Time-Restricted Eating Without Exercise Enhances Anaerobic Power and Reduces Body Weight: A Randomized Crossover Trial in Untrained Adults
Abstract
1. Introduction
2. Methods
2.1. Participants
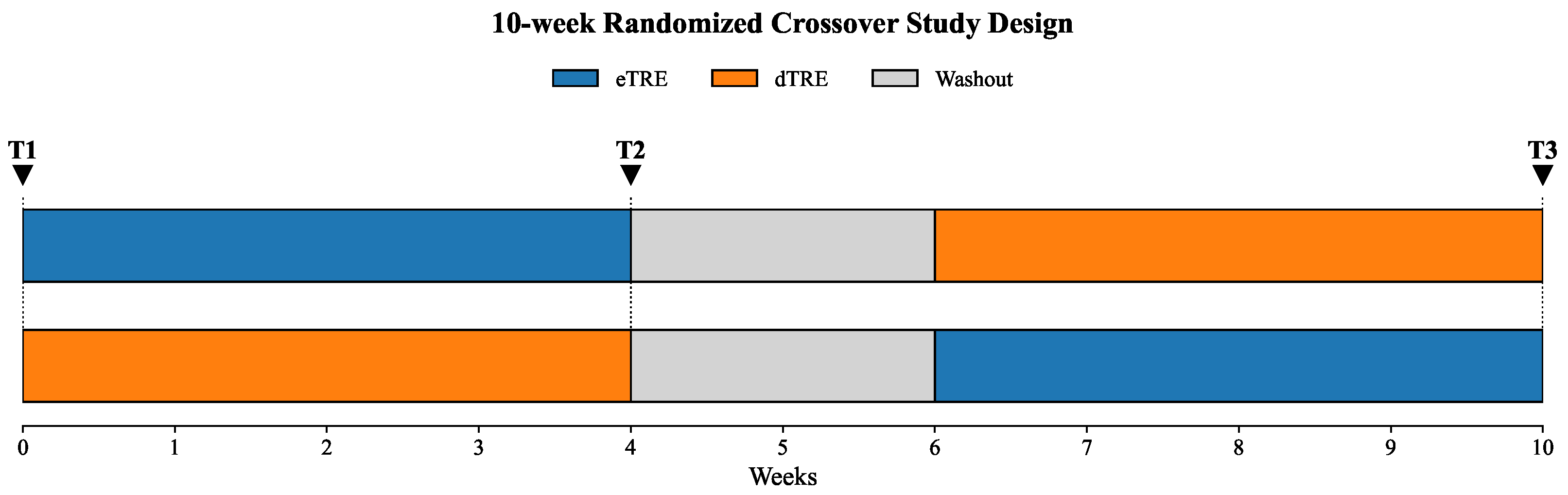
2.2. Study Design
2.3. Dietary Intervention and Compliance
- eTRE Phase:First meal between 08:00 and 09:00; last meal between 14:00 and 15:00.
- dTRE Phase: First meal between 12:00 and 13:00; last meal between 18:00 and 19:00.
2.4. Outcome Measures
2.4.1. Body Weight
2.4.2. Anaerobic Power
2.4.3. Aerobic Endurance
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics and Summary of Outcomes
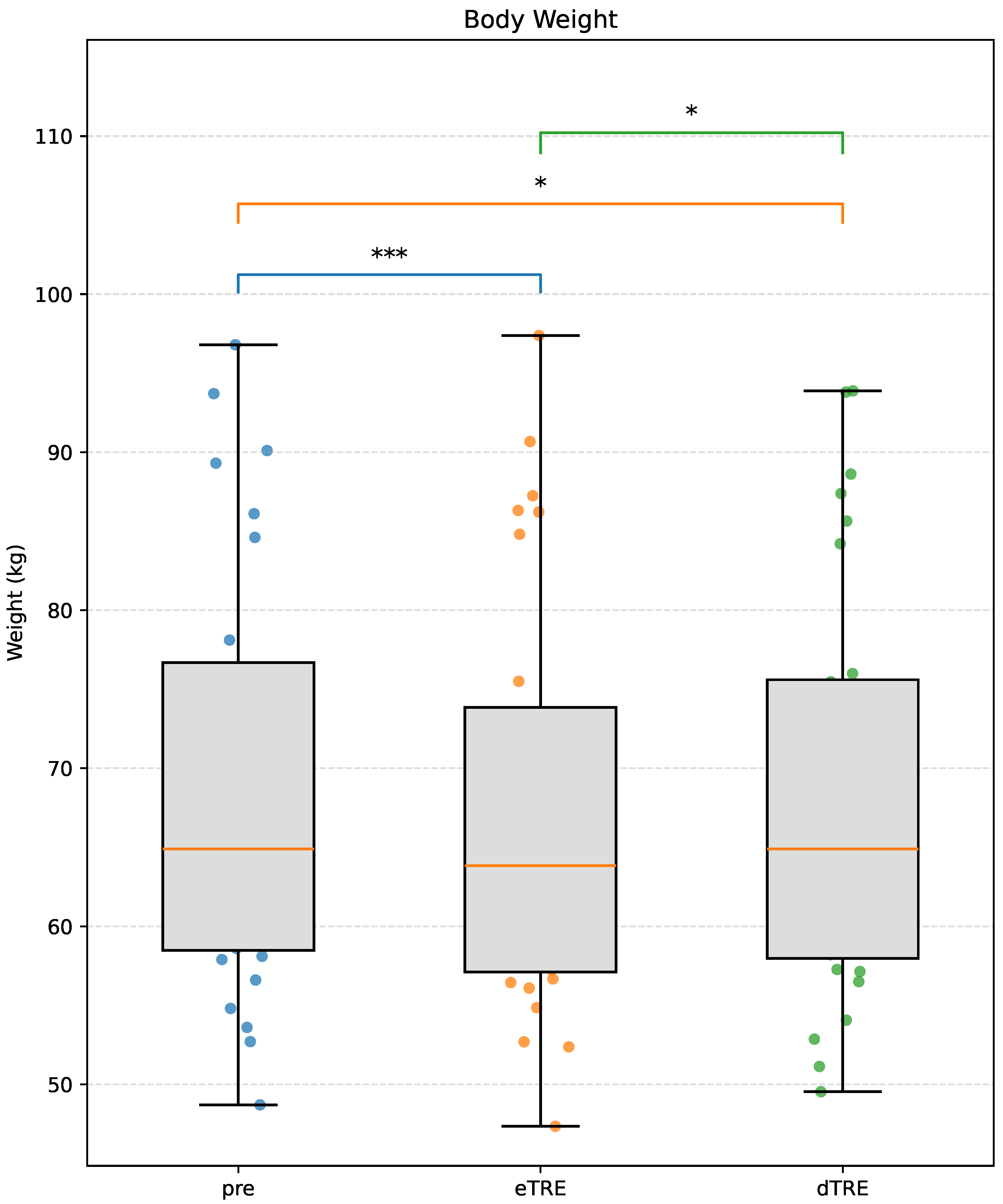
3.2. Body Weight
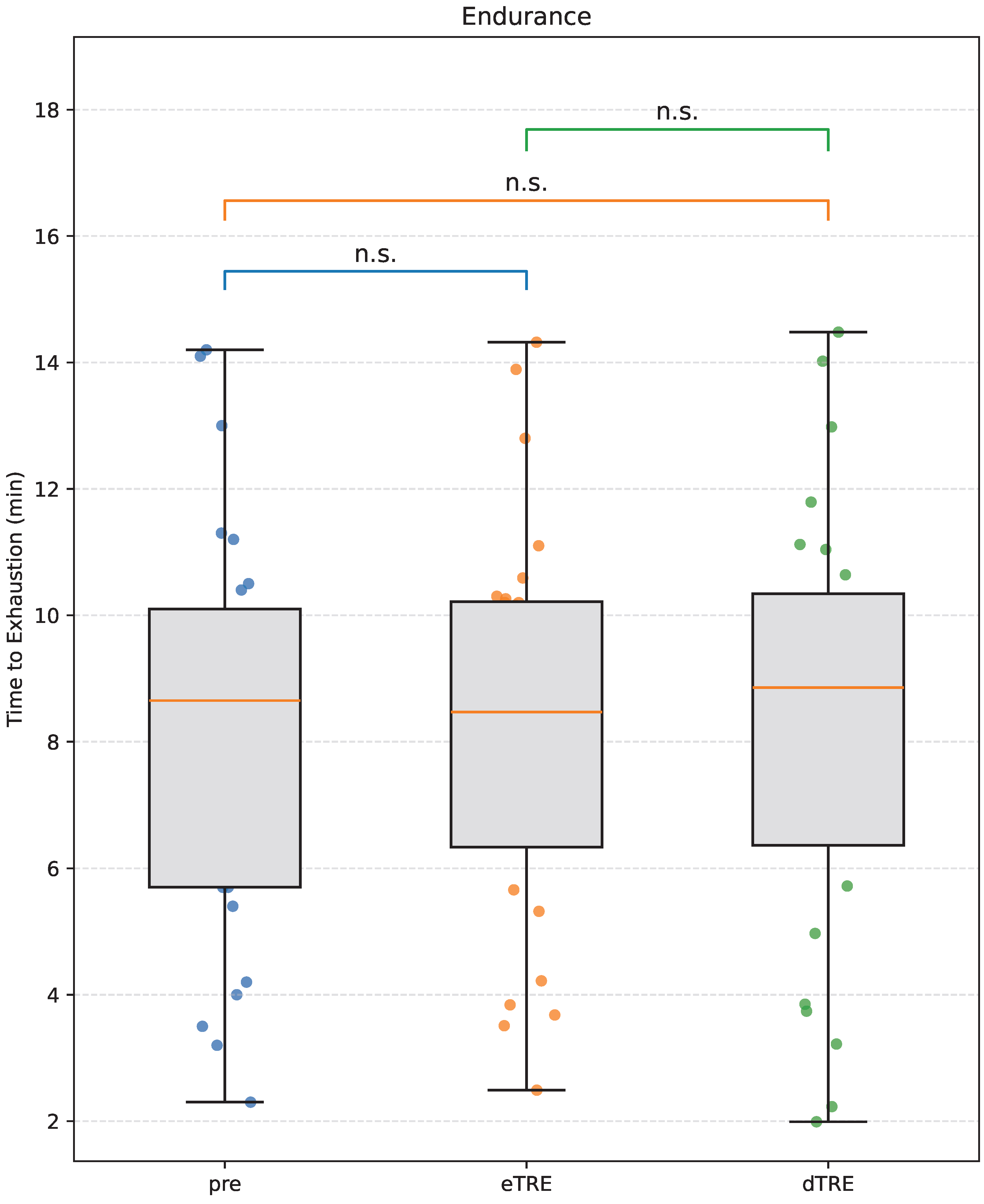
3.3. Aerobic Endurance
3.4. Anaerobic Power
4. Discussion
4.1. eTRE Leads to Greater Weight Loss than dTRE
4.2. Aerobic Endurance Is Maintained During TRE
4.3. Anaerobic Power Gains Without Structured Training
4.4. Sex-Specific Considerations
4.5. Practical Implications
4.6. Limitations and Directions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TRE | time-restricted eating |
| eTRE | early time-restricted eating |
| dTRE | delayed time-restricted eating |
| HIIT | high-intensity interval training |
| rpm | revolutions per minute |
| LMMs | linear mixed-effects models |
References
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef]
- Kodama, S.; Tanaka, S.; Saito, K.; Shu, M.; Sone, Y.; Onitake, F.; Suzuki, E.; Shimano, H.; Yamamoto, S.; Kondo, K.; et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: A meta-analysis. Arch. Intern. Med. 2007, 167, 999–1008. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Clevel. Clin. J. Med. 2017, 84, S15. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. Bmj Open Sport Exerc. Med. 2017, 2, e000143. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef]
- Reid, K.F.; Fielding, R.A. Skeletal muscle power: A critical determinant of physical functioning in older adults. Exerc. Sport Sci. Rev. 2012, 40, 4–12. [Google Scholar] [CrossRef]
- Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food timing, circadian rhythm and chrononutrition: A systematic review of time-restricted eating’s effects on human health. Nutrients 2020, 12, 3770. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves 24-h glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar] [CrossRef]
- Villanueva, J.E.; Livelo, C.; Trujillo, A.S.; Chandran, S.; Woodworth, B.; Andrade, L.; Le, H.D.; Manor, U.; Panda, S.; Melkani, G.C. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat. Commun. 2019, 10, 2700. [Google Scholar] [CrossRef]
- Marosi, K.; Moehl, K.; Navas-Enamorado, I.; Mitchell, S.J.; Zhang, Y.; Lehrmann, E.; Aon, M.A.; Cortassa, S.; Becker, K.G.; Mattson, M.P. Metabolic and molecular framework for the enhancement of endurance by intermittent food deprivation. Faseb J. 2018, 32, 3844. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Longo, G.; Grigoletto, D.; Bianco, A.; Ferraris, C.; Guglielmetti, M.; Veneto, A.; Tagliabue, A.; Marcolin, G.; et al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Diab, R.; Dimachkie, L.; Zein, O.; Dakroub, A.; Eid, A.H. Intermittent fasting regulates metabolic homeostasis and improves cardiovascular health. Cell Biochem. Biophys. 2024, 82, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Lundell, L.S.; Parr, E.B.; Devlin, B.L.; Ingerslev, L.R.; Altıntaş, A.; Sato, S.; Sassone-Corsi, P.; Barrès, R.; Zierath, J.R.; Hawley, J.A. Time-restricted feeding alters lipid and amino acid metabolite rhythmicity without perturbing clock gene expression. Nat. Commun. 2020, 11, 4643. [Google Scholar] [CrossRef] [PubMed]
- Hepler, C.; Weidemann, B.J.; Waldeck, N.J.; Marcheva, B.; Cedernaes, J.; Thorne, A.K.; Kobayashi, Y.; Nozawa, R.; Newman, M.V.; Gao, P.; et al. Time-restricted feeding mitigates obesity through adipocyte thermogenesis. Science 2022, 378, 276–284. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Jones, R.; Pabla, P.; Mallinson, J.; Nixon, A.; Taylor, T.; Bennett, A.; Tsintzas, K. Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am. J. Clin. Nutr. 2020, 112, 1015–1028. [Google Scholar] [CrossRef]
- Conde-Pipó, J.; Mora-Fernandez, A.; Martinez-Bebia, M.; Gimenez-Blasi, N.; Lopez-Moro, A.; Latorre, J.A.; Almendros-Ruiz, A.; Requena, B.; Mariscal-Arcas, M. Intermittent fasting: Does it affect sports performance? A systematic review. Nutrients 2024, 16, 168. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; De Cabo, R. A time to fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., III; Leeuwenburgh, C.; Mattson, M.P. Flipping the metabolic switch: Understanding and applying the health benefits of fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4-and 6-h time-restricted feeding on weight and cardiometabolic health: A randomized controlled trial in adults with obesity. Cell Metab. 2020, 32, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, J.; Zhao, Y.; He, Y.; Sun, N. Intermittent fasting, fatty acid metabolism reprogramming, and neuroimmuno microenvironment: Mechanisms and application prospects. Front. Nutr. 2024, 11, 1485632. [Google Scholar] [CrossRef]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef]
- Manoogian, E.N.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef]
- Regmi, P.; Heilbronn, L.K. Time-restricted eating: Benefits, mechanisms, and challenges in translation. iScience 2020, 23, 101161. [Google Scholar] [CrossRef]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2019, 29, 303–319. [Google Scholar] [CrossRef]
- Menzies, F.M.; Fleming, A.; Caricasole, A.; Bento, C.F.; Andrews, S.P.; Ashkenazi, A.; Füllgrabe, J.; Jackson, A.; Sanchez, M.J.; Karabiyik, C.; et al. Autophagy and neurodegeneration: Pathogenic mechanisms and therapeutic opportunities. Neuron 2017, 93, 1015–1034. [Google Scholar] [CrossRef]
- Sebastian, D.; Zorzano, A. Self-eating for muscle fitness: Autophagy in the control of energy metabolism. Dev. Cell 2020, 54, 268–281. [Google Scholar] [CrossRef]
- Witt, C.R.; Grozier, C.D.; Killen, L.G.; Renfroe, L.G.; O’Neal, E.K.; Waldman, H.S. A self-selected 16: 8 time-restricted eating protocol improves fat oxidation rates, markers of cardiometabolic health, and 10-km cycling performance in middle-age male cyclists. J. Strength Cond. Res. 2023, 37, 1117–1123. [Google Scholar] [CrossRef]
- De Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Abaidia, A.E.; Daab, W.; Bouzid, M.A. Effects of Ramadan fasting on physical performance: A systematic review with meta-analysis. Sports Med. 2020, 50, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Meckel, Y.; Ismaeel, A.; Eliakim, A. The effect of the Ramadan fast on physical performance and dietary habits in adolescent soccer players. Eur. J. Appl. Physiol. 2008, 102, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Kordi, R.; Abdollahi, M.; Memari, A.H.; Najafabadi, M.G. Investigating two different training time frames during Ramadan fasting. Asian J. Sports Med. 2011, 2, 205. [Google Scholar] [CrossRef]
- Maughan, R.; Shirreffs, S. Hydration and performance during Ramadan. J. Sports Sci. 2012, 30, S33–S41. [Google Scholar] [CrossRef]
- Liu, J.; Yi, P.; Liu, F. The effect of early time-restricted eating vs later time-restricted eating on weight loss and metabolic health. J. Clin. Endocrinol. Metab. 2023, 108, 1824–1834. [Google Scholar] [CrossRef]
- Shimizu, H.; Hanzawa, F.; Kim, D.; Sun, S.; Laurent, T.; Umeki, M.; Ikeda, S.; Mochizuki, S.; Oda, H. Delayed first active-phase meal, a breakfast-skipping model, led to increased body weight and shifted the circadian oscillation of the hepatic clock and lipid metabolism-related genes in rats fed a high-fat diet. PLoS ONE 2018, 13, e0206669. [Google Scholar] [CrossRef]
- Petridi, F.; Geurts, J.M.; Nyakayiru, J.; Schaafsma, A.; Schaafsma, D.; Meex, R.C.; Singh-Povel, C.M. Effects of Early and Late Time-Restricted Feeding on Parameters of Metabolic Health: An Explorative Literature Assessment. Nutrients 2024, 16, 1721. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Regmi, P.; Chaudhary, R.; Page, A.J.; Hutchison, A.T.; Vincent, A.D.; Liu, B.; Heilbronn, L. Early or delayed time-restricted feeding prevents metabolic impact of obesity in mice. J. Endocrinol. 2021, 248, 75–86. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Zupan, M.F.; Arata, A.W.; Dawson, L.H.; Wile, A.L.; Payn, T.L.; Hannon, M.E. Wingate anaerobic test peak power and anaerobic capacity classifications for men and women intercollegiate athletes. J. Strength Cond. Res. 2009, 23, 2598–2604. [Google Scholar] [CrossRef]
- Coakley, S.L.; Passfield, L. Cycling performance is superior for time-to-exhaustion versus time-trial in endurance laboratory tests. J. Sports Sci. 2018, 36, 1228–1234. [Google Scholar] [CrossRef]
- Xiao, Q.; Garaulet, M.; Scheer, F.A. Meal timing and obesity: Interactions with macronutrient intake and chronotype. Int. J. Obes. 2019, 43, 1701–1711. [Google Scholar] [CrossRef]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The influence of meal frequency and timing on health in humans: The role of fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef]
- Richter, J.; Herzog, N.; Janka, S.; Baumann, T.; Kistenmacher, A.; Oltmanns, K.M. Twice as high diet-induced thermogenesis after breakfast vs dinner on high-calorie as well as low-calorie meals. J. Clin. Endocrinol. Metab. 2020, 105, e211–e221. [Google Scholar] [CrossRef]
- Boege, H.L.; Bhatti, M.Z.; St-Onge, M.P. Circadian rhythms and meal timing: Impact on energy balance and body weight. Curr. Opin. Biotechnol. 2021, 70, 1–6. [Google Scholar] [CrossRef]
- Teo, W.; Newton, M.J.; McGuigan, M.R. Circadian rhythms in exercise performance: Implications for hormonal and muscular adaptation. J. Sports Sci. Med. 2011, 10, 600. [Google Scholar]
- Martin-López, J.; Pérez-López, A.; Varillas-Delgado, D.; López-Samanes, Á. Influence of time-of-day on neuromuscular performance in team sport athletes: A systematic review and meta-analysis. Front. Sports Act. Living 2025, 6, 1466050. [Google Scholar] [CrossRef]
- Souissi, A.; Yousfi, N.; Souissi, N.; Haddad, M.; Driss, T. The effect of diurnal variation on the performance of exhaustive continuous and alternated-intensity cycling exercises. PLoS ONE 2020, 15, e0244191. [Google Scholar] [CrossRef]
- Malo-Vintimilla, L.; Aguirre, C.; Vergara, A.; Fernández-Verdejo, R.; Galgani, J.E. Resting energy metabolism and sweet taste preference during the menstrual cycle in healthy women. Br. J. Nutr. 2024, 131, 384–390. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Corapi, S.; Gabel, K.; Ezpeleta, M.; Kalam, F.; Lin, S.; Pavlou, V.; Varady, K.A. Effect of intermittent fasting on reproductive hormone levels in females and males: A review of human trials. Nutrients 2022, 14, 2343. [Google Scholar] [CrossRef]

| Variable | Mean ± SD |
|---|---|
| Age (years) | 23.47 ± 2.87 |
| Body Weight (kg) | 68.71 ± 13.52 |
| Height (cm) | 171.45 ± 10.16 |
| BMI (kg/m2) | 23.37 ± 5.37 |
| Aerobic Endurance (min) | 8.22 ± 3.21 |
| Anaerobic Power (W) | 581.79 ± 152.93 |
| Variable | Phase | Mean ± SD | Comparison | p-Value | Cohen’s |
|---|---|---|---|---|---|
| Body Weight (kg) | Pre | 68.71 ± 13.52 | Pre vs. eTRE | <0.001 *** | 1.17 |
| eTRE | 67.15 ± 13.35 | Pre vs. dTRE | 0.022 * | 0.55 | |
| dTRE | 68.10 ± 13.22 | eTRE vs. dTRE | 0.020 * | 0.56 | |
| Aerobic Endurance (min) | Pre | 8.22 ± 3.21 | Pre vs. eTRE | n.s. | — |
| eTRE | 8.23 ± 3.13 | Pre vs. dTRE | n.s. | — | |
| dTRE | 8.33 ± 3.36 | eTRE vs. dTRE | n.s. | — | |
| Anaerobic Power (W) | Pre | 581.79 ± 152.93 | Pre vs. eTRE | <0.001 *** | 1.10 |
| eTRE | 603.04 ± 153.41 | Pre vs. dTRE | <0.001 *** | 1.20 | |
| dTRE | 617.21 ± 156.83 | eTRE vs. dTRE | 0.025 * | 0.54 | |
| Energy Intake (kcal) | Pre | 2163.96 ± 513.66 | Pre vs. eTRE | <0.001 *** | 0.51 |
| eTRE | 1902.57 ± 525.62 | Pre vs. dTRE | <0.001 *** | 0.46 | |
| dTRE | 1967.04 ± 567.11 | eTRE vs. dTRE | 0.040 * | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Ueda, T. Time-Restricted Eating Without Exercise Enhances Anaerobic Power and Reduces Body Weight: A Randomized Crossover Trial in Untrained Adults. Nutrients 2025, 17, 3011. https://doi.org/10.3390/nu17183011
Yu Z, Ueda T. Time-Restricted Eating Without Exercise Enhances Anaerobic Power and Reduces Body Weight: A Randomized Crossover Trial in Untrained Adults. Nutrients. 2025; 17(18):3011. https://doi.org/10.3390/nu17183011
Chicago/Turabian StyleYu, Zifu, and Takeshi Ueda. 2025. "Time-Restricted Eating Without Exercise Enhances Anaerobic Power and Reduces Body Weight: A Randomized Crossover Trial in Untrained Adults" Nutrients 17, no. 18: 3011. https://doi.org/10.3390/nu17183011
APA StyleYu, Z., & Ueda, T. (2025). Time-Restricted Eating Without Exercise Enhances Anaerobic Power and Reduces Body Weight: A Randomized Crossover Trial in Untrained Adults. Nutrients, 17(18), 3011. https://doi.org/10.3390/nu17183011






