Effects of Fermented Soy on Cognition in Older Adults: Outcomes of a Randomized, Controlled Trial
Abstract
1. Introduction
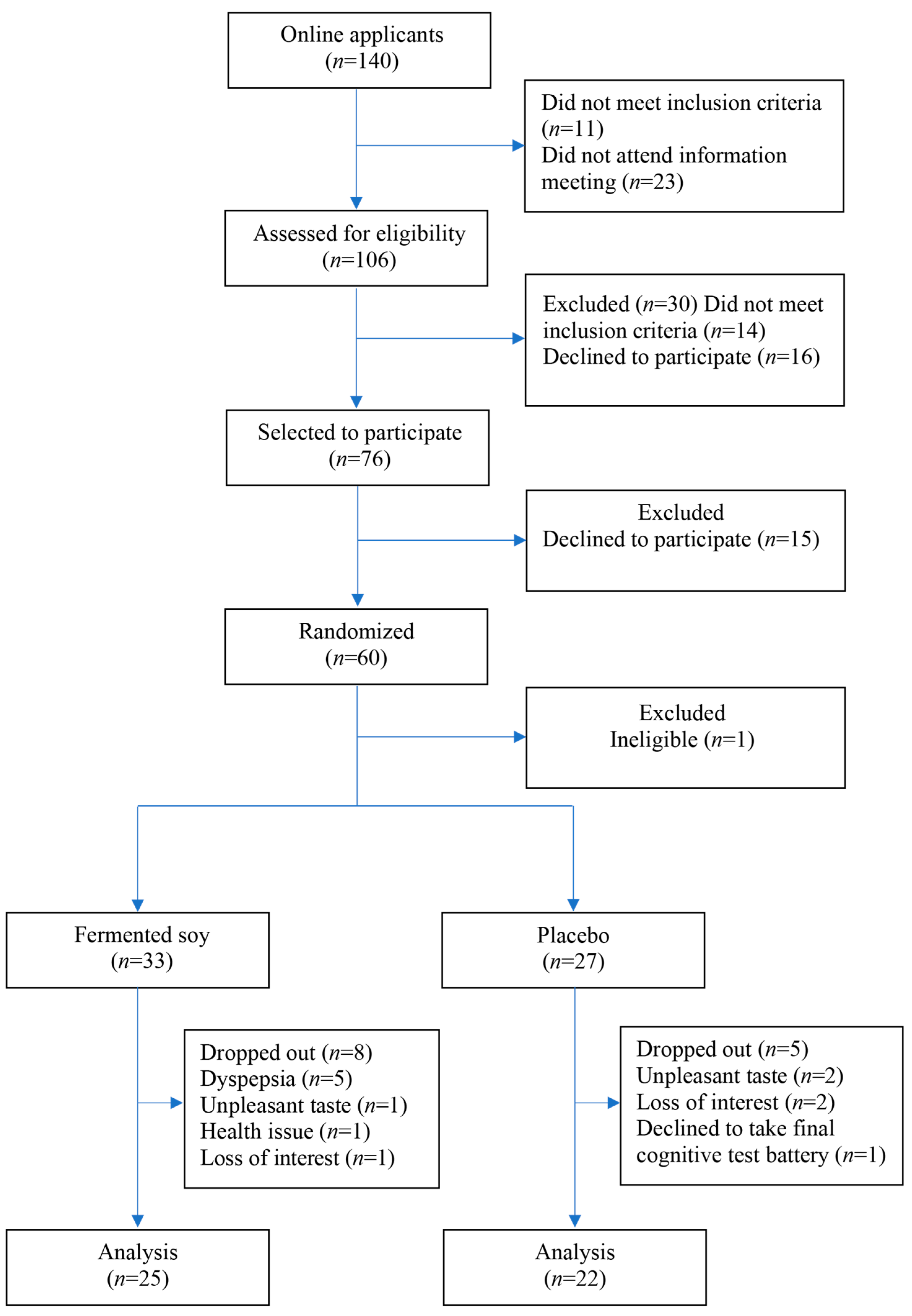
2. Materials and Methods
2.1. Study Protocol
2.2. Statistical Analyses
3. Results
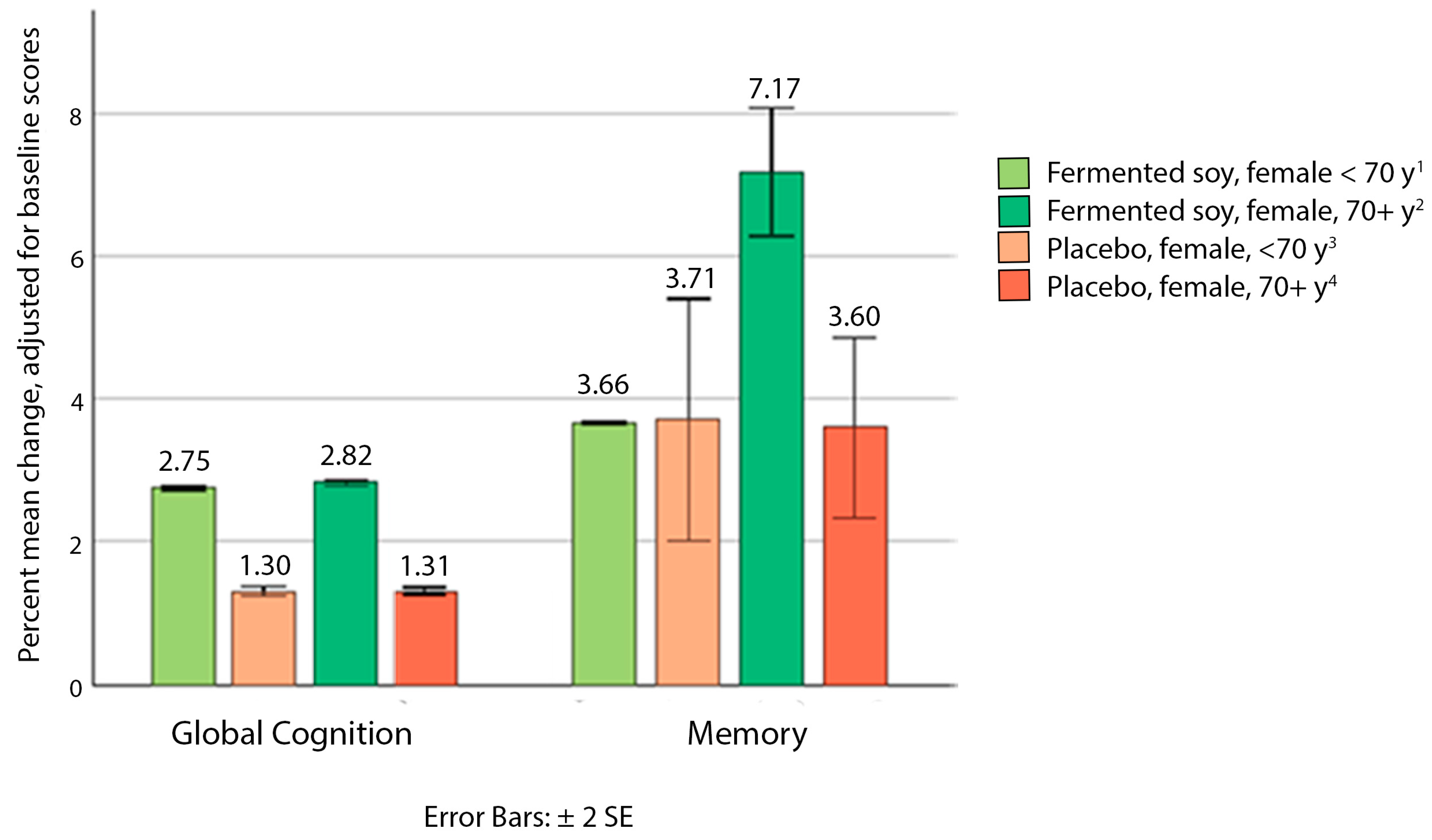
Cognition Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARCD | Age-related cognitive decline |
| MCI | Mild cognitive impairment |
| BDNF | Brain-derived neurotrophic factor |
| RCT | Randomized, controlled trial |
| TMT | Trail Making Test |
| ANCOVA | Analysis of covariance |
| ERβ | Estrogen receptor beta |
References
- GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, Physical Activity, and Other Lifestyle Factors in the Prevention of Cognitive Decline and Dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef]
- Fieldhouse, J.L.P.; Doorduijn, A.S.; De Leeuw, F.A.; Verhaar, B.J.H.; Koene, T.; Wesselman, L.M.P.; De Van Der Schueren, M.A.; Visser, M.; Van De Rest, O.; Scheltens, P.; et al. A Suboptimal Diet is Associated with Poorer Cognition: The NUDAD Project. Nutrients 2020, 12, 703. [Google Scholar] [CrossRef]
- Mcgrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; De La Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Cognitive performance among the elderly in relation to the intake of plant foods. The Hordaland Health Study. Br. J. Nutr. 2010, 104, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019, 10 (Suppl. S4), S422–S436. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Inagaki, H.; Gondo, Y.; Kamide, K.; Ikebe, K.; Masui, Y.; Arai, Y.; Ishizaki, T.; Sasaki, S.; Nakagawa, T.; et al. Association between dietary patterns and cognitive function among 70-year-old Japanese elderly: A cross-sectional analysis of the SONIC study. Nutr. J. 2017, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Soy Isoflavones Inhibit Endothelial Cell Dysfunction and Prevent Cardiovascular Disease. J. Cardiovasc. Pharmacol. 2019, 74, 201–209. [Google Scholar] [CrossRef]
- Zheng, X.; Lee, S.K.; Chun, O.K. Soy Isoflavones and Osteoporotic Bone Loss: A Review with an Emphasis on Modulation of Bone Remodeling. J. Med. Food 2016, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Otsuka, R.; Nishita, Y.; Tange, C.; Tomida, M.; Kato, Y.; Imai, T.; Sakai, T.; Ando, F.; Shimokata, H. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur. J. Clin. Nutr. 2018, 72, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Szczerba, E.; Koch, M.; Schlesinger, S. Soy consumption, cognitive function, and dementia. Curr. Opin. Lipidol. 2022, 33, 68–75. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Ho, C.-T.; Pan, M.-H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- do Prado, F.G.; Pagnoncelli, M.G.B.; Pereira, G.V.d.M.; Karp, S.G.; Soccol, C.R. Fermented Soy Products and Their Potential Health Benefits: A Review. Microorganisms 2022, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Xu, B. An insight into the health benefits of fermented soy products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, M.J.; Park, S.; Song, E.; Nam, Y.; Ahn, J.; Jang, Y.; Ha, T.; Jung, C.H. Bioavailability of Isoflavone Metabolites After Korean Fermented Soybean Paste (Doenjang) Ingestion in Estrogen-Deficient Rats. J. Food Sci. 2018, 83, 2212–2221. [Google Scholar] [CrossRef]
- Zhou, X.; Du, H.-H.; Jiang, M.; Zhou, C.; Deng, Y.; Long, X.; Zhao, X. Antioxidant Effect of Lactobacillus fermentum CQPC04-Fermented Soy Milk on D-Galactose-Induced Oxidative Aging Mice. Front. Nutr. 2021, 8, 727467. [Google Scholar] [CrossRef]
- Ahmad, A.; Ramasamy, K.; Jaafar, S.M.; Majeed, A.B.A.; Mani, V. Total isoflavones from soybean and tempeh reversed scopolamine-induced amnesia, improved cholinergic activities and reduced neuroinflammation in brain. Food Chem. Toxicol. 2014, 65, 120–128. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Lee, I.-T.; Wang, M.-F.; Yeh, W.-C.; Liang, B.-C. Tempeh attenuates cognitive deficit, antioxidant imbalance, and amyloid β of senescence-accelerated mice by modulating Nrf2 expression via MAPK pathway. J. Funct. Foods 2018, 50, 112–119. [Google Scholar] [CrossRef]
- Kridawati, A.; Hardinsyah, H.; Sulaeman, A.; Rahardjo, T.B.W.; Hogervorst, E. Tempe, Tofu, and Amyloid-β 1–40 Serum Levels in Ovariectomized Rats. J. Alzheimer’s Dis. 2020, 76, 159–163. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Wang, D.; Zhang, L.; Lv, J.; Jiang, N.; Fan, B.; Liu, X.; Wang, F. Neuroprotective Effects of Soy Isoflavones on Scopolamine-Induced Amnesia in Mice. Nutrients 2018, 10, 853. [Google Scholar] [CrossRef]
- Yang, H.J.; Zhang, T.; Yue, Y.; Jeong, S.-J.; Ryu, M.-S.; Wu, X.; Li, C.; Jeong, D.-Y.; Park, S. Protective Effect of Long-Term Fermented Soybeans with Abundant Bacillus subtilis on Glucose and Bone Metabolism and Memory Function in Ovariectomized Rats: Modulation of the Gut Microbiota. Foods 2023, 12, 2958. [Google Scholar] [CrossRef]
- Zhang, T.; Ryu, M.-S.; Wu, X.; Yang, H.-J.; Jeong, S.J.; Seo, J.-W.; Jeong, D.-Y.; Park, S. Alleviation of Neuronal Cell Death and Memory Deficit with Chungkookjang Made with Bacillus amyloliquefaciens and Bacillus subtilis Potentially through Promoting Gut-Brain Axis in Artery-Occluded Gerbils. Foods 2021, 10, 2697. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yasuda, M.; Yamao, M.; Gokan, T.; Sejima, Y.; Nishikawa, T.; Katayama, S. Fermented soybean foods (natto) ameliorate age-related cognitive decline by hippocampal TAAR1-mediated activation of the CaMKII/CREB/BDNF signaling pathway in senescence-accelerated mouse prone 8 (SAMP8). Food Funct. 2023, 14, 10097–10106. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H.; et al. Efficacy and Safety of Lactobacillus plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Handajani, Y.S.; Turana, Y.; Yogiara, Y.; Widjaja, N.T.; Sani, T.P.; Christianto, G.A.M.; Suwanto, A. Tempeh Consumption and Cognitive Improvement in Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. 2020, 49, 497–502. [Google Scholar] [CrossRef]
- Jung, S.M.; Haddad, E.H.; Kaur, A.; Sirirat, R.; Kim, A.Y.; Oda, K.; Rajaram, S.; Sabaté, J. A Non-Probiotic Fermented Soy Product Reduces Total and LDL Cholesterol: A Randomized Controlled Crossover Trial. Nutrients 2021, 13, 535. [Google Scholar] [CrossRef]
- Jung, S.M.; Kaur, A.; Amen, R.I.; Oda, K.; Rajaram, S.; Sabatè, J.; Haddad, E.H. Effect of the Fermented Soy Q-CAN(®) Product on Biomarkers of Inflammation and Oxidation in Adults with Cardiovascular Risk, and Canonical Correlations between the Inflammation Biomarkers and Blood Lipids. Nutrients 2023, 15, 3195. [Google Scholar] [CrossRef] [PubMed]
- Luca, M.; Luca, A.; Calandra, C. The Role of Oxidative Damage in the Pathogenesis and Progression of Alzheimer’s Disease and Vascular Dementia. Oxidative Med. Cell. Longev. 2015, 2015, 504678. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, L.T.; Christoffersen, M.; Frikke-Schmidt, R. Shared Risk Factors between Dementia and Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 9777. [Google Scholar] [CrossRef]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef]
- Xu, L.; Du, B.; Xu, B. A systematic, comparative study on the beneficial health components and antioxidant activities of commercially fermented soy products marketed in China. Food Chem. 2015, 174, 202–213. [Google Scholar] [CrossRef]
- Dioletis, E.; Paiva, R.S.; Kaffe, E.; Secor, E.R.; Weiss, T.R.; Fields, M.R.; Ouyang, X.; Ali, A. The fermented soy beverage Q-CAN® plus induces beneficial changes in the oral and intestinal microbiome. BMC Nutr. 2021, 7, 6. [Google Scholar] [CrossRef]
- Asaoka, D.; Xiao, J.; Takeda, T.; Yanagisawa, N.; Yamazaki, T.; Matsubara, Y.; Sugiyama, H.; Endo, N.; Higa, M.; Kasanuki, K.; et al. Effect of Probiotic Bifidobacterium breve in Improving Cognitive Function and Preventing Brain Atrophy in Older Patients with Suspected Mild Cognitive Impairment: Results of a 24-Week Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimer's Dis. 2022, 88, 75–95. [Google Scholar] [CrossRef]
- Li, T.; Chu, C.; Yu, L.; Zhai, Q.; Wang, S.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease. Nutrients 2022, 14, 4678. [Google Scholar] [CrossRef]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef] [PubMed]
- Toward, R.; Montandon, S.; Walton, G.; Gibson, G.R. Effect of prebiotics on the human gut microbiota of elderly persons. Gut Microbes 2012, 3, 57–60. [Google Scholar] [CrossRef]
- Rajaram, S.; Valls-Pedret, C.; Cofán, M.; Sabaté, J.; Serra-Mir, M.; Pérez-Heras, A.M.; Arechiga, A.; Casaroli-Marano, R.P.; Alforja, S.; Sala-Vila, A.; et al. The Walnuts and Healthy Aging Study (WAHA): Protocol for a nutritional intervention trial with walnuts on brain aging. Front. Aging Neurosci. 2016, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.J.; Nagata, C.; Wu, A.H. Estimated Asian Adult Soy Protein and Isoflavone Intakes. Nutr. Canter 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Valls-Pedret, C.; Rajaram, S.; Coll-Padrós, N.; Cofán, M.; Serra-Mir, M.; Pérez-Heras, A.M.; Roth, I.; Freitas-Simoes, T.M.; Doménech, M.; et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts and Healthy Aging (WAHA) study: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Lee, H. Is the center for epidemiologic studies depression scale as useful as the geriatric depression scale in screening for late-life depression? A systematic review. J. Affect. Disord. 2021, 292, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Moeller, J. A word on standardization in longitudinal studies: Don’t. Front. Psychol. 2015, 6, 1389. [Google Scholar] [CrossRef]
- Kenny, A.M.; Mangano, K.M.; Abourizk, R.H.; Bruno, R.S.; E Anamani, D.; Kleppinger, A.; Walsh, S.J.; Prestwood, K.M.; E Kerstetter, J. Soy proteins and isoflavones affect bone mineral density in older women: A randomized controlled trial. Am. J. Clin. Nutr. 2009, 90, 234–242. [Google Scholar] [CrossRef]
- Turana, Y.; Handajani, Y.S.; Barus, T.; Kristian, K.; Theodoraliu, E.; Suswanti, I. Comparison of the effects of mixed tempeh with soy tempeh on cognitive function in older people. Front. Nutr. 2025, 12, 1551211. [Google Scholar] [CrossRef]
- Messinis, L.; Nasios, G.; Mougias, A.; Politis, A.; Zampakis, P.; Tsiamaki, E.; Malefaki, S.; Gourzis, P.; Papathanasopoulos, P. Age and education adjusted normative data and discriminative validity for Rey’s Auditory Verbal Learning Test in the elderly Greek population. J. Clin. Exp. Neuropsychol. 2016, 38, 23–39. [Google Scholar] [CrossRef]
- Cui, C.; Birru, R.L.; E Snitz, B.; Ihara, M.; Kakuta, C.; Lopresti, B.J.; Aizenstein, H.J.; Lopez, O.L.; A Mathis, C.; Miyamoto, Y.; et al. Effects of soy isoflavones on cognitive function: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2020, 78, 134–144. [Google Scholar] [CrossRef]
- de Oliveira Silva, F.; Lemos, T.C.; Sandôra, D.; Monteiro, M.; Perrone, D. Fermentation of soybean meal improves isoflavone metabolism after soy biscuit consumption by adults. J. Sci. Food Agric. 2020, 100, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Ketnawa, S.; Ogawa, Y. Evaluation of protein digestibility of fermented soybeans and changes in biochemical characteristics of digested fractions. J. Funct. Foods 2019, 52, 640–647. [Google Scholar] [CrossRef]
- Jang, C.H.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.-S. Fermented Soy Products: Beneficial Potential in Neurodegenerative Diseases. Foods 2021, 10, 636. [Google Scholar] [CrossRef]
- Ju, D.-T.; K, A.K.; Kuo, W.-W.; Ho, T.-J.; Chang, R.-L.; Lin, W.-T.; Day, C.H.; Viswanadha, V.V.P.; Liao, P.-H.; Huang, C.-Y. Bioactive Peptide VHVV Upregulates the Long-Term Memory-Related Biomarkers in Adult Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2019, 20, 3069. [Google Scholar] [CrossRef]
- Weiser, M.J.; Foradori, C.D.; Handa, R.J. Estrogen receptor beta in the brain: From form to function. Brain Res. Rev. 2008, 57, 309–320. [Google Scholar] [CrossRef]
- Almeida, J.; Martins, A.R.; Amaral, L.; Valério, D.; Bukhari, Q.; Schu, G.; Nogueira, J.; Spínola, M.; Soleimani, G.; Fernandes, F.; et al. The cerebellum is causally involved in episodic memory under aging. GeroScience 2023, 45, 2267–2287. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, B.; Kuri, L.; Ferri, M.; Doykos, P.; Fazeli, M.S.; Hofer, K.; Andonova, A.; Ferri, L. Understanding Hispanic/Latino Participation in Clinical Trials and Observational Studies, and Strategies to Increase Participation: A Targeted Literature Review. J. Health Care Poor Underserved 2023, 34, 399–424. [Google Scholar] [CrossRef] [PubMed]
| Fermented Soy | Placebo | p-Value | |
|---|---|---|---|
| (n = 25) | (n = 22) | ||
| Sex | . | ||
| Female, n (%) | 18 (72%) | 18 (82%) | 0.505 2 |
| Age, y | 74.1 ± 4.5 | 74.0 ± 5.7 | 0.911 3 |
| Female ≥ 70 y, n (%) | 16 (64%) | 13 (59%) | 0.482 2 |
| Race/ethnicity, n (%) | 0.962 4 | ||
| Caucasian/White | 17 (68%) | 15 (68%) | |
| Hispanic | 4 (16%) | 3 (14%) | |
| Other | 4 (16%) | 4 (18%) | |
| Education, number of y | 15.8 ± 2.2 | 16.2 ± 1.80 | 0.429 3 |
| More than 12 y, n (%) | 24 (96%) | 23 (100%) | |
| Height, cm | 166.0 ± 11.4 | 165.0 ± 8.5 | 0.753 3 |
| Weight, kg | 81.5 ± 19.1 | 74.1 ± 13.9 | 0.145 3 |
| BMI | 29.4 ± 5.5 | 27.2 ± 5.0 | 0.164 3 |
| Coffee consumption, n (%) | 0.536 4 | ||
| None | 13 (52%) | 10 (46%) | |
| Low | 12 (48%) | 11 (50%) | |
| Moderate | 0 (0%) | 1 (5%) | |
| Alcohol consumption 5, n (%) | 0.136 4 | ||
| None | 14 (58%) | 7 (33%) | |
| Consumers | 10 (42%) | 14 (67%) | |
| Never smoker 5, n (%) | 17 (71%) | 16 (76%) | 0.746 2 |
| Physical activity 5, n (%) | 0.935 4 | ||
| Sedentary | 5 (21%) | 2 (9%) | |
| Light | 4 (17%) | 9 (41%) | |
| Moderate | 15 (62%) | 11 (50%) | |
| Center for Epidemiological Studies Depression Scale: 0–60 scale | 9.1 ± 7.1 | 10.5 ± 9.1 | 0.576 3 |
| At risk of depression: score ≥ 16, n (%) | 6 (24%) | 4 (18%) | 0.451 2 |
| Stress, perceived, n (%) | 0.770 4 | ||
| Low | 13 (52%) | 13 (59%) | |
| Moderate to high | 12 (48%) | 9 (41%) |
| Cognitive Composites | Fermented Soy | Placebo | p Value |
|---|---|---|---|
| (n = 25) | (n = 22) | ||
| Global cognition | |||
| Baseline | 57.83 (54.51, 61.15) | 57.71 (54.17, 61.25) | 0.961 |
| 12 weeks | 59.66 (55.95, 63.37) | 58.85 (54.90, 62.81) | 0.765 |
| Unadjusted change | 1.84 (0.41, 3.26) | 1.15 (−0.37, 2.66) | 0.507 |
| Adjusted change 3 | 1.06 (−0.48, 2.60) | 0.86 (−1.05, 2.78) | 0.200 |
| Memory | |||
| Baseline | 57.91 (52.38 63.43) | 52.34 (46.45, 58.22) | 0.172 |
| 12 weeks | 61.62 (55.75, 67.49) | 55.23 (48.97, 61.48) | 0.140 |
| Unadjusted change | 3.71 (−0.01, 7.44) | 2.89 (−1.08, 6.86) | 0.763 |
| Adjusted change 3 | 1.81 (−2.10, 5.72) | 1.16 (−3.64, 5.97) | 0.041 |
| Verbal fluency | |||
| Baseline | 42.43 (38.69, 46.17) | 42.52 (38.54, 46.51) | 0.973 |
| 12 weeks | 42.31 (38.12, 46.50) | 44.26 (39.80, 48.73) | 0.524 |
| Unadjusted change | −0.12 (−2.23, 1.99) | 1.74 (−0.51, 3.99) | 0.231 |
| Adjusted change 3 | −0.15 (−2.57, 2.27) | 2.44 (−0.56, 5.45) | 0.720 |
| Processing speed | |||
| Baseline | 63.94 (60.10, 67.79) | 67.21 (63.12, 71.31) | 0.247 |
| 12 weeks | 65.96 (62.02, 69.90) | 67.77 (63.58, 71.97) | 0.528 |
| Unadjusted change | 2.02 (0.11, 3.93) | 0.56 (−1.48, 2.59) | 0.298 |
| Adjusted change 3 | 1.51 (0.57, 3.77) | 0.37 (−2.29, 3.03) | 0.478 |
| Executive function | |||
| Baseline | 67.03 (62.84, 71.22) | 69.76 (64.29, 73.22) | 0.573 |
| 12 weeks | 68.76 (64.18, 73.34) | 68.15 (63.26, 73.03) | 0.855 |
| Unadjusted change | 1.73 (−0.27, 3.73) | −0.61 (−2.74, 1.52) | 0.114 |
| Adjusted change 3 | 1.28 (−1.02, 3.57) | −0.85 (−3.70, 2.00) | 0.506 |
| Fermented Soy Group (n = 25) | Placebo Group (n = 22) | p-Value | |
|---|---|---|---|
| Memory | |||
| Rey Auditory Verbal Learning Test, immediate recall | −0.08 (9.25) | −0.36 (7.22) | 0.908 |
| Rey Auditory Verbal Learning Test, delayed recall | −0.24 (2.19) | 0.50 (2.82) | 0.317 |
| Brief Visuospatial Memory Test-Revised, immediate recall | 2.84 (4.51) | 1.50 (4.03) | 0.291 |
| Brief Visuospatial Memory Test-Revised, delayed recall | 1.04 (2.30) | 0.55 (1.50) | 0.395 |
| Verbal Fluency | |||
| FAS Test | 1.16 (6.49) | 0.64 (6.33) | 0.781 |
| Animals Naming Test | −0.56 (2.93) | 1.14 (2.93) | 0.054 |
| Processing Speed | |||
| Symbol Digit Modalities Test | 2.48 (5.68) | 2.86 (4.98) | 0.808 |
| Trail Making Test A 3 | −2.96 (7.57) | −1.72 (9.09) | 0.611 |
| Stroop Word | 0.40 (8.58) | −2.18 (6.99) | 0.268 |
| Stroop Color | 1.20 (5.61) | −0.82 (6.08) | 0.243 |
| Executive Function | |||
| Trail Making Test B 3 | −7.01 (21.99) | 11.31 (47.13) | 0.088 |
| Stroop Color and Word | 1.32 (4.96) | −0.91 (4.83) | 0.127 |
| Digit Span Test | −0.32 (3.01) | −0.09 (2.93) | 0.793 |
| Automated Cognitive Test | 1.63 (4.65) | 2.27 (5.57) | 0.671 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
West, L.M.; Sabaté, J.; Nwachukwu, I.D.; Lee, G.J.; Sirirat, R.; Wright, A.; Rajaram, S. Effects of Fermented Soy on Cognition in Older Adults: Outcomes of a Randomized, Controlled Trial. Nutrients 2025, 17, 2936. https://doi.org/10.3390/nu17182936
West LM, Sabaté J, Nwachukwu ID, Lee GJ, Sirirat R, Wright A, Rajaram S. Effects of Fermented Soy on Cognition in Older Adults: Outcomes of a Randomized, Controlled Trial. Nutrients. 2025; 17(18):2936. https://doi.org/10.3390/nu17182936
Chicago/Turabian StyleWest, Laura M., Joan Sabaté, Ifeanyi D. Nwachukwu, Grace J. Lee, Rawiwan Sirirat, Amandeep Wright, and Sujatha Rajaram. 2025. "Effects of Fermented Soy on Cognition in Older Adults: Outcomes of a Randomized, Controlled Trial" Nutrients 17, no. 18: 2936. https://doi.org/10.3390/nu17182936
APA StyleWest, L. M., Sabaté, J., Nwachukwu, I. D., Lee, G. J., Sirirat, R., Wright, A., & Rajaram, S. (2025). Effects of Fermented Soy on Cognition in Older Adults: Outcomes of a Randomized, Controlled Trial. Nutrients, 17(18), 2936. https://doi.org/10.3390/nu17182936







