Avenanthramide-C Mitigates High-Fat Diet-Accelerated Alzheimer’s Pathologies via NOD1-Driven Neuroinflammation in 5×FAD Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. High-Fat Diet and Avn-C Treatment
2.3. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.4. Intraperitoneal Insulin Tolerance Test (IPITT)
2.5. ELISA for Insulin and Cytokines
2.6. Novel Objective Recognition Test
2.7. Morris Water Maze Test
2.8. Hippocampal Slice Preparation
2.9. Electrophysiological Recordings
2.10. Golgi–Cox Staining and Spine Density Analysis
2.11. Western Blots of Hippocampus
2.12. Immunofluorescence
2.13. Microarray Analysis and Differential Gene Expression Analysis
2.14. C12-iE-DAP Administration
2.15. Data Analysis
3. Results
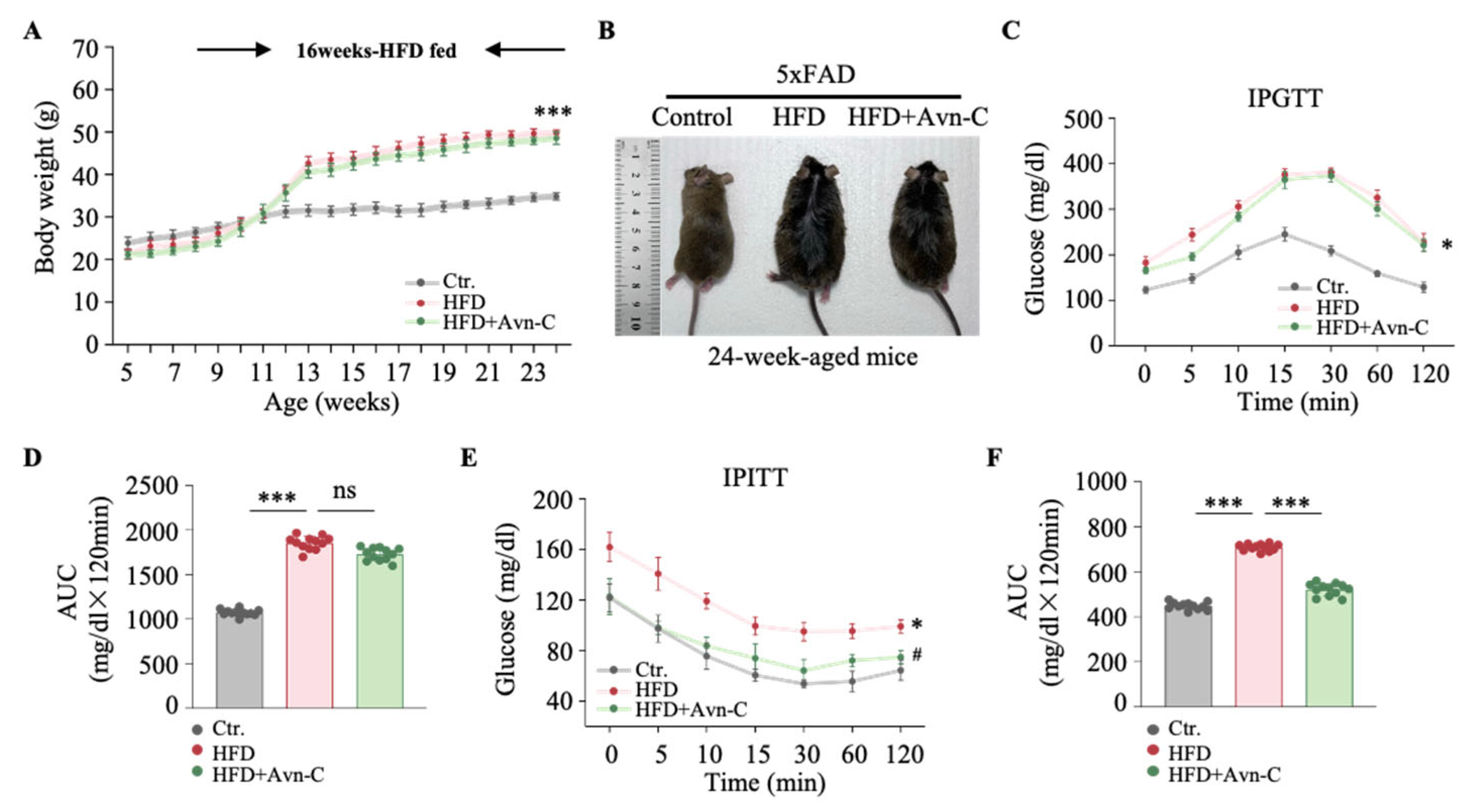
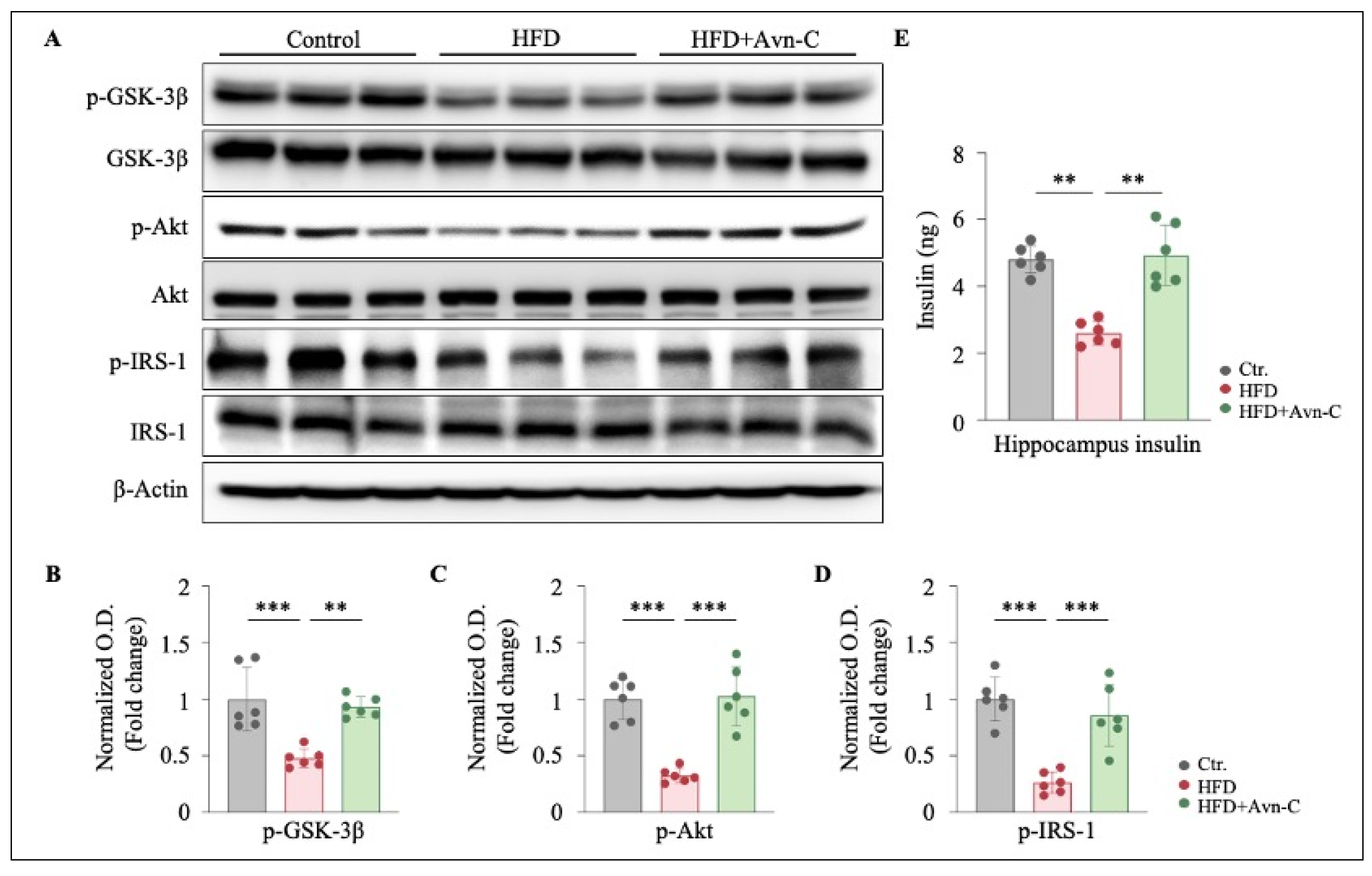
3.1. Avn-C Does Not Reverse Body Weight Gain or Glucose Tolerance but Does Alleviate Insulin Resistance in Obese 5×FAD Mouse Hippocampi
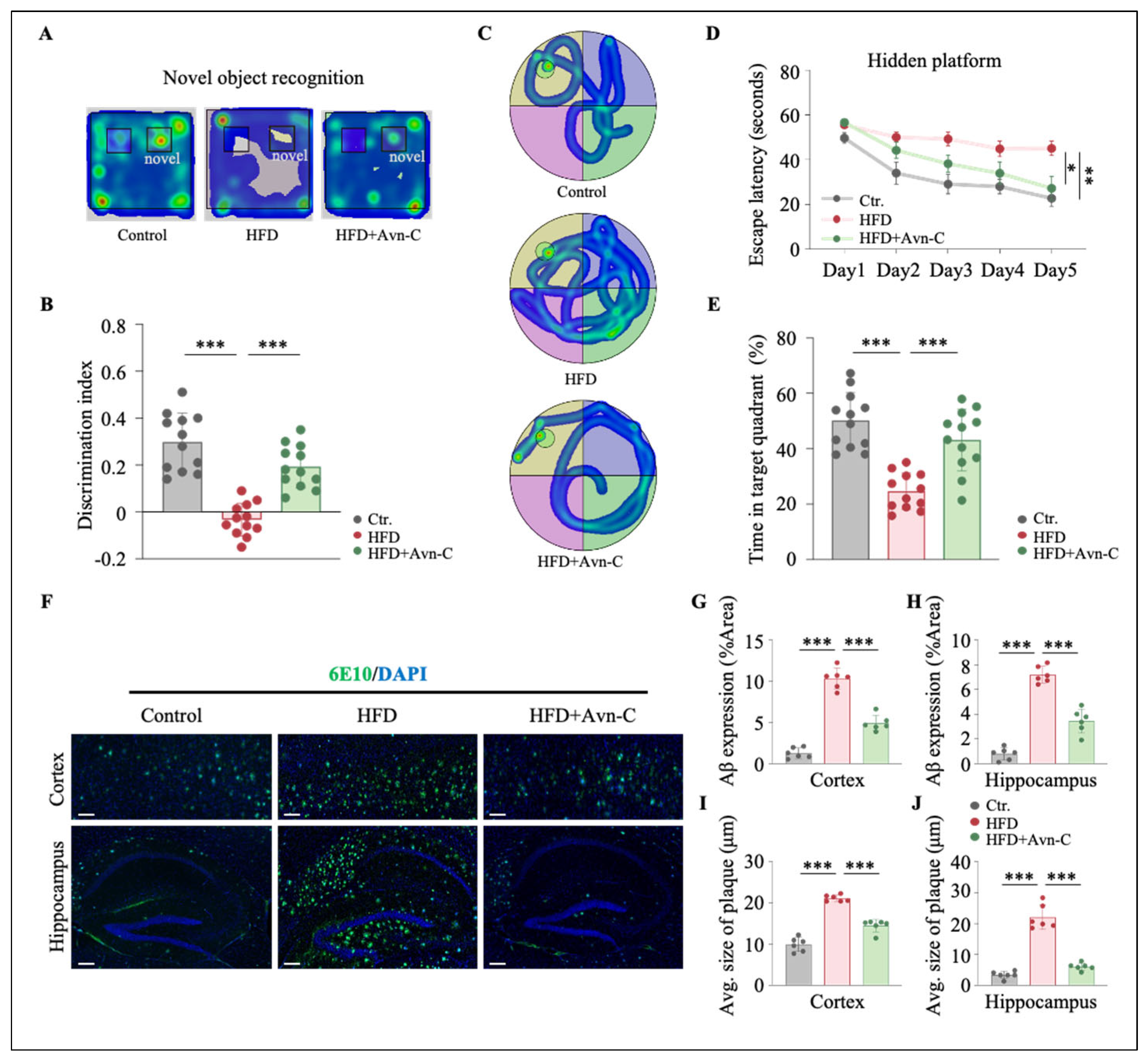
3.2. Avn-C Reduces Recognition and Spatial Memory Impairments in Obese 5×FAD Mice
3.3. Avn-C Treatment Reduces Aβ Aggravation in Obese 5×FAD Mice
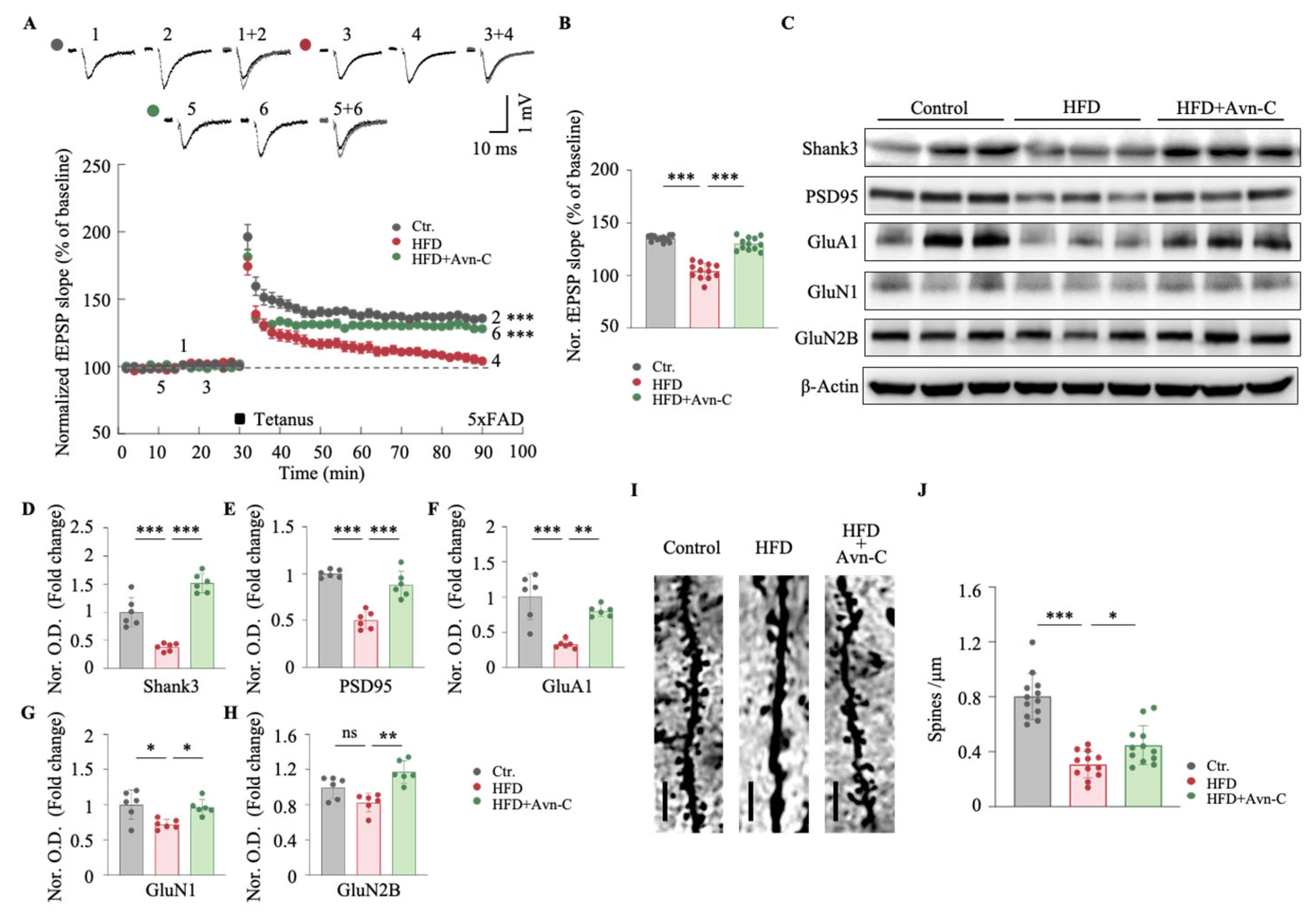
3.4. Avn-C Improves Synaptic Plasticity and Dendritic Spine Density in Obese 5×FAD Mice
3.5. Avn-C Inhibits Neuronal Inflammation Induced by HFD
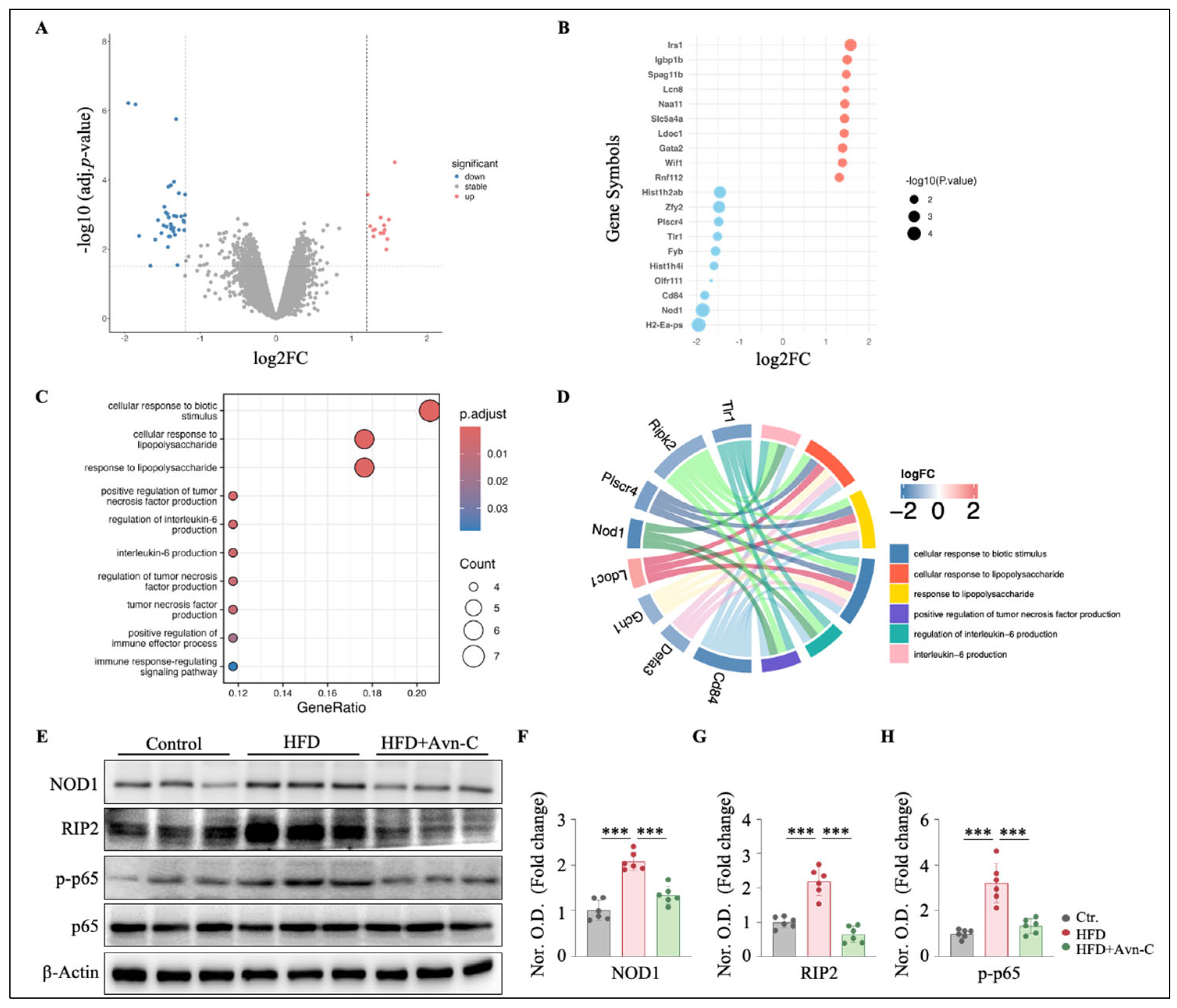
3.6. Avn-C Modulates Inflammation-Related Gene Expression and Downregulates NOD1 Signaling in the Hippocampus
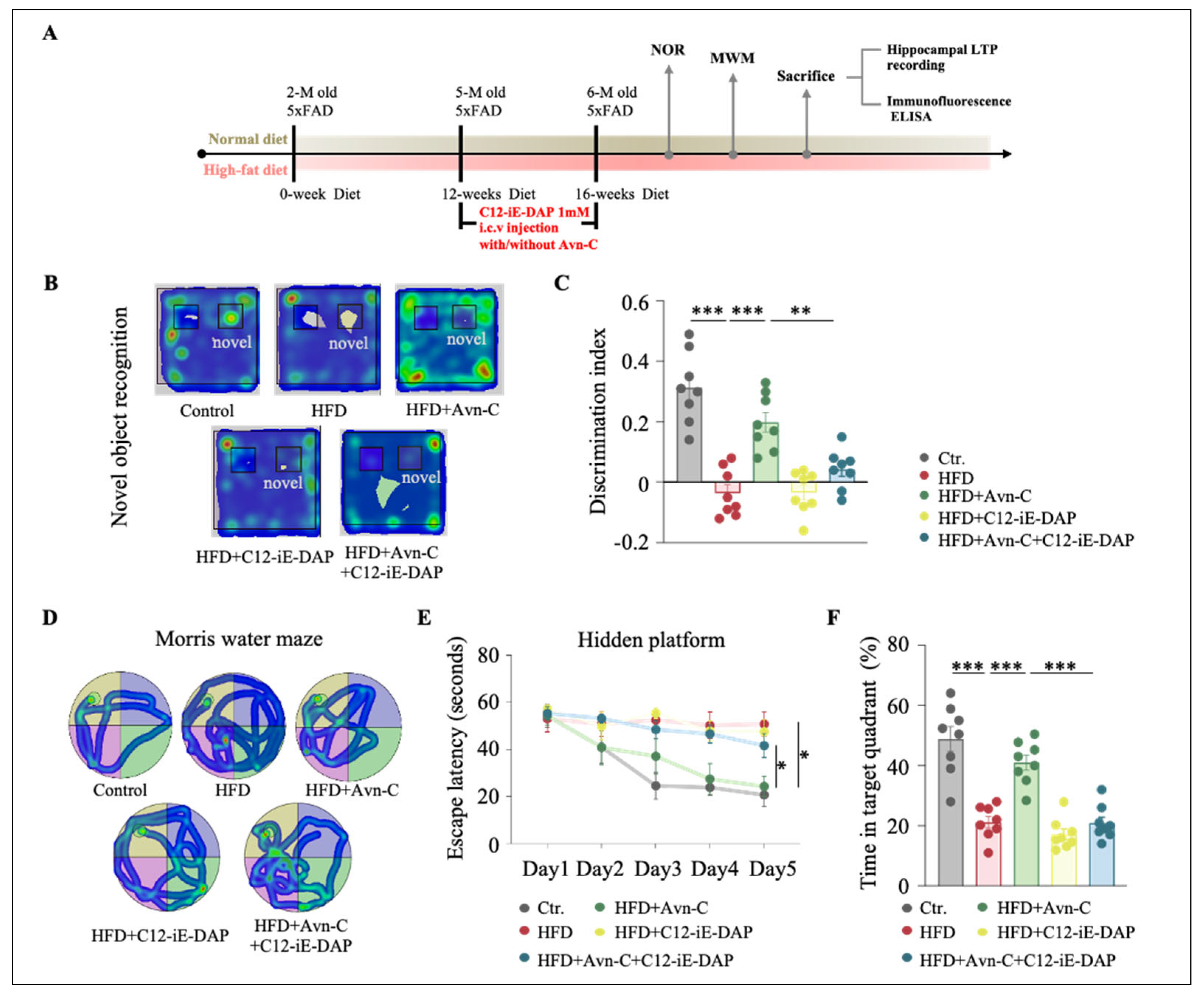
3.7. NOD1 Activation Abolishes Avn-C-Mediated Memory Improvements in Obese 5×FAD Mice
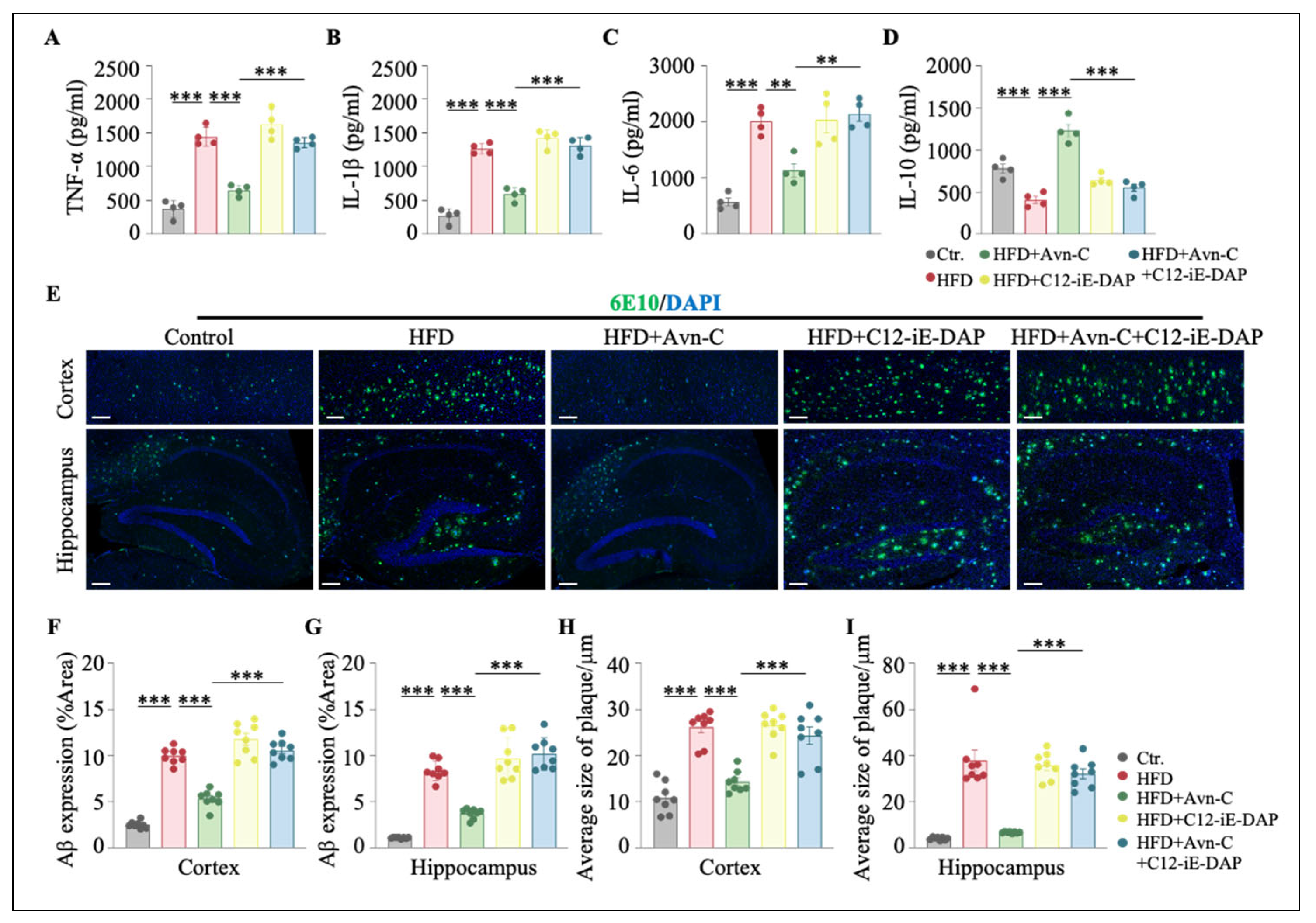
3.8. Activation of NOD1 Attenuates the Therapeutic Efficacy of Avn-C in HFD-Fed 5×FAD Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Forstl, H.; Burns, A.; Levy, R.; Cairns, N.; Luthert, P.; Lantos, P. Neuropathological correlates of behavioural disturbance in confirmed Alzheimer’s disease. Br. J. Psychiatry 1993, 163, 364–368. [Google Scholar] [CrossRef]
- Jahn, H. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 2013, 15, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Olmo, B.M.G.; Bettes, M.N.; DeMarsh, J.W.; Zhao, F.; Askwith, C.; Barrientos, R.M. Short-term high-fat diet consumption impairs synaptic plasticity in the aged hippocampus via IL-1 signaling. NPJ Sci. Food 2023, 7, 35. [Google Scholar] [CrossRef]
- Wu, X.; Gao, Y.; Shi, C.; Tong, J.; Ma, D.; Shen, J.; Yang, J.; Ji, M. Complement C1q drives microglia-dependent synaptic loss and cognitive impairments in a mouse model of lipopolysaccharide-induced neuroinflammation. Neuropharmacology 2023, 237, 109646. [Google Scholar] [CrossRef]
- Chapman, T.R.; Barrientos, R.M.; Ahrendsen, J.T.; Maier, S.F.; Patterson, S.L. Synaptic correlates of increased cognitive vulnerability with aging: Peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J. Neurosci. 2010, 30, 7598–7603. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Powell, T.L.; Barrett, E.S.; Hardy, D.B. Developmental origins of metabolic diseases. Physiol. Rev. 2021, 101, 739–795. [Google Scholar] [CrossRef]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Yamaguchi, K.; Matsui, K.; Sano, T.; Kubota, T.; Hashimoto, T.; Mano, A.; Yamada, K.; Matsuo, Y.; Kubota, N.; et al. Differential effects of diet- and genetically-induced brain insulin resistance on amyloid pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 15. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Wilson, R.S. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology 2004, 62, 1573–1579. [Google Scholar] [CrossRef]
- Nguyen, J.C.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef]
- Amelianchik, A.; Sweetland-Martin, L.; Norris, E.H. The effect of dietary fat consumption on Alzheimer’s disease pathogenesis in mouse models. Transl. Psychiatry 2022, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Honkura, N.; Ellis-Davies, G.C.; Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 2004, 429, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Staging of Alzheimer-related cortical destruction. Int. Psychogeriatr. 1997, 9 (Suppl. S1), 257–261, discussion 269–272. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Murlimanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and its involvement in Alzheimer’s disease: A review. 3 Biotech 2022, 12, 55. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Schneider, J.A.; Wilson, R.S.; Scherr, P.A. Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Arch. Neurol. 2006, 63, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Dey, A.; Yu, X.; Stranahan, A.M. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav. Immun. 2016, 51, 230–239. [Google Scholar] [CrossRef]
- Attuquayefio, T.; Stevenson, R.J.; Oaten, M.J.; Francis, H.M. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS ONE 2017, 12, e0172645. [Google Scholar] [CrossRef]
- Spencer, S.J.; D’Angelo, H.; Soch, A.; Watkins, L.R.; Maier, S.F.; Barrientos, R.M. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol. Aging 2017, 58, 88–101. [Google Scholar] [CrossRef]
- Cortez, M.; Carmo, L.S.; Rogero, M.M.; Borelli, P.; Fock, R.A. A high-fat diet increases IL-1, IL-6, and TNF-alpha production by increasing NF-kappaB and attenuating PPAR-gamma expression in bone marrow mesenchymal stem cells. Inflammation 2013, 36, 379–386. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-kappaB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Djorgbenoo, R.; Hu, J.; Hu, C.; Sang, S. Fermented Oats as a Novel Functional Food. Nutrients 2023, 15, 3521. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, Y.; Yerke, A.; Wise, M.L.; Johnson, J.; Chu, Y.; Sang, S. Oat avenanthramides induce heme oxygenase-1 expression via Nrf2-mediated signaling in HK-2 cells. Mol. Nutr. Food Res. 2015, 59, 2471–2479. [Google Scholar] [CrossRef]
- Zhouyao, H.; Malunga, L.N.; Chu, Y.F.; Eck, P.; Ames, N.; Thandapilly, S.J. The inhibition of intestinal glucose absorption by oat-derived avenanthramides. J. Food Biochem. 2022, 46, e14324. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Koenig, R.; Dickman, J.R.; Kang, C.; Zhang, T.; Chu, Y.F.; Ji, L.L. Avenanthramide supplementation attenuates exercise-induced inflammation in postmenopausal women. Nutr. J. 2014, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, S.H.; Quang, B.D.; Tran, T.T.; Kim, Y.G.; Ko, J.; Choi, W.Y.; Lee, S.Y.; Ryu, J.H. Avenanthramide-C Shows Potential to Alleviate Gingival Inflammation and Alveolar Bone Loss in Experimental Periodontitis. Mol. Cells 2023, 46, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, T.; Zhang, Y.; Liu, T.; Gagnon, G.; Ebrahim, J.; Johnson, J.; Chu, Y.F.; Ji, L.L. Avenanthramide supplementation reduces eccentric exercise-induced inflammation in young men and women. J. Int. Soc. Sports Nutr. 2020, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, H.; Yang, E.J.; Lee, S.; Kim, M.J.; Baek, M.C.; Lee, B.; Park, P.H.; Kwon, T.K.; Khang, D.; Song, K.S.; et al. Avenanthramide C from germinated oats exhibits anti-allergic inflammatory effects in mast cells. Sci. Rep. 2019, 9, 6884. [Google Scholar] [CrossRef]
- Kang, C.; Shin, W.S.; Yeo, D.; Lim, W.; Zhang, T.; Ji, L.L. Anti-inflammatory effect of avenanthramides via NF-kappaB pathways in C2C12 skeletal muscle cells. Free Radic. Biol. Med. 2018, 117, 30–36. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, Y.; Cao, X.; Zhang, Y.; Song, T. Avenanthramide-C Activates Nrf2/ARE Pathway and Inhibiting Ferroptosis Pathway to Improve Cognitive Dysfunction in Aging Rats. Neurochem. Res. 2023, 48, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, V.S.; Samidurai, M.; Park, H.J.; Wang, M.; Park, R.Y.; Yu, S.Y.; Kang, H.K.; Hong, S.; Choi, W.S.; Lee, Y.Y.; et al. Avenanthramide-C Restores Impaired Plasticity and Cognition in Alzheimer’s Disease Model Mice. Mol. Neurobiol. 2020, 57, 315–330. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; DeKosky, S.T.; Mufson, E.J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef]
- Nordengen, K.; Kirsebom, B.E.; Henjum, K.; Selnes, P.; Gisladottir, B.; Wettergreen, M.; Torsetnes, S.B.; Grontvedt, G.R.; Waterloo, K.K.; Aarsland, D.; et al. Glial activation and inflammation along the Alzheimer’s disease continuum. J. Neuroinflamm. 2019, 16, 46. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Kure, C.; Timmer, J.; Stough, C. The Immunomodulatory Effects of Plant Extracts and Plant Secondary Metabolites on Chronic Neuroinflammation and Cognitive Aging: A Mechanistic and Empirical Review. Front. Pharmacol. 2017, 8, 117. [Google Scholar] [CrossRef]
- Park, H.; Yoo, J.S.; Kim, J.Y.; Hwang, B.Y.; Han, J.S.; Yeon, S.W.; Kang, J.H. Anti-amyloidogenic effects of ID1201, the ethanolic extract of the fruits of Melia toosendan, through activation of the phosphatidylinositol 3-kinase/Akt pathway. Environ. Toxicol. Pharmacol. 2014, 37, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Jimenez, E.; Carrillo, I.J.-F.; Pedraza-Escalona, M.; Ramirez-Serrano, C.E.; Alvarez-Arellano, L.; Cortes-Mendoza, J.; Herrera-Ruiz, M.; Jimenez-Ferrer, E.; Zamilpa, A.; Tortoriello, J.; et al. Malva parviflora extract ameliorates the deleterious effects of a high fat diet on the cognitive deficit in a mouse model of Alzheimer’s disease by restoring microglial function via a PPAR-gamma-dependent mechanism. J. Neuroinflamm. 2019, 16, 143. [Google Scholar] [CrossRef]
- Callahan, M.J.; Lipinski, W.J.; Bian, F.; Durham, R.A.; Pack, A.; Walker, L.C. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am. J. Pathol. 2001, 158, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.S.; Bu, X.L.; Liu, Y.H.; Zhu, C.; Wang, Q.H.; Shen, L.L.; Liu, C.H.; Wang, Y.R.; Yao, X.Q.; Wang, Y.J. Sex Dimorphism Profile of Alzheimer’s Disease-Type Pathologies in an APP/PS1 Mouse Model. Neurotox. Res. 2016, 29, 256–266. [Google Scholar] [CrossRef]
- Gallagher, J.J.; Minogue, A.M.; Lynch, M.A. Impaired performance of female APP/PS1 mice in the Morris water maze is coupled with increased Abeta accumulation and microglial activation. Neurodegener. Dis. 2013, 11, 33–41. [Google Scholar] [CrossRef]
- Jankowsky, J.L.; Zheng, H. Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Foy, M.R.; Baudry, M.; Brinton, R.D.; Thompson, R.F. Estrogen and hippocampal plasticity in rodent models. J. Alzheimers Dis. 2008, 15, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; de la Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J. Alzheimers Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef]
- Wang, L.; Peng, W.; Zhao, Z.; Zhang, M.; Shi, Z.; Song, Z.; Zhang, X.; Li, C.; Huang, Z.; Sun, X.; et al. Prevalence and Treatment of Diabetes in China, 2013–2018. JAMA 2021, 326, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Ishikawa, T.; Griep, A.; Axt, D.; Kummer, M.P.; Heneka, M.T. PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 2012, 32, 17321–17331. [Google Scholar] [CrossRef]
- Lim, G.P.; Yang, F.; Chu, T.; Gahtan, E.; Ubeda, O.; Beech, W.; Overmier, J.B.; Hsiao-Ashec, K.; Frautschy, S.A.; Cole, G.M. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol. Aging 2001, 22, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Gurgul, A.A.; Najjar, Y.; Chee, A.; An, H.; Che, C.T.; Park, T.J.; Warpeha, K.M. Phenylpropanoid-enriched broccoli seedling extract can reduce inflammatory markers and pain behavior. J. Transl. Med. 2023, 21, 922. [Google Scholar] [CrossRef]
- Philpott, D.J.; Sorbara, M.T.; Robertson, S.J.; Croitoru, K.; Girardin, S.E. NOD proteins: Regulators of inflammation in health and disease. Nat. Rev. Immunol. 2014, 14, 9–23. [Google Scholar] [CrossRef]
- Girardin, S.E.; Tournebize, R.; Mavris, M.; Page, A.L.; Li, X.; Stark, G.R.; Bertin, J.; DiStefano, P.S.; Yaniv, M.; Sansonetti, P.J.; et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001, 2, 736–742. [Google Scholar] [CrossRef]
- Shiny, A.; Regin, B.; Balachandar, V.; Gokulakrishnan, K.; Mohan, V.; Babu, S.; Balasubramanyam, M. Convergence of innate immunity and insulin resistance as evidenced by increased nucleotide oligomerization domain (NOD) expression and signaling in monocytes from patients with type 2 diabetes. Cytokine 2013, 64, 564–570. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahren, B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53 (Suppl. S3), S215–S219. [Google Scholar] [CrossRef]
- Fernandez-Garcia, V.; Gonzalez-Ramos, S.; Avendano-Ortiz, J.; Martin-Sanz, P.; Gomez-Coronado, D.; Delgado, C.; Castrillo, A.; Bosca, L. High-fat diet activates splenic NOD1 and enhances neutrophil recruitment and neutrophil extracellular traps release in the spleen of ApoE-deficient mice. Cell Mol. Life Sci. 2022, 79, 396. [Google Scholar] [CrossRef]
- Petrov, D.; Pedros, I.; Artiach, G.; Sureda, F.X.; Barroso, E.; Pallas, M.; Casadesus, G.; Beas-Zarate, C.; Carro, E.; Ferrer, I.; et al. High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiences contribute to Alzheimer disease pathology in rodents. Biochim. Biophys. Acta 2015, 1852, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Ferreira, S.T. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 2014, 63, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Szalai, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: Effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, T.R.; Forny-Germano, L.; Sathler, L.B.; Brito-Moreira, J.; Houzel, J.C.; Decker, H.; Silverman, M.A.; Kazi, H.; Melo, H.M.; McClean, P.L.; et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Abeta oligomers. J. Clin. Investig. 2012, 122, 1339–1353. [Google Scholar] [CrossRef]
- Biessels, G.J.; Reagan, L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015, 16, 660–671. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Liu, C.; Li, C.L.; Song, Y.L.; Tang, Y.S.; Zhou, H.; Li, A.; Li, Y.; Weng, Y.; Zheng, F.P. Increased NOD1, but not NOD2, activity in subcutaneous adipose tissue from patients with metabolic syndrome. Obesity 2015, 23, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, P.; Zhou, Y.; Purohit, J.; Hwang, D. NOD1 activation induces proinflammatory gene expression and insulin resistance in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E587–E598. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 206. [Google Scholar] [CrossRef]
- Knight, E.M.; Martins, I.V.; Gumusgoz, S.; Allan, S.M.; Lawrence, C.B. High-fat diet-induced memory impairment in triple-transgenic Alzheimer’s disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol. Aging 2014, 35, 1821–1832. [Google Scholar] [CrossRef]
- Vandal, M.; White, P.J.; Tremblay, C.; St-Amour, I.; Chevrier, G.; Emond, V.; Lefrancois, D.; Virgili, J.; Planel, E.; Giguere, Y.; et al. Insulin reverses the high-fat diet-induced increase in brain Abeta and improves memory in an animal model of Alzheimer disease. Diabetes 2014, 63, 4291–4301. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Norman, E.D.; Lee, K.; Cutler, R.G.; Telljohann, R.S.; Egan, J.M.; Mattson, M.P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008, 18, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Sturchler-Pierrat, C.; Abramowski, D.; Duke, M.; Wiederhold, K.H.; Mistl, C.; Rothacher, S.; Ledermann, B.; Burki, K.; Frey, P.; Paganetti, P.A.; et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 1997, 94, 13287–13292. [Google Scholar] [CrossRef]
- Fu, M.; Zuo, Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 2011, 34, 177–187. [Google Scholar] [CrossRef]
- Nimchinsky, E.A.; Sabatini, B.L.; Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002, 64, 313–353. [Google Scholar] [CrossRef]
- Penzes, P.; Cahill, M.E.; Jones, K.A.; VanLeeuwen, J.E.; Woolfrey, K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011, 14, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yang, X.; Zhang, H.; Wang, H.; Huang, W.; Xu, F.; Zhuang, C.; Wang, X.; Li, Y. Aluminum chloride induces neuroinflammation, loss of neuronal dendritic spine and cognition impairment in developing rat. Chemosphere 2016, 151, 289–295. [Google Scholar] [CrossRef]
- Espinosa-Jimenez, T.; Cano, A.; Sanchez-Lopez, E.; Olloquequi, J.; Folch, J.; Bullo, M.; Verdaguer, E.; Auladell, C.; Pont, C.; Munoz-Torrero, D.; et al. A novel rhein-huprine hybrid ameliorates disease-modifying properties in preclinical mice model of Alzheimer’s disease exacerbated with high fat diet. Cell Biosci. 2023, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Sarroca, S.; Gatius, A.; Rodriguez-Farre, E.; Vilchez, D.; Pallas, M.; Grinan-Ferre, C.; Sanfeliu, C.; Corpas, R. Resveratrol confers neuroprotection against high-fat diet in a mouse model of Alzheimer’s disease via modulation of proteolytic mechanisms. J. Nutr. Biochem. 2021, 89, 108569. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Jin, B.; Xu, J.; Wang, C. Avenanthramide-C Mitigates High-Fat Diet-Accelerated Alzheimer’s Pathologies via NOD1-Driven Neuroinflammation in 5×FAD Mice. Nutrients 2025, 17, 2679. https://doi.org/10.3390/nu17162679
Wang M, Jin B, Xu J, Wang C. Avenanthramide-C Mitigates High-Fat Diet-Accelerated Alzheimer’s Pathologies via NOD1-Driven Neuroinflammation in 5×FAD Mice. Nutrients. 2025; 17(16):2679. https://doi.org/10.3390/nu17162679
Chicago/Turabian StyleWang, Ming, Baoyuan Jin, Jia Xu, and Chuang Wang. 2025. "Avenanthramide-C Mitigates High-Fat Diet-Accelerated Alzheimer’s Pathologies via NOD1-Driven Neuroinflammation in 5×FAD Mice" Nutrients 17, no. 16: 2679. https://doi.org/10.3390/nu17162679
APA StyleWang, M., Jin, B., Xu, J., & Wang, C. (2025). Avenanthramide-C Mitigates High-Fat Diet-Accelerated Alzheimer’s Pathologies via NOD1-Driven Neuroinflammation in 5×FAD Mice. Nutrients, 17(16), 2679. https://doi.org/10.3390/nu17162679





