Hydroxytyrosol Bioavailability: Unraveling Influencing Factors and Optimization Strategies for Dietary Supplements
Abstract
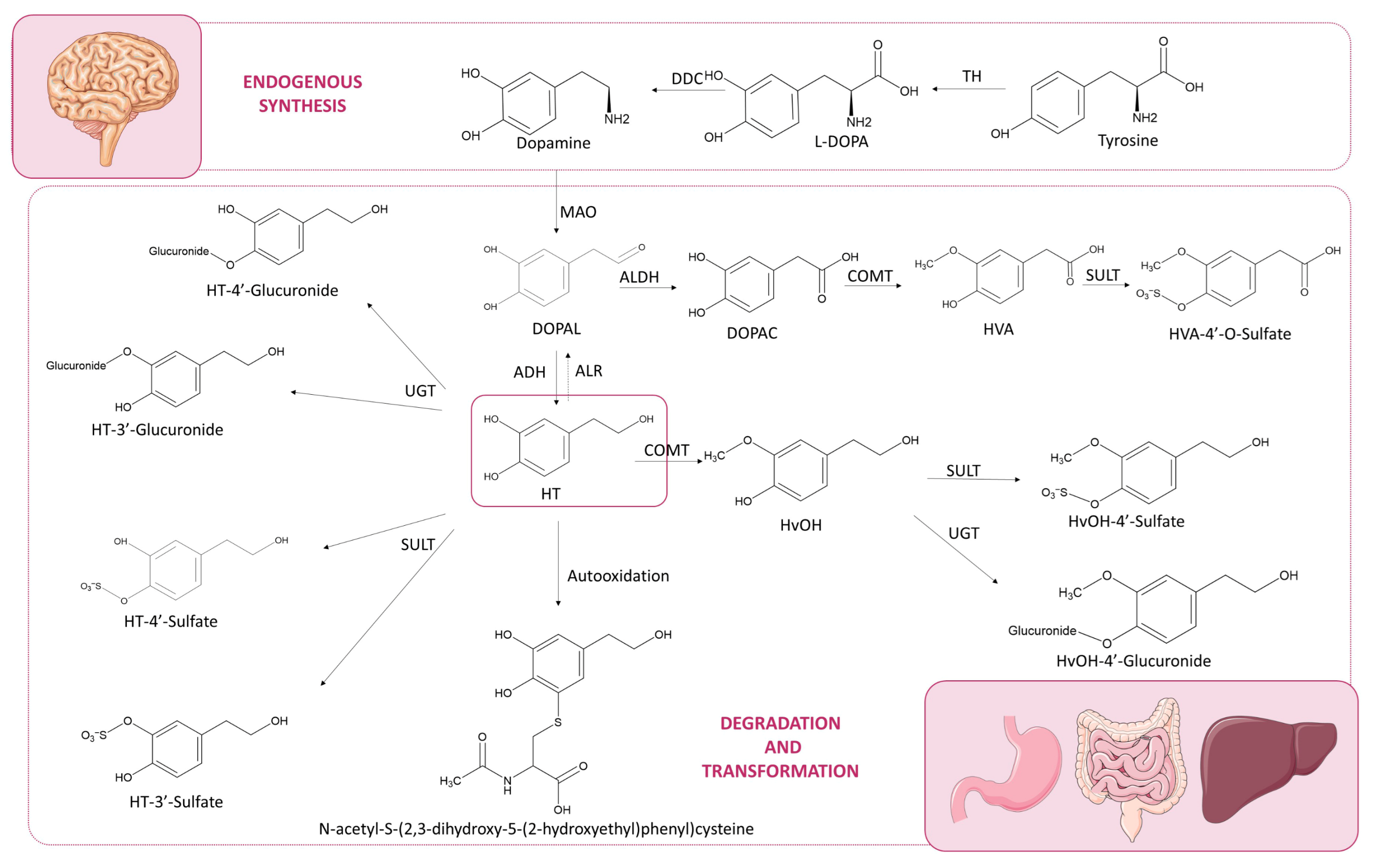
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Hydroxytyrosol Bioavailability from Olive Oil
| Ref. | Overview | HT Source | Administered Dose | Plasma Presence | Urine | ||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Peak Time | Maximum Concentration | Metabolites | Peak Time | Total Amounts/Concentrations | ||||
| [21] | Bioavailability study after methodological development for plasma and urine sample analysis for quantification of HT and 3-O-methyl HT and the evaluation of extensive intestinal and hepatic metabolism | Virgin olive oil (VOO) | 25 mL VOO | HT 3-O-methyl-HT | 32 min (0.58 h) 53 min (0.88 h) | 25.83 μg/L 3.94 ug/L | HT 3-O-methyl-HT | 0–2 h | 300–400 ug ** ~100 ug |
| [22] | Characterization of olive polyphenol absorption and metabolism, with detection of glucuronide conjugates and DOPAC, early peak concentrations in plasma, and enhancement of systemic antioxidant status (n = 8) | kalamon olives | 100 g kalamon olives | HT HvOH HVA DOPAC | 60 min 60 min 60 min 60 min | 3.145 ± 0.341 mg/mL 0.122 ± 0.013 mg/mL 2.263 ± 0.170 mg/mL 14.742 ± 0.941 mg/mL | HT HvOH HVA DOPAC | 0–4 h 0–4 h 0–4 h 0–4 h | 4.762 ± 1.296 mg/h ** 9.116 ± 3.596 mg/h 0.025 ± 0.004 mg/h 17.866 ± 11.275 mg/h |
| [23] | Method development to detect free HT in extra virgin olive oil allowed to detect EVOO CMax peak at 15 min | Extra Virgin olive oil (EVOO) | 1.38 mg HT (25 mL) | Not determined Only free HT | 15 min | 4.4 ng/mL | |||
| [15] | Evaluation of the enrichment of EVOO through a bioavailability study. Results indicated higher levels of HT sulfate and vanillin sulfate | Fortified (EVOO) and VOO | VOO (Control) 30 mL Fortified EVOO: 30 mL (6× phenol content) | HT-S HVA derivative HVA-S HT-S HVA derivative HVA-S | 60 min 125 min | 0.53 ± 0.30 µmol/L 0.53 ± 0.29 µmol/L 0.78 ± 0.46 µmol/L 0.86 ± 0.24 µmol/L 0.34 ± 0.10 µmol/L 0.96 ± 0.88 µmol/L | |||
| [18] | Demonstration of matrix-dependent bioavailability, with extra virgin olive oil identified as the optimal matrix Results are summarized in different corresponding tables. HT maximum concentration peak was detected at 30 min. (n = 20) | HT added to EVOO HT added to refined olive oil | EVOO 5 mg HT in 20 mg refined olive oil | HT HT | 30 min 30 min | 3.79 ng/mL 1.5 ng/mL | HT HT-Acetate DOPAC HvOH HT HT-Acetate DOPAC HvOH | 0.86 μg/mg creatinine ~50 μg/mg creatinine ~40 μg/mg creatinine ~25 μg/mg creatinine 0.63 μg/mg creatinine ~100 μg/mg creatinine ~50 μg/mg creatinine ~20 μg/mg creatinine | |
| [24] | Characterization of HT bioavailability, demonstrating COMT-mediated metabolism and dose-dependent formation of metabolites, as assessed by homovanillic alcohol and acid levels. | Four VOO samples accompanied by 40 g of bread | 7 mg HT in 50 mL oil 13.3 mg HT in 50 mL oil 19.2 mg HT in 50 mL oil 23.2 mg HT in 50 mL oil | HT HvOH HVA HT HvOH HVA HT HvOH HVA HT HvOH HVA | 267 μg (16.8%) * 367 μg (22%) 1027 μg (61.8%) 1328 μg (29.8%) 749 μg (16.8%) 2380 μg (53.4%) 1112 μg (21.8%) 1137 μg (22.3%) 2837 μg (55.8%) 1653 μg (23.7%) 1567 μg (22.4%) 3752 μg (53.9%) | ||||
| [30] | Trial-based investigation (n = 7) of tyrosol and HT absorption after moderate and sustained virgin olive oil intake, and evaluation of their potential as intake biomarkers. Higher HT recovery was observed after sustained virgin olive oil intake compared to a single-dose administration | VOO | Single dose: 50 mL VOO (1370 µg HT) (1720 µg tyrosol) Sustained doses: 25 mL/day during a week (685 µg HT) (860 µg tyrosol) | Single dose: HT Sustained doses: HT | 78.5 ± 14.1% 121 ± 45.9% | ||||
| [31] | Intervention study (n = 11) carried out to identify the main conjugated metabolites derived from free phenols, with the aim of assessing their chemical and in vitro biological antioxidant activities at physiologically relevant concentrations. Glucuronide conjugates of HT and homovanillic alcohol (HvOH) were measured. However, these metabolites did not exhibit significant antioxidant activity under the tested concentrations (0.01–10 µM) | VOO | 50 mL | T-4-G HvOH-4-G HT-4-G HT-3-G | 0–6 h 0–6 h 0–6 h 0–6 h | 6.65 ± 2.91 μM 2.66 ± 1.18 μM 3.89 ± 1.69 μM 2.32 ± 1.31 μM | |||
| [32] | Validation of a UPLC-MRM method for the simultaneous quantification of glucuronidated metabolites of olive oil phenols in human urine, showing that approximately 13% of the ingested dose was excreted within 24 h, mainly as glucuronidated forms | VOO | 50 mL VOO with bread (22.0 ± 1.4 µmoles HT ≈ 3.38 ± 0.22 mg HT) | HT HT-4-G HT-3-G HvOH HvOH-4-G | 0–6 h 0–6 h 0–6 h 0–6 h 0–6 h | 0.39 µmol 0.67 µmol 0.95 µmol 0.31 µmol 0.63 µmol | |||
| [33] | A randomized cross-over study in 12 healthy volunteers evaluated the pharmacokinetics of phenolic metabolites from three phenol-enriched virgin olive oils revealing a dose-dependent increase in HT sulfate (main plasma metabolite) and identifying HT acetate sulfate as a key novel metabolite. | Three phenol enriched VOO 250, 500, 750 mg/kg total phenols | 2.23 mg total oleuropein derivatives (30 mL oil) 6.27 mg total oleuropein derivatives (30 mL oil) 10.87 mg total oleuropein derivatives (30 mL oil) | HT-S HT- Ac-S HVA HVA-S HT-S HT-Ac-S HVA HVA-S HT-S HT- Ac-S HVA HVA-S | 1 h 1 h 1.5 h 1 h 1 h 2 h 1 h 1 h 1.5 h 1 h 1 h 1 h | 1.35 ± 0.72 µmol/L 0.46 ± 0.26 µmol/L 0.17 ± 0.19 µmol/L 0.12 ± 0.15 µmol/L 3.32 ± 1.56 µmol/L 1.89 ± 1.63 µmol/L 0.63 ± 0.30 µmol/L 0.27 ± 0.25 µmol/L 4.09 ± 1.99 µmol/L 2.24 ± 0.51 µmol/L 0.65 ± 0.41 µmol/L 0.53 ± 0.62 µmol/L | |||
| [34] | Bioavailability study of fortified oil vs. other aqueous enriched extracts. HVA and DOPAC were the main metabolites found in urine followed by HT-3-S | HT-Fortified olive oil | 25 mL oil | HT-3-S HT-4-S HT-3-G HT-4-G HVA DOPAC | 2.57 ± 1.17 μmol * 0.05 ± 0.19 µmol 0.06 ± 0.09 µmol 0.01 ± 0.02 µmol 4.82 ± 3.93 µmol 4.53 ± 1.76 µmol | ||||
| [35] | Evaluation of HT pharmacokinetics showed that an enteric coated EVOO based capsule significantly improved its bioavailability, highlighting the impact of formulation (n = 20) | HT enteric coated EVOOO based capsule | 7.5 mg HT | HT | 123 min | 4.493 ± 0.106 ng/mL | Sulfo-conjugated derivatives of HT HVA Glucurono-conjugated derivatives DOPAC HT | 6 h 6 h 6 h 6 h 6 h | 19.46 µmol 18.39 μmol 11.48 μmol 9.93 μmol 4.67 μmol |
3.2. Hydroxytyrosol Bioavailability from Supplements and Non-Olive Oil Matrixes
3.3. Approaches to Modulate Bioavailability, Stability and Antioxidant Capacity of Hydroxytyrosol
3.3.1. Emulsions
3.3.2. Liposomes
3.3.3. Encapsulation Systems
3.3.4. Chemical Modification: Phenolipids
3.4. Gut Microbiota and Other Interindividual Factors Influencing Hydroxytyrosol Bioavailability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and Other Biological Activities of Phenols from Olives and Olive Oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Fernández-Prior, Á.; Bermúdez-Oria, A.; Millán-Linares, M.D.C.; Fernández-Bolaños, J.; Espejo-Calvo, J.A.; Rodríguez-Gutiérrez, G. Anti-Inflammatory and Antioxidant Activity of Hydroxytyrosol and 3,4-Dihydroxyphenyglycol Purified from Table Olive Effluents. Foods 2021, 10, 227. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Galli, C. Free Radical-Scavenging Properties of Olive Oil Polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef]
- Carrera-González, M.P.; Ramírez-Expósito, M.J.; Mayas, M.D.; Martínez-Martos, J.M. Protective Role of Oleuropein and Its Metabolite Hydroxytyrosol on Cancer. Trends Food Sci. Technol. 2013, 31, 92–99. [Google Scholar] [CrossRef]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of Phenols, Flavones, and Lignans in Virgin Olive Oils by Solid-Phase Extraction and High-Performance Liquid Chromatography with Diode Array Ultraviolet Detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL-Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper Respiratory Tract Health” (ID 3468), “Can Help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- Djaoudene, O.; Romano, A.; Bradai, Y.D.; Zebiri, F.; Ouchene, A.; Yousfi, Y.; Amrane-Abider, M.; Sahraoui-Remini, Y.; Madani, K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients 2023, 15, 3320. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of Hydroxytyrosol as a Novel Food Pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e04728. [Google Scholar] [CrossRef]
- Kundisova, I.; Colom, H.; Juan, M.E.; Planas, J.M. Pharmacokinetics of Hydroxytyrosol and Its Sulfate and Glucuronide Metabolites after the Oral Administration of Table Olives to Sprague-Dawley Rats. J. Agric. Food Chem. 2024, 72, 2154–2164. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Partitioning of Antioxidants in Edible Oil–Water Binary Systems and in Oil-in-Water Emulsions. Antioxidants 2023, 12, 828. [Google Scholar] [CrossRef]
- Micheli, L.; Bertini, L.; Bonato, A.; Villanova, N.; Caruso, C.; Caruso, M.; Bernini, R.; Tirone, F. Role of Hydroxytyrosol and Oleuropein in the Prevention of Aging and Related Disorders: Focus on Neurodegeneration, Skeletal Muscle Dysfunction and Gut Microbiota. Nutrients 2023, 15, 1767. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, Toxicity, and Clinical Applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive Oil Phenolics Are Dose-dependently Absorbed in Humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Bonanome, A.; Pagnan, A.; Caruso, D.; Toia, A.; Xamin, A.; Fedeli, E.; Berra, B.; Zamburlini, A.; Ursini, F.; Galli, G. Evidence of Postprandial Absorption of Olive Oil Phenols in Humans. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 111–120. [Google Scholar] [PubMed]
- Suárez, M.; Valls, R.M.; Romero, M.P.; MacIà, A.; Fernández, S.; Giralt, M.; Solà, R.; Motilva, M.J. Bioavailability of Phenols from a Phenol-Enriched Olive Oil. Br. J. Nutr. 2011, 106, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Macia, A.; Valls, R.M.; Pedret, A.; Romero, M.-P.; Sola, R.; Motilva, M.-J. A New Hydroxytyrosol Metabolite Identified in Human Plasma: Hydroxytyrosol Acetate Sulphate. Food Chem. 2012, 134, 1132–1136. [Google Scholar] [CrossRef]
- de Bock, M.; Thorstensen, E.B.; Derraik, J.G.B.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human Absorption and Metabolism of Oleuropein and Hydroxytyrosol Ingested as Olive (Olea europaea L.) Leaf Extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, Á. Pharmacokinetics and Bioavailability of Hydroxytyrosol Are Dependent on the Food Matrix in Humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Galmés, S.; Reynés, B.; Palou, M.; Palou-March, A.; Palou, A. Absorption, Distribution, Metabolism, and Excretion of the Main Olive Tree Phenols and Polyphenols: A Literature Review. J. Agric. Food Chem. 2021, 69, 5281–5296. [Google Scholar] [CrossRef]
- Nikou, T.; Sakavitsi, M.E.; Kalampokis, E.; Halabalaki, M. Metabolism and Bioavailability of Olive Bioactive Constituents Based on In Vitro, In Vivo and Human Studies. Nutrients 2022, 14, 3773. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E.; Covas, M.-I.; Farre, M.; Fito, M.; Ortuño, J.; Weinbrenner, T.; Roset, P.; De La Torre, R. Hydroxytyrosol Disposition in Humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef]
- Kountouri, A.M.; Mylona, A.; Kaliora, A.C.; Andrikopoulos, N.K. Bioavailability of the Phenolic Compounds of the Fruits (Drupes) of Olea europaea (Olives): Impact on Plasma Antioxidant Status in Humans. Phytomedicine 2007, 14, 659–667. [Google Scholar] [CrossRef]
- Pastor, A.; Rodriguez-Morato, J.; Olesti, E.; Pujadas, M.; Perez-Mana, C.; Khymenets, O.; Fito, M.; Covas, M.-I.; Sola, R.; Motilva, M.-J.; et al. Analysis of Free Hydroxytyrosol in Human Plasma Following the Administration of Olive Oil. J. Chromatogr. A 2016, 1437, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Visioli, F.; Patelli, R.; Galli, C.; Galli, G. Urinary Excretion of Olive Oil Phenols and Their Metabolites in Humans. Metab. Clin. Exp. 2001, 50, 1426–1428. [Google Scholar] [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and Metabolism of Hydroxytyrosol, a Natural Antioxidant from Olive Oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Raneva, V.; Shimasaki, H.; Ishida, Y.; Ueta, N.; Niki, E. Antioxidative Activity of 3,4-Dihydroxyphenylacetic Acid and Caffeic Acid in Rat Plasma. Lipids 2001, 36, 1111–1116. [Google Scholar] [CrossRef]
- Kotronoulas, A.; Pizarro, N.; Serra, A.; Robledo, P.; Joglar, J.; Rubió, L.; Hernaéz, Á.; Tormos, C.; Motilva, M.J.; Fitó, M.; et al. Dose-Dependent Metabolic Disposition of Hydroxytyrosol and Formation of Mercapturates in Rats. Pharmacol. Res. 2013, 77, 47–56. [Google Scholar] [CrossRef]
- Serreli, G.; Le Sayec, M.; Diotallevi, C.; Teissier, A.; Deiana, M.; Corona, G. Conjugated Metabolites of Hydroxytyrosol and Tyrosol Contribute to the Maintenance of Nitric Oxide Balance in Human Aortic Endothelial Cells at Physiologically Relevant Concentrations. Molecules 2021, 26, 7480. [Google Scholar] [CrossRef]
- Atzeri, A.; Lucas, R.; Incani, A.; Penalver, P.; Zafra-Gomez, A.; Paola Melis, M.; Pizzala, R.; Morales, J.C.; Deiana, M. Hydroxytyrosol and Tyrosol Sulfate Metabolites Protect against the Oxidized Cholesterol Pro-Oxidant Effect in Caco-2 Human Enterocyte-like Cells. Food Funct. 2016, 7, 337–346. [Google Scholar] [CrossRef]
- Miró-Casas, E.; Covas, M.-I.; Fitó, M.; Farré-Albadalejo, M.; Marrugat, J.; De La Torre, R. Short Communication Tyrosol and Hydroxytyrosol Are Absorbed from Moderate and Sustained Doses of Virgin Olive Oil in Humans. Eur. J. Clin. Nutr. 2003, 57, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Khymenets, O.; Fito, M.; Tourino, S.; Munoz-Aguayo, D.; Pujadas, M.; Torres, J.L.; Joglar, J.; Farre, M.; Covas, M.-I.; De La Torre, R. Antioxidant Activities of Hydroxytyrosol Main Metabolites Do Not Contribute to Beneficial Health Effects after Olive Oil Ingestion. Drug Metab. Dispos. 2010, 38, 1417–1421. [Google Scholar] [CrossRef]
- Khymenets, O.; Farré, M.; Pujadas, M.; Ortiz, E.; Joglar, J.; Covas, M.I.; De La Torre, R. Direct Analysis of Glucuronidated Metabolites of Main Olive Oil Phenols in Human Urine after Dietary Consumption of Virgin Olive Oil. Food Chem. 2011, 126, 306–314. [Google Scholar] [CrossRef]
- Rubió, L.; Valls, R.-M.; Macià, A.; Pedret, A.; Giralt, M.; Romero, M.-P.; De La Torre, R.; Covas, M.-I.; Solà, R.; Motilva, M.-J. Impact of Olive Oil Phenolic Concentration on Human Plasmatic Phenolic Metabolites. Food Chem. 2012, 135, 2922–2929. [Google Scholar] [CrossRef]
- Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef]
- Di Renzo, L.; Smeriglio, A.; Ingegneri, M.; Gualtieri, P.; Trombetta, D. The Pharmaceutical Formulation Plays a Pivotal Role in Hydroxytyrosol Pharmacokinetics. Pharmaceutics 2023, 15, 743. [Google Scholar] [CrossRef]
- Lopez de las Hazas, M.-C.; Rubio, L.; Kotronoulas, A.; de la Torre, R.; Sola, R.; Motilva, M.-J. Dose Effect on the Uptake and Accumulation of Hydroxytyrosol and Its Metabolites in Target Tissues in Rats. Mol. Nutr. Food Res. 2015, 59, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, F.; Pojero, F. Use of Oleuropein and Hydroxytyrosol for Cancer Prevention and Treatment: Considerations about How Bioavailability and Metabolism Impact Their Adoption in Clinical Routine. Biomedicines 2024, 12, 502. [Google Scholar] [CrossRef] [PubMed]
- González-Santiago, M.; Fonollá, J.; Lopez-Huertas, E. Human Absorption of a Supplement Containing Purified Hydroxytyrosol, a Natural Antioxidant from Olive Oil, and Evidence for Its Transient Association with Low-Density Lipoproteins. Pharmacol. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Human Nutrition and Metabolism Olive Oil Phenols Are Absorbed in Humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Khymenets, O.; Crespo, M.C.; Dangles, O.; Rakotomanomana, N.; Andres-Lacueva, C.; Visioli, F. Human Hydroxytyrosol’s Absorption and Excretion from a Nutraceutical. J. Funct. Foods 2016, 23, 278–282. [Google Scholar] [CrossRef]
- Mateos, R.; Martínez-López, S.; Baeza Arévalo, G.; Amigo-Benavent, M.; Sarriá, B.; Bravo-Clemente, L. Hydroxytyrosol in Functional Hydroxytyrosol-Enriched Biscuits Is Highly Bioavailable and Decreases Oxidised Low Density Lipoprotein Levels in Humans. Food Chem. 2016, 205, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Galletti, P.; Maisto, G.; Cucciolla, V.; D’Angelo, S.; Zappia, V. Transport Mechanism and Metabolism of Olive Oil Hydroxytyrosol in Caco-2 Cells. Febs Lett. 2000, 470, 341–344. [Google Scholar] [CrossRef]
- Zafra-Gómez, A.; Luzón-Toro, B.; Capel-Cuevas, S.; Morales, J.C. Stability of Hydroxytyrosol in Aqueous Solutions at Different Concentration, Temperature and with Different Ionic Content: A Study Using UPLC-MS. Food Nutr. Sci. 2011, 2, 1114–1120. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Iacovino, S. Delivery Systems for Hydroxytyrosol Supplementation: State of the Art. Colloids Interfaces 2020, 4, 25. [Google Scholar] [CrossRef]
- Lisete-Torres, P.; Losada-Barreiro, S.; Albuquerque, H.; Sánchez-Paz, V.; Paiva-Martins, F.; Bravo-Díaz, C. Distribution of Hydroxytyrosol and Hydroxytyrosol Acetate in Olive Oil Emulsions and Their Antioxidant Efficiency. J. Agric. Food Chem. 2012, 60, 7318–7325. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Arik, N.; Monteil, J.; Papadimitriou, V.; Leal-Calderon, F.; Xenakis, A. Microemulsion versus Emulsion as Effective Carrier of Hydroxytyrosol. Colloids Surf. B-Biointerfaces 2016, 137, 146–151. [Google Scholar] [CrossRef]
- Flaiz, L.; Freire, M.; Cofrades, S.; Mateos, R.; Weiss, J.; Jimenez-Colmenero, F.; Bou, R. Comparison of Simple, Double and Gelled Double Emulsions as Hydroxytyrosol and n-3 Fatty Acid Delivery Systems. Food Chem. 2016, 213, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ghelichi, S.; Hajfathalian, M.; Yesiltas, B.; Sørensen, A.M.; García-Moreno, P.J.; Jacobsen, C. Oxidation and Oxidative Stability in Emulsions. Comp. Rev. Food Sci. Food Safe 2023, 22, 1864–1901. [Google Scholar] [CrossRef]
- Cofrades, S.; Bou, R.; Flaiz, L.; Garcimartín, A.; Benedí, J.; Mateos, R.; Sánchez-Muniz, F.J.; Olivero-David, R.; Jiménez-Colmenero, F. Bioaccessibility of Hydroxytyrosol and N-3 Fatty Acids as Affected by the Delivery System: Simple, Double and Gelled Double Emulsions. J. Food Sci. Technol. 2017, 54, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Jordânia Silva, T.; Ramírez-Carrasco, P.; Romero-Hasler, P.; Soto-Bustamante, E.; Barrera-Arellano, D.; Robert, P.; Giménez, B. Soybean Oil Organogelled Emulsions as Oral Delivery Systems of Hydroxytyrosol and Hydroxytyrosol Alkyl Esters. Food Chem. 2022, 379, 132182. [Google Scholar] [CrossRef]
- Baréa, B.; Barouh, N.; Fenaghra, A.; Colosetti, P.; Lecomte, J.; Durand, E.; Mey, A.; Laugerette, F.; Michalski, M.C.; Bourlieu-Lacanal, C.; et al. Comparison of Antioxidant Efficiencies in Oil-in-Water Emulsion Using Extracellular Vesicles from Olive Co-Products or Liposomes as Antioxidants Carriers. JAOCS J. Am. Oil Chem. Soc. 2025, 102, 323–337. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting Liposomes for Oral Drug Delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Daniello, V.; Di Gioia, S.; Conese, M.; Ingrosso, C.; Ciriaco, F.; Catucci, L. Polymer Encapsulated Liposomes for Oral Co-Delivery of Curcumin and Hydroxytyrosol. Int. J. Mol. Sci. 2023, 24, 790. [Google Scholar] [CrossRef]
- Patel, A.R.; Velikov, K.P. Colloidal Delivery Systems in Foods: A General Comparison with Oral Drug Delivery. LWT 2011, 44, 1958–1964. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.; Sun, J.; Zhang, W.; Zhang, X.; Liu, Y.; Zhou, X.; Li, X. Preparation, Characterization, and in Vivo Evaluation of Glycyrrhetinic Acid-Mediated Nano-Drug Delivery System Co-Loaded with Syringopicroside and Hydroxytyrosol. J. Biomater. Appl. 2023, 38, 392–411. [Google Scholar] [CrossRef]
- Hendawy, O.M.; Al-Sanea, M.M.; Elbargisy, R.M.; Rahman, H.U.; Mohamed, A.A.B.; Kamal, I.; Elshaarawy, R.F.M.; Khedr, A.I.M.; El-Fattah, W.A. Phenylboronic Acid-Grafted Chitosan Nanocapsules for Effective Delivery and Controllable Release of Natural Antioxidants: Olive Oil and Hydroxytyrosol. Pharmaceutics 2023, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Revisiting the Polar Paradox Theory: A Critical Overview. J. Agric. Food Chem. 2011, 59, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Mateos, R.; De Teran, L.C.; Espartero, J.L.; Cert, R.; Jover, M.; Alcudia, F.; Bautista, J.; Cert, A.; Parrado, J. Lipophilic Hydroxytyrosyl Esters. Antioxidant Activity in Lipid Matrices and Biological Systems. J. Agric. Food Chem. 2006, 54, 3779–3785. [Google Scholar] [CrossRef]
- Alcudia, F.; Cert, A.; Espartero, J.L.; Mateos, R.; Trujillo, M. Procedimiento Para La Preparación de Ésteres de Hidroxitirosol, Ésteres Obtenidos y Utilización. WO2004005237B1, 25 March 2004. Available online: https://patents.google.com/patent/WO2004005237B1 (accessed on 12 August 2025).
- Torres de Pinedo, A.; Peñalver, P.; Pérez-Victoria, I.; Rondón, D.; Morales, J.C. Synthesis of New Phenolic Fatty Acid Esters and Their Evaluation as Lipophilic Antioxidants in an Oil Matrix. Food Chem. 2007, 105, 657–665. [Google Scholar] [CrossRef]
- Medina, I.; Lois, S.; Alcantara, D.; Lucas, R.; Morales, J.C. Effect of Lipophilization of Hydroxytyrosol on Its Antioxidant Activity in Fish Oils and Fish Oil-in-Water Emulsions. J. Agric. Food Chem. 2009, 57, 9773–9779. [Google Scholar] [CrossRef]
- Tofani, D.; Balducci, V.; Gasperi, T.; Incerpi, S.; Gambacorta, A. Fatty Acid Hydroxytyrosyl Esters: Structure/Antioxidant Activity Relationship by Abts and in Cell-Culture DCF Assays. J. Agric. Food Chem. 2010, 58, 5292–5299. [Google Scholar] [CrossRef]
- Madrona, A.; Pereira-Caro, G.; Bravo, L.; Mateos, R.; Espartero, J.L. Preparation and Antioxidant Activity of Tyrosyl and Homovanillyl Ethers. Food Chem. 2011, 129, 1169–1178. [Google Scholar] [CrossRef]
- Madrona, A.; Pereira-Caro, G.; Mateos, R.; Rodríguez, G.; Trujillo, M.; Fernández-Bolaños, J.; Espartero, J.L. Synthesis of Hydroxytyrosyl Alkyl Ethers from Olive Oil Waste Waters. Molecules 2009, 14, 1762–1772. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Madrona, A.; Bravo, L.; Espartero, J.L.; Alcudia, F.; Cert, A.; Mateos, R. Antioxidant Activity Evaluation of Alkyl Hydroxytyrosyl Ethers, a New Class of Hydroxytyrosol Derivatives. Food Chem. 2009, 115, 86–91. [Google Scholar] [CrossRef]
- Mateos, R.; Pereira-Caro, G.; Saha, S.; Cert, R.; Redondo-Horcajo, M.; Bravo, L.; Kroon, P.A. Acetylation of Hydroxytyrosol Enhances Its Transport across Differentiated Caco-2 Cell Monolayers. Food Chem. 2011, 125, 865–872. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Yu, J.; Guo, X.; Tong, P.; Yin, F.; Liu, X.; Zhou, D. The Potential of Hydroxytyrosol Fatty Acid Esters to Enhance Oral Bioavailabilities of Hydroxytyrosol and Fatty Acids: Continuous and Slow-Release Ability in Small Intestine and Blood. Food Chem. 2023, 422, 136246. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Mateos, R.; Saha, S.; Madrona, A.; Luis Espartero, J.; Bravo, L.; Kroon, P.A. Transepithelial Transport and Metabolism of New Lipophilic Ether Derivatives of Hydroxytyrosol by Enterocyte-like Caco-2/TC7 Cells. J. Agric. Food Chem. 2010, 58, 11501–11509. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Sarriá, B.; Madrona, A.; Espartero, J.L.; Goya, L.; Bravo, L.; Mateos, R. Alkyl Hydroxytyrosyl Ethers Show Protective Effects against Oxidative Stress in HepG2 Cells. J. Agric. Food Chem. 2011, 59, 5964–5976. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, M.; Sarria, B.; Largo, C.; Martinez-Lopez, S.; Madrona, A.; Espartero, J.L.; Bravo, L.; Mateos, R. Comparative Evaluation of the Metabolic Effects of Hydroxytyrosol and Its Lipophilic Derivatives (Hydroxytyrosyl Acetate and Ethyl Hydroxytyrosyl Ether) in Hypercholesterolemic Rats. Food Funct. 2014, 5, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Costa, M.; Ferreira, M.; Gameiro, P.; Paiva-Martins, F. A New Family of Hydroxytyrosol Phenolipids for the Antioxidant Protection of Liposomal Systems. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183505. [Google Scholar] [CrossRef]
- Mai, V. Dietary Modification of the Intestinal Microbiota. Nutr. Rev. 2004, 62, 235–242. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Akkermans, A.D.L.; Akkermans-van Vliet, W.M.; De Visser, J.A.G.M.; De Vos, W.M. The Host Genotype Affects the Bacterial Community in the Human Gastrointestinal Tract. Microb. Ecol. Health Dis. 2001, 13, 129–134. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors Affecting Intake, Metabolism and Health Benefits of Phenolic Acids: Do We Understand Individual Variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Xing, X.; Wang, S. Health Benefits of Dietary Polyphenols: Insight into Interindividual Variability in Absorption and Metabolism. Curr. Opin. Food Sci. 2022, 48, 100941. [Google Scholar] [CrossRef]
- De Bruyne, T.; Steenput, B.; Roth, L.; De Meyer, G.R.Y.; Santos, C.N.D.; Valentová, K.; Dambrova, M.; Hermans, N. Dietary Polyphenols Targeting Arterial Stiffness: Interplay of Contributing Mechanisms and Gut Microbiome-Related Metabolism. Nutrients 2019, 11, 578. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Mosele, J.I.; Martín-Peláez, S.; Macià, A.; Farràs, M.; Valls, R.M.; Catalán, Ú.; Motilva, M.J. Faecal Microbial Metabolism of Olive Oil Phenolic Compounds: In Vitro and in Vivo Approaches. Mol. Nutr. Food Res. 2014, 58, 1809–1819. [Google Scholar] [CrossRef]
- Fan, L.; Peng, Y.; Wang, J.; Ma, P.; Zhao, L.; Li, X. Total Glycosides from Stems of Cistanche Tubulosa Alleviate Depression-like Behaviors: Bidirectional Interaction of the Phytochemicals and Gut Microbiota. Phytomedicine 2021, 83, 153471. [Google Scholar] [CrossRef] [PubMed]
- López de las Hazas, M.C.; Piñol, C.; Macià, A.; Romero, M.P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.J. Differential Absorption and Metabolism of Hydroxytyrosol and Its Precursors Oleuropein and Secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Rodriguez-Morato, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Perez-Mana, C.; Khymenets, O.; Fito, M.; Farre, M.; et al. Metabolic Disposition and Biological Significance of Simple Phenols of Dietary Origin: Hydroxytyrosol and Tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Kalampokis, E.; Nikou, T.; Beteinakis, S.; Halabalaki, M. Multiscale Workflow for the Profiling and Identification of Urinary Food Bioactives Metabolites Part I: Optimizing Urine Extraction. Anal. Chim. Acta 2025, 1353, 343947. [Google Scholar] [CrossRef] [PubMed]
- Conterno, L.; Martinelli, F.; Tamburini, M.; Fava, F.; Mancini, A.; Sordo, M.; Pindo, M.; Martens, S.; Masuero, D.; Vrhovsek, U.; et al. Measuring the Impact of Olive Pomace Enriched Biscuits on the Gut Microbiota and Its Metabolic Activity in Mildly Hypercholesterolaemic Subjects. Eur. J. Nutr. 2019, 58, 63–81. [Google Scholar] [CrossRef]
- Castillo-Luna, A.; Ledesma-Escobar, C.A.; Gómez-Díaz, R.; Priego-Capote, F. The Secoiridoid Profile of Virgin Olive Oil Conditions Phenolic Metabolism. Food Chem. 2022, 395, 133585. [Google Scholar] [CrossRef] [PubMed]
- Sakavitsi, M.E.; Breynaert, A.; Nikou, T.; Lauwers, S.; Pieters, L.; Hermans, N.; Halabalaki, M. Availability and Metabolic Fate of Olive Phenolic Alcohols Hydroxytyrosol and Tyrosol in the Human GI Tract Simulated by the In Vitro GIDM-Colon Model. Metabolites 2022, 12, 391. [Google Scholar] [CrossRef]
- Wen, X.; Wan, F.; Zhong, R.; Chen, L.; Zhang, H. Hydroxytyrosol Alleviates Intestinal Oxidative Stress by Regulating Bile Acid Metabolism in a Piglet Model. Int. J. Mol. Sci. 2024, 25, 5590. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Yang, H.; Ouyang, Z.; Lv, C.; Geng, Z.; Zhao, J. The Bile Acid-Gut Microbiota Axis: A Central Hub for Physiological Regulation and a Novel Therapeutic Target for Metabolic Diseases. Biomed. Pharmacother. 2025, 188, 118182. [Google Scholar] [CrossRef]
- Song, Y.; Li, M.; Liu, J.; Wang, J.; Zhou, A.; Cao, Y.; Duan, S.; Wang, Q. Screening Study of Hydroxytyrosol Metabolites from in Vitro Fecal Fermentation and Their Interaction with Intestinal Barrier Repair Receptor AhR. J. Food Sci. 2024, 89, 10134–10151. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Cai, D.; Guo, X.; Tong, P.; Yin, F.; Liu, X.; Zhou, D. In Vitro Gastrointestinal Digestion and Microbial Hydrolysis of Hydroxytyrosol-SCFA and Tyrosol-SCFA Acyl Esters: Controlled-Release of SCFAs and Polyphenols. J. Agric. Food Chem. 2023, 71, 9361–9369. [Google Scholar] [CrossRef]
- Lauwers, S.; Weyns, A.S.; Breynaert, A.; Van Rillaer, T.; Van Huynegem, V.; Fransen, E.; Bittremieux, W.; Lebeer, S.; Tuenter, E.; Hermans, N. Comparison of In Vitro Biotransformation of Olive Polyphenols Between Healthy Young and Elderly. Metabolites 2025, 15, 26. [Google Scholar] [CrossRef]
- Roselli, L.; Cicia, G.; Cavallo, C.; Del Giudice, T.; Carlucci, D.; Clodoveo, M.L.; De Gennaro, B.C. Consumers’ Willingness to Buy Innovative Traditional Food Products: The Case of Extra-Virgin Olive Oil Extracted by Ultrasound. Food Res. Int. 2018, 108, 482–490. [Google Scholar] [CrossRef] [PubMed]

| Ref. | Overview | HT Source | Administered Dose | Plasma Presence | Urine | ||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Peak Time | Maximum Concentration | Metabolites | Peak Time | Total Amounts/Concentrations | ||||
| [38] | HT bioavailability study and lipoprotein binding dynamics assessment, following aqueous supplementation, showing rapid plasma appearance, excretion of metabolites (HVA, DOPAC-G), transient LDL association and absence of measurable antioxidant activity (n = 10) | Pure HT (99.5%) as supplement in aqueous solution | 2.5 mg of HT per kg of bodyweight | HT HvOH | 13.0 ± 1.5 min (10–20) min 16.7 ± 2.4 min (10–30) min | 1.11 ± 0.20 µmol/L (0.47–2.23) µmol/L 0.49 ± 0.14 µmol/L (0.00–1.48) µmol/L | HT HT-G HT-S HvOH HvOH-G HvOH-S HVA HVAG HVA S DOPAC DOPAC-G DOPAC-S | 0.1 ± 0.04 (0.00–0.35) µM (0.1%) * 0.91 ± 0.45 (0.00–4.92) µM (1.2%) 2.95 ± 1.93 (0.00–19.89) µM (3.8%) 0.00 ± 0.00 (000–0.01) µM (0.0%) 0.29 ± 0.19 (0.00–1.97) µM (0.4%) 0.20 ± 0.17 (0.00–1.98) µM (0.2%) 24.29 ± 14.98 (0.00–163.25) µM (31%) 7.93 ± 3.48 (0.00 ± 32.43) µM (10.1%) 9.08 ± 5.54 (0.00–55.90) µM (11.6%) 10.35 ± 5.63 (0.00–55.82) µM (13.2%) 17.76 ± 6.87 (0.00 ± 63.00) µM (22.7%) 4.41 ± 3.33 (0.00–36.41) (5.6%) | |
| [39] | In a controlled crossover trial involving 8 ileostomy patients and 12 healthy volunteers, between 55 and 66% of ingested olive oil phenols were absorbed primarily in the small intestine, underwent metabolic transformation, and only 5–6% was recovered in urine | Watery fluid rich in polar phenols | Ileostomy volunteers: 498 µmol Healthy volunteers: 526 µmol | HT Tyrosol | Ileostomy Effluent: ** 1.8 µmol 1.4 µmol | HT Tyrosol HT Tyrosol | Dose: 498 µmol (Ileostomy volunteers) ** 24.7 ± 10.9 µmol 4.1 ± 3.1 µmol Dose: 526 µmol (healthy volunteers) * 21.6 ± 4.8 µmol 5.6 ± 8.8 µmol | ||
| [17] | Bioavailability assessment of oleuropein and HT from olive leaf extract, showing higher absorption, faster HT peak, and greater exposure in liquid form compared to capsules and male gender (n = 9) | Olive leaf extract (OLE) taken as capsule Olive leaf extract (OLE) taken as liquid | 51.1 mg oleuropein, 9.7 mg HT 76.6 mg oleuropein, 14.5 mg HT 51.1 mg oleuropein, 9.7 mg HT 76.6 mg oleuropein, 14.5 mg HT | Sulphated derivatives of HT and HVA Sulphated derivatives of HT and HVA Sulphated derivatives of HT and HVA Sulphated derivatives of HT and HVA | 90 ± 0 min 96 ± 13 min 53 ± 29 min 75 ± 17 min | ||||
| [40] | Dose-dependent HT bioavailability was hypothesized and confirmed HT-3S was the most abundant metabolite | HT-based product from olives, called Hytolive (HT, 10%) | 5 mg HT 25 mg HT | HT HT-4-G HT-3-G HT-3-S HT-4-S HT HT-G-4 HT-G-3 HT-S-3 HT-S-4 | Dose: 5 mg * 0 mg 0.11 mg 0.14 mg 1.18 mg (16.6%) 0.01 mg Dose: 25 mg 0 mg 0.46 mg 0.72 mg 4.15 mg (23.1%) 0.07 mg | ||||
| [34] | Bioavailability study was performed with an enriched supplement with different doses. HT-3-S, and DOPAC were the main metabolites found in plasma and urine. (n = 12) | Two olive-derived watery supplements containing different doses | 30.58 mg HT 61.48 mg HT | HT-3-S HVA DOPAC HT-3-S HVA DOPAC | 30 min 30 min 30 min 30 min 30 min 30 min | 384.96 ± 62.63 nmol/L 868.86 ± 111.86 nmol/L 301.07 ± 13.43 nmol/L 406.28 ± 32.68 nmol/L 948.76 ± 160.19 nmol/L 466 ± 147.68 nmol/L | HT-3-S HT-4-S HT-3-G HT-4-G HVA DOPAC HT-3-S HT-4-S HT-3-G HT-4-G HVA DOPAC | Dose: 30.58 mg * 16.58 ± 6.0 µmol 5.32 ± 16.23 µmol 0.46 ± 0.35 µmol 0.15 ± 0.10 µmol 36.1 ± 20.08 µmol 44.31 ± 3.93 µmol Dose: 61.48 mg 20.05 ± 1.55 µmol 0.16 ± 0.05 µmol 0.87 ± 0.13 µmol 0.41 ± 0.10 µmol 46.16 ± 5.37 µmol 59.74 ± 3.00 µmol | |
| Ref. | Overview | HT Source | Administered Dose | Plasma Presence Urine | Urine | ||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Peak Time | Maximum Concentration | Metabolites | Peak Time | Total Amounts/Concentrations | ||||
| [41] | Crossover study is realized, demonstrating HT (HT) in HT-enriched biscuits is highly bioavailable, with metabolites peaking within 0.5–1 h post-consumption, and significant reduction in postprandial oxidized-LDL levels. Major metabolites found were sulfated derivatives of HT and DOPAC | HT enriched biscuits and non-enriched ones | 30 g biscuits (5.25 mg HT) | HT-G (2) DOPAC-G DOPAC HT-S DOPAC-S HVA-G HVA HVA-S | 36 ± 13 min 60 ± 21 min 36 ± 13 min 47 ± 25 min 56 ± 23 min 66 ± 24 min 53 ± 24 min 53 ± 24 min | 0.002 ± 0.001 µmol/L 0.009 ± 0.003 µmol/L 0.03 ± 0.01 µmol/L 1.0 ± 0.03 µmol/L 0.5 ± 0.1 µmol/L 0.007 ± 0.003 µmol/L 0.10 ± 0.02 µmol/L 0.07 ± 0.02 µmol/L | HT-G (1) HT-G (2) DOPAC-G DOPAC HT-S DOPAC-S HVA-G HVA HVA-S | 0–3 h 0–3 h 12–24 h 0–3 h 0–3 h 0–3 h 0–3 h 0–3 h - | 2.6 ± 0.8 ng * 3 ± 1 ng 98 ± 28 ng 18 ± 4 ng 2168 ± 547 ng 1457 ± 439 ng 28 ± 6 301 ± 48 n.d. |
| [18] | Demonstration of matrix-dependent bioavailability, with extra virgin olive oil identified as the optimal matrix HT peak was reached at 30 min. (n = 20) | HT added to flax oil HT added to grapeseed oil HT added to margarine HT added to pineapple juice | 5 mg/20 g 5 mg/20 g 5 mg/20 g 5 mg/20 g | HT HT HT HT | n.d. n.d. n.d. n.d. | HT HT-Acetate DOPAC HvOH HT HT-Acetate DOPAC HvOH HT HT-Acetate DOPAC HvOH HT HT-Acetate DOPAC HvOH | 0–8 h 0–8 h 0–8 h 0–8 h | ~0.3 μg/mg creatinine ~20 μg/mg creatinine ~18 μg/mg creatinine ~30 μg/mg creatinine ~0.3 μg/mg creatinine ~20 μg/mg creatinine ~20 μg/mg creatinine ~20 μg/mg creatinine ~0.3 μg/mg creatinine ~40 μg/mg creatinine ~40 μg/mg creatinine ~5 μg/mg creatinine ~0.3 μg/mg creatinine ~20 μg/mg creatinine ~20 μg/mg creatinine ~30 μg/mg creatinine | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordán, M.; García-Acosta, N.; Espartero, J.L.; Goya, L.; Mateos, R. Hydroxytyrosol Bioavailability: Unraveling Influencing Factors and Optimization Strategies for Dietary Supplements. Nutrients 2025, 17, 2937. https://doi.org/10.3390/nu17182937
Jordán M, García-Acosta N, Espartero JL, Goya L, Mateos R. Hydroxytyrosol Bioavailability: Unraveling Influencing Factors and Optimization Strategies for Dietary Supplements. Nutrients. 2025; 17(18):2937. https://doi.org/10.3390/nu17182937
Chicago/Turabian StyleJordán, Marta, Natalia García-Acosta, José Luis Espartero, Luis Goya, and Raquel Mateos. 2025. "Hydroxytyrosol Bioavailability: Unraveling Influencing Factors and Optimization Strategies for Dietary Supplements" Nutrients 17, no. 18: 2937. https://doi.org/10.3390/nu17182937
APA StyleJordán, M., García-Acosta, N., Espartero, J. L., Goya, L., & Mateos, R. (2025). Hydroxytyrosol Bioavailability: Unraveling Influencing Factors and Optimization Strategies for Dietary Supplements. Nutrients, 17(18), 2937. https://doi.org/10.3390/nu17182937







