Predicting Metabolic and Cardiovascular Healthy from Nutritional Patterns and Psychological State Among Overweight and Obese Young Adults: A Neural Network Approach
Abstract
1. Introduction
Justification and Objectives
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Procedure
2.4. Neural Networks
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Sample
3.2. Neural Network Model for Women Predicting the Presence of MUO (HOMA-IR Criterion)
3.3. Neural Network Model for Men Predicting the Presence of MUO (HOMA-IR Criterion)
3.4. Neural Network Model for Women Predicting the Presence of MUO (IDF Criterion)
3.5. Neural Network Model for Men Predicting the Presence of MUO (IDF Criterion)
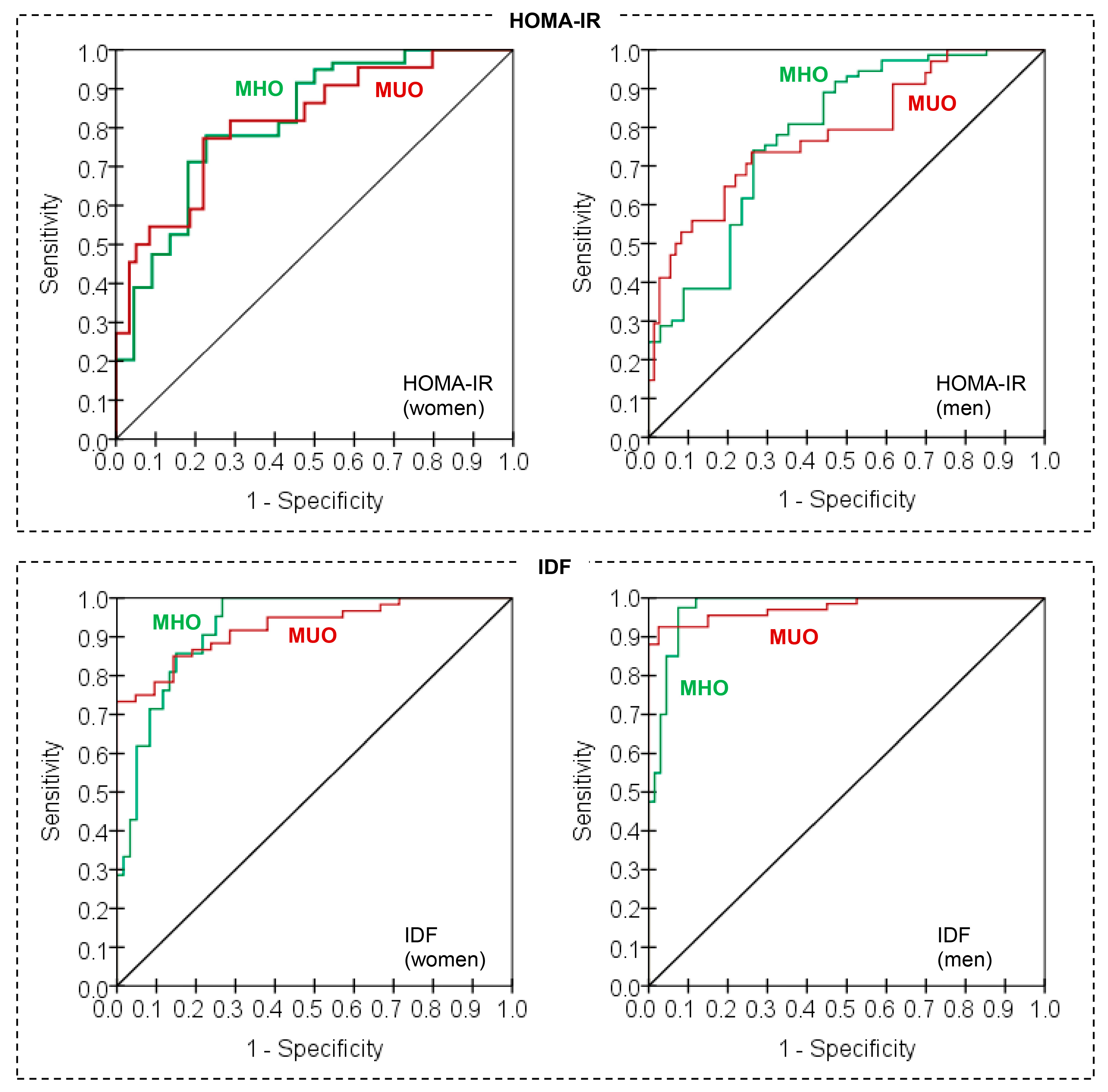
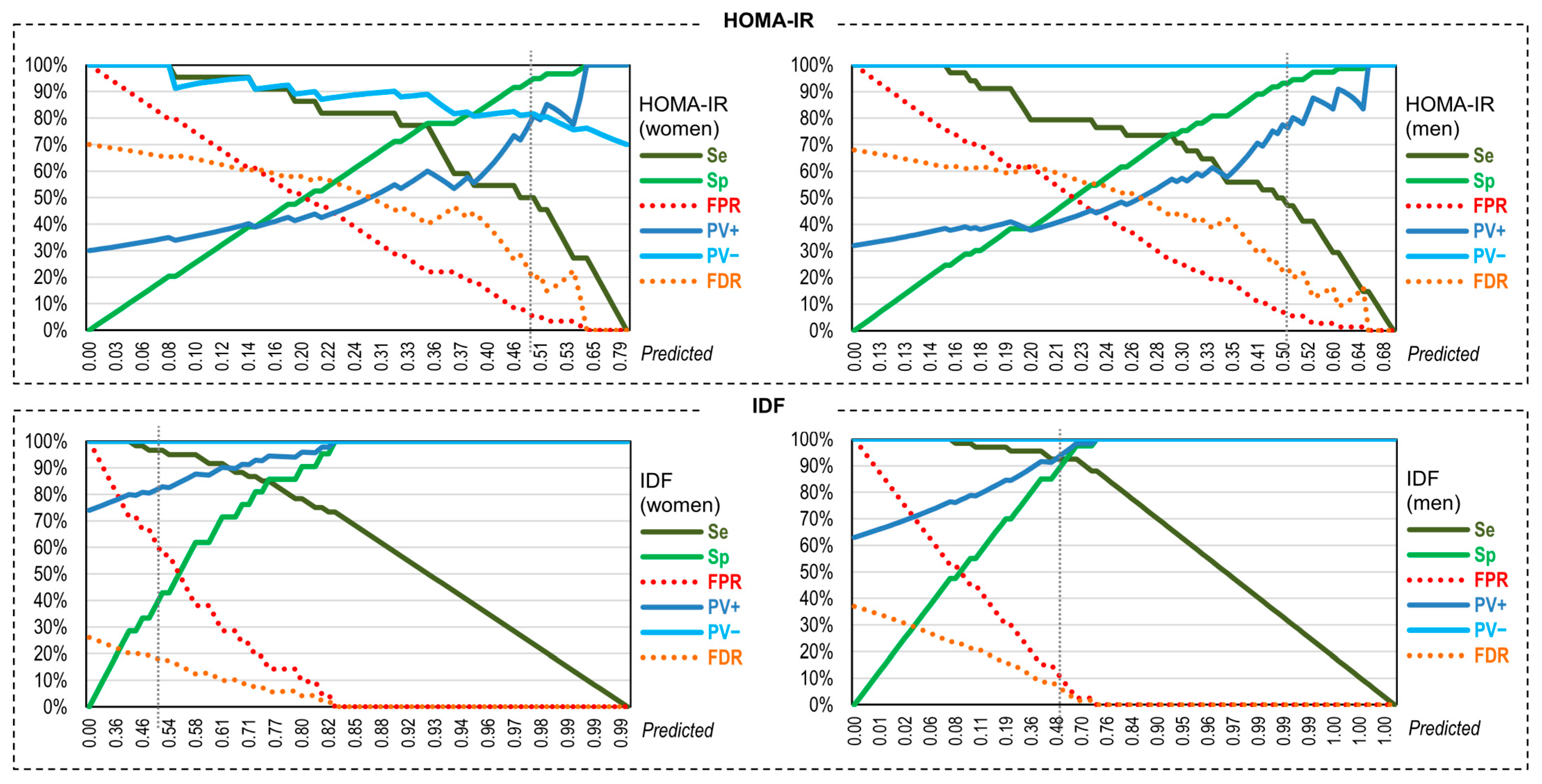
3.6. Assessmen of the Validity of the Neural Predictive Models for Discriminating MUO/MHO
4. Discussion
4.1. Limitations and Proposals for Future Studies
4.2. Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, R.K.; Lister, N.B.; Paxton, S.J.; Maguire, S.; Libesman, S.; Seidler, A.L.; Cooper, K.; Quigley, F.; Yourell, J.; Baur, L.A.; et al. Potential pathways to the onset and development of eating disorders in people with overweight and obesity: A scoping review. Obes. Rev. 2025, 26, e13840. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.; Seidell, J.C.; Meinders, A.E. De waarde en de beperkingen van de ‘body mass index’ (BMI) voor het bepalen van het gezondheidsrisico van overgewicht en obesitas [The value and limitations of the body mass index (BMI) in the assessment of the health risks of overweight and obesity]. Ned. Tijdschr. Voor Geneeskd. 2004, 148, 2379–2382. [Google Scholar]
- World Health Organization. (WHO). Overweight and Obesity. 2024. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 September 2024).
- Wyatt, S.B.; Winters, K.P.; Dubbert, P.M. Overweight and obesity: Prevalence, consequences, and causes of a growing public health problem. Am. J. Med. Sci. 2006, 331, 166–174. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.; Mandes, T.; Crimarco, A. A plant-based diet for overweight and obesity prevention and treatment. J. Geriatr. Cardiol. 2017, 14, 369–374. [Google Scholar] [CrossRef]
- Moradi, S.; Entezari, M.H.; Mohammadi, H.; Jayedi, A.; Lazaridi, A.V.; Kermani, M.A.H.; Miraghajani, M. Ultra-processed food consumption and adult obesity risk: A systematic review and dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 63, 249–260. [Google Scholar] [CrossRef]
- Jebb, S.A.; Moore, M.S. Contribution of a sedentary lifestyle and inactivity to the etiology of overweight and obesity: Current evidence and research issues. Med. Sci. Sports Exerc. 1999, 31 (Suppl. S11), S534–S541. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef] [PubMed]
- Dakanalis, A.; Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Vasios, G.K.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. The association of emotional eating with overweight/obesity, depression, anxiety/stress, and dietary patterns: A review of the current clinical evidence. Nutrients 2023, 15, 1173. [Google Scholar] [CrossRef]
- van Reedt Dortland, A.K.; Vreeburg, S.A.; Giltay, E.J.; Licht, C.M.; Vogelzangs, N.; van Veen, T.; de Geus, E.J.C.; Penninx, B.W.J.H.; Zitman, F.G. The impact of stress systems and lifestyle on dyslipidemia and obesity in anxiety and depression. Psychoneuroendocrinology 2013, 38, 209–218. [Google Scholar] [CrossRef]
- Ler, P.; Ojalehto, E.; Zhan, Y.; Finkel, D.; Dhal Aslan, A.K.; Karlsson, I.K. Conversions between metabolically unhealthy and healthy obesity from midlife to late-life. Int. J. Obes. 2024, 48, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef]
- Kabat, G.C.; Wu, W.Y.; Bea, J.W.; Chen, C.; Qi, L.; Stefanick, M.L.; Chlebowski, R.T.; Lane, D.S.; Wactawski-Wende, J.; Wassertheil-Smoller, S.; et al. Metabolic phenotypes of obesity: Frequency, correlates and change over time in a cohort of postmenopausal women. Int. J. Obes. 2017, 41, 170–177. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, X.; Hu, D.; Li, G.; Song, G. Transition patterns of metabolism-weight phenotypes over time: A longitudinal study using the multistate Markov model in China. Front. Public Health 2022, 10, 1026751. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Kim, J.H.; Sumerlin, T.S.; Feng, Q.; Yu, J. Metabolic health and adiposity transitions and risks of type 2 diabetes and cardiovascular diseases: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2023, 15, 60. [Google Scholar] [CrossRef]
- Bae, J.C.; Cho, N.H.; Kim, J.H.; Hur, K.Y.; Jin, S.M.; Lee, M.K. Association of Body Mass Index with the Risk of Incident Type 2 Diabetes, Cardiovascular Disease, and All-Cause Mortality: A Community-Based Prospective Study. Endocrinol. Metab. 2020, 35, 416–424. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Tazzeo, C.; Zucchelli, A.; Vetrano, D.L.; Demurtas, J.; Smith, L.; Schoene, D.; Sanchez-Rodriguez, D.; Onder, G.; Balci, C.; Bonetti, S.; et al. Risk factors for multimorbidity in adulthood: A systematic review. Ageing Res. Rev. 2023, 91, 102039. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab. Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Abbasifard, M.; Bazmandegan, G.; Ostadebrahimi, H.; Aimiri, M.; Kamiab, Z. General and central obesity prevalence in young adult: A study based on the Rafsanjan youth cohort study. Sci. Rep. 2023, 13, 17259. [Google Scholar] [CrossRef]
- Anekwe, C.V.; Jarrell, A.R.; Townsend, M.J.; Gaudier, G.I.; Hiserodt, J.M.; Stanford, F.C. Socioeconomics of obesity. Curr. Obes. Rep. 2020, 9, 272–279. [Google Scholar] [CrossRef]
- Sobal, J. Obesity and socioeconomic status: A framework for examining relationships between physical and social variables. Med. Anthropol. 1991, 13, 231–247. [Google Scholar] [CrossRef]
- Craig, P. Obesity and culture. In Clinical Obesity in Adults and Children; Kopelman, P.G., Caterson, I.D., Dietz, W.H., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2009; pp. 41–57. [Google Scholar] [CrossRef]
- Sobal, J. Social and cultural influences on obesity. In International Textbook of Obesity; Björntorp, P., Ed.; John Wiley Sons: Hoboken, NJ, USA, 2001; pp. 305–322. [Google Scholar] [CrossRef]
- Bianchi, A.; Pagan-Pomar, A.; Jimenez-Segovia, M.; Martinez-Corcoles, J.A.; Gonzalez-Argenté, F.X. Biliopancreatic diversion in the surgical treatment of morbid obesity: Long-term results and metabolic consequences. Obes. Surg. 2020, 30, 4234–4242. [Google Scholar] [CrossRef]
- Iacobini, C.; Pugliese, G.; Blasetti Fantauzzi, C.; Federici, M.; Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metab. Clin. Exp. 2019, 92, 51–60. [Google Scholar] [CrossRef]
- Tsatsoulis, A.; Paschou, S.A. Metabolically healthy obesity: Criteria, epidemiology, controversies, and consequences. Curr. Obes. Rep. 2020, 9, 109–120. [Google Scholar] [CrossRef]
- Mathew, H.; Farr, O.M.; Mantzoros, C.S. Metabolic health and weight: Understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metab. Clin. Exp. 2016, 65, 73–80. [Google Scholar] [CrossRef]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; De Alteriis, G.; Colao, A.; Savastano, S. Metabolically Healthy Obesity (MHO) vs. Metabolically Unhealthy Obesity (MUO) Phenotypes in PCOS: Association with Endocrine-Metabolic Profile, Adherence to the Mediterranean Diet, and Body Composition. Nutrients 2021, 13, 3925. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Dillon, C.; Harrington, J.M.; McCarthy, V.J.; Kearney, P.M.; Fitzgerald, A.P.; Perry, I.J. Defining metabolically healthy obesity: Role of dietary and lifestyle factors. PLoS ONE 2013, 8, e76188. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; Zimmet, P.; Alberti, K.G.; Rubino, F.; International Diabetes Federation Taskforce on Epidemiology and Prevention. Bariatric surgery: An IDF statement for obese Type 2 diabetes. Diabet. Med. 2011, 28, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, M.; Xu, L.; Wang, Y.; Cheng, H.; Zhao, X.; Mi, J. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol. Metab. Syndr. 2013, 5, 71. [Google Scholar] [CrossRef]
- Horáková, D.; Štěpánek, L.; Janout, V.; Janoutová, J.; Pastucha, D.; Kollárová, H.; Petráková, A.; Štěpánek, L.; Husár, R.; Martiník, K. Optimal Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) Cut-Offs: A Cross-Sectional Study in the Czech Population. Medicina 2019, 55, 158. [Google Scholar] [CrossRef]
- Wiebe, N.; Muntner, P.; Tonelli, M. Associations of body mass index, fasting insulin, and inflammation with mortalityu: A prospective cohort study. Int. J. Obes. 2022, 46, 2107–2113. [Google Scholar] [CrossRef]
- Sahay, M.; Kalra, S.; Badani, R.; Bantwal, G.; Bhoraskar, A.; Das, A.K.; Dhorepatil, B.; Ghosh, S.; Jeloka, T.; Khandelwal, D.; et al. Diabetes and Anemia: International Diabetes Federation (IDF)–Southeast Asian Region (SEAR) position statement. Diabetes Metab. Syndr. 2017, 11, S685–S695. [Google Scholar] [CrossRef]
- Esteghamati, A.; Ashraf, H.; Khalilzadeh, O.; Zandieh, A.; Nakhjavani, M.; Rashidi, A.; Haghazali, M.; Asgari, F. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: Third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr. Metab. 2010, 7, 26. [Google Scholar] [CrossRef]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Investig. 2019, 129, 3978–3989. [Google Scholar] [CrossRef]
- Manu, P.; Ionescu-Tirgoviste, C.; Tsang, J.; Napolitano, B.A.; Lesser, M.L.; Correll, C.U. Dysmetabolic Signals in “Metabolically Healthy” Obesity. Obes. Res. Clin. Pract. 2012, 6, e9–e20. [Google Scholar] [CrossRef]
- Pujia, A.; Gazzaruso, C.; Ferro, Y.; Mazza, E.; Maurotti, S.; Russo, C.; Lazzaro, V.; Romeo, S.; Montalcini, T. Individuals with metabolically healthy overweight/obesity have higher fat utilization than metabolically unhealthy individuals. Nutrients 2016, 8, 2. [Google Scholar] [CrossRef]
- Iyer, A.; Kauter, K.; Brown, L. Gender differences in metabolic syndrome: A key research issue? Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 182–188. [Google Scholar] [CrossRef]
- Jokela, M.; Hamer, M.; Singh-Manoux, A.; Batty, G.D.; Kivimäki, M. Association of metabolically healthy obesity with depressive symptoms: Pooled analysis of eight studies. Mol. Psychiatry 2014, 19, 910–914. [Google Scholar] [CrossRef]
- Ooi, D.S.Q.; Ong, S.G.; Chia, J.M.X.; Lim, Y.Y.; Ho, C.W.L.; Tay, V.; Vijaya, K.; Loke, K.Y.; Sng, A.A.; Griva, K.; et al. Quality of life and psychosocial outcomes among children with metabolically healthy and unhealthy obesity. Pediatr. Res. 2023, 94, 1089–1097. [Google Scholar] [CrossRef]
- Manippa, V.; Padulo, C.; van der Laan, L.N.; Brancucci, A. Gender Differences in Food Choice: Effects of Superior Temporal Sulcus Stimulation. Front. Hum. Neurosci. 2017, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Camhi, S.M.; Crouter, S.E.; Hayman, L.L.; Must, A.; Lichtenstein, A.H. Lifestyle behaviors in metabolically healthy and unhealthy overweight and obese women: A preliminary study. PLoS ONE 2015, 10, e0138548. [Google Scholar] [CrossRef]
- Durstewitz, D.; Koppe, G.; Meyer-Lindenberg, A. Deep neural networks in psychiatry. Mol. Psychiatry 2019, 24, 1583–1598. [Google Scholar] [CrossRef]
- Shatte, A.B.R.; Hutchinson, D.M.; Teague, S.J. Machine learning in mental health: A scoping review of methods and applications. Psychol. Med. 2019, 49, 1426–1448. [Google Scholar] [CrossRef]
- Walter, M.; Alizadeh, S.; Jamalabadi, H.; Lueken, U.; Dannlowski, U.; Walter, H.; Olbrich, S.; Colic, L.; Kambeitz, J.; Koutsouleris, N.; et al. Translational machine learning for psychiatric neuroimaging. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 91, 113–121. [Google Scholar] [CrossRef]
- Chen, Z.S.; Kulkarni, P.P.; Galatzer-Levy, I.R.; Bigio, B.; Nasca, C.; Zhang, Y. Modern views of machine learning for precision psychiatry. Patterns 2022, 3, 100602. [Google Scholar] [CrossRef]
- Reivan Ortiz, G.G.; Granero, R.; Aranda-Ramírez, M.P.; Aguirre-Quezada, M.A. Association Between Nutrition Patterns and Metabolic and Psychological State Among Young Adults. Eur. Eat. Disord. Rev. 2025. [Google Scholar] [CrossRef]
- Morejón, Y.; Manzano, A.; Betancourt, S.; Ulloa, V. Development of a Food Consumption Frequency Questionnaire for Ecuadorian Adults, cross-sectional study. Rev. Española Nutr. Humana Dietética 2021, 25, 394–402. [Google Scholar]
- Bauce, G.; Moya-Sifontes, M.Z. Waist circumference weight index as a complementary indicator of overweight and obesity in different groups of subjects. Rev. Digit. Postgrado 2020, 9, e195. [Google Scholar] [CrossRef]
- Gómez-León Mandujano, A.; Morales López, S.; Álvarez Díaz CD, J. Correct technique for taking blood pressure in the outpatient. Rev. La Fac. Med. 2016, 59, 49–55. [Google Scholar]
- Buccini Graciela, G.; Wolfthal, D. Cut-off values for insulin resistance, insulin sensitivity and insulin secretion indices derived from the HOMA formula and the HOMA2 program, Interpretation of the data. Rev. Argent. Endocrinol. Metab. 2008, 45, 3–21. [Google Scholar]
- Román, F.; Santibáñez, P.; Vinet, E.V. Use of the Depression Anxiety Stress Scales (DASS-21) as a Screening Instrument in Young People with Clinical Problems. Acta Investig. Psicológica 2016, 6, 2325–2336. [Google Scholar] [CrossRef]
- Hollingshead, A.B. Four factor index of social status. Yale J. Sociol. 2011, 8, 21–51. [Google Scholar]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. Available online: https://tvst.arvojournals.org/article.aspx?articleid=2762344 (accessed on 25 September 2024).
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Mentzou, A.; Rogers, A.; Carvalho, E.; Daly, A.; Malone, M.; Kerasidou, X. Artificial Intelligence in Digital Self-Diagnosis Tools: A Narrative Overview of Reviews. Mayo Clin. Proc. Digit. Health 2025, 3, 100242. [Google Scholar] [CrossRef]
- Guo, Z.; Lai, A.; Thygesen, J.H.; Farrington, J.; Keen, T.; Li, K. Large Language Models for Mental Health Applications: Systematic Review. JMIR Ment. Health 2024, 11, e57400. [Google Scholar] [CrossRef]
- Zhou, X.H.; Obuchowski, N.A.; McClish, D.K. Statistical Methods in Diagnostic Medicine, 2nd ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Pepe, M.S. The Statistical Evaluation of Medical Tests for Classification and Prediction; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Pataky, M.W.; Young, W.F.; Nair, K.S. Hormonal and Metabolic Changes of Aging and the Influence of Lifestyle Modifications. Mayo Clin. Proc. 2021, 96, 788–814. [Google Scholar] [CrossRef]
- Simpson, J.L.; Bailey, L.B.; Pietrzik, K.; Shane, B.; Holzgreve, W. Micronutrients and women of reproductive potential: Required dietary intake and consequences of dietary deficienty or excess. Part II-Vitamin D, Vitamin A, Iron, Zinc, Iodine, Essential Fatty Acids. J. Matern.-Fetal Neonatal Med. 2011, 24, 1–24. [Google Scholar] [CrossRef]
- Via, M. The malnutrition of obesity: Micronutrient deficiencies that promote diabetes. ISRN Endocrinol. 2012, 2012, 103472. [Google Scholar] [CrossRef]
- He, J.; Ogden, L.G.; Vupputuri, S.; Bazzano, L.A.; Loria, C.; Whelton, P.K. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA 1999, 282, 2027–2034. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Dixit, V.D.; Kahn, C.R.; Leibel, R.L.; Lin, X.; Nieuwdorp, M.; Pietiläinen, K.H.; Rabasa-Lhoret, R.; Roden, M.; Scherer, P.E.; et al. Metabolically healthy and unhealthy obese–the 2013 S tock Conference report. Obes. Rev. 2014, 15, 697–708. [Google Scholar] [CrossRef]
- Rudzka, A.; Kapusniak, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Kapusniak, J.; Barczyńska-Felusiak, R. The Importance of Micronutrient Adequacy in Obesity and the Potential of Microbiota Interventions to Support It. Appl. Sci. 2024, 14, 4489. [Google Scholar] [CrossRef]
- Epel, E.S.; McEwen, B.; Seeman, T.; Matthews, K.; Castellazzo, G.; Brownell, K.D.; Bell, J.; Ickovics, J.R. Stress and body shape: Stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom. Med. 2000, 62, 623–632. [Google Scholar] [CrossRef]
- Kukucka, T.; Ferencova, N.; Visnovcova, Z.; Ondrejka, I.; Hrtanek, I.; Kovacova, V.; Macejova, A.; Mlyncekova, Z.; Tonhajzerova, I. Mechanisms Involved in the Link between Depression, Antidepressant Treatment, and Associated Weight Change. Int. J. Mol. Sci. 2024, 25, 4511. [Google Scholar] [CrossRef]
- Simmons, W.K.; Burrows, K.; Avery, J.A.; Kerr, K.L.; Taylor, A.; Bodurka, J.; Potter, W.; Teague, T.K.; Drevets, W.C. Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol. Psychiatry 2020, 25, 1457–1468. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, X.; Zou, Z.; Zhou, Y.; Liu, A.; Li, X.; Du, Y.; Ji, X.; Li, Z.; Wu, X.; et al. Association between visceral fat area and metabolic syndrome in individuals with normal body weight: Insights from a Chinese health screening dataset. Lipids Health Dis. 2025, 24, 57. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Leroith, D.; Karnieli, E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt. Sinai J. Med. N. Y. 2010, 77, 511–523. [Google Scholar] [CrossRef]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef]
- Thomas, M.K.; Demay, M.B. Vitamin D deficiency and disorders of vitamin D metabolism. Endocrinol. Metab. Clin. N. Am. 2000, 29, 611–627. [Google Scholar] [CrossRef]
- Speakman, J.R.; Hall, K.D. Carbohydrates, insulin, and obesity. Science 2021, 372, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Abate, N. Body fat distribution and insulin resistance. Nutrients 2013, 5, 2019–2027. [Google Scholar] [CrossRef]
- Mao, Y.; Zhu, Z.; Pan, S.; Lin, W.; Liang, J.; Huang, H.; Li, L.; Wen, J.; Chen, G. Value of machine learning algorithms for predicting diabetes risk: A subset analysis from a real-world retrospective cohort study. J. Diabetes Investig. 2023, 14, 309–320. [Google Scholar] [CrossRef] [PubMed]
- DeGregory, K.W.; Kuiper, P.; DeSilvio, T.; Pleuss, J.D.; Miller, R.; Roginski, J.W.; Fisher, C.B.; Harness, D.; Viswanath, S.; Heymsfield, S.B.; et al. A review of machine learning in obesity. Obes. Rev. 2018, 19, 668–685. [Google Scholar] [CrossRef] [PubMed]
- Nieto, O.; Cardona, E.; Ramírez, D.; González, M.; Castaño, J. Obesity and inflammation in students of a Colombian public university. Rev. Public Health 2020, 22, 582–588. [Google Scholar] [CrossRef]
- Isasi, C.R.; Parrinello, C.M.; Ayala, G.X.; Delamater, A.M.; Perreira, K.M.; Daviglus, M.L.; Elder, J.P.; Marchante, A.N.; Bangdiwala, S.I.; Van Horn, L.; et al. Sex Differences in Cardiometabolic Risk Factors among Hispanic/Latino Youth. J. Pediatr. 2016, 176, 121–127.e1. [Google Scholar] [CrossRef]
- Katz, S.F.; Rodriguez, F.; Knowles, J.W. Health disparities in cardiometabolic risk among Black and Hispanic youth in the United States. Am. J. Prev. Cardiol. 2021, 6, 100175. [Google Scholar] [CrossRef]

| Total Sample; N = 188 | Women; N = 81 | Men; N = 107 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p | ||
| Marital | Single | 148 | 78.7% | 59 | 72.8% | 89 | 83.2% | 0.055 |
| Married—couple | 35 | 18.6% | 21 | 25.9% | 14 | 13.1% | ||
| Divorced—separated | 5 | 2.7% | 1 | 1.2% | 4 | 3.7% | ||
| Social | Low | 71 | 37.8% | 35 | 43.2% | 36 | 33.6% | 0.163 |
| Mean | 62 | 33.0% | 28 | 34.6% | 34 | 31.8% | ||
| High | 55 | 29.3% | 18 | 22.2% | 37 | 34.6% | ||
| Mean | SD | Mean | SD | Mean | SD | p | ||
| Age (years-old) | 20.76 | 2.57 | 20.40 | 2.62 | 21.04 | 2.51 | 0.090 | |
| BMI (kg/m2) | 28.36 | 2.94 | 28.27 | 2.92 | 28.44 | 2.97 | 0.694 | |
| Nutritional pattern 1 (NP1) | 7066.55 | 1883.53 | 6685.09 | 1813.72 | 7355.33 | 1892.24 | 0.015 | |
| Nutritional pattern 2 (NP2) | 271.37 | 45.46 | 268.97 | 46.28 | 273.18 | 44.96 | 0.531 | |
| Nutritional pattern 3 (NP3) | 77.05 | 18.36 | 75.60 | 16.78 | 78.15 | 19.47 | 0.347 | |
| Depression | 5.89 | 1.30 | 6.12 | 1.51 | 5.71 | 1.08 | 0.030 | |
| Anxiety | 5.21 | 1.55 | 5.56 | 1.60 | 4.95 | 1.46 | 0.008 | |
| Stress | 10.69 | 2.62 | 11.11 | 2.55 | 10.37 | 2.64 | 0.056 | |
| Glucose | 98.89 | 11.20 | 98.40 | 10.02 | 99.26 | 12.04 | 0.604 | |
| Insulin | 10.41 | 2.93 | 10.39 | 3.36 | 10.41 | 2.57 | 0.961 | |
| Cholesterol-T | 193.29 | 52.11 | 191.12 | 44.11 | 194.94 | 57.58 | 0.620 | |
| Triacylglycerol (TAG) | 147.80 | 52.43 | 148.46 | 52.33 | 147.30 | 52.75 | 0.880 | |
| n | % | n | % | n | % | p | ||
| Hypertension (HTN) | No | 140 | 74.5% | 66 | 81.5% | 74 | 69.2% | 0.055 |
| Yes | 48 | 25.5% | 15 | 18.5% | 33 | 30.8% | ||
| HOMA-IR criterion | MHO | 132 | 70.2% | 59 | 72.8% | 73 | 68.2% | 0.493 |
| MUO | 56 | 29.8% | 22 | 27.2% | 34 | 31.8% | ||
| IDF criterion | MHO | 61 | 32.4% | 21 | 25.9% | 40 | 37.4% | 0.097 |
| MUO | 127 | 67.6% | 60 | 74.1% | 67 | 62.6% | ||
| Input Layer | Hidden Layer 1 | Output Layer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HOMA-IR, women | H(1:1) | H(1:2) | H(1:3) | Hidden Layer 1 | [MHO = 0] | [MUO = 1] | |||
| (Bias) | 0.284 | 0.099 | −0.432 | (Bias) | |||||
| Age (years) | −0.609 | 0.148 | 0.151 | H(1:1) | 0.497 | −0.327 | |||
| BMI (kg/m2) | −0.015 | −0.550 | 0.335 | H(1:2) | 0.322 | 0.051 | |||
| NP1 | 0.598 | 0.925 | −0.357 | H(1:3) | 0.600 | −0.930 | |||
| NP2 | 0.641 | 0.546 | 0.354 | ||||||
| NP3 | −0.455 | −0.043 | −0.221 | ||||||
| Depression | 0.291 | −0.795 | −0.403 | ||||||
| Anxiety | 0.222 | 0.057 | −0.034 | ||||||
| Stress | −0.123 | 0.054 | −0.683 | ||||||
| HOMA-IR, men | H(1:1) | H(1:2) | H(1:3) | H(1:4) | H(1:5) | H(1:6) | Hidden Layer 1 | [MHO = 0] | [MUO = 1] |
| (Bias) | −0.370 | 0.153 | −0.047 | 0.084 | 0.129 | −0.286 | (Bias) | 0.386 | −0.376 |
| Age (years) | −0.495 | −0.257 | 0.053 | 0.167 | 0.119 | 0.190 | H(1:1) | −0.293 | −0.080 |
| BMI (kg/m2) | 0.440 | 0.719 | 0.150 | −0.385 | −0.534 | −0.012 | H(1:2) | 0.079 | 0.533 |
| NP1 | −0.149 | 0.305 | 0.073 | −0.031 | 0.055 | −0.220 | H(1:3) | −0.451 | −0.089 |
| NP2 | −0.218 | −0.056 | 0.126 | 0.223 | 0.139 | 0.119 | H(1:4) | −0.599 | −0.645 |
| NP3 | 0.166 | −0.124 | −0.501 | 0.473 | −0.344 | −0.004 | H(1:5) | 0.459 | −0.273 |
| Depression | −0.022 | 0.582 | −0.006 | 0.062 | −0.459 | −0.422 | H(1:6) | −0.386 | −0.527 |
| Anxiety | −0.433 | −0.122 | 0.039 | −0.200 | −0.025 | 0.023 | |||
| Stress | 0.079 | 0.588 | 0.294 | −0.331 | 0.304 | −0.453 | |||
| IDF, women | H(1:1) | H(1:2) | H(1:3) | H(1:4) | Hidden Layer 1 | [MHO = 0] | [MUO = 1] | ||
| (Bias) | −0.377 | −0.531 | 1.089 | −0.951 | (Bias) | 0.020 | 0.818 | ||
| Age (years) | 0.685 | −0.244 | −0.421 | −0.786 | H(1:1) | 0.119 | −0.477 | ||
| BMI (kg/m2) | −0.731 | −0.822 | 2.018 | −0.972 | H(1:2) | 0.376 | −0.398 | ||
| NP1 | −0.366 | 0.640 | 0.273 | 0.602 | H(1:3) | −0.884 | 0.671 | ||
| NP2 | 0.244 | 0.192 | −0.471 | −0.308 | H(1:4) | 0.211 | −0.678 | ||
| NP3 | 0.142 | 0.331 | 0.263 | 0.410 | |||||
| Depression | −0.580 | 0.051 | 0.404 | −0.593 | |||||
| Anxiety | −0.100 | −0.776 | −1.052 | 0.630 | |||||
| Stress | 0.040 | 0.445 | 0.302 | −1.362 | |||||
| IDF, men | H(1:1) | H(1:2) | H(1:3) | H(1:4) | Hidden Layer 1 | [MHO = 0] | [MUO = 1] | ||
| (Bias) | 0.547 | −0.728 | 1.035 | −1.076 | (Bias) | −1.057 | 1.345 | ||
| Age (years) | 0.645 | 0.320 | 0.855 | 0.033 | H(1:1) | 2.112 | −2.484 | ||
| BMI (kg/m2) | −1.968 | 0.264 | 1.704 | −3.141 | H(1:2) | 0.573 | −1.228 | ||
| NP1 | 2.429 | 1.169 | 0.853 | 1.782 | H(1:3) | −1.229 | 1.929 | ||
| NP2 | 1.054 | −0.279 | −0.099 | 0.654 | H(1:4) | −3.064 | 2.220 | ||
| NP3 | −0.035 | 0.651 | 0.339 | −0.299 | |||||
| Depression | −0.168 | 0.544 | −0.290 | 1.985 | |||||
| Anxiety | 0.945 | 0.557 | −0.710 | 0.599 | |||||
| Stress | −1.666 | 0.126 | −0.172 | 0.623 | |||||
| Predicted (Set Training) | Predicted (Set Testing) | ||||||
|---|---|---|---|---|---|---|---|
| Model | Observed | MHO | MUO | Correct | MHO | MUO | Correct |
| HOMA-IR | MHO | 48 | 2 | 96.0% | 7 | 2 | 77.8% |
| Women | MUO | 11 | 7 | 38.9% | 0 | 4 | 100.0% |
| Overall | 86.8% | 13.2% | 80.9% | 53.8% | 46.2% | 84.6% | |
| HOMA-IR | MHO | 54 | 3 | 94.7% | 15 | 1 | 93.8% |
| Men | MUO | 16 | 13 | 44.8% | 2 | 3 | 60.0% |
| Overall | 81.4% | 18.6% | 77.9% | 81.0% | 19.0% | 85.7% | |
| IDF | MHO | 8 | 12 | 40.0% | 1 | 0 | 100.0% |
| Women | MUO | 2 | 46 | 95.8% | 0 | 12 | 100.0% |
| Overall | 14.7% | 85.3% | 79.4% | 7.7% | 92.3% | 100.0% | |
| IDF | MHO | 29 | 3 | 90.6% | 8 | 0 | 100.0% |
| Men | MUO | 3 | 51 | 94.4% | 2 | 11 | 84.6% |
| Overall | 37.2% | 62.8% | 93.0% | 47.6% | 52.4% | 90.5% | |
| Sp | Se | PV+ | PV− | FPR | FDR | AUC | Kappa | |
|---|---|---|---|---|---|---|---|---|
| HOMA-IR, women | 93.22% | 50.00% | 73.33% | 83.33% | 6.78% | 16.67% | 0.817 | 0.480 |
| HOMA-IR, men | 94.52% | 47.06% | 80.00% | 79.31% | 5.48% | 20.69% | 0.785 | 0.467 |
| IDF, women | 42.86% | 96.67% | 82.86% | 81.82% | 57.14% | 18.18% | 0.925 | 0.468 |
| IDF, men | 92.50% | 92.54% | 95.38% | 88.10% | 7.50% | 11.90% | 0.975 | 0.842 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reivan Ortiz, G.G.; Maraver-Capdevila, L.; Granero, R. Predicting Metabolic and Cardiovascular Healthy from Nutritional Patterns and Psychological State Among Overweight and Obese Young Adults: A Neural Network Approach. Nutrients 2025, 17, 2651. https://doi.org/10.3390/nu17162651
Reivan Ortiz GG, Maraver-Capdevila L, Granero R. Predicting Metabolic and Cardiovascular Healthy from Nutritional Patterns and Psychological State Among Overweight and Obese Young Adults: A Neural Network Approach. Nutrients. 2025; 17(16):2651. https://doi.org/10.3390/nu17162651
Chicago/Turabian StyleReivan Ortiz, Geovanny Genaro, Laura Maraver-Capdevila, and Roser Granero. 2025. "Predicting Metabolic and Cardiovascular Healthy from Nutritional Patterns and Psychological State Among Overweight and Obese Young Adults: A Neural Network Approach" Nutrients 17, no. 16: 2651. https://doi.org/10.3390/nu17162651
APA StyleReivan Ortiz, G. G., Maraver-Capdevila, L., & Granero, R. (2025). Predicting Metabolic and Cardiovascular Healthy from Nutritional Patterns and Psychological State Among Overweight and Obese Young Adults: A Neural Network Approach. Nutrients, 17(16), 2651. https://doi.org/10.3390/nu17162651








