, C.C.; Paschoalin, V.M.F. A Critical Review on Vasoactive Nutrients for the Management of Endothelial Dysfunction and Arterial Stiffness in Individuals under Cardiovascular Risk. Nutrients 2023, 15, 2618. [Google Scholar] [CrossRef]
- Baião, D.S.; da Silva, D.V.T.; Paschoalin, V.M.F. Beetroot, a Remarkable Vegetable: Its Nitrate and Phytochemical Contents Can be Adjusted in Novel Formulations to Benefit Health and Support Cardiovascular Disease Therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef]
- Vong, L.B.; Nagasaki, Y. Nitric oxide nano-delivery systems for cancer therapeutics: Advances and challenges. Antioxidants 2020, 9, 791. [Google Scholar] [CrossRef]
- Keh, D.; Thieme, I.; Kürer, K.; Falke, J.; Gerlach, H. Inactivation of platelet glycoprotein IIb/IIIa receptor by nitric oxide donor 3-morpholino-sydnonimine. Blood Coagul. Fibrinolysis 2003, 14, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Gence, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Baião, D.S.; da Silva, D.V.T.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, Bioactive and Physicochemical Characteristics of Different Beetroot Formulations. Food Addit. 2017, 2, 20–44. [Google Scholar] [CrossRef]
- Baião, D.S.; d’El-Rei, J.; Alves, G.; Neves, M.F.; Perrone, D.; Del Aguila, E.M.; Paschoalin, V.M.F. Chronic effects of nitrate supplementation with a newly designed beetroot formulation on biochemical and hemodynamic parameters of individuals presenting risk factors for cardiovascular diseases: A pilot study. J. Funct. Foods 2019, 58, 85–94. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef]
- Shafe, M.O.; Gumede, N.M.; Nyakudya, T.T.; Chivandi, E. Lycopene: A Potent Antioxidant with Multiple Health Benefits. J. Nutr. Metab. 2024, 2024, 6252426. [Google Scholar] [CrossRef] [PubMed]
- Bufka, J.; Vaňková, L.; Sýkora, J.; Křížková, V. Exploring carotenoids: Metabolism, antioxidants, and impacts on human health. J. Funct. Foods 2024, 118, 106284. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Treggiari, D.; Dalbeni, A.; Meneguzzi, A.; Delva, P.; Fava, C.; Molesini, B.; Pandolfini, T.; Minuz, P. Lycopene inhibits endothelial cells migration induced by vascular endothelial growth factor A increasing nitric oxide bioavailability. J. Funct. Foods 2018, 42, 312–318. [Google Scholar] [CrossRef]
- Ebadi, M.; Mohammadi, M.; Pezeshki, A.; Jafari, S.M. Health Benefits of Beta-Carotene. In Handbook of Food Bioactive Ingredients; Jafari, S.M., Rashidinejad, A., Simal-Gandara, J., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Rougé, C.; Des Robert, C.; Robins, A.; Le Bacquer, O.; Volteau, C.; De La Cochetière, M.F.; Darmaun, D. Manipulation of citrulline availability in humans. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 293, G1061–G1067. [Google Scholar] [CrossRef]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Perkins-Veazie, P.M.; Arjmandi, B.H. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: A pilot study. Am. J. Hypertens. 2011, 24, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Wong, A.; Arjmandi, B.H. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese adults with prehypertension or hypertension. Am. J. Hypertens. 2012, 25, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Wong, A.; Kalfon, R. Effects of watermelon supplementation on aortic hemodynamic responses to the cold pressor test in obese hypertensive adults. Am. J. Hypertens. 2014, 27, 899–906. [Google Scholar] [CrossRef]
- Fan, J.; Park, E.; Zhang, L.; Edirisinghe, I.; Burton-Freeman, B.; Sandhu, A.K. Pharmacokinetic Parameters of Watermelon (Rind, Flesh, and Seeds) Bioactive Components in Human Plasma: A Pilot Study to Investigate the Relationship to Endothelial Function. J. Agric. Food Chem. 2020, 68, 7393–7403. [Google Scholar] [CrossRef] [PubMed]
- Vincellette, C.M.; Losso, J.; Early, K.; Spielmann, G.; Irving, B.A.; Allerton, T.D. Supplemental Watermelon Juice Attenuates Acute Hyperglycemia-Induced Macro-and Microvascular Dysfunction in Healthy Adults. J. Nutr. 2021, 151, 3450–3458. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.C.; Mehta, T.; Nagabooshanam, V.A.; Dudenbostel, T.; Locher, J.L.; Crowe-White, K.M. Daily 100% watermelon juice consumption and vascular function among postmenopausal women: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Fujie, S.; Iemitsu, K.; Inoue, K.; Ogawa, T.; Nakashima, A.; Suzuki, K.; Iemitsu, M. Wild Watermelon-Extracted Juice Ingestion Reduces Peripheral Arterial Stiffness with an Increase in Nitric Oxide Production: A Randomized Crossover Pilot Study. Nutrients 2022, 14, 5199. [Google Scholar] [CrossRef]
- Volino-Souza, M.; Oliveira, G.V.; Tavares, A.C.; Souza, K.; Alvares, T. The effect of microencapsulated watermelon rind (Citrullus lanatus) and beetroot (Beta vulgaris L.) ingestion on ischemia/reperfusion-induced endothelial dysfunction: A randomised clinical trial. Food Funct. 2023, 14, 7959–7968. [Google Scholar] [CrossRef]
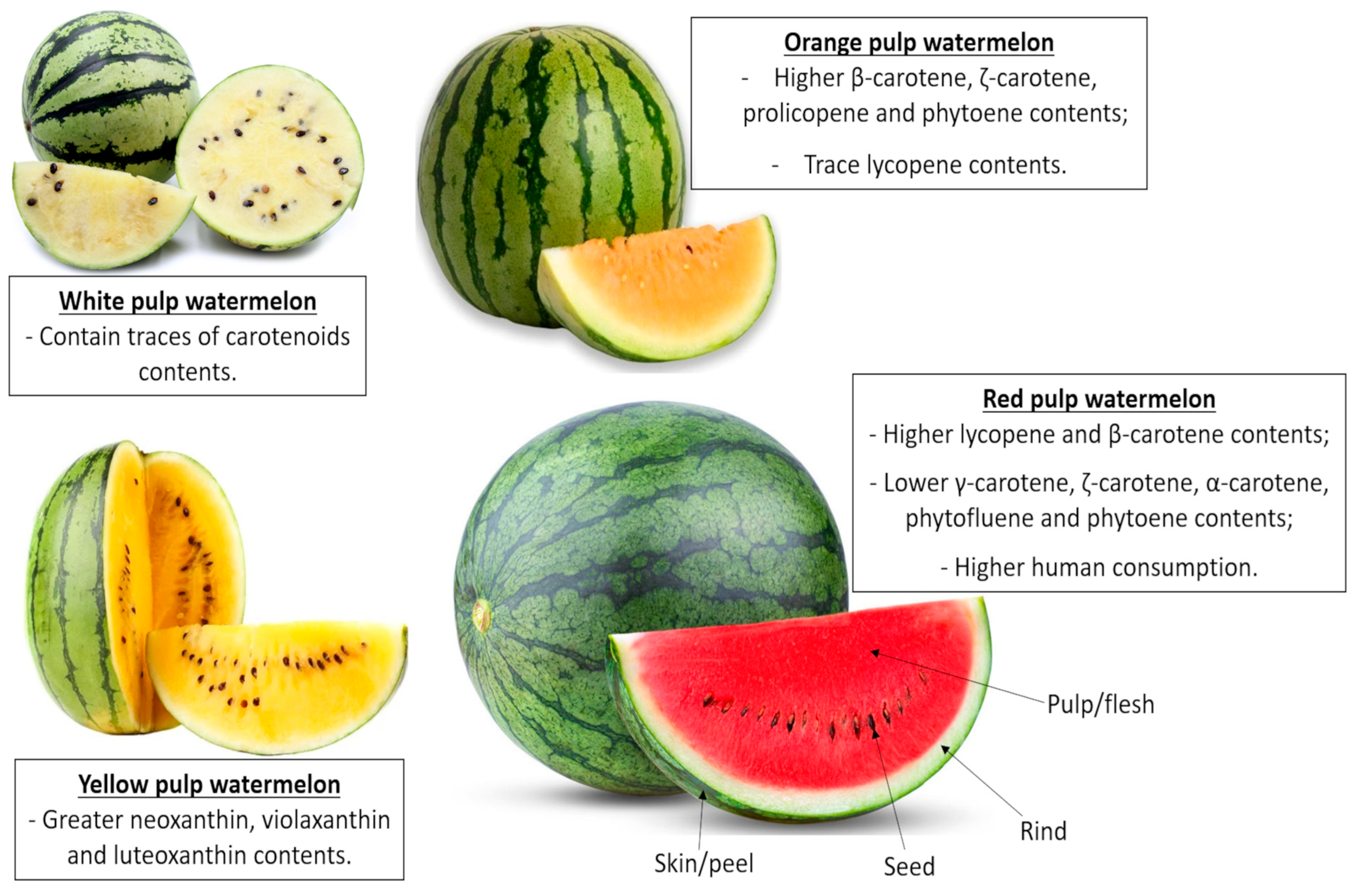
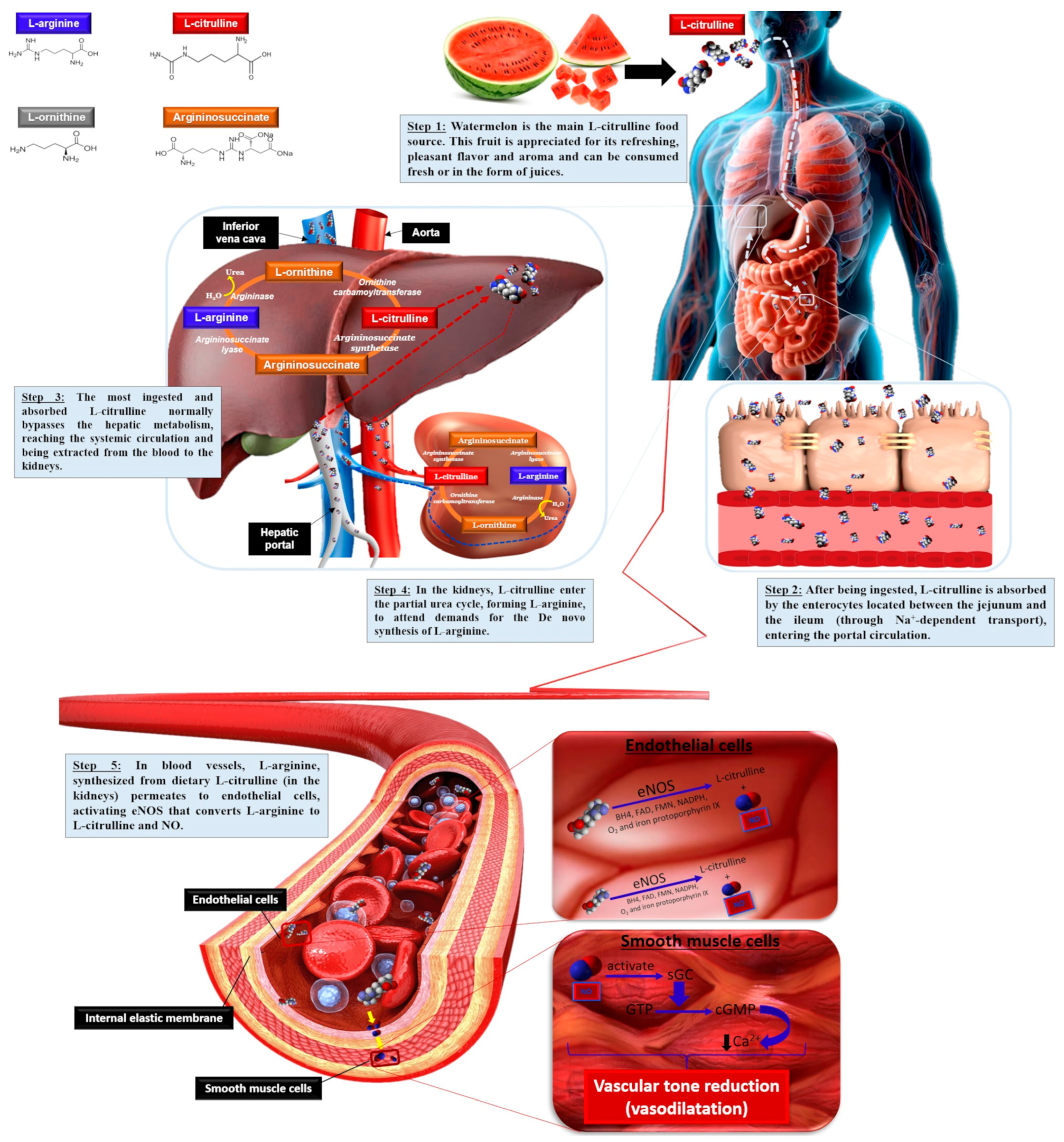
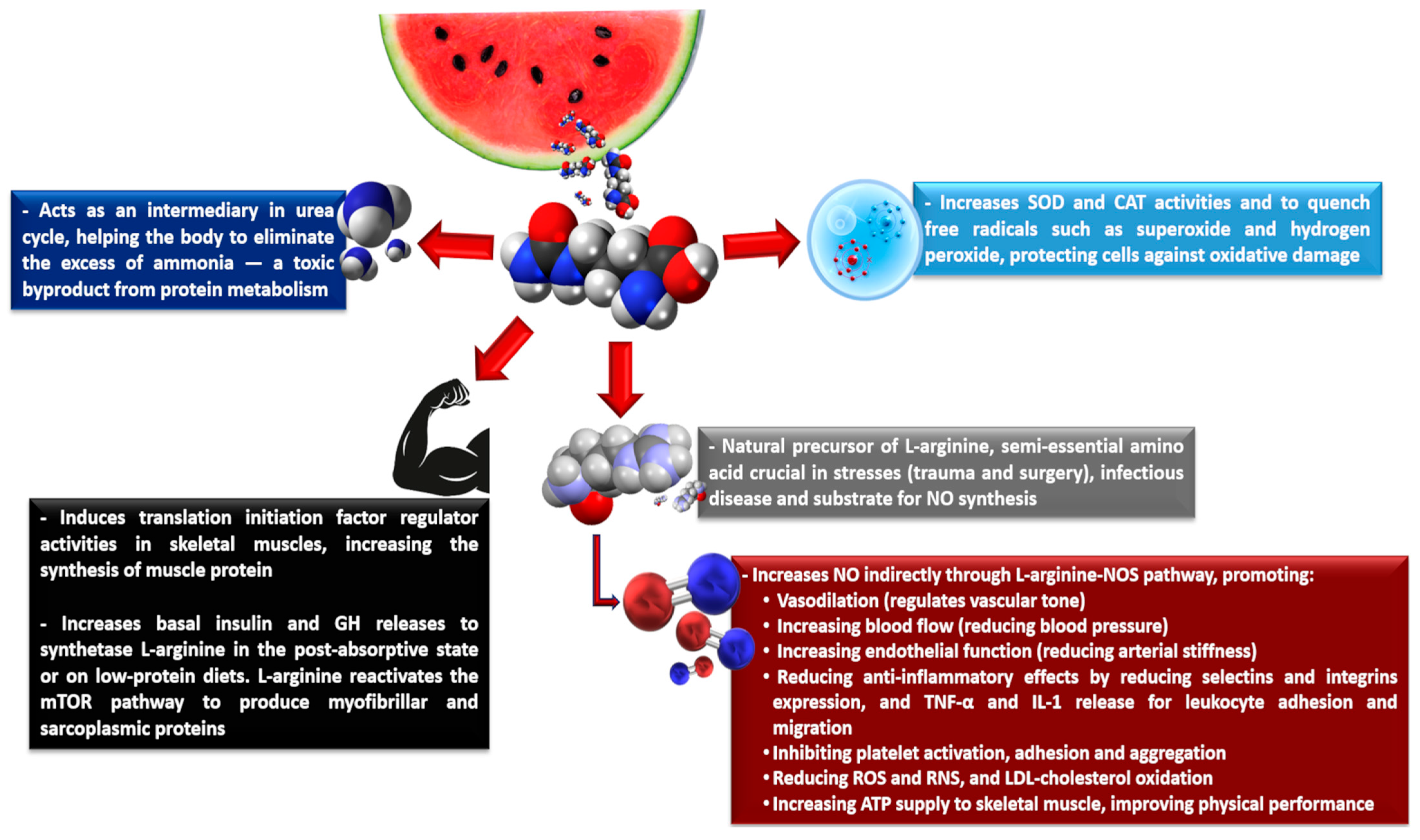
| Compounds | Pulp/Flesh (fwb) | Rind (fwb) | Seeds (dwb) |
|---|---|---|---|
| Ashes (%) | 0.25–0.50 | 1.00–3.84 | 2.30–5.10 |
| Moisture (%) | 85–95.2 | 65.00–85.00 | 5.05–10.06 |
| Energy (kilocalorie) | 30–46 | 130 | 354–560 |
| Carbohydrate (%) | 4.23–11.60 | 15.50–35.5 | 10.00–25.30 |
| Total sugars (%) | 5.74–9.20 | 5.39 | 3.23 |
| Fructose (%) | 2.72–4.11 | 0.20–0.75 | ND |
| Glucose (%) | 0.67–2.13 | 0.15–0.60 | ND |
| Sucrose (%) | 0.11–2.87 | 0.10–0.45 | ND |
| Maltose (%) | 0.02–0.14 | tr | ND |
| Fiber (%) | 0.2–0.73 | 3.00–5.59 | 14.50–20.10 |
| L-citrulline (%) | 0.04–0.16 | 0.06–0.5 | ND |
| Total protein (%) | 0.73–0.98 | 0.53–2.51 | 31.99–49.70 |
| Glutamic acid (%) | 0.063–0.080 | ND | 5.70–8.10 |
| L-arginine (%) | 0.055–0.075 | 0.054 | 5.00–7.00 |
| Aspartate acid (%) | 0.040–0.055 | 0.007 | 2.76–5.30 |
| L-glycine | 0.010–0.015 | tr | 1.20–2.50 |
| L-phenylalanine (%) | 0.015–0.022 | 0.003 | 2.03–3.00 |
| L-valine (%) | 0.015–0.022 | 0.003 | 1.56–2.10 |
| L-serine (%) | 0.018–0.025 | 0.018 | 1.51–2.1 |
| L-threonine (%) | 0.025–0.035 | ND | 1.11–1.80 |
| L-leucine (%) | 0.018–0.025 | tr | 2.15–2.80 |
| L-isoleucine (%) | 0.018–0.025 | 0.002 | 1.34–2.00 |
| L-histidine (%) | 0.010–0.015 | tr | 0.77–1.20 |
| L-lysine (%) | 0.055–0.075 | tr | 0.90–2.00 |
| L-alanine (%) | 0.018–0.025 | 0.007 | 1.48–2.11 |
| L-methionine (%) | 0.010–0.015 | ND | 0.83–2.17 |
| L-tyrosine (%) | 0.012–0.018 | ND | 1.30–1.60 |
| L-proline (%) | 0.022–0.030 | 0.01 | 1.25–2.00 |
| Total lipids (%) | 0.20–0.25 | 0.10–0.83 | 22.00–50.00 |
| Myristic acid—C14:0 (%) | 0.003–0.004 | 0.004 | 0.14–14.4 |
| Palmitic acid—C16:0 (%) | 0.0419–0.052 | 0.24 | 2.64–5.81 |
| Margaric acid—C17:0 (%) | ND | ND | 0.03–0.07 |
| Stearic acid—C18:0 (%) | 0.0118–0.014 | 0.045 | 1.90–6.55 |
| Arachidic acid—C20:0 (%) | 0.011–0.0125 | 0.005 | 0.1–1.19 |
| Palmitoleic acid—C16:1 (%) | 0.003–0.004 | 0.003 | 0.01–0.090 |
| Oleic acid—C18:1 (%) | 0.057–0.072 | 0.032 | 3.28–7.32 |
| Gadoleic acid—C20:1 (%) | 0.003–0.004 | 0.001 | 0.02–0.09 |
| Linoleic acid—C18:2 (%) | 0.048–0.060 | 0.252 | 13.14–28.1 |
| Linolenic acid—C18:3 (%) | 0.020–0.025 | 0.251 | 0.04–0.05 |
| Pulp/Flesh (fwb) | Rind (dwb) | Seeds (dwb) | |
|---|---|---|---|
| Vitamins (mg·100 g−1) | |||
| Retinol activity equivalents (A) | 0.028 | 50.15–52.13 | ND |
| Tocopherol (E) | 0.03–0.04 | 0.02–0.04 | 37.50–53.00 |
| Thiamine (B1) | 0.03–0.05 | 0.03–1.23 | 0.13–2.20 |
| Riboflavin (B2) | 0.02–0.06 | 0.02–2.71 | 0.05–0.15 |
| Niacin (B3) | 0.18–0.3 | 0.04–4.25 | 0.33–3.55 |
| Pantothenic acid (B5) | 0.22 | ND | 0.27–0.35 |
| Pyridoxin (B6) | 0.05–0.07 | 5.34 | 0.1–3.2 |
| Choline (B8) | 4.10–4.50 | ND | ND |
| Folate (B9) | ND | ND | 0.3–0.10 |
| Ascorbic acid (C) | 8.10–12.31 | 5.35–8.46 | 4.21–6.81 |
| Minerals (mg·100 g−1) | |||
| Calcium (Ca) | 5.60–11.00 | 28.00–29.15 | 57.20–758.20 |
| Potassium (K) | 100.50–200 | 21.70–447.33 | 482.30–1036.68 |
| Magnesium (Mg) | 10.00–15.00 | 1.48–35.00 | 86.20–515.00 |
| Sodium (Na) | 0.60–1.00 | 11.40–12.65 | 7.10–99.00 |
| Iron (Fe) | 0.19–1.0 | 1.30–4.63 | 4.50–8.40 |
| Phosphorus (P) | 11.0–15.0 | 129.70–135.24 | 107.70–787.00 |
| Zinc (Zn) | 0.1–1.5 | 1.29–5.10 | 4.10–10.40 |
| Copper (Cu) | 0.04–1.4 | 0.40–0.45 | 12.1–75.51 |
| Selenium (Se) | ND | ND | ND |
| Manganese (Mn) | 0.04–0.20 | 1.30–1.42 | 2.60–24.00 |
| Cadmium (Cd) | ND | ND | ND |
| Bioactive compounds (mg·100 g−1) | |||
| Lycopene | 4.40–8.00 | 0.73–1.57 | ND |
| β-carotene | 0.61–1.02 | 0.12–0.65 | ND |
| Total phenolic content (GAE) | 1.00–1.10 | 2.60–3.00 | 4.20–4.50 |
| Watermelon (L-Citrulline Content) | Subjects/Age | Intervention Period | Clinical Trial Design | Main Outcomes | Reported by |
|---|---|---|---|---|---|
| Watermelon powder (2.7 g) | 9 prehypertensive individuals (4 male/5 female) (54 y) | 6 weeks | Crossover Placebo-controlled | ↓ AIx, ↓ bPP, ↓ aSBP, ↓ aPP and ↓ P2 cfPWV—no effect | Figueroa et al. [117] |
| Watermelon powder (4 g) | 14 obese prehypertensive or stage-1 hypertensive individuals (11 female/3 male) (58 y) | 6 weeks | Randomized Placebo-controlled Two-periods crossover | ↓ cAIx, ↓ SBP, ↓ DBP, ↓ MAP HR and ABI—no effects | Figueroa et al. [118] |
| Watermelon powder (4 g) | 12 postmenopausal women (57 y) | 6 weeks | Randomized Placebo-controlled Crossover | ↑ plasma Cit, ↑ plasma Arg, ↓ baPWV, ↓ SBP, ↓ DBP and ↓ aortic SBP aortic and radial SBP—no effect | Figueroa et al. [119] |
| Fresh watermelon rind (387.4 mg) Fresh watermelon flesh (471.5 mg) Fresh watermelon seed (231.3 mg) | 6 overweight/obese individuals (32.2 ± 7.6 y) | 1 week | Randomized Placebo-controlled Crossover | FMD—no effect | Fan et al. [120] |
| Watermelon juice (795 mg) | 17 healthy young adults (21–25 y) (6 males/11 females) | 2 weeks | Randomized Placebo-controlled Double-blind Crossover | plasma Cit and Arg—no effects ↑ FMD | Vincellette et al. [121] |
| Watermelon juice (1.63 g) | 21 healthy postmenopausal females (55–70 y) | 4 weeks | Randomized Placebo-controlled Double-blind Crossover | plasma Cit and Arg—no effects FMD, PWV, MAP, SBP and DBP—no effects | Ellis et al. [122] |
| Watermelon juice (162 mg) | 20 healthy females (20 y) | Single intake | Randomized Placebo-controlled Double-blind Crossover | ↑ plasma NOx, ↑ tibial blood flow and ↓ faPWV SBP, DBP, baPWV, and cfPWV—no effects | Fujie et al. [123] |
| Microencapsulated watermelon rind (4 g) | 12 healthy adults | Single intake | Randomized Placebo-controlled Single-blind Crossover | ↑ plasma Cit, ↑ plasma Arg, ↑ plasma NOx and ↑ FMD | Volino-Souza et al. [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baião, D.d.S.; da Silva, D.V.T.; Paschoalin, V.M.F. Watermelon Nutritional Composition with a Focus on L-Citrulline and Its Cardioprotective Health Effects—A Narrative Review. Nutrients 2025, 17, 3221. https://doi.org/10.3390/nu17203221
Baião DdS, da Silva DVT, Paschoalin VMF. Watermelon Nutritional Composition with a Focus on L-Citrulline and Its Cardioprotective Health Effects—A Narrative Review. Nutrients. 2025; 17(20):3221. https://doi.org/10.3390/nu17203221
Chicago/Turabian StyleBaião, Diego dos Santos, Davi V. T. da Silva, and Vania M. F. Paschoalin. 2025. "Watermelon Nutritional Composition with a Focus on L-Citrulline and Its Cardioprotective Health Effects—A Narrative Review" Nutrients 17, no. 20: 3221. https://doi.org/10.3390/nu17203221
APA StyleBaião, D. d. S., da Silva, D. V. T., & Paschoalin, V. M. F. (2025). Watermelon Nutritional Composition with a Focus on L-Citrulline and Its Cardioprotective Health Effects—A Narrative Review. Nutrients, 17(20), 3221. https://doi.org/10.3390/nu17203221









