Effects of Oral Anthocyanin Supplementation on In Vitro Neurogenesis, Hippocampus-Dependent Cognition, and Blood-Based Dementia Biomarkers: Results from a 24-Week Randomized Controlled Trial in Older Adults At Risk for Dementia (ACID)
Abstract
1. Introduction
2. Materials and Methods
2.1. ACID Clinical Trial
2.2. ApoE Genotyping
2.3. Cognitive Performance
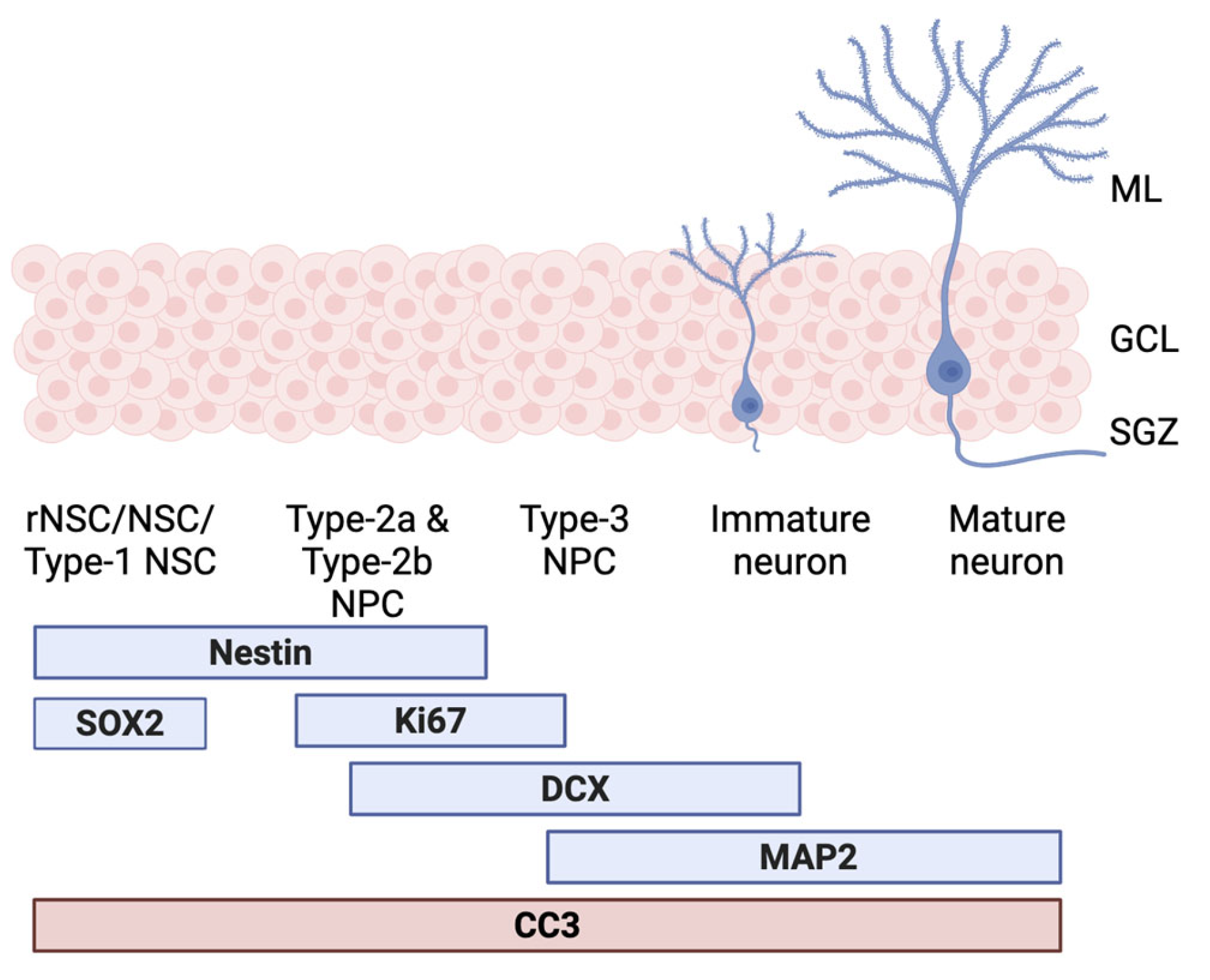
2.4. In Vitro Neurogenesis Model
2.5. Blood-Based Biomarkers
2.6. Statistical Analysis
3. Results
3.1. ACN Intervention Impacts SOX2
3.2. ACN Intervention Does Not Impact Cognition
3.3. NFL GFAP pTAU at Baseline Are Associated with Neurogenesis Markers but Do Not Impact ACN Intervention Effects
3.4. Markers of Apoptotic Cell Death Are Significantly Associated with Hippocampal-Dependent Cognitive Performance
3.5. BMI and ApoE Have a Moderator Effect on Neurogenesis in Cognition
4. Discussion
4.1. Decrease in Stem Cell Integrity Following Oral ACN Supplementation
4.2. Intervention and Hippocampal Cognition
4.3. Blood-Based Biomarkers Are Associated with the Neurogenic Process at Baseline
4.4. Neurogenesis Markers and AD Blood-Based Biomarkers Can Predict Cognitive Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| ACID | AnthoCyanins In people at risk for Dementia |
| ACNs | Anthocyanins |
| AHN | Adult Hippocampal Neurogenesis |
| AIC | Akaike Information Criterion |
| BBMs | Blood-Based Biomarkers |
| CMB | Cognitive Combination Score |
| DPICNACC | Picture Recognition New Stimuli Accuracy |
| DPICOACC | Picture Recognition Original Stimuli Accuracy |
| FDR | False Discovery Rate |
| GFAP | Glial Fibrillary Acidic Protein |
| HPCs | Hippocampal Progenitor Cells |
| NFL | Neurofilament Light Chain |
| NSCs | Neural Stem Cells |
References
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, 974–982.e973. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Villeda, S.A.; Plambeck, K.E.; Middeldorp, J.; Castellano, J.M.; Mosher, K.I.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef]
- de Lucia, C.; Murphy, T.; Maruszak, A.; Wright, P.; Powell, T.; Hartopp, N.; de Jong, S.; Sullivan, M.O.; Breen, G.; Price, J.; et al. Serum from Older Adults Increases Apoptosis and Molecular Aging Markers in Human Hippocampal Progenitor Cells. Aging Dis. 2021, 12, 2151–2172. [Google Scholar] [CrossRef]
- Maruszak, A.; Silajdžić, E.; Lee, H.; Murphy, T.; Liu, B.; Shi, L.; de Lucia, C.; Douiri, A.; Salta, E.; Nevado, A.J.; et al. Predicting progression to Alzheimer’s disease with human hippocampal progenitors exposed to serum. Brain 2023, 146, 2045–2058. [Google Scholar] [CrossRef]
- de Lucia, C.; Murphy, T.; Steves, C.J.; Dobson, R.J.B.; Proitsi, P.; Thuret, S. Lifestyle mediates the role of nutrient-sensing pathways in cognitive aging: Cellular and epidemiological evidence. Commun. Biol. 2020, 3, 157. [Google Scholar] [CrossRef]
- Du Preez, A.; Lefèvre-Arbogast, S.; Houghton, V.; de Lucia, C.; Low, D.Y.; Helmer, C.; Féart, C.; Delcourt, C.; Proust-Lima, C.; Pallàs, M.; et al. The serum metabolome mediates the concert of diet, exercise, and neurogenesis, determining the risk for cognitive decline and dementia. Alzheimer’s Dement. 2022, 18, 654–675. [Google Scholar] [CrossRef]
- Borsini, A.; Stangl, D.; Jeffries, A.R.; Pariante, C.M.; Thuret, S. The role of omega-3 fatty acids in preventing glucocorticoid-induced reduction in human hippocampal neurogenesis and increase in apoptosis. Transl. Psychiatry 2020, 10, 219. [Google Scholar] [CrossRef]
- Du Preez, A.; Lefèvre-Arbogast, S.; González-Domínguez, R.; Houghton, V.; de Lucia, C.; Lee, H.; Low, D.Y.; Helmer, C.; Féart, C.; Delcourt, C.; et al. Association of dietary and nutritional factors with cognitive decline, dementia, and depressive symptomatology in older individuals according to a neurogenesis-centred biological susceptibility to brain ageing. Age Ageing 2024, 53 (Suppl. S2), ii47–ii59. [Google Scholar] [CrossRef] [PubMed]
- Bacalini, M.G.; Friso, S.; Olivieri, F.; Pirazzini, C.; Giuliani, C.; Capri, M.; Santoro, A.; Franceschi, C.; Garagnani, P. Present and future of anti-ageing epigenetic diets. Mech. Ageing Dev. 2014, 136–137, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, K.; Bergland, A.K.; Soennesyn, H.; Oppedal, K.; Oesterhus, R.; Dalen, I.; Larsen, A.I.; Fladby, T.; Brooker, H.; Wesnes, K.A.; et al. Effects of Purified Anthocyanins in People at Risk for Dementia: Study Protocol for a Phase II Randomized Controlled Trial. Front. Neurol. 2020, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- Le Sayec, M.; Carregosa, D.; Khalifa, K.; de Lucia, C.; Aarsland, D.; Santos, C.N.; Rodriguez-Mateos, A. Identification and quantification of (poly)phenol and methylxanthine metabolites in human cerebrospinal fluid: Evidence of their ability to cross the BBB. Food Funct. 2023, 14, 8893–8902. [Google Scholar] [CrossRef]
- Shohayeb, B.; Diab, M.; Ahmed, M.; Ng, D.C.H. Factors that influence adult neurogenesis as potential therapy. Transl. Neurodegener. 2018, 7, 4. [Google Scholar] [CrossRef]
- Chen, W.L.; Zhao, J. Association between dietary anthocyanidins intake and depression among US adults: A cross-sectional study (NHANES, 2007–2010 and 2017–2018). BMC Psychiatry 2023, 23, 525. [Google Scholar] [CrossRef]
- Agarwal, P.; Holland, T.M.; Wang, Y.; Bennett, D.A.; Morris, M.C. Association of Strawberries and Anthocyanidin Intake with Alzheimer’s Dementia Risk. Nutrients 2019, 11, 3060. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef]
- Aarsland, D.; Khalifa, K.; Bergland, A.K.; Soennesyn, H.; Oppedal, K.; Holteng, L.B.A.; Oesterhus, R.; Nakling, A.; Jarholm, J.A.; de Lucia, C.; et al. A Randomised Placebo-Controlled Study of Purified Anthocyanins on Cognition in Individuals at Increased Risk for Dementia. Am. J. Geriatr. Psychiatry 2023, 31, 141–151. [Google Scholar] [CrossRef]
- Wesnes, K.A.; Brooker, H.; Ballard, C.; McCambridge, L.; Stenton, R.; Corbett, A. Utility, reliability, sensitivity and validity of an online test system designed to monitor changes in cognitive function in clinical trials. Int. J. Geriatr. Psychiatry 2017, 32, e83–e92. [Google Scholar] [CrossRef] [PubMed]
- Farmand, S.; Du Preez, A.; Kim, C.; de Lucia, C.; Ruepp, M.-D.; Stubbs, B.; Thuret, S. Cognition on the move: Examining the role of physical exercise and neurogenesis in counteracting cognitive aging. Ageing Res. Rev. 2025, 107, 102725. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021, 141, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Graham, V.; Khudyakov, J.; Ellis, P.; Pevny, L. SOX2 Functions to Maintain Neural Progenitor Identity. Neuron 2003, 39, 749–765. [Google Scholar] [CrossRef]
- Gebara, E.; Bonaguidi, M.A.; Beckervordersandforth, R.; Sultan, S.; Udry, F.; Gijs, P.-J.; Lie, D.C.; Ming, G.-L.; Song, H.; Toni, N. Heterogeneity of Radial Glia-Like Cells in the Adult Hippocampus. Stem Cells 2016, 34, 997–1010. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. GFAP as a Potential Biomarker for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef]
- Benedet, A.L.; Milà-Alomà, M.; Vrillon, A.; Ashton, N.J.; Pascoal, T.A.; Lussier, F.; Karikari, T.K.; Hourregue, C.; Cognat, E.; Dumurgier, J.; et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol. 2021, 78, 1471–1483. [Google Scholar] [CrossRef]
- Fuster-Matanzo, A.; Llorens-Martín, M.; Hernández, F.; Avila, J. Role of neuroinflammation in adult neurogenesis and Alzheimer disease: Therapeutic approaches. Mediat. Inflamm. 2013, 2013, 260925. [Google Scholar] [CrossRef]
- Ashton, N.J.; Brum, W.S.; Di Molfetta, G.; Benedet, A.L.; Arslan, B.; Jonaitis, E.; Langhough, R.E.; Cody, K.; Wilson, R.; Carlsson, C.M.; et al. Diagnostic Accuracy of a Plasma Phosphorylated Tau 217 Immunoassay for Alzheimer Disease Pathology. JAMA Neurol. 2024, 81, 255–263. [Google Scholar] [CrossRef]
- Mielke, M.M.; Syrjanen, J.A.; Blennow, K.; Zetterberg, H.; Vemuri, P.; Skoog, I.; Machulda, M.M.; Kremers, W.K.; Knopman, D.S.; Jack, C., Jr.; et al. Plasma and CSF neurofilament light: Relation to longitudinal neuroimaging and cognitive measures. Neurology 2019, 93, e252–e260. [Google Scholar] [CrossRef] [PubMed]
- Levi, O.; Michaelson, D.M. Environmental enrichment stimulates neurogenesis in apolipoprotein E3 and neuronal apoptosis in apolipoprotein E4 transgenic mice. J. Neurochem. 2007, 100, 202–210. [Google Scholar] [CrossRef]
- Hansen, C.P.; Overvad, K.; Kyrø, C.; Olsen, A.; Tjønneland, A.; Johnsen, S.P.; Jakobsen, M.U.; Dahm, C.C. Adherence to a Healthy Nordic Diet and Risk of Stroke. Stroke 2017, 48, 259–264. [Google Scholar] [CrossRef]
- Bere, E.; Brug, J. Towards health-promoting and environmentally friendly regional diets—A Nordic example. Public Health Nutr. 2009, 12, 91–96. [Google Scholar] [CrossRef] [PubMed]

| Active (N = 90) | Placebo (N = 91) | Overall (N = 181) | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 69.2 (5.25) | 69.5 (5.60) | 69.4 (5.41) |
| Median [Min, Max] | 68.0 [60.0, 79.0] | 69.0 [60.0, 80.0] | 69.0 [60.0, 80.0] |
| Sex | |||
| Female | 45 (50.0%) | 45 (49.5%) | 90 (49.7%) |
| Male | 45 (50.0%) | 46 (50.5%) | 91 (50.3%) |
| Education | |||
| Mean (SD) | 14.6 (3.42) | 13.8 (2.97) | 14.2 (3.22) |
| Median [Min, Max] | 15.0 [8.00, 22.0] | 14.0 [8.00, 20.0] | 14.0 [8.00, 22.0] |
| ApoE | |||
| E4 non-carrier | 54 (60.0%) | 54 (59.3%) | 108 (59.7%) |
| E4 carrier | 36 (40.0%) | 37 (40.7%) | 73 (40.3%) |
| Risk | |||
| CMD | 64 (71.1%) | 58 (63.7%) | 122 (67.4%) |
| MCI | 26 (28.9%) | 33 (36.3%) | 59 (32.6%) |
| BMI | |||
| Mean (SD) | 27.3 (3.86) | 28.3 (4.61) | 27.8 (4.28) |
| Median [Min, Max] | 26.9 [19.9, 38.4] | 28.7 [19.6, 41.4] | 27.7 [19.6, 41.4] |
| Site | |||
| Stavanger | 48 (53.3%) | 51 (56.0%) | 99 (54.7%) |
| Bergen | 30 (33.3%) | 31 (34.1%) | 61 (33.7%) |
| Akershus | 12 (13.3%) | 9 (9.9%) | 21 (11.6%) |
| Outcome | Intervention * Visit | Covariates | ||
|---|---|---|---|---|
| Estimate | p | q | ||
| Sox2 | −0.42 | <0.0001 | 0.0008 | BMI, Education, ApoE, Plate Location, Test Site, Staining Batch |
| Nestin | −0.04 | 0.30 | 0.53 | BMI, MCI, Plate Location, and Staining Batch |
| Ki67 proliferation | 0.02 | 0.53 | 0.71 | Staining Batch and Test Site |
| CC3 proliferation | −0.005 | 0.86 | 0.93 | Staining Batch, Plate Location, and Test Site |
| Ki67 differentiation | −0.039 | 0.24 | 0.53 | Staining Batch, Risk Type, and Test Site |
| DCX | 0.008 | 0.93 | 0.93 | Staining Batch and Test Site |
| Map2 | −0.13 | 0.04 | 0.16 | Staining Batch, Plate Location, and Test Site |
| CC3 differentiation | 0.0428 | 0.33 | 0.53 | Age, Staining Batch, and Test Site |
| Outcome | Intervention * Visit | Covariates | ||
|---|---|---|---|---|
| Chisq | p | q | ||
| DPIOACC | 0.19 | 0.66 | 0.20 | Age, Education, Risk Type, and ApoE |
| DPINACC | 0.73 | 0.39 | 0.59 | Age and Test Site |
| CMB | 0.10 | 0.75 | 0.75 | Age, Education, MCI, and ApoE |
| Sox2 Adj R = 0.05 F = 3 p = 0.03 | Nestin Adj R = 0.14 F = 5 p = 0.0003 | CC3p Adj R = 0.19 F = 10 p ≤ 0.0001 | Ki67p Adj R = 0.08 F = 12 p = 0.0006 | DCX Adj R = 0.45 F = 26 p < 0.0001 | CC3d Adj R = 0.65 F = 115 p ≤ 0.0001 | Ki67d Adj R = 0.19 F = 6 p ≤ 0.0001 | Map2 Adj R = 0.40 F = 16 p < 0.0001 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | q | β | p | q | β | p | q | β | p | q | β | p | q | β | p | q | β | p | q | β | p | q | |
| BMI | - | - | - | 0.0004 | 0.04 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Risk Type | 0.009 | 0.03 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.013 | 0.15 | - | - | - | - |
| Age | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.002 | 0.057 | - | - | - | - |
| ApoE4 status | - | - | - | - | - | - | −0.004 | 0.04 | - | - | - | - | - | - | - | 0.019 | 0.08 | - | - | - | - | - | - | - |
| Staining batch | −0.001 | 0.10 | - | - | - | - | - | - | 0.005 | 0.0006 | - | 0.013 | <0.0001 | −0.06 | <0.0001 | - | 0.009 | 0.005 | - | −0.07 | <0.0001 | - | ||
| Plate location | - | - | - | 0.002 | 0.006 | - | - | - | - | - | - | 0.003 | 0.08 | - | - | - | - | - | - | - | - | - | ||
| Test site | - | - | - | - | - | - | −0.005 | 0.003 | - | - | - | 0.01 | 0.03 | - | - | - | - | - | - | −0.04 | 0.10 | - | ||
| GFAP | - | - | - | - | - | - | 0.00008 | <0.0001 | 0.001 | - | - | - | - | - | - | - | - | - | −0.0002 | 0.0089 | 0.030 | −0.0007 | 0.03 | 0.067 |
| p-tau217 | −0.012 | 0.06 | 0.067 | −0.004 | 0.06 | 0.067 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.12 | 0.04 | 0.067 |
| p-tau231 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.002 | 0.0006 | 0.003 | - | - | - |
| NFL | - | - | - | - | - | - | - | - | - | - | - | - | 0.0005 | 0.06 | 0.067 | - | - | - | 0.001 | 0.08 | 0.080 | 0.004 | 0.06 | 0.067 |
| DPIOACC Adj R = 0.49, F statistic = 14, p < 0.0001 | DPINACC Adj R = 0.43, F statistic = 27, p < 0.0001 | CMB Adj R = 0.51, F statistic = 13, p < 0.0001 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p | q | β | p | q | β | p | q | |
| BL cognition | 0.56 | <0.0001 | - | 0.62 | <0.0001 | - | 0.64 | <0.0001 | - |
| ApoE | −76,520 | 0.037 | - | - | - | - | −7.55 | 0.046 | - |
| BMI | −17,230 | 0.0002 | - | - | - | - | −0.87 | 0.06 | - |
| Risk Type | −131,600 | 0.003 | - | - | - | - | −6.86 | 0.12 | - |
| Map2 | 166,300 | 0.65 | 0.72 | - | - | - | - | - | - |
| CC3d | −8,810,000 | 0.045 | 0.061 | - | - | - | −9.29 | 0.91 | 0.91 |
| CC3p | - | - | - | 466.70 | 0.01 | 0.046 | 4288.24 | 0.01 | 0.046 |
| GFAP | - | - | - | −0.08 | 0.02 | 0.046 | −0.09 | 0.06 | 0.07 |
| CC3d * BMI | 340,800 | 0.02 | 0.046 | - | - | - | - | - | - |
| ApoE * Map2 | −1,560,000 | 0.008 | 0.046 | - | - | - | - | - | - |
| CC3p * BMI | - | - | - | - | - | - | −133.36 | 0.03 | 0.046 |
| ApoE * CC3d | - | - | - | - | - | - | 216.98 | 0.04 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lucia, C.; Tovar-Rios, D.A.; Khalifa, K.; Kvernberg, S.M.; Pola, I.; Bergland, A.K.; Maple-Grødem, J.; Siow, R.; Ashton, N.; Ballard, C.; et al. Effects of Oral Anthocyanin Supplementation on In Vitro Neurogenesis, Hippocampus-Dependent Cognition, and Blood-Based Dementia Biomarkers: Results from a 24-Week Randomized Controlled Trial in Older Adults At Risk for Dementia (ACID). Nutrients 2025, 17, 2680. https://doi.org/10.3390/nu17162680
de Lucia C, Tovar-Rios DA, Khalifa K, Kvernberg SM, Pola I, Bergland AK, Maple-Grødem J, Siow R, Ashton N, Ballard C, et al. Effects of Oral Anthocyanin Supplementation on In Vitro Neurogenesis, Hippocampus-Dependent Cognition, and Blood-Based Dementia Biomarkers: Results from a 24-Week Randomized Controlled Trial in Older Adults At Risk for Dementia (ACID). Nutrients. 2025; 17(16):2680. https://doi.org/10.3390/nu17162680
Chicago/Turabian Stylede Lucia, Chiara, Diego Alejandro Tovar-Rios, Khadija Khalifa, Silje Meihack Kvernberg, Ilaria Pola, Anne Katrine Bergland, Jodi Maple-Grødem, Richard Siow, Nicholas Ashton, Clive Ballard, and et al. 2025. "Effects of Oral Anthocyanin Supplementation on In Vitro Neurogenesis, Hippocampus-Dependent Cognition, and Blood-Based Dementia Biomarkers: Results from a 24-Week Randomized Controlled Trial in Older Adults At Risk for Dementia (ACID)" Nutrients 17, no. 16: 2680. https://doi.org/10.3390/nu17162680
APA Stylede Lucia, C., Tovar-Rios, D. A., Khalifa, K., Kvernberg, S. M., Pola, I., Bergland, A. K., Maple-Grødem, J., Siow, R., Ashton, N., Ballard, C., Thuret, S., & Aarsland, D. (2025). Effects of Oral Anthocyanin Supplementation on In Vitro Neurogenesis, Hippocampus-Dependent Cognition, and Blood-Based Dementia Biomarkers: Results from a 24-Week Randomized Controlled Trial in Older Adults At Risk for Dementia (ACID). Nutrients, 17(16), 2680. https://doi.org/10.3390/nu17162680







