Impact of Vitamin D Status and Supplementation on Brain-Derived Neurotrophic Factor and Mood–Cognitive Outcomes: A Structured Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
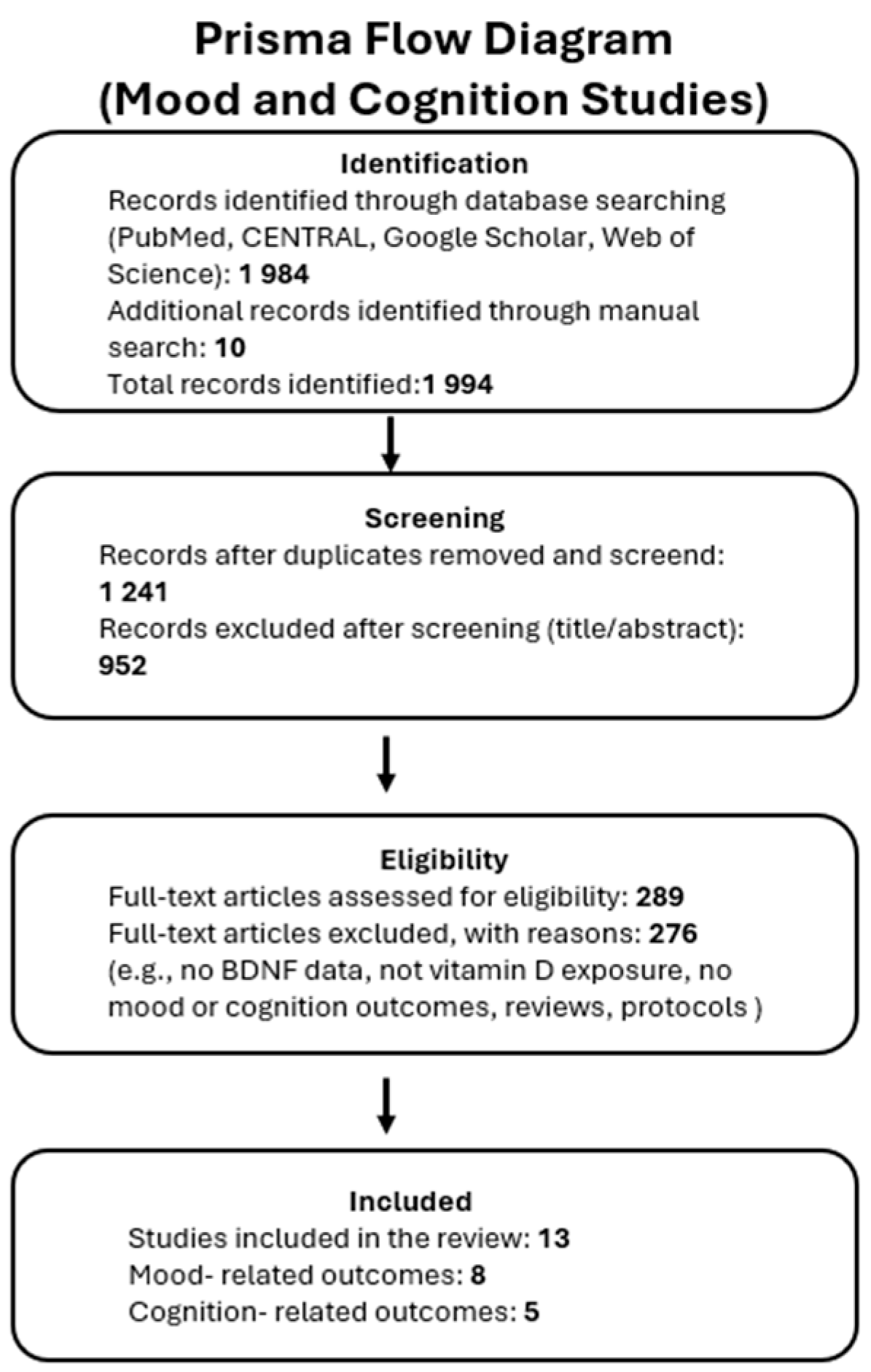
2.3. Search Strategy and Study Selection
2.4. Data Extraction
2.5. Quality Assessment and Data Synthesis
3. Results
3.1. Effects of Vitamin D on BDNF and Mood
| No | Author (Year) | Design/Population | Vitamin D Exposure | Vitamin D Dose/Status/Baseline 25(OH)D ng/mL | BDNF Outcomes | Mood Assessments | Key Findings |
|---|---|---|---|---|---|---|---|
| 1 | Yosaee et al. (2020) [39] | RCT/Obese adults with mild/mod depression n = 140 >20 y | Vitamin D alone or with Zn | 2000 IU/day for 12 weeks/26.07 ± 13.27 ng/mL | ↔ in serum BDNF | ↓ BDI-II | Vitamin D and Zn and vitamin D supplementation improved mood scale scores; no mediation tested. |
| 2 | Abiri et al. (2022) [40] | RCT/Obese women with mild/mod depression n = 102 20–45 y | Vitamin D alone or with Mg | 50,000 IU/week for 8 weeks/16.33 ± 9.67 ng/mL | ↑ in serum BDNF in vit D and Mg group | ↓ BDI-II | Co-supplementation vitamin D and Mg improved mood scale scores and level of BDNF; no mediation tested. |
| 3 | Vyas et al. (2023) [41] | RCT/Older adults (late-life depression prevention) n = 400 ≥60 y | Vitamin D + omega-3 fatty acids | 2000 IU/day for 2 years/baseline not reported | ↔ in serum BDNF after 2 years | ↔ MINI (DSM-IV) for incident MDD; PHQ-9 for symptoms | No effect on depression incidence or symptoms; BDNF did not change or mediate effect. |
| 4 | Goltz et al. (2018) [42] | Cross-sectional/Adults from general population (SHIP-TREND cohort) n = 3926 36–67 y | Vitamin D status | Mean 21.1 (14.4–29.9) ng/mL | ↔ BDNF | ↑ vitamin D–↓ PHQ-9 | Higher level of vitamin D associated with lower depression severity. |
| 5 | Yousefian et al. (2018) [47] | Animal/CMS model in rats n = 42 ♂ | Vitamin D i.p. | 5 or 10 µg/kg, 2×/week for 5 weeks/baseline not reported | ↔ BDNF in hippocampus (NS) | ↑ Sucrose preference SPT | Vitamin D presented antidepressant-like effect—reversal of anhedonia; BDNF did not change or mediate effect. |
| 6 | Yang et al. (2024) [30] | Animal/UCMS adolescent model in mice n = 75 ♂ | Vitamin D i.m. | 400/800/1600 IU/week for 8 weeks/baseline not reported | ↔ BDNF in hippocampus expression | ↓ Immobility in FST; ↑ activity in OFT | Vitamin D prevented depression-like behavior; BDNF did not change or mediate effect. |
| 7 | Xu and Liang (2021) [46] | Animal study/PSD, MCAO + UCMS model in mice n = 32 ♂ | Active vitamin D (calcitriol) i.c.v. | 25 μg/kg/day for 4 weeks/baseline not reported | ↑ Hippocampal BDNF expression (↑ protein and mRNA) | ↑ SPT; ↓ immobility in FST | Vitamin D injection reversed depression-like behavior via ↑ hippocampal BDNF; blocked by TrkB-IgG ↓ anhedonia. |
| 8 | Koshkina et al. (2019) [45] | Animal study/UCMS and menopausal model in rats n = 49 ♀ | Vitamin D s.c. | 1.0, 2.5, 5.0 mg/kg/day × for 4 weeks/baseline 25(OH)D ≈ 15 µg L−1 (~15 ng mL−1) | 5.0 mg/kg↑ hippocampal BDNF but 1.0 mg/kg ↓ hippocampal BDNF | 5.0 mg/kg ↑ SPT, ↓ immobility in FST; various doses ↑ activity in OFT | High-dose of vitamin D normalized BDNF and fully reversed anhedonia- and depressive-like behavior, but low-dose worsened mood and ↓ BDNF, indicating dose-dependent role of hippocampal neurotrophins in vitamin D-linked mood regulation. |
3.2. Effects of Vitamin D on BDNF and Cognitive Function
| No | Author (Year) | Design/Population | Vitamin D Exposure | Vitamin D Dose/Status/Baseline 25(OH)D ng/mL | BDNF Outcomes | Cognition Assessments | Key Findings |
|---|---|---|---|---|---|---|---|
| 1 | Quialheiro et al. (2023) [19] | Cross-sectional/older adults n = 576 ≥ 60 y | Vitamin D status | Mean ~26.5 ng/mL; categorized: <20, 21–29, ≥30 ng/mL | ↑ vitamin D–↑ in serum BDNF | ↑ vitamin D–↑ MMSE | Higher level of vitamin D associated with higher BDNF and better cognitive performance; BDNF not mediator. |
| 2 | Dewi et al. (2025) [20] | Cross-sectional/children n = 85 <2 y | Vitamin D status | Mean 27.65 ng/mL (10.5–39.8 ng/mL); cutoff ≤ 32.7 vs. >32.7 ng/mL | ↑ vitamin D → ↑ serum BDNF | ↑ vitamin D → ↑ gross motor, social, problem solving (ASQ-3) | Higher level of vitamin D associated with higher BDNF and better cognitive development; BDNF not mediator. |
| 3 | Khairy and Attia (2021) [29] | Animal study/Rats n = 60 ♂ | Vitamin D oral supplementation | 500 IU/kg/day for 5 weeks/baseline not reported | ↑ BNFS in brain | Biochemical markers (BDNF, AChE, oxidative stress, caspase-3) | Vitamin D showed neuroprotective effects and improved biochemical markers of aging. |
| 4 | Mansouri et al. (2021) [43] | Animal study/scopolamine-induced cognitive deficit n = 50 ♂ | Vitamin D i.p. (with scopolamine) | 100, 1000, and 10,000 IU/kg for 3 weeks/baseline not reported | ↑ BNFS in hippocampus | ↑MWM, ↑PA | Vitamin D improved cognitive outcomes and BDNF levels; no mediation tested. |
| 5 | Medhat et al. (2019) [44] | Animal study/LPS-induced AD-like rats n = 50 ♀ | Active vitamin D (calcitriol) i.p. and calcitriol and exercise | 1 μg per kg of body weight/2× day for 4 weeks | ↑ BDNF in brain | T-maze: ↓ time, ↑ % alternation | Vitamin D and vitamin D and exercise improved cognitive outcomes and BDNF levels; no mediation tested. |
4. Discussion
5. Conclusions
- Vitamin D may increase BDNF. High-dose protocols (≥2000 IU daily or 50,000 IU weekly) increase circulating or hippocampal BDNF levels by 7% in deficient humans and stressed rodents.
- The clinical effects of vitamin D supplementation and its concentration on BDNF are more pronounced in their effects on mood than on cognition impairment. Each increase in BDNF levels corresponds to a decrease of several points on depression scales, while cognitive improvement is smaller and occurs only after longer-term supplementation or combined supplementation.
- Targeted correction to levels of 30–40 ng/mL is a cost-effective and effective strategy. Vitamin D deficiency affects nearly half of women, the elderly, and those living above 49° north latitude. Achieving healthy vitamin D levels in these groups will offer the greatest public health benefits.
- Co-supplementation may prove crucial. Combining vitamin D with magnesium, zinc, omega-3 fatty acids, probiotics, or structured exercise doubles the neurotrophic and symptomatic response.
- Future research must consider important determinants. Seasonality, the role of genotype, gender, and objective monitoring of adherence should be considered in future projects, along with repeated BDNF testing and the use of objective methods and tools to provide definitive evidence of the effectiveness of vitamin D in neuropsychiatric therapy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 25(OH)D | 25-hydroxycholecalciferol (25-hydroxyvitamin D) |
| AD | Alzheimer’s disease |
| ASQ-3 | Ages and Stages Questionnaire, Third Edition |
| BDI-II | Beck Depression Inventory, Second Edition |
| BDNF | Brain-derived neurotrophic factor |
| BMI | Body mass index |
| CI | Confidence interval |
| CREB | cAMP (cyclic adenosine monophosphate) response element-binding protein |
| DALYs | Disability-adjusted life years |
| DASH | Dietary Approaches to Stop Hypertension |
| DNA | Deoxyribonucleic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| GDS | Geriatric Depression Scale |
| IU | International unit |
| LTP | Long-term potentiation |
| MCI | Mild cognitive impairment |
| MeDi | Mediterranean diet |
| MIND | Mediterranean-DASH Intervention for Neurodegenerative Delay |
| MMSE | Mini-mental state examination |
| MRI | Magnetic resonance imaging |
| NHANES | National Health and Nutrition Examination Survey |
| NOS | Newcastle–Ottawa Scale |
| PHQ-9 | Patient Health Questionnaire-9 |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PUFA | Polyunsaturated fatty acids |
| RCT | Randomized controlled trial |
| RoB 2 | Risk of Bias 2 |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| SMD | Standardized mean difference |
| SYRCLE | Systematic Review Centre for Laboratory animal Experimentation |
| TrkB | Tropomyosin receptor kinase B |
| VDR | Vitamin D receptor |
| WHO | World Health Organization |
References
- Meshkin, A.; Badiee, F.; Salari, N.; Hassanabadi, M.; Khaleghi, A.A.; Mohammadi, M. The Global Prevalence of Vitamin D Deficiency in the Elderly: A Meta-Analysis. Indian J. Orthop. 2024, 58, 223–230. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, A.; Ji, J.S. A Comparison Study of Vitamin D Deficiency among Older Adults in China and the United States. Sci. Rep. 2019, 9, 19713. [Google Scholar] [CrossRef]
- Boettger, S.F.; Angersbach, B.; Klimek, C.N.; Wanderley, A.L.M.; Shaibekov, A.; Sieske, L.; Wang, B.; Zuchowski, M.; Wirth, R.; Pourhassan, M. Prevalence and Predictors of Vitamin D-Deficiency in Frail Older Hospitalized Patients. BMC Geriatr. 2018, 18, 219. [Google Scholar] [CrossRef] [PubMed]
- Gracious, B.L.; Finucane, T.L.; Friedman-Campbell, M.; Messing, S.; Parkhurst, M.N. Vitamin D Deficiency and Psychotic Features in Mentally ill adolescents: A Cross-Sectional Study. BMC Psychiatry 2012, 12, 38. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clinic Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Burne, T.H.J.; McGrath, J.J. Vitamin D, Effects on Brain Development, Adult Brain Function and the Links between Low Levels of Vitamin D and Neuropsychiatric Disease. Front. Neuroendocrinol. 2013, 34, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Tuohimaa, P. Neurosteroid Hormone Vitamin D and Its Utility in Clinical Nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 12–19. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-Derived Neurotrophic Factor and Its Clinical Implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Lee, C.H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef]
- Chan, V.K.Y.; Leung, M.Y.M.; Chan, S.S.M.; Yang, D.; Knapp, M.; Luo, H.; Craig, D.; Chen, Y.; Bishai, D.M.; Wong, G.H.Y.; et al. Projecting the 10-Year Costs of Care and Mortality Burden of Depression until 2032: A Markov Modelling Study Developed from Real-World Data. Lancet Reg. Health West. Pac. 2024, 45, 101026. [Google Scholar] [CrossRef]
- Cheruvu, V.K.; Chiyaka, E.T. Prevalence of Depressive Symptoms among Older Adults Who Reported Medical Cost as a Barrier to Seeking Health Care: Findings from a Nationally Representative Sample. BMC Geriatr. 2019, 19, 192. [Google Scholar] [CrossRef]
- Zenebe, Y.; Akele, B.; W/Selassie, M.; Necho, M. Prevalence and Determinants of Depression among Old Age: A Systematic Review and Meta-Analysis. Ann. Gen. Psychiatry 2021, 20, 55. [Google Scholar] [CrossRef]
- Morimoto, S.S.; Kanellopoulos, D.; Alexopoulos, G.S. Cognitive Impairment in Depressed Older Adults: Implications for Prognosis and Treatment. Psychiatr. Ann. 2014, 44, 138–142. [Google Scholar] [CrossRef]
- Lee, J.S.; Potter, G.G.; Wagner, H.R.; Welsh-Bohmer, K.A.; Steffens, D.C. Persistent Mild Cognitive Impairment in Geriatric Depression. Int. Psychogeriatr. 2007, 19, 125–135. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J. Dementia Associated with Psychiatric Disorders. Int. Psychogeriatr. 2005, 17, S207–S221. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W. Evidence-Based Applications of Combination Psychotherapy and Pharmacotherapy for Depression. Focus Am. Psychiatr. Publ. 2016, 14, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Niarchou, E.; Roberts, L.; Naughton, B.D. What Is the Impact of Antidepressant Side Effects on Medication Adherence among Adult Patients Diagnosed with Depressive Disorder: A Systematic Review. J. Psychopharmacol. 2024, 38, 127–136. [Google Scholar] [CrossRef]
- Quialheiro, A.; D Orsi, E.; Moreira, J.D.; Xavier, A.J.; Peres, M.A. The Association between Vitamin D and BDNF on Cognition in Older Adults in Southern Brazil. Rev. Saude. Publica. 2023, 56, 109. [Google Scholar] [CrossRef]
- Dewi, M.M.; Imron, A.; Risan, N.A.; Mediana, G.; Judistiani, R.T.D.; Setiabudiawan, B. The Association of Vitamin D, Nerve Growth Factor (NGF), Brain-Derived Neurotrophic Factor (BDNF), and Glial Cell-Derived Neurotrophic Factor (GDNF) with Development in Children. Children 2025, 12, 60. [Google Scholar] [CrossRef]
- Wilson, V.K.; Houston, D.K.; Kilpatrick, L.; Lovato, J.; Yaffe, K.; Cauley, J.A.; Harris, T.B.; Simonsick, E.M.; Ayonayon, H.N.; Kritchevsky, S.B.; et al. Relationship Between 25-Hydroxyvitamin D and Cognitive Function in Older Adults: The Health ABC Study. J. Am. Geriatr. Soc. 2014, 62, 636–641. [Google Scholar] [CrossRef]
- Kilpatrick, L.; Houston, D.K.; Wilson, V.K.; Lovato, J.; Ayonayon, H.N.; Cauley, J.A.; Harris, T.; Simonsick, E.M.; Yaffe, K.; Kritchevsky, S.B.; et al. Low 25-Hydroxyvitamin D Concentrations and Risk of Incident Cognitive Impairment in Black and White Older Adults: The Health ABC Study. J. Nutr. Gerontol. Geriatr. 2018, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xue, W.; Li, J.; Fu, K.; Shi, H.; Zhang, B.; Teng, W.; Tian, L. 25-Hydroxyvitamin D Levels and the Risk of Dementia and Alzheimer’s Disease: A Dose–Response Meta-Analysis. Front. Aging Neurosci. 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Roșian, A.; Zdrîncă, M.; Dobjanschi, L.; Vicaș, L.G.; Mureșan, M.E.; Dindelegan, C.M.; Platona, R.I.; Marian, E. The Role of Vitamin D in the Management of Major Depressive Disorder: A Systematic Review. Pharmaceuticals 2025, 18, 792. [Google Scholar] [CrossRef]
- Mikola, T.; Marx, W.; Lane, M.M.; Hockey, M.; Loughman, A.; Rajapolvi, S.; Rocks, T.; O’Neil, A.; Mischoulon, D.; Valkonen-Korhonen, M.; et al. The Effect of Vitamin D Supplementation on Depressive Symptoms in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 11784–11801. [Google Scholar] [CrossRef] [PubMed]
- Latimer, C.S.; Brewer, L.D.; Searcy, J.L.; Chen, K.-C.; Popović, J.; Kraner, S.D.; Thibault, O.; Blalock, E.M.; Landfield, P.W.; Porter, N.M. Vitamin D Prevents Cognitive Decline and Enhances Hippocampal Synaptic Function in Aging Rats. Proc. Natl. Acad. Sci. USA 2014, 111, E4359–E4366. [Google Scholar] [CrossRef]
- Nadimi, H.; Djazayery, A.; Javanbakht, M.H.; Dehpour, A.; Ghaedi, E.; Derakhshanian, H.; Mohammadi, H.; Mousavi, S.N.; Djalali, M. Effect of Vitamin D Supplementation on CREB-TrkB-BDNF Pathway in the Hippocampus of Diabetic Rats. Iran. J. Basic Med. Sci. 2020, 23, 117–123. [Google Scholar] [CrossRef]
- Morello, M.; Landel, V.; Lacassagne, E.; Baranger, K.; Annweiler, C.; Féron, F.; Millet, P. Vitamin D Improves Neurogenesis and Cognition in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6463–6479. [Google Scholar] [CrossRef]
- Khairy, E.Y.; Attia, M.M. Protective Effects of Vitamin D on Neurophysiologic Alterations in Brain Aging: Role of Brain-Derived Neurotrophic Factor (BDNF). Nutr. Neurosci. 2021, 24, 650–659. [Google Scholar] [CrossRef]
- Yang, X.; Miao, J.; Huang, Y.; Li, L.; Zhuang, G. Preventive and Therapeutic Effect of Vitamin D on Depression-like Behavior in a Mouse Adolescent Depression Model and Its Association with BDNF Protein Expression. Front. Psychiatry 2024, 15, 1425681. [Google Scholar] [CrossRef]
- Lachat, C.; Hawwash, D.; Ocké, M.C.; Berg, C.; Forsum, E.; Hörnell, A.; Larsson, C.; Sonestedt, E.; Wirfält, E.; Åkesson, A.; et al. Strengthening the Reporting of Observational Studies in Epidemiology-Nutritional Epidemiology (STROBE-Nut): An Extension of the STROBE Statement. PLoS Med. 2016, 13, e1002036. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 22–23, Quiz 34–57. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Danks, M.T.; Gray, P.H.; Hurrion, E.M. Diagnostic Accuracy of Ages and Stages Questionnaire, Third Edition to Identify Abnormal or Delayed Gross Motor Development in High-Risk Infants. J. Paediatr. Child Health 2024, 60, 709–715. [Google Scholar] [CrossRef]
- Yosaee, S.; Soltani, S.; Esteghamati, A.; Motevalian, S.A.; Tehrani-Doost, M.; Clark, C.C.T.; Jazayeri, S. Effects of Zinc, Vitamin D, and Their Co-Supplementation on Mood, Serum Cortisol, and Brain-Derived Neurotrophic Factor in Patients with Obesity and Mild to Moderate Depressive Symptoms: A Phase II, 12-Wk, 2 × 2 Factorial Design, Double-Blind, Randomized, Placebo-Controlled Trial. Nutrition 2020, 71, 110601. [Google Scholar] [CrossRef]
- Abiri, B.; Sarbakhsh, P.; Vafa, M. Randomized Study of the Effects of Vitamin D and/or Magnesium Supplementation on Mood, Serum Levels of BDNF, Inflammation, and SIRT1 in Obese Women with Mild to Moderate Depressive Symptoms. Nutr. Neurosci. 2022, 25, 2123–2135. [Google Scholar] [CrossRef]
- Vyas, C.M.; Mischoulon, D.; Chang, G.; Reynolds, C.F.; Cook, N.R.; Weinberg, A.; Copeland, T.; Bubes, V.; Bradwin, G.; Lee, I.-M.; et al. Relation of Serum BDNF to Major Depression and Exploration of Mechanistic Roles of Serum BDNF in a Study of Vitamin D3 and Omega-3 Supplements for Late-Life Depression Prevention. J. Psychiatr. Res. 2023, 163, 357–364. [Google Scholar] [CrossRef]
- Goltz, A.; Janowitz, D.; Hannemann, A.; Nauck, M.; Hoffmann, J.; Seyfart, T.; Völzke, H.; Terock, J.; Grabe, H.J. Association of Brain-Derived Neurotrophic Factor and Vitamin D with Depression and Obesity: A Population-Based Study. Neuropsychobiology 2018, 76, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, F.; Ghanbari, H.; Marefati, N.; Arab, Z.; Salmani, H.; Beheshti, F.; Hosseini, M. Protective Effects of Vitamin D on Learning and Memory Deficit Induced by Scopolamine in Male Rats: The Roles of Brain-Derived Neurotrophic Factor and Oxidative Stress. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Medhat, E.; Rashed, L.; Abdelgwad, M.; Aboulhoda, B.E.; Khalifa, M.M.; El-Din, S.S. Exercise Enhances the Effectiveness of Vitamin D Therapy in Rats with Alzheimer’s Disease: Emphasis on Oxidative Stress and Inflammation. Metab. Brain Dis. 2020, 35, 111–120. [Google Scholar] [CrossRef]
- Koshkina, A.; Dudnichenko, T.; Baranenko, D.; Fedotova, J.; Drago, F. Effects of Vitamin D3 in Long-Term Ovariectomized Rats Subjected to Chronic Unpredictable Mild Stress: BDNF, NT-3, and NT-4 Implications. Nutrients 2019, 11, 1726. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, L. Vitamin D3/Vitamin D Receptor Signaling Mitigates Symptoms of Post-Stroke Depression in Mice by Upregulating Hippocampal BDNF Expression. Neurosci Res. 2021, 170, 306–313. [Google Scholar] [CrossRef]
- Yousefian, Z.; Khaleghian, A.; Parsaei, H.; Vafaei, A.A.; Rashidy-Pour, A.; Sedaghat, K. Effect of Vitamin D on Hippocampus Brain-Derived Neurotrophic Factor Level in Chronic Mild Stress Model of Depression in Rats. Middle East J. Rehabil. Health 2018, 5, e63901. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 August 2025).
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Wang, H.; Ma, L.; Zhang, M.; Zu, Z.; Wei, W.; Li, N.; Yang, L.; Chen, F.; Fan, C.; Wang, K.; et al. Risk Factors, Protective Factors, Peripheral Biomarkers, and Neurocognitive Markers Associated with Mood Disorders: An Umbrella Review of 103 Meta-Analyses and Systematic Reviews. Brain Behav. Immun. Health 2025, 48, 101068. [Google Scholar] [CrossRef]
- Polyakova, M.; Stuke, K.; Schuemberg, K.; Mueller, K.; Schoenknecht, P.; Schroeter, M.L. BDNF as a Biomarker for Successful Treatment of Mood Disorders: A Systematic & Quantitative Meta-Analysis. J. Affect Disord. 2015, 174, 432–440. [Google Scholar] [CrossRef]
- Ghaemi, S.; Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. The Effect of Vitamin D Supplementation on Depression: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Psychol. Med. 2024, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Musazadeh, V.; Keramati, M.; Ghalichi, F.; Kavyani, Z.; Ghoreishi, Z.; Alras, K.A.; Albadawi, N.; Salem, A.; Albadawi, M.I.; Salem, R.; et al. Vitamin D Protects against Depression: Evidence from an Umbrella Meta-Analysis on Interventional and Observational Meta-Analyses. Pharmacol. Res. 2023, 187, 106605. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Huang, Y.-C.; Huang, W.-L. The Effect of Vitamin D Supplement on Negative Emotions: A Systematic Review and Meta-Analysis. Depress. Anxiety 2020, 37, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Khoraminya, N.; Tehrani-Doost, M.; Jazayeri, S.; Hosseini, A.; Djazayery, A. Therapeutic Effects of Vitamin D as Adjunctive Therapy to Fluoxetine in Patients with Major Depressive Disorder. Aust. N. Zealand J. Psychiatry 2013, 47, 271–275. [Google Scholar] [CrossRef]
- Penckofer, S.; Byrn, M.; Adams, W.; Emanuele, M.A.; Mumby, P.; Kouba, J.; Wallis, D.E. Vitamin D Supplementation Improves Mood in Women with Type 2 Diabetes. J. Diabetes Res. 2017, 2017, 8232863. [Google Scholar] [CrossRef]
- Srifuengfung, M.; Srifuengfung, S.; Pummangura, C.; Pattanaseri, K.; Oon-Arom, A.; Srisurapanont, M. Efficacy and Acceptability of Vitamin D Supplements for Depressed Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrition 2023, 108, 111968. [Google Scholar] [CrossRef]
- Ates Bulut, E.; Soysal, P.; Yavuz, I.; Kocyigit, S.E.; Isik, A.T. Effect of Vitamin D on Cognitive Functions in Older Adults: 24-Week Follow-Up Study. Am. J. Alzheimers Dis. Other Demen. 2019, 34, 112–117. [Google Scholar] [CrossRef]
- Pirotta, S.; Kidgell, D.J.; Daly, R.M. Effects of Vitamin D Supplementation on Neuroplasticity in Older Adults: A Double-Blinded, Placebo-Controlled Randomised Trial. Osteoporos. Int. 2015, 26, 131–140. [Google Scholar] [CrossRef]
- Ilboudo, Y.; Yoshiji, S.; Lu, T.; Butler-Laporte, G.; Zhou, S.; Richards, J.B. Vitamin D, Cognition, and Alzheimer’s Disease: Observational and Two-Sample Mendelian Randomization Studies. J. Alzheimer’s Dis. 2024, 99, 1243–1260. [Google Scholar] [CrossRef]
- Kalra, A.; Teixeira, A.L.; Diniz, B.S. Association of Vitamin D Levels with Incident All-Cause Dementia in Longitudinal Observational Studies: A Systematic Review and Meta-Analysis. J. Prev. Alzheimer’s Dis. 2020, 7, 14–20. [Google Scholar] [CrossRef]
- Zeqaj, I.; Piffero, R.; Calzaducca, E.; Pirisi, M.; Bellan, M. The Potential Role of Vitamin D Supplementation in Cognitive Impairment Prevention. CNS Neurol. Disord. Drug Targets 2024, 23, 628–637. [Google Scholar] [CrossRef]
- Chakkera, M.; Ravi, N.; Ramaraju, R.; Vats, A.; Nair, A.R.; Bandhu, A.K.; Koirala, D.; Pallapothu, M.R.; Quintana Mariñez, M.G.; Khan, S. The Efficacy of Vitamin D Supplementation in Patients With Alzheimer’s Disease in Preventing Cognitive Decline: A Systematic Review. Cureus 2022, 14, e31710. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, M.; Smith, E.E.; Chen, H.-Y.; Creese, B.; Goodarzi, Z.; Ismail, Z. Vitamin D Supplementation and Incident Dementia: Effects of Sex, APOE, and Baseline Cognitive Status. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12404. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.J.; Fabian, C.J. How I Treat Vitamin D Deficiency. J. Oncol. Pr. 2010, 6, 97–101. [Google Scholar] [CrossRef]
- Orwoll, E.; Nielson, C.M.; Marshall, L.M.; Lambert, L.; Holton, K.F.; Hoffman, A.R.; Barrett-Connor, E.; Shikany, J.M.; Dam, T.; Cauley, J.A. Vitamin D Deficiency in Older Men. J. Clin. Endocrinol. Metab. 2009, 94, 1214–1222. [Google Scholar] [CrossRef]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Dobrzańska, A.; Franek, E.; et al. Practical Guidelines for the Supplementation of Vitamin D and the Treatment of Deficits in Central Europe—Recommended Vitamin D Intakes in the General Population and Groups at Risk of Vitamin D Deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef]
- Melamed, M.L.; Kumar, J. Low Levels of 25-Hydroxyvitamin D in the Pediatric Populations: Prevalence and Clinical Outcomes. Ped Health 2010, 4, 89–97. [Google Scholar] [CrossRef]
- Pozzi, F.; Aloe, L.; Frajese, G.; Frajese, G. Vitamin D (Calcifediol) Supplementation Modulates NGF and BDNF and Improves Memory Function in Postmenopausal Women: A Pilot Study. Res. Endocrinol. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Babaei, P.; Damirchi, A.; Hoseini, Z.; Hoseini, R. Co-Treatment of Vitamin D Supplementation and Aerobic Training Improves Memory Deficit in Ovariectomized Rat. Int. J. Neurosci. 2020, 130, 595–600. [Google Scholar] [CrossRef]
- Mruczyk, K.; Molska, M.; Wójciak, R.W.; Śliwicka, E.; Cisek-Woźniak, A. Associated between Cognition, Brain-Derived Neurotrophic Factor (BDNF) and Macronutrients in Normal and Overweight Postmenopausal Women. Exp. Gerontol. 2024, 192, 112449. [Google Scholar] [CrossRef] [PubMed]
- Mruczyk, K.; Wójciak, R.W.; Molska, M.; Śliwicka, E.; Podgórski, T.; Skoczek-Rubińska, A.; Borowiecka, A.; Cisek-Woźniak, A. The Impact of Physical Activity on Metabolic Health and Cognitive Function in Postmenopausal Women: A Cross-Sectional Study. Metabolites 2025, 15, 420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, Z.; Pan, J.; Yuan, M.; Lang, Y.; Wei, X.; Zhang, C. The Interaction of BDNF with Estrogen in the Development of Hypertension and Obesity, Particularly during Menopause. Front. Endocrinol. 2024, 15, 1384159. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, O.; Deveci, A.; Taneli, F. The Effect of Chronic Antidepressant Treatment on Serum Brain-Derived Neurotrophic Factor Levels in Depressed Patients: A Preliminary Study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 261–265. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Li, T.; Xu, B.; Fu, B. Fluoxetine May Enhance VEGF, BDNF and Cognition in Patients with Vascular Cognitive Impairment No Dementia: An Open-Label Randomized Clinical Study. Neuropsychiatr. Dis. Treat. 2021, 17, 3819–3825. [Google Scholar] [CrossRef]
- Fu, Q.; DeJager, J.; Gardner, E.M. Supplementation and Mitigating Cognitive Decline in Older Adults With or Without Mild Cognitive Impairment or Dementia: A Systematic Review. Nutrients 2024, 16, 3567. [Google Scholar] [CrossRef]
- Borges-Vieira, J.G.; Cardoso, C.K.S. Efficacy of B-Vitamins and Vitamin D Therapy in Improving Depressive and Anxiety Disorders: A Systematic Review of Randomized Controlled Trials. Nutr. Neurosci. 2023, 26, 187–207. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Ye, Z.; Ding, G.; Li, F.; Ma, J.; Hua, W. The Neurocognitive and BDNF Changes of Multicomponent Exercise for Community-Dwelling Older Adults with Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Aging 2020, 12, 4907–4917. [Google Scholar] [CrossRef]
- Dehghani, F.; Abdollahi, S.; Shidfar, F.; Clark, C.C.T.; Soltani, S. Probiotics Supplementation and Brain-Derived Neurotrophic Factor (BDNF): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Neurosci. 2023, 26, 942–952. [Google Scholar] [CrossRef]
- Molska, M.; Mruczyk, K.; Cisek-Woźniak, A.; Prokopowicz, W.; Szydełko, P.; Jakuszewska, Z.; Marzec, K.; Trocholepsza, M. The Influence of Intestinal Microbiota on BDNF Levels. Nutrients 2024, 16, 2891. [Google Scholar] [CrossRef]
- Barnestein-Fonseca, P.; Guerrero-Pertiñez, G.; Gúzman-Parra, J.; Valera-Moreno, E.; Mayoral-Cleries, F. Is It Possible to Diagnose Therapeutic Adherence in Mild Cognitive Impairment and Dementia Patients in Clinical Practice? Front. Pharmacol. 2024, 15, 1362168. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Lovell, J.; Weller, C.; Kennedy, B.; Winbolt, M.; Young, C.; Ibrahim, J. A Systematic Review of Medication Non-Adherence in Persons with Dementia or Cognitive Impairment. PLoS ONE 2017, 12, e0170651. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pang, X.; Huang, Y. Higher Dietary Vitamin D Intake Influences Brain and Mental Function in Elderly Americans: A Cross-Sectional Analysis. Front. Nutr. 2025, 12, 1564568. [Google Scholar] [CrossRef] [PubMed]
- Bertone-Johnson, E.R.; Powers, S.I.; Spangler, L.; Brunner, R.L.; Michael, Y.L.; Larson, J.C.; Millen, A.E.; Bueche, M.N.; Salmoirago-Blotcher, E.; Liu, S.; et al. Vitamin D Intake from Foods and Supplements and Depressive Symptoms in a Diverse Population of Older Women1234. Am. J. Clin. Nutr. 2011, 94, 1104–1112. [Google Scholar] [CrossRef]
- Key, M.N.; Szabo-Reed, A.N. Impact of Diet and Exercise Interventions on Cognition and Brain Health in Older Adults: A Narrative Review. Nutrients 2023, 15, 2495. [Google Scholar] [CrossRef]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and Bioactives in Green Leafy Vegetables and Cognitive Decline: Prospective Study. Neurology 2018, 90, e214–e222. [Google Scholar] [CrossRef]
- Contreras-Rodriguez, O.; Reales-Moreno, M.; Fernández-Barrès, S.; Cimpean, A.; Arnoriaga-Rodríguez, M.; Puig, J.; Biarnés, C.; Motger-Albertí, A.; Cano, M.; Fernández-Real, J.M. Consumption of Ultra-Processed Foods Is Associated with Depression, Mesocorticolimbic Volume, and Inflammation. J. Affect. Disord. 2023, 335, 340–348. [Google Scholar] [CrossRef]
- Kuningas, M.; Mooijaart, S.P.; Jolles, J.; Slagboom, P.E.; Westendorp, R.G.J.; van Heemst, D. VDR Gene Variants Associate with Cognitive Function and Depressive Symptoms in Old Age. Neurobiol. Aging 2009, 30, 466–473. [Google Scholar] [CrossRef]
- Notaras, M.; Hill, R.; van den Buuse, M. The BDNF Gene Val66Met Polymorphism as a Modifier of Psychiatric Disorder Susceptibility: Progress and Controversy. Mol. Psychiatry 2015, 20, 916–930. [Google Scholar] [CrossRef]
- Gulej, R.; Patai, R.; Ungvari, A.; Kallai, A.; Tarantini, S.; Yabluchanskiy, A.; Huffman, D.M.; Conboy, M.J.; Conboy, I.M.; Kivimäki, M.; et al. Impacts of Systemic Milieu on Cerebrovascular and Brain Aging: Insights from Heterochronic Parabiosis, Blood Exchange, and Plasma Transfer Experiments. Geroscience 2025, 1–170. [Google Scholar] [CrossRef]
- Fekete, M.; Lehoczki, A.; Szappanos, Á.; Zábó, V.; Kaposvári, C.; Horváth, A.; Farkas, Á.; Fazekas-Pongor, V.; Major, D.; Lipécz, Á.; et al. Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications. Nutrients 2025, 17, 1351. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Major, D.; Feher, A.; Fazekas-Pongor, V.; Lehoczki, A. Geroscience and Pathology: A New Frontier in Understanding Age-Related Diseases. Pathol. Oncol. Res. 2024, 30, 1611623. [Google Scholar] [CrossRef] [PubMed]
- Nyúl-Tóth, Á.; Patai, R.; Csiszar, A.; Ungvari, A.; Gulej, R.; Mukli, P.; Yabluchanskiy, A.; Benyo, Z.; Sotonyi, P.; Prodan, C.I.; et al. Linking Peripheral Atherosclerosis to Blood-Brain Barrier Disruption: Elucidating Its Role as a Manifestation of Cerebral Small Vessel Disease in Vascular Cognitive Impairment. Geroscience 2024, 46, 6511–6536. [Google Scholar] [CrossRef] [PubMed]
- Pál, É.; Ungvári, Z.; Benyó, Z.; Várbíró, S. Role of Vitamin D Deficiency in the Pathogenesis of Cardiovascular and Cerebrovascular Diseases. Nutrients 2023, 15, 334. [Google Scholar] [CrossRef]
| SANRA Criterion (0–5) | Mood Section | Cognition Section |
|---|---|---|
| 1. Clearly defined aim | 5 | 5 |
| 2. Search strategy | 4 | 4 |
| 3. Presentation of studies | 4 | 3 |
| 4. Critical appraisal | 4 | 3 |
| 5. Interpretation/conclusions | 4 | 4 |
| 6. Relevance/significance | 5 | 4 |
| Total (max 30) | 26 | 23 |
| Section/Item | Description | Completed |
|---|---|---|
| Title | Identify the report as a review | Yes |
| Abstract | Structured summary (PRISMA-Abstract) | Yes |
| Rationale | Describe rationale | Yes |
| Objectives | Provide explicit statement of objectives | Yes |
| Eligibility criteria | Specify study characteristics | Yes |
| Information sources | All databases, date of last search | Yes |
| Search strategy | Full search strings | Yes |
| Selection process | Methods and independent reviewers | Yes |
| Data collection process | Methods of extraction | Yes |
| Risk of bias | Specify tools used | Yes |
| Synthesis methods | Methods of synthesis | Yes |
| Reporting bias assessment | Assess risk of reporting bias | Partial |
| Certainty assessment | Certainty of evidence (GRADE) | NA |
| Results—Study selection | Flow diagram | Yes |
| Results—Study characteristics | Tables | Yes |
| Results—Risk of bias | Presentation of risk of bias | Yes |
| Results—Synthesis | Narrative synthesis | Yes |
| Discussion | Interpretation, limitations | Yes |
| Other info—Registration | Registration and protocol | No (narrative) |
| Other info—Funding | Sources of support | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoczek-Rubińska, A.; Cisek-Woźniak, A.; Molska, M.; Heyser, M.; Trocholepsza, M.; Pietrzak, S.; Mruczyk, K. Impact of Vitamin D Status and Supplementation on Brain-Derived Neurotrophic Factor and Mood–Cognitive Outcomes: A Structured Narrative Review. Nutrients 2025, 17, 2655. https://doi.org/10.3390/nu17162655
Skoczek-Rubińska A, Cisek-Woźniak A, Molska M, Heyser M, Trocholepsza M, Pietrzak S, Mruczyk K. Impact of Vitamin D Status and Supplementation on Brain-Derived Neurotrophic Factor and Mood–Cognitive Outcomes: A Structured Narrative Review. Nutrients. 2025; 17(16):2655. https://doi.org/10.3390/nu17162655
Chicago/Turabian StyleSkoczek-Rubińska, Aleksandra, Angelika Cisek-Woźniak, Marta Molska, Martyna Heyser, Martyna Trocholepsza, Sebastian Pietrzak, and Kinga Mruczyk. 2025. "Impact of Vitamin D Status and Supplementation on Brain-Derived Neurotrophic Factor and Mood–Cognitive Outcomes: A Structured Narrative Review" Nutrients 17, no. 16: 2655. https://doi.org/10.3390/nu17162655
APA StyleSkoczek-Rubińska, A., Cisek-Woźniak, A., Molska, M., Heyser, M., Trocholepsza, M., Pietrzak, S., & Mruczyk, K. (2025). Impact of Vitamin D Status and Supplementation on Brain-Derived Neurotrophic Factor and Mood–Cognitive Outcomes: A Structured Narrative Review. Nutrients, 17(16), 2655. https://doi.org/10.3390/nu17162655






