The Neuroprotective Potential of Vitamin D3
Abstract
1. Introduction
2. Materials and Methods
3. Neuroplasticity and Vitamin D
4. The Neuroprotective Properties of Vitamin D
5. Parkinson’s Disease
6. Autism Spectrum Disorder
7. Alzheimer’s Disease
8. Multiple Sclerosis
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 25(OH)D/25(OH)D3 | 25-Hydroxyvitamin D |
| 1,25(OH)2D/1,25(OH)2D3 | 1,25-Dihydroxyvitamin D3 (Calcitriol) |
| AD | Alzheimer’s Disease |
| ADHD | Attention-Deficit/Hyperactivity Disorder |
| APC | Antigen-Presenting Cell |
| APP | Amyloid Precursor Protein |
| Aβ/Aβ40/Aβ42 | Amyloid-β Peptides |
| ARE | Antioxidant Response Element |
| ASD | Autism Spectrum Disorder |
| ATEC | Autism Treatment Evaluation Checklist |
| Bax | Bcl-2-Associated X Protein |
| BDNF | Brain-Derived Neurotrophic Factor |
| BMI | Body Mass Index |
| Cai | Intracellular Calcium |
| CAMK2A | Calcium/Calmodulin-Dependent Protein Kinase II Alpha |
| CARS | Childhood Autism Rating Scale |
| CaATPase | Calcium-Transporting ATPase |
| CD4+ | Cluster of Differentiation 4 Positive T Cells |
| CIS | Clinically Isolated Syndrome |
| CNS | Central Nervous System |
| CNTF | Ciliary Neurotrophic Factor |
| CSF | Cerebrospinal Fluid |
| CYP2R1 | Cytochrome P450 Family 2 Subfamily R Member 1 |
| CYP24A1 | Cytochrome P450 Family 24 Subfamily A Member 1 |
| CYP27B1 | Cytochrome P450 Family 27 Subfamily B Member 1 |
| DAP1α | Death-Associated Protein 1 Alpha |
| DBP | Vitamin D-Binding Protein |
| DBS | Deep Brain Stimulation |
| DDR | DNA Damage Response |
| EBV | Epstein–Barr Virus |
| ERK1/2 MAPKs | Extracellular Signal-Regulated Kinases 1/2, Mitogen-Activated Protein Kinases |
| FGF23 | Fibroblast Growth Factor 23 |
| GABA | Gamma-Aminobutyric Acid |
| GADD45α | Growth Arrest and DNA-Damage-Inducible Protein Alpha |
| GDNF | Glial Cell Line-Derived Neurotrophic Factor |
| IFN-β | Interferon Beta |
| IFN-γ | Interferon Gamma |
| IL/IL-1β/IL-2/IL-4/IL-6/IL-10/IL-17 | Interleukins |
| iNOS | Inducible Nitric Oxide Synthase |
| IP3 | Inositol 1,4,5-Triphosphate |
| IU | International Units |
| LCPUFA | Long-Chain Polyunsaturated Fatty Acids |
| L-VGCCs | L-Type Voltage-Gated Calcium Channels |
| LVSCC-A1C | L-Type Voltage-Sensitive Calcium Channel A1C |
| MAPK | Mitogen-Activated Protein Kinase |
| MCI | Mild Cognitive Impairment |
| MoCA | Montreal Cognitive Assessment |
| MS | Multiple Sclerosis |
| MPTP | 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine |
| MYH | MutY DNA Glycosylase |
| MTH1 | MutT Homolog 1 |
| NGF | Nerve Growth Factor |
| NMDA | N-Methyl-D-Aspartate |
| NO | Nitric Oxide |
| NRF2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| OGG1 | 8-oxoguanine DNA Glycosylase |
| P16INK4a | Cyclin-Dependent Kinase Inhibitor 2A |
| PARP | Poly(ADP-Ribose) Polymerase |
| PASAT | Paced Auditory Serial Addition Test |
| PCNA | Proliferating Cell Nuclear Antigen |
| PD | Parkinson’s Disease |
| PDIA3 | Protein Disulfide-Isomerase A3 |
| PI3K | Phosphatidylinositol 3-Kinase |
| PKC | Protein Kinase C |
| PLA2 | Phospholipase A2 |
| PLC | Phospholipase C |
| PMCA1b | Plasma Membrane Calcium ATPase Isoform 1b |
| PS1/PS2 | Presenilin-1/Presenilin-2 |
| PTH | Parathyroid Hormone |
| RAD23B | RAD23 Homolog B |
| RRMS | Relapsing–Remitting Multiple Sclerosis |
| RXR | Retinoid X Receptor |
| Th1/Th2/Th17/Th9/Th22 | T Helper Cell Subtypes |
| TGF-β | Transforming Growth Factor Beta |
| TNF-α | Tumor Necrosis Factor Alpha |
| TRH | Thyrotropin-Releasing Hormone |
| TSH | Thyroid-Stimulating Hormone |
| Treg/Tregs | Regulatory T Cell(s) |
| UCP-5 | Uncoupling Protein-5 |
| VDR | Vitamin D Receptor |
| VDRE | Vitamin D Response Element |
| 8-OXO-dG | 8-oxo-2′-deoxyguanosine |
References
- Wongdee, K.; Chanpaisaeng, K.; Teerapornpuntakit, J.; Charoenphandhu, N. Intestinal calcium absorption. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 2047–2073. [Google Scholar] [CrossRef]
- Plachot, J.J.; Du Bois, M.B.; Halpern, S.; Cournot-Witmer, G.; Garabedian, M.; Balsan, S. In vitro action of 1,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol on matrix organization and mineral distribution in rabbit growth plate. Metab. Bone Dis. Relat. Res. 1982, 4, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Abe, E. Role of vitamin D in bone resorption. J. Cell Biochem. 1992, 49, 53–58. [Google Scholar] [CrossRef]
- Tenenhouse, H.S. Cellular and molecular mechanisms of renal phosphate transport. J. Bone Miner. Res. 1997, 12, 159–164. [Google Scholar] [CrossRef]
- Peterlik, M.; Wasserman, R. Regulation by vitamin D of intestinal phosphate absorption. Horm. Metab. Res. 1980, 12, 216–219. [Google Scholar] [CrossRef]
- Bouhtiauy, I.; Lajeunesse, D.; Christakos, S.; Brunette, M.G. Two vitamin D3-dependent calcium binding proteins increase calcium reabsorption by different mechanisms. I. Effect of CaBP 28K. Kidney Int. 1994, 45, 461–468. [Google Scholar] [CrossRef]
- Roth, J.; Thorens, B.; Hunziker, W.; Norman, A.W.; Orci, L. Vitamin D–dependent calcium binding protein: Immunocytochemical localization in chick kidney. Science 1981, 214, 197–200. [Google Scholar] [CrossRef]
- Cantley, L.K.; Russell, J.; Lettieri, D.; Sherwood, L.M. 1,25-Dihydroxyvitamin D3 suppresses parathyroid hormone secretion from bovine parathyroid cells in tissue culture. Endocrinology 1985, 117, 2114–2119. [Google Scholar] [CrossRef]
- Kadowaki, S.; Norman, A.W. Demonstration that the vitamin D metabolite 1,25(OH)2-vitamin D3 and not 24R,25(OH)2-vitamin D3 is essential for normal insulin secretion in the perfused rat pancreas. Diabetes 1985, 34, 315–320. [Google Scholar] [CrossRef]
- Sooy, K.; Schermerhorn, T.; Noda, M.; Surana, M.; Rhoten, W.B.; Meyer, M.; Fleischer, N.; Sharp, G.W.G.; Christakos, S. Calbindin-D28k controls [Ca2+] and insulin release. J. Biol. Chem. 1999, 274, 34343–34349. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.L.; Celli, A.; Mauro, T.; Chang, W. Calcium-sensing receptor regulates epidermal intracellular Ca2+ signaling and re-epithelialization after wounding. J. Investig. Dermatol. 2019, 139, 919–929. [Google Scholar] [CrossRef]
- Li, Y.C.; Pirro, A.E.; Amling, M.; Delling, G.; Baron, R.; Bronson, R.; Demay, M.B. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA 1997, 94, 9831–9835. [Google Scholar] [CrossRef]
- Schauber, J.; Dorschner, R.A.; Coda, A.B.; Büchau, A.S.; Liu, P.T.; Kiken, D.; Helfrich, Y.R.; Kang, S.; Elalieh, H.Z.; Steinmeyer, A.; et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D–dependent mechanism. J. Clin. Investig. 2007, 117, 803–811. [Google Scholar] [CrossRef]
- Van Etten, E.; Mathieu, C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef]
- Daniel, C.; Sartory, N.A.; Zahn, N.; Radeke, H.H.; Stein, J.M. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008, 324, 23–33. [Google Scholar] [CrossRef]
- Selles, J.; Boland, R. Rapid stimulation of calcium uptake and protein phosphorylation in isolated cardiac muscle by 1,25-dihydroxyvitamin D3. Mol Cell Endocrinol. 1991, 77, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Garami, M.; Cao, L.; Li, Q.; Gardner, D.G. 1,25(OH)2D3 suppresses expression and secretion of atrial natriuretic peptide from cardiac myocytes. Am. J. Physiol. Endocrinol. Metab. 1995, 268, E1108–E1113. [Google Scholar] [CrossRef]
- Törnquist, K. Pretreatment with 1,25-dihydroxycholecalciferol enhances thyrotropin-releasing hormone- and inositol 1,4,5-trisphosphate-induced release of sequestered Ca2+ in permeabilized GH4C1 pituitary cells. Endocrinology 1992, 131, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shu, X.B.; Yao, Z.; Ji, G.; Zhang, L. Is vitamin D receptor a druggable target for non-alcoholic steatohepatitis? World J. Gastroenterol. 2020, 26, 5812–5821. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Guillozo, H.; Marin, L.; Dufour, M.E.; Tordet, C.; Pike, J.W.; Garabedian, M. 1,25-Dihydroxyvitamin D3 receptors in rat lung during the perinatal period: Regulation and immunohistochemical localization. Endocrinology 1990, 127, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P. Supplementing vitamin D in different patient groups to reduce deficiency. Nutrients 2023, 15, 3725. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Saba, A.; Zucchi, R. An update on vitamin D metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Li, L.; Tuckey, R.C. Inactivation of vitamin D2 metabolites by human CYP24A1. J. Steroid Biochem. Mol. Biol. 2023, 233, 106368. [Google Scholar] [CrossRef]
- Mianesaz, H.; Göczi, L.; Nagy, G.; Póliska, S.; Fadel, L.; Bojcsuk, D.; Penyige, A.; Szirák, K.; AlHaman, F.; Nagy, L.; et al. Genomic regions occupied by both RARα and VDR are involved in the convergence and cooperation of retinoid and vitamin D signaling pathways. Nucleic Acids Res. 2025, 53, gkaf230. [Google Scholar] [CrossRef]
- Haussler, M.R.; Norman, A.W. Chromosomal receptor for a vitamin D metabolite. Proc. Natl. Acad. Sci. USA 1969, 62, 155–162. [Google Scholar] [CrossRef]
- Chen, S.; Cui, J.; Nakamura, K.; Ribeiro, R.C.J.; West, B.L.; Gardner, D.G. Coactivator–vitamin D receptor interactions mediate inhibition of the atrial natriuretic peptide promoter. J. Biol. Chem. 2000, 275, 15039–15048. [Google Scholar] [CrossRef] [PubMed]
- Casaletto, K.B.; Rentería, M.A.; Pa, J.; Tom, S.E.; Harrati, A.; Armstrong, N.M.; Rajan, K.B.; Mungas, D.; Walters, S.; Kramer, J.; et al. Late-life physical and cognitive activities independently contribute to brain and cognitive resilience. J. Alzheimers Dis. 2020, 74, 363–376. [Google Scholar] [CrossRef]
- Lisakovska, O.; Labudzynskyi, D.; Khomenko, A.; Isaev, D.; Savotchenko, A.; Kasatkina, L.; Savosko, S.; Veliky, M.; Shymanskyi, I. Brain vitamin D3 auto/paracrine system in relation to structural, neurophysiological, and behavioral disturbances associated with glucocorticoid-induced neurotoxicity. Front. Cell. Neurosci. 2023, 17, 1133400. [Google Scholar] [CrossRef]
- Xue, M.; Sheng, W.; Song, X.; Shi, Y.; Geng, Z.; Shen, L.; Wang, R.; Lü, H.; Hu, J. Astrocytes expressing vitamin D-activating enzyme identify Parkinson’s disease. CNS Neurosci. Ther. 2022, 28, 703–713. [Google Scholar] [CrossRef]
- Kermpatsou, D.; Olsson, F.; Wåhlén, E.; Söderberg, O.; Lennartsson, J.; Norlin, M. Cellular responses to silencing of PDIA3 (protein disulfide-isomerase A3): Effects on proliferation, migration, and genes in control of active vitamin D. J. Steroid Biochem. Mol. Biol. 2024, 240, 106497. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Arora, J. Two lineages of immune cells that differentially express the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2023, 228, 106253. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.V.; Al-Jaberi, F.A.H.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Kongsbak-Wismann, M.; Geisler, C. Macrophages control the bioavailability of vitamin D and vitamin D-regulated T cell responses. Front. Immunol. 2021, 12, 722806. [Google Scholar] [CrossRef]
- Minton, K. Vitamin D shuts down T cell-mediated inflammation. Nat. Rev. Immunol. 2022, 22, 1. [Google Scholar] [CrossRef]
- Fangal, V.D.; Kılıç, A.; Mirzakhani, H.; Litonjua, A.A.; Demay, M.B.; Levy, B.D.; Weiss, S.T. Vitamin D exerts endogenous control over Th2 cell fate and immune plasticity. iScience 2025, 28, 112117. [Google Scholar] [CrossRef]
- Švajger, U.; Rožman, P.J. Synergistic effects of interferon-γ and vitamin D3 signaling in induction of ILT-3highPDL-1high tolerogenic dendritic cells. Front. Immunol. 2019, 10, 2627. [Google Scholar] [CrossRef]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus statement on vitamin D status assessment and supplementation: Whys, whens, and hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for preventing and treating vitamin D deficiency: A 2023 update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]
- Nordt, S.P.; Williams, S.R.; Clark, R.F. Pharmacologic misadventure resulting in hypercalcemia from vitamin D intoxication. J. Emerg. Med. 2002, 22, 301–303. [Google Scholar] [CrossRef]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Tregub, P.P.; Komleva, Y.K.; Kukla, M.V.; Averchuk, A.S.; Vetchinova, A.S.; Rozanova, N.A.; Illarioshkin, S.N.; Salmina, A.B. Brain plasticity and cell competition: Immediate early genes are the focus. Cells 2025, 14, 143. [Google Scholar] [CrossRef]
- Almeras, L.; Eyles, D.; Benech, P.; Laffite, D.; Villard, C.; Patatian, A.; Boucraut, J.; Mackay-Sim, A.; McGrath, J.; Féron, F. Developmental vitamin D deficiency alters brain protein expression in the adult rat: Implications for neuropsychiatric disorders. Proteomics 2007, 7, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.J.; Hashimoto, T.; Lewis, D.A. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 2006, 11, 557–566. [Google Scholar] [CrossRef]
- Moretti, R.; Morelli, M.E.; Caruso, P. Vitamin D in neurological diseases: A rationale for a pathogenic impact. Int. J. Mol. Sci. 2018, 19, 2245. [Google Scholar] [CrossRef]
- Máčová, L.; Kancheva, R.; Bičíková, M. Molecular regulation of the CNS by vitamin D. Physiol. Res. 2023, 72, S339–S356. [Google Scholar] [CrossRef]
- Żmijewski, M.A. Nongenomic activities of vitamin D. Nutrients 2022, 14, 5104. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Yang, S.; Joyce, M.K.P.; Woo, E.; McCarroll, S.A.; Gonzalez-Burgos, G.; Perone, I.; Uchendu, S.; Ling, E.; Goldman, M.; et al. Key roles of CACNA1C/Cav1.2 and CALB1/Calbindin in prefrontal neurons altered in cognitive disorders. JAMA Psychiatry 2024, 81, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.J.; Alenezi, S.K.; Alhowail, A.H. Molecular insights into the pathogenic impact of vitamin D deficiency in neurological disorders. Biomed. Pharmacother. 2023, 162, 114718. [Google Scholar] [CrossRef]
- Cui, X.; McGrath, J.J.; Burne, T.H.J.; Eyles, D.W. Vitamin D and schizophrenia: 20 years on. Mol. Psychiatry 2021, 26, 2708–2720. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.M.; Yuan, X.Z.; Wang, F.Y.; Shen, J.; Wang, Y. Association between serum 25-hydroxyvitamin D level and cognitive impairment in patients with white matter lesions: A cross-sectional study. Med. Princ. Pract. 2020, 29, 451–457. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Hu, T.; Huang, G. Serum 25-hydroxy vitamin D level is associated with cognitive impairment in people aged 65 years and older. Ann. Palliat. Med. 2021, 10, 7479–7485. [Google Scholar] [CrossRef]
- Schneider, A.L.; Lutsey, P.L.; Alonso, A.; Gottesman, R.F.; Sharrett, A.R.; Carson, K.A.; Gross, M.; Post, W.S.; Knopman, D.S.; Mosley, T.H.; et al. Vitamin D and cognitive function and dementia risk in a biracial cohort: The ARIC brain MRI study. Eur. J. Neurol. 2014, 21, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.R.F.; Pettersen, J.A. Vitamin D is associated with visual memory in young northern adolescents. Nutr. Neurosci. 2024, 27, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, P.L. Structural basis of VDR–DNA interactions on direct repeat response elements. EMBO J. 2002, 21, 2242–2252. [Google Scholar] [CrossRef]
- Carlberg, C. Genome-wide (over)view on the actions of vitamin D. Front. Physiol. 2014, 5, 167. [Google Scholar] [CrossRef]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Rostami, A.; Zhang, G.X. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp. Mol. Pathol. 2015, 98, 240–245. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. The role of brain-derived neurotrophic factor as an essential mediator in neuronal functions and the therapeutic potential of its mimetics for neuroprotection in neurologic and psychiatric disorders. Molecules 2025, 30, 848. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D Receptor and 1α-Hydroxylase in Human Brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. The Effects of Vitamin D Receptor Silencing on the Expression of LVSCC-A1C and LVSCC-A1D and the Release of NGF in Cortical Neurons. PLoS ONE 2011, 6, e17553. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.D.; Thibault, V.; Chen, K.C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D Hormone Confers Neuroprotection in Parallel with Downregulation of L-Type Calcium Channel Expression in Hippocampal Neurons. J. Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef]
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. Vitamin D and the Brain: Genomic and Non-Genomic Actions. Mol. Cell. Endocrinol. 2017, 453, 131–143. [Google Scholar] [CrossRef]
- Barsony, J.; Prufer, K. Vitamin D Receptor and Retinoid X Receptor Interactions in Motion. In Vitamins & Hormones; Litwack, G., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 345–376. [Google Scholar]
- Chen, J.; Doroudi, M.; Cheung, J.; Grozier, A.L.; Schwartz, Z.; Boyan, B.D. Plasma Membrane Pdia3 and VDR Interact to Elicit Rapid Responses to 1α,25(OH)2D3. Cell. Signal. 2013, 25, 2362–2373. [Google Scholar] [CrossRef]
- Larriba, M.J.; González-Sancho, J.M.; Bonilla, F.; Muñoz, A. Interaction of Vitamin D with Membrane-Based Signaling Pathways. Front. Physiol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Doroudi, M.; Schwartz, Z.; Boyan, B.D. Phospholipase A2 Activating Protein Is Required for 1α,25-Dihydroxyvitamin D3 Dependent Rapid Activation of Protein Kinase C via PDIA3. J. Steroid Biochem. Mol. Biol. 2012, 132, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, S.; Messina, G.; Villano, I.; Messina, A.; Valenzano, A.; Moscatelli, F.; Salerno, M.; Sullo, A.; Avola, R.; Monda, V.; et al. Neuroprotective Effects of Physical Activity: Evidence from Human and Animal Studies. Front. Neurol. 2017, 8, 188. [Google Scholar] [CrossRef]
- Messina, G.; Viggiano, E.; Monda, V.; Messina, A.; Moscatelli, F.; Valenzano, A.; Tafuri, D.; Cibelli, G.; De Luca, B.; Monda, M. Cortical Spreading Depression Produces a Neuroprotective Effect Activating Mitochondrial Uncoupling Protein-5. Neuropsychiatr. Dis. Treat. 2016, 12, 1705–1710. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Ma, S.Y.; Röyttä, M.; Rinne, J.O.; Collan, Y.; Rinne, U.K. Correlation between Neuromorphometry in the Substantia Nigra and Clinical Features in Parkinson’s Disease Using Disector Counts. J. Neurol. Sci. 1997, 151, 83–87. [Google Scholar] [CrossRef]
- Pertile, R.A.N.; Brigden, R.; Raman, V.; Cui, X.; Du, Z.; Eyles, D. Vitamin D: A Potent Regulator of Dopaminergic Neuron Differentiation and Function. J. Neurochem. 2023, 166, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Fullard, M.E.; Duda, J.E. A Review of the Relationship between Vitamin D and Parkinson Disease Symptoms. Front. Neurol. 2020, 11, 454. [Google Scholar] [CrossRef]
- Gooch, H.; Cui, X.; Anggono, V.; Trzaskowski, M.; Tan, M.C.; Eyles, D.W.; Burne, T.H.J.; Jang, S.E.; Mattheisen, M.; Hougaard, D.M.; et al. 1,25-Dihydroxyvitamin D Modulates L-Type Voltage-Gated Calcium Channels in a Subset of Neurons in the Developing Mouse Prefrontal Cortex. Transl. Psychiatry 2019, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef]
- Bytowska, Z.K.; Korewo-Labelle, D.; Kowalski, K.; Libionka, W.; Przewłócka, K.; Kloc, W.; Kaczor, J.J. Impact of 12 Weeks of Vitamin D3 Administration in Parkinson’s Patients with Deep Brain Stimulation on Kynurenine Pathway and Inflammatory Status. Nutrients 2023, 15, 3839. [Google Scholar] [CrossRef]
- Saechua, C.; Sarachana, T.; Chonchaiya, W.; Trairatvorakul, P.; Yuwattana, W.; Poolcharoen, C.; Sangritdech, M.; Saeliw, T.; van Erp, M.L.; Sangsuthum, S.; et al. Impact of Gene Polymorphisms Involved in the Vitamin D Metabolic Pathway on the Susceptibility to and Severity of Autism Spectrum Disorder. Sci. Rep. 2024, 14, 28333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, H.; Liu, C.; Zhang, Y.; Wang, W.; Zou, Z.; Yang, L.; He, X.; Wu, J.; Ma, J.; et al. Research Progress on the Role of Vitamin D in Autism Spectrum Disorder. Front. Behav. Neurosci. 2022, 16, 859151. [Google Scholar] [CrossRef]
- Sultan, S.; Alhejin, N.; Serafi, R.; Abu Alrahi, M.; Afifi, G.; Al-Adawi, L.; Serafi, M.; El Madhoun, N. Does Vitamin D Deficiency Increase the Risk of Autism Spectrum Disorder? Linking Evidence with Theory—A Narrative Review. Psychiatry Int. 2025, 6, 22. [Google Scholar] [CrossRef]
- Trifonova, E.A.; Klimenko, A.I.; Mustafin, Z.S.; Lashin, S.A.; Kochetov, A.V. The mTOR Signaling Pathway Activity and Vitamin D Availability Control the Expression of Most Autism Predisposition Genes. Int. J. Mol. Sci. 2019, 20, 6332. [Google Scholar] [CrossRef]
- Pertile, R.A.N.; Cui, X.; Hammond, L.; Eyles, D.W. Vitamin D Regulation of GDNF/Ret Signaling in Dopaminergic Neurons. FASEB J. 2018, 32, 819–828. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Lu, Z.; Song, M.; Huang, X.; Mi, L.; Yang, J.; Cui, X. Effects of Vitamin D Supplementation on Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Clin. Psychopharmacol. Neurosci. 2023, 21, 240–251. [Google Scholar] [CrossRef]
- DeLuca, H.F. Vitamin D. In Vitamins & Hormones; Litwack, G., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 1–20. [Google Scholar]
- Halicka, H.D.; Zhao, H.; Li, J.; Traganos, F.; Studzinski, G.P.; Darzynkiewicz, Z. Attenuation of Constitutive DNA Damage Signaling by 1,25-Dihydroxyvitamin D3. Aging 2012, 4, 270–278. [Google Scholar] [CrossRef]
- Cui, X.; Eyles, D.W. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients 2022, 14, 4353. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, N.; Bonthu, M.G.; Gayatri, A.P.; Metri, S.; Kumar, P.K.; Addanki, A. Exploring the Role of Vitamin D in Alzheimer’s Disease: Mechanistic Insights and Implications. J. Pharmacol. Pharmacother. 2025, 16, 164–171. [Google Scholar] [CrossRef]
- Walawska-Hrycek, A.; Hrycek, E.; Galus, W.; Jędrzejowska-Szypułka, H.; Krzystanek, E. Does Systematic Use of Small Doses of Vitamin D Have Anti-Inflammatory Effects and Effectively Correct Deficiency among Healthy Adults? Nutrients 2025, 17, 352. [Google Scholar] [CrossRef]
- Javadfar, Z.; Abdollahzad, H.; Moludi, J.; Rezaeian, S.; Amirian, H.; Foroughi, A.A.; Nachvak, S.M.; Goharmehr, N.; Mostafai, R. Effects of Vitamin D Supplementation on Core Symptoms, Serum Serotonin, and Interleukin-6 in Children with Autism Spectrum Disorders: A Randomized Clinical Trial. Nutrition 2020, 79–80, 110986. [Google Scholar] [CrossRef] [PubMed]
- Mazahery, H.; Conlon, C.A.; Beck, K.L.; Mugridge, O.; Kruger, M.C.; Stonehouse, W.; Camargo, C.A.; Meyer, B.J.; Tsang, B.; von Hurst, P.R. Inflammation (IL-1β) Modifies the Effect of Vitamin D and Omega-3 Long Chain Polyunsaturated Fatty Acids on Core Symptoms of Autism Spectrum Disorder—An Exploratory Pilot Study. Nutrients 2020, 12, 661. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Guxens, M.; Llop, S.; Rodríguez-Bernal, C.L.; Tardón, A.; Riaño, I.; Ibarluzea, J.; Lertxundi, N.; Espada, M.; Rodriguez, A.; et al. Circulating 25-Hydroxyvitamin D3 in Pregnancy and Infant Neuropsychological Development. Pediatrics 2012, 130, e913–e920. [Google Scholar] [CrossRef]
- Chawla, D.; Fuemmeler, B.; Benjamin-Neelon, S.E.; Hoyo, C.; Murphy, S.; Daniels, J.L. Early Prenatal Vitamin D Concentrations and Social-Emotional Development in Infants. J. Matern.-Fetal Neonatal Med. 2019, 32, 1441–1448. [Google Scholar] [CrossRef]
- Whitehouse, A.J.O.; Holt, B.J.; Serralha, M.; Holt, P.G.; Kusel, M.M.H.; Hart, P.H. Maternal Serum Vitamin D Levels during Pregnancy and Offspring Neurocognitive Development. Pediatrics 2012, 129, 485–493. [Google Scholar] [CrossRef] [PubMed]
- García-Serna, A.M.; Morales, E. Neurodevelopmental Effects of Prenatal Vitamin D in Humans: Systematic Review and Meta-Analysis. Mol. Psychiatry 2020, 25, 2468–2481. [Google Scholar] [CrossRef]
- Aagaard, K.; Jepsen, J.R.M.; Sevelsted, A.; Horner, D.; Vinding, R.; Rosenberg, J.B.; Brustad, N.; Eliasen, A.; Mohammadzadeh, P.; Følsgaard, N.; et al. High-Dose Vitamin D3 Supplementation in Pregnancy and Risk of Neurodevelopmental Disorders in the Children at Age 10: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2024, 119, 362–370. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in Physiology and Pathology. Nat. Rev. Neurosci. 2016, 17, 22–35. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondrial Dysfunction in the Pathogenesis of Alzheimer’s Disease: Recent Advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Jia, J.; Hu, J.; Huo, X.; Miao, R.; Zhang, Y.; Ma, F. Effects of Vitamin D Supplementation on Cognitive Function and Blood Aβ-Related Biomarkers in Older Adults with Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1347–1352. [Google Scholar] [CrossRef]
- Thal, D.R.; Griffin, W.S.T.; Braak, H. Parenchymal and Vascular Aβ Deposition and Its Effects on the Degeneration of Neurons and Cognition in Alzheimer’s Disease. J. Cell. Mol. Med. 2008, 12, 1848–1862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Wang, H.R.; Meng-Wei Hu, Y.Z.; Sun, H.M.; Feng, Y.X.; Jia, J.J. Association of Vitamin D Levels with Risk of Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of Prospective Studies. J. Alzheimer’s Dis. 2024, 98, 373–385. [Google Scholar] [CrossRef]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D Deficiency as a Risk Factor for Dementia and Alzheimer’s Disease: An Updated Meta-Analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef]
- Gressner, O.A.; Schifflers, M.C.; Kim, P.; Heuts, L.; Lahme, B.; Gressner, A.M. Questioning the Role of Actin-Free Gc-Globulin as Actin Scavenger in Neurodegenerative Central Nervous System Disease: Relationship to S-100B Levels and Blood–Brain Barrier Function. Clin. Chim. Acta 2009, 400, 86–90. [Google Scholar] [CrossRef]
- Meier, U.; Gressner, O.; Lammert, F.; Gressner, A.M. Gc-Globulin: Roles in Response to Injury. Clin. Chem. 2006, 52, 1247–1253. [Google Scholar] [CrossRef]
- Thu, V.T.A.; Hoang, T.X.; Kim, J.Y. 1,25-Dihydroxy Vitamin D3 Facilitates the M2 Polarization and β-Amyloid Uptake by Human Microglia in a TREM2-Dependent Manner. Biomed. Res. Int. 2023, 2023, 3483411. [Google Scholar] [CrossRef]
- Dursun, E.; Gezen-Ak, D.; Yilmazer, S. A Novel Perspective for Alzheimer’s Disease: Vitamin D Receptor Suppression by Amyloid-β and Preventing the Amyloid-β-Induced Alterations by Vitamin D in Cortical Neurons. J. Alzheimer’s Dis. 2011, 23, 207–219. [Google Scholar] [CrossRef]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review. Cells 2023, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, H.; Xiong, Y.; Chen, C.; Duan, K.; Jia, J.; Ma, F. Vitamin D Supplementation Improves Cognitive Function Through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J. Alzheimers Dis. 2020, 78, 1509–1518. [Google Scholar] [CrossRef]
- Forero, D.A.; González-Giraldo, Y.; López-Quintero, C.; Castro-Vega, L.J.; Barreto, G.E.; Perry, G. Meta-Analysis of Telomere Length in Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Calugaru, K.; Yu, E.Y.; Huang, S.; González-Rodríguez, N.; Coloma, J.; Lue, N.F. The Yeast CST and Polα/Primase Complexes Act in Concert to Ensure Proper Telomere Maintenance and Protection. Nucleic Acids Res. 2025, 53, gkaf245. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Gängler, S.; Wieczorek, M.; Belsky, D.W.; Ryan, J.; Kressig, R.W.; Stähelin, H.B.; Theiler, R.; Dawson-Hughes, B.; Rizzoli, R.; et al. Individual and Additive Effects of Vitamin D, Omega-3 and Exercise on DNA Methylation Clocks of Biological Aging in Older Adults from the DO-HEALTH Trial. Nat. Aging 2025, 5, 376–385. [Google Scholar] [CrossRef]
- Behboudi, H.; Noureini, S.K.; Ghazanfari, T.; Ardestani, S.K. DNA Damage and Telomere Length Shortening in the Peripheral Blood Leukocytes of 20 Years SM-Exposed Veterans. Int. Immunopharmacol. 2018, 61, 37–44. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, X.-C.; Song, Y.; Pan, X.-D.; Dai, X.-M.; Zhang, J.; Cui, X.-L.; Wu, X.-L.; Zhu, Y.-G. Amyloid β Protein Aggravates Neuronal Senescence and Cognitive Deficits in 5XFAD Mouse Model of Alzheimer’s Disease. Chin. Med. J. 2016, 129, 1835–1844. [Google Scholar] [CrossRef]
- Ba, X.; Aguilera-Aguirre, L.; Rashid, Q.T.A.N.; Bacsi, A.; Radak, Z.; Sur, S.; Hosoki, K.; Hegde, M.L.; Boldogh, I. The Role of 8-Oxoguanine DNA Glycosylase-1 in Inflammation. Int. J. Mol. Sci. 2014, 15, 16975–16997. [Google Scholar] [CrossRef]
- Rolf, L.; Muris, A.-H.; Mathias, A.; Du Pasquier, R.; Koneczny, I.; Disanto, G.; Kuhle, J.; Ramagopalan, S.; Damoiseaux, J.; Smolders, J.; et al. Exploring the Effect of Vitamin D3 Supplementation on the Anti-EBV Antibody Response in Relapsing-Remitting Multiple Sclerosis. Mult. Scler. 2018, 24, 1280–1287. [Google Scholar] [CrossRef]
- Bjornevik, K.; Münz, C.; Cohen, J.I.; Ascherio, A. Epstein–Barr Virus as a Leading Cause of Multiple Sclerosis: Mechanisms and Implications. Nat. Rev. Neurol. 2023, 19, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.E.; Williamson, A.J.; Disanto, G.; Handunnetthi, L.; Giovannoni, G.; Ramagopalan, S.V. An Updated Meta-Analysis of Risk of Multiple Sclerosis Following Infectious Mononucleosis. PLoS ONE 2010, 5, e12496. [Google Scholar] [CrossRef] [PubMed]
- Lünemann, J.D.; Tintoré, M.; Messmer, B.; Strowig, T.; Rovira, Á.; Perkal, H.; Caballero, E.; Münz, C.; Montalban, X.; Comabella, M. Elevated Epstein–Barr Virus-Encoded Nuclear Antigen-1 Immune Responses Predict Conversion to Multiple Sclerosis. Ann. Neurol. 2010, 67, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Thouvenot, E.; Laplaud, D.; Lebrun-Frenay, C.; Derache, N.; Le Page, E.; Maillart, E.; Froment-Tilikete, C.; Castelnovo, G.; Casez, O.; Coustans, M.; et al. High-Dose Vitamin D in Clinically Isolated Syndrome Typical of Multiple Sclerosis: The D-Lay MS Randomized Clinical Trial. JAMA 2025, 333, 1413. [Google Scholar] [CrossRef]
- Amirinejad, R.; Shirvani-Farsani, Z.; Naghavi Gargari, B.; Sahraian, M.A.; Mohammad Soltani, B.; Behmanesh, M. Vitamin D Changes Expression of DNA Repair Genes in the Patients with Multiple Sclerosis. Gene 2021, 781, 145488. [Google Scholar] [CrossRef]
- Spiezia, A.L.; Falco, F.; Manganelli, A.; Carotenuto, A.; Petracca, M.; Novarella, F.; Iacovazzo, C.; Servillo, G.; Lanzillo, R.; Morra, V.B.; et al. Low Serum 25-Hydroxy-Vitamin D Levels Are Associated with Cognitive Impairment in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2023, 79, 105044. [Google Scholar] [CrossRef]
- Muris, A.H.; Smolders, J.; Rolf, L.; Thewissen, M.; Hupperts, R.; Damoiseaux, J. Immune Regulatory Effects of High Dose Vitamin D3 Supplementation in a Randomized Controlled Trial in Relapsing Remitting Multiple Sclerosis Patients Receiving IFNβ: The SOLARIUM Study. J. Neuroimmunol. 2016, 300, 47–56. [Google Scholar] [CrossRef]
- Tonev, D.; Momchilova, A. Oxidative Stress and the Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Pathway in Multiple Sclerosis: Focus on Certain Exogenous and Endogenous Nrf2 Activators and Therapeutic Plasma Exchange Modulation. Int. J. Mol. Sci. 2023, 24, 17223. [Google Scholar] [CrossRef]
- Maldonado, P.P.; Guevara, C.; Olesen, M.A.; Orellana, J.A.; Quintanilla, R.A.; Ortiz, F.C. Neurodegeneration in Multiple Sclerosis: The Role of Nrf2-Dependent Pathways. Antioxidants 2022, 11, 1146. [Google Scholar] [CrossRef]
- Hashemi, R.; Hosseini-Asl, S.S.; Arefhosseini, S.R.; Morshedi, M. The Impact of Vitamin D3 Intake on Inflammatory Markers in Multiple Sclerosis Patients and Their First-Degree Relatives. PLoS ONE 2020, 15, e0231145. [Google Scholar] [CrossRef]
- Naghavi Gargari, B.; Behmanesh, M.; Shirvani Farsani, Z.; Pahlevan Kakhki, M.; Azimi, A.R. Vitamin D Supplementation Up-Regulates IL-6 and IL-17A Gene Expression in Multiple Sclerosis Patients. Int. Immunopharmacol. 2015, 28, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-Y.; Kim, J.-Y.; Kim, K.-W.; Park, M.-K.; Moon, Y.; Kim, W.-U.; Kim, H.-Y. IL-17 Induces Production of IL-6 and IL-8 in Rheumatoid Arthritis Synovial Fibroblasts via NF-κB- and PI3-Kinase/Akt-Dependent Pathways. Arthritis Res. Ther. 2004, 6, R120. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine Vitamin D Signaling Switches off Pro-Inflammatory Programs of TH1 Cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef]

| Target Tissue | Response |
|---|---|
| Intestine | stimulation of transcellular calcium transport in duodenum and caecum; inducing basolateral CaATPase, PMCA1b [1] |
| Bone | optimal endochondral bone formation, increasing the number and activity of osteoclasts [2,3] |
| Kidney | regulation of calcium (distal tubule) and phosphate (proximal tubule) transport, stimulating VDR, calcium pump and calbindin [4,5,6,7] |
| Parathyroid gland | inhibition of production of parathormon—suppression of the PTH promoter [8] |
| Pancreatic-cells | stimulation of insulin secretion; calbindin-D28k modulates depolarization-stimulated insulin release [9,10] |
| Skin | calcium induced regulation of keratinocyte differentiation; stimulation of wound healing, regulation of hair follicle cycle [11,12,13] |
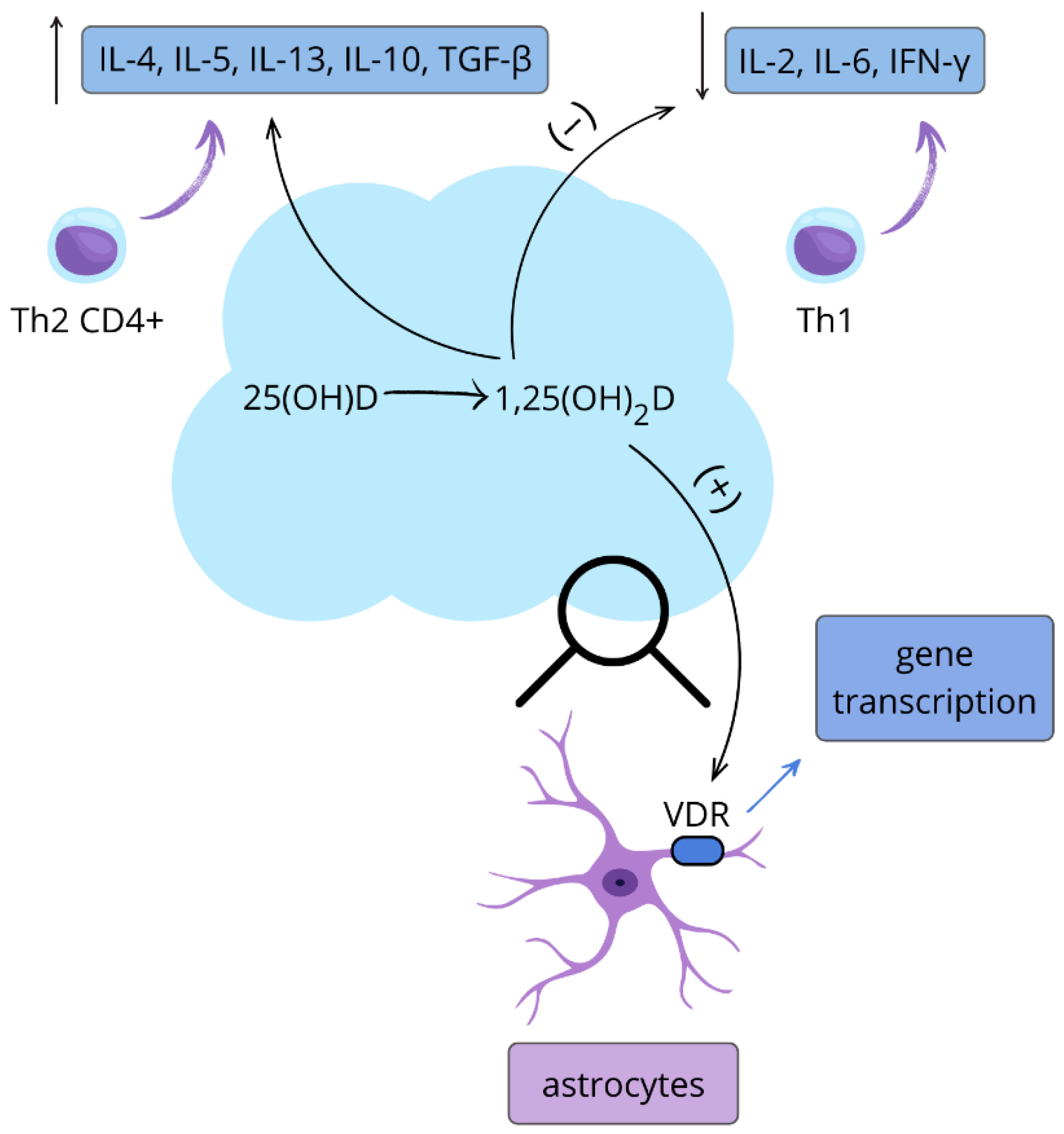
| Immune system | stimulation of cathelicidin, inhibition of the maturation of dendritic cells; reduction in T cell proliferation and shifting the balance of T cell differentiation from the Th1 and Th17 pathways to Th2 and Treg pathways [14,15,16] |
| Heart | increase in contractility of cardiac muscle, stimulation of calcium uptake; inhibition of atrial natriuretic factor’s promoter [17,18] |
| Pituitary gland | increase in the TRH-stimulated TSH secretion through increased Cai and IP3 production [19] |
| Liver | stimulation of hepatic regeneration [20] |
| Lung | stimulation of type TT epithelial pneumocytes through increased phospholipid production and surfactant release [21] |
| Disease | Mechanism of Action of Vitamin D3 | Effect of Supplementation |
|---|---|---|
| Parkinson’s Disease | modulation of microglia towards an anti-inflammatory phenotype [76]; decrease in pro-inflammatory markers and increase in anti-inflammatory markers [76] | Improve motor outcomes and functional capacity [77] |
| Autism Spectrum Disorder | Increased GABA synthesis, support for dopaminergic activity, and increased GDNF levels [82] Increased glutathione production and decreased NO synthesis [83,84] Inhibition of pro-inflammatory cytokine production [88] | Improvement in social functioning, communication, better results in CARS and ATEC tests [90] |
| Alzheimer’s Disease | Reduced beta-amyloid marker concentration [103] Leukocyte telomere elongation [111] Telomere structure stabilization [114] Reduced oxidative stress [111,116] | Improved cognitive function [103] |
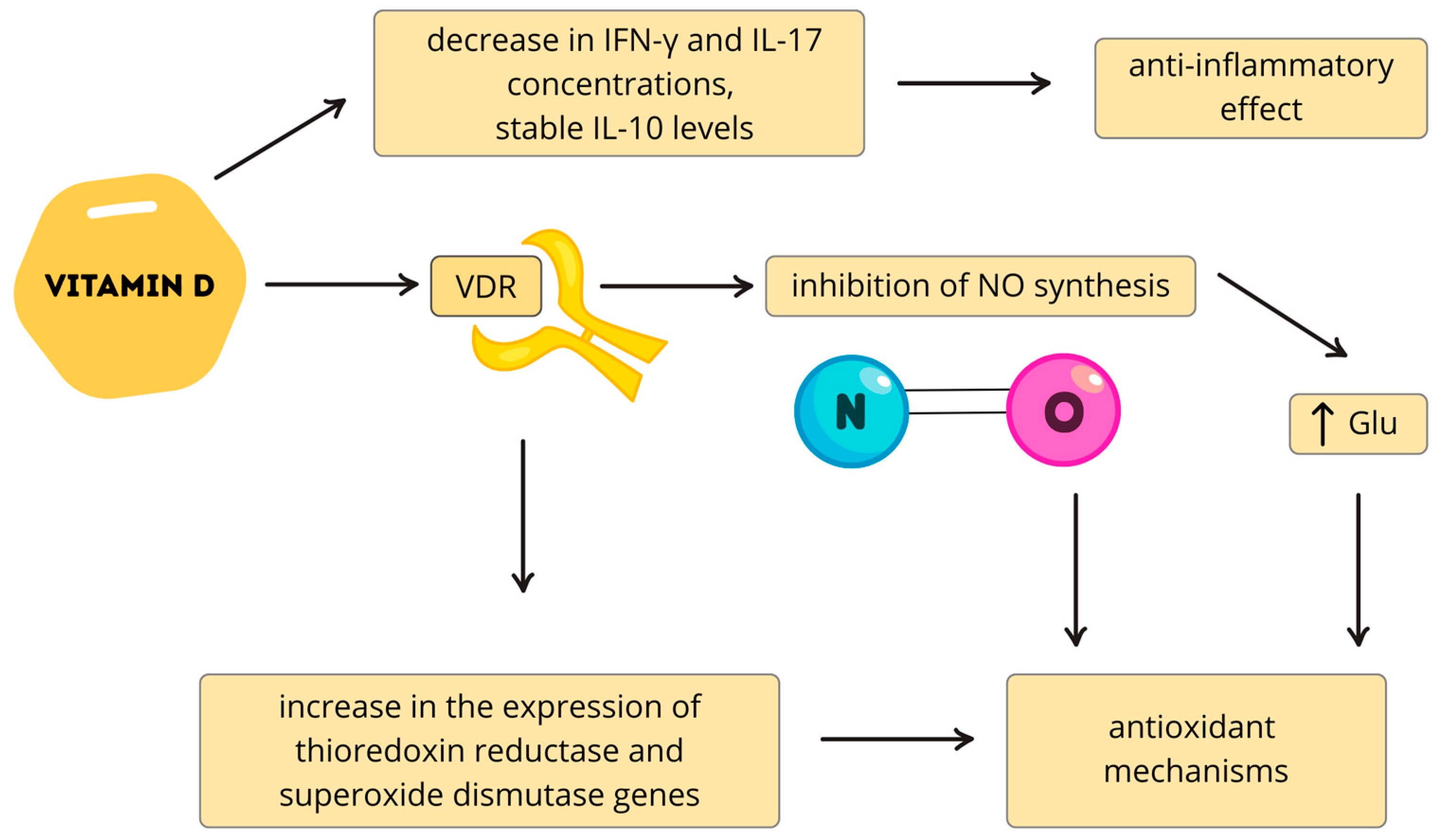
| Multiple Sclerosis | Reduction in EBV antibody levels [119,120]; Inhibition of Th17 lymphocyte activity and IL-17 transcription [89]; reduction in cytokine synthesis produced by: Th1, Th9, and Th22 [131,132] | Reduced risk of CIS → RRMS progression [119,120,121] Reduced relapse frequency in RRMS [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietruszkiewicz, J.; Mrozek, K.; Zwierz, M.; Wińska, A.; Suprunowicz, M.; Oracz, A.J.; Waszkiewicz, N. The Neuroprotective Potential of Vitamin D3. Nutrients 2025, 17, 3202. https://doi.org/10.3390/nu17203202
Pietruszkiewicz J, Mrozek K, Zwierz M, Wińska A, Suprunowicz M, Oracz AJ, Waszkiewicz N. The Neuroprotective Potential of Vitamin D3. Nutrients. 2025; 17(20):3202. https://doi.org/10.3390/nu17203202
Chicago/Turabian StylePietruszkiewicz, Jacek, Katarzyna Mrozek, Mateusz Zwierz, Agata Wińska, Maria Suprunowicz, Aleksandra Julia Oracz, and Napoleon Waszkiewicz. 2025. "The Neuroprotective Potential of Vitamin D3" Nutrients 17, no. 20: 3202. https://doi.org/10.3390/nu17203202
APA StylePietruszkiewicz, J., Mrozek, K., Zwierz, M., Wińska, A., Suprunowicz, M., Oracz, A. J., & Waszkiewicz, N. (2025). The Neuroprotective Potential of Vitamin D3. Nutrients, 17(20), 3202. https://doi.org/10.3390/nu17203202









