Postbiotics Derived from Lactococcus lactis and Streptococcus thermophilus Attenuate Experimental Periodontitis by Modulating Macrophage Polarization and Osteoclastogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Preparation of Postbiotics
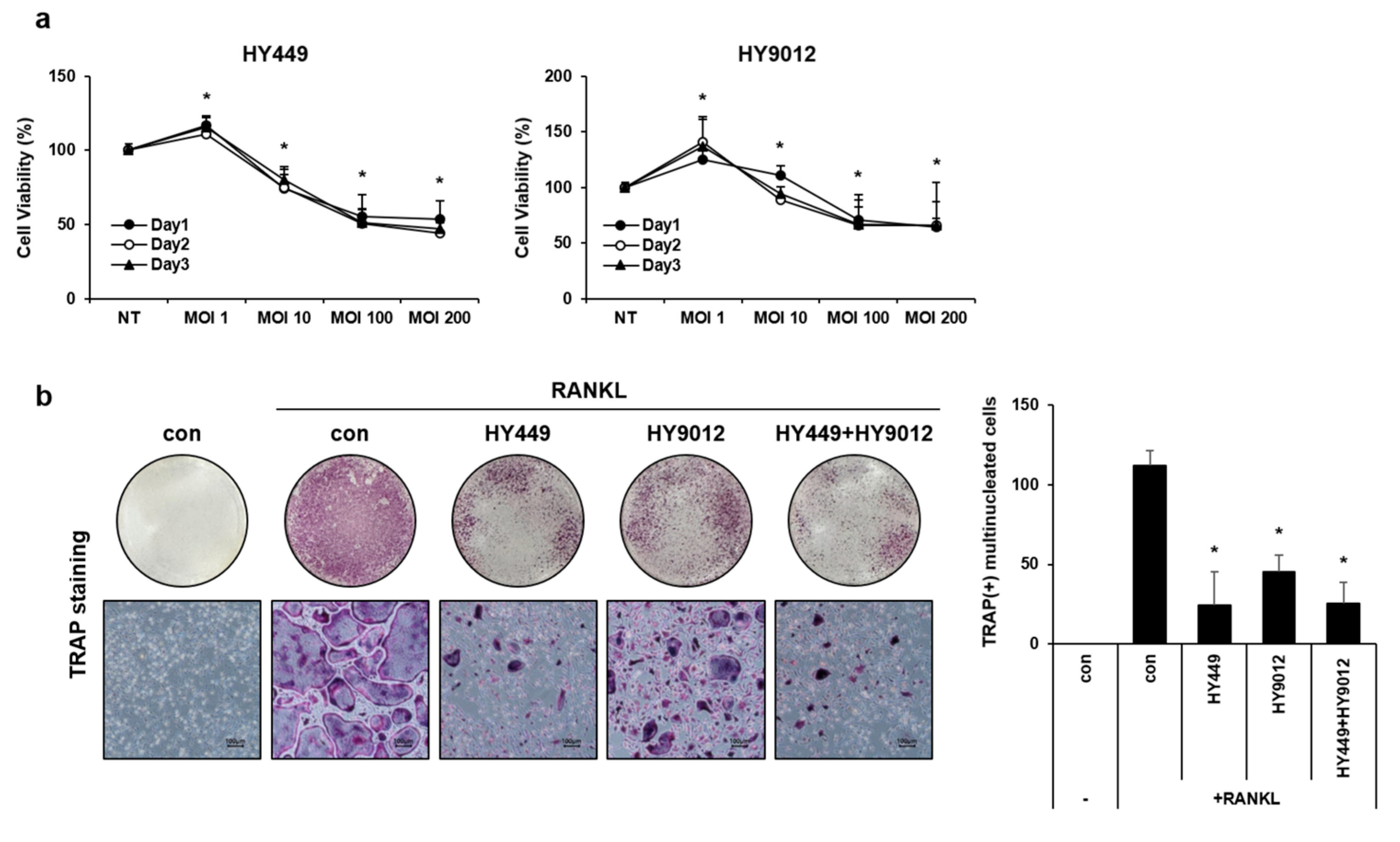
2.3. MTT Assay
2.4. Macrophage Polarization
2.5. In Vitro Osteoclast Differentiation
2.6. Reverse Transcription-Quantitative PCR (RT-qPCR)
2.7. Western Immunoblot Assay
2.8. Flow Cytometry Analysis
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Ligature-Induced Periodontitis in Mice
2.11. Statistical Analysis
3. Results
3.1. HK-HY449 and HK-HY9012 Alter M1/M2 Marker Expression During Macrophage Polarization
3.2. HK-HY449 and HK-HY9012 Differentially Modulate M1/M2 Cytokine Expression and Polarization-Associated Signaling Pathways
3.3. HK-HY449 and HK-HY9012 Suppress Osteoclast Differentiation by Modulating Osteoclastogenic Gene Expression and MAPK Signaling Pathways
3.4. HK-HY449 and HK-HY9012 Attenuate Alveolar Bone Loss and Inflammation in a Ligature-Induced Periodontitis Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMM | Bone marrow-derived macrophages |
| CTK | Cathepsin K |
| DC-STAMP | Dendritic cell-specific transmembrane protein |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK | Extracellular signal-regulated kinase |
| IFN | Interferon |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| M-CSF | Macrophage colony-stimulating factor |
| MAPK | Mitogen-activated protein kinase |
| MOI | Multiplicity of infection |
| NFATc1 | Nuclear factor of activated T-cells cytoplasmic 1 |
| PBS | Phosphate-buffered saline |
| PMA | Phorbol 12-myristate 13-acetate |
| RANKL | Receptor activator of nuclear factor κB ligand |
| RT-qPCR | Reverse transcription-quantitative PCR |
| TNF-α | Tumor necrosis factor-α |
| TRAP | Tartrate-resistant acid phosphatase |
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal Diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Afzali, H.; Graves, D.T. An Update on Periodontal Inflammation and Bone Loss. Front. Immunol. 2024, 15, 1385436. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Haque, M.M.; Yerex, K.; Kelekis-Cholakis, A.; Duan, K. Advances in Novel Therapeutic Approaches for Periodontal Diseases. BMC Oral Health 2022, 22, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, X.; Hou, J. Macrophages in Periodontitis: A Dynamic Shift between Tissue Destruction and Repair. Jpn. Dent. Sci. Rev. 2022, 58, 336–347. [Google Scholar] [CrossRef]
- Sun, X.; Gao, J.; Meng, X.; Lu, X.; Zhang, L.; Chen, R. Polarized Macrophages in Periodontitis: Characteristics, Function, and Molecular Signaling. Front. Immunol. 2021, 12, 763334. [Google Scholar] [CrossRef]
- Choi, Y.; Heo, S.C.; Kim, Y.N.; Joo, J.; Hwang, J.J.; Bae, M.; Kim, H.J. Gastrin-Releasing Peptide (GRP) Stimulates Osteoclastogenesis in Periodontitis. Cells 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- AlQranei, M.S.; Chellaiah, M.A. Osteoclastogenesis in Periodontal Diseases: Possible Mediators and Mechanisms. J. Oral Biosci. 2020, 62, 123–130. [Google Scholar] [CrossRef]
- Liao, W.; Ni, C.; Ge, R.; Li, Y.; Jiang, S.; Yang, W.; Yan, F. Nel-Like Molecule Type 1 Combined with Gold Nanoparticles Modulates Macrophage Polarization, Osteoclastogenesis, and Oral Microbiota in Periodontitis. ACS Appl. Mater. Interfaces 2024, 16, 8442–8458. [Google Scholar] [CrossRef]
- Di Stefano, M.; Santonocito, S.; Polizzi, A.; Mauceri, R.; Troiano, G.; Lo Giudice, A.; Romano, A.; Mascitti, M.; Isola, G. A Reciprocal Link between Oral, Gut Microbiota during Periodontitis: The Potential Role of Probiotics in Reducing Dysbiosis-Induced Inflammation. Int. J. Mol. Sci. 2023, 24, 1084. [Google Scholar] [CrossRef]
- Moraes, R.M.; Schlagenhauf, U.; Anbinder, A.L. Outside the Limits of Bacterial Viability: Postbiotics in the Management of Periodontitis. Biochem. Pharmacol. 2022, 201, 115072. [Google Scholar] [CrossRef]
- Lee, S.; Baek, D. Effects of Streptococcus Thermophilus on Volatile Sulfur Compounds Produced by Porphyromonas gingivalis. Arch. Oral Biol. 2014, 59, 1205–1210. [Google Scholar] [CrossRef]
- Oh, S.; Kim, S.; Ko, Y.; Sim, J.; Kim, K.S.; Lee, S.; Park, S.; Kim, Y.J. Effect of Bacteriocin Produced by Lactococcus Sp. HY 449 on Skin-Inflammatory Bacteria. Food Chem. Toxicol. 2006, 44, 1184–1190. [Google Scholar] [CrossRef]
- Shin, H.; Baek, D.; Lee, S. Inhibitory Effect of Lactococcus Lactis on the Bioactivity of Periodontopathogens. J. Gen. Appl. Microbiol. 2018, 64, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, N.; Johnson, J.; Apostolopoulos, V. Streptococcus Thermophilus Alters the Expression of Genes Associated with Innate and Adaptive Immunity in Human Peripheral Blood Mononuclear Cells. PLoS ONE 2020, 15, e0228531. [Google Scholar] [CrossRef] [PubMed]
- Holowacz, S.; Guinobert, I.; Guilbot, A.; Hidalgo, S.; Bisson, J.F.A. Mixture of Five Bacterial Strains Attenuates Skin Inflammation in Mice. J. Appl. Microbiol. 2004, 96, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, M.; Wang, L.; Chen, W. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar]
- Wang, H.; Lou, J.; Liu, H.; Liu, Y.; Xie, B.; Zhang, W.; Xie, J.; Pan, H.; Han, W. TRIM59 Deficiency Promotes M1 Macrophage Activation and Inhibits Colorectal Cancer through the STAT1 Signaling Pathway. Sci. Rep. 2024, 14, 16081. [Google Scholar] [CrossRef]
- Liu, L.; Guo, H.; Song, A.; Huang, J.; Zhang, Y.; Jin, S.; Li, S.; Zhang, L.; Yang, C.; Yang, P. Progranulin Inhibits LPS-Induced Macrophage M1 Polarization Via NF-кB and MAPK Pathways. BMC Immunol. 2020, 21, 32. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Chen, H.; Hu, Y.; Tang, L.; Zhou, X.; Tao, M.; Lv, Z.; Chen, S.; Qiu, A.; et al. Pharmacologic Inhibition of Histone Deacetylase 6 Prevents the Progression of Chlorhexidine Gluconate-Induced Peritoneal Fibrosis by Blockade of M2 Macrophage Polarization. Front. Immunol. 2022, 13, 899140. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, M.; Lei, W.; Yang, R.; Fu, S.; Fan, Z.; Yang, Y.; Zhang, T. Advances in the Role of STAT3 in Macrophage Polarization. Front. Immunol. 2023, 14, 1160719. [Google Scholar] [CrossRef]
- Fan, Z.; Cui, Y.; Chen, L.; Liu, P.; Duan, W. 23-Hydroxybetulinic Acid Attenuates 5-Fluorouracil Resistance of Colorectal Cancer by Modulating M2 Macrophage Polarization Via STAT6 Signaling. Cancer Immunol. Immunother. 2024, 73, 83. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin Ligand is a Cytokine that Regulates Osteoclast Differentiation and Activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Yagi, M.; Ninomiya, K.; Fujita, N.; Suzuki, T.; Iwasaki, R.; Morita, K.; Hosogane, N.; Matsuo, K.; Toyama, Y.; Suda, T.; et al. Induction of DC-STAMP by Alternative Activation and Downstream Signaling Mechanisms. J. Bone Miner. Res. 2007, 22, 992–1001. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast Differentiation and Activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and Activation of the Transcription Factor NFATc1 (NFAT2) Integrate RANKL Signaling in Terminal Differentiation of Osteoclasts. Dev. Cell. 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Huang, H.; Chang, E.; Ryu, J.; Lee, Z.H.; Lee, Y.; Kim, H. Induction of C-Fos and NFATc1 during RANKL-Stimulated Osteoclast Differentiation is Mediated by the p38 Signaling Pathway. Biochem. Biophys. Res. Commun. 2006, 351, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Matsubara, T.; Tsurukai, T.; Hata, K.; Nishimura, R.; Yoneda, T. JNK/C-Jun Signaling Mediates an Anti-Apoptotic Effect of RANKL in Osteoclasts. J. Bone Miner. Res. 2008, 23, 907–914. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, H.S.; Yeon, J.; Choi, S.; Chun, C.H.; Kwak, H.B.; Oh, J. GM-CSF Regulates Fusion of Mononuclear Osteoclasts into Bone-Resorbing Osteoclasts by Activating the Ras/ERK Pathway. J. Immunol. 2009, 183, 3390–3399. [Google Scholar] [CrossRef]
- Lin, P.; Niimi, H.; Ohsugi, Y.; Tsuchiya, Y.; Shimohira, T.; Komatsu, K.; Liu, A.; Shiba, T.; Aoki, A.; Iwata, T.; et al. Application of Ligature-Induced Periodontitis in Mice to Explore the Molecular Mechanism of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 8900. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The Role of the Microbiota in Periodontal Disease. Periodontology 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Rebelo, M.B.; Oliveira, C.S.; Tavaria, F.K. Novel Strategies for Preventing Dysbiosis in the Oral Cavity. Front. Biosci. (Elite Ed) 2023, 15, 23. [Google Scholar] [CrossRef]
- Yamazaki, K. Oral-Gut Axis as a Novel Biological Mechanism Linking Periodontal Disease and Systemic Diseases: A Review. Jpn. Dent. Sci. Rev. 2023, 59, 273–280. [Google Scholar] [CrossRef]
- Wang, A.; Zhai, Z.; Ding, Y.; Wei, J.; Wei, Z.; Cao, H. The Oral-Gut Microbiome Axis in Inflammatory Bowel Disease: From Inside to Insight. Front. Immunol. 2024, 15, 1430001. [Google Scholar] [CrossRef] [PubMed]
- Gan, G.; Lin, S.; Luo, Y.; Zeng, Y.; Lu, B.; Zhang, R.; Chen, S.; Lei, H.; Cai, Z.; Huang, X. Unveiling the Oral-Gut Connection: Chronic Apical Periodontitis Accelerates Atherosclerosis Via Gut Microbiota Dysbiosis and Altered Metabolites in apoE(-/-) Mice on a High-Fat Diet. Int. J. Oral Sci. 2024, 16, 39. [Google Scholar] [CrossRef]
- Kunath, B.J.; De Rudder, C.; Laczny, C.C.; Letellier, E.; Wilmes, P. The Oral-Gut Microbiome Axis in Health and Disease. Nat. Rev. Microbiol. 2024, 22, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Ferrillo, M.; Giudice, A.; Migliario, M.; Renó, F.; Lippi, L.; Calafiore, D.; Marotta, N.; de Sire, R.; Fortunato, L.; Ammendolia, A.; et al. Oral-Gut Microbiota, Periodontal Diseases, and Arthritis: Literature Overview on the Role of Probiotics. Int. J. Mol. Sci. 2023, 24, 4626. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Jhong, J.; Tsai, W.; Yang, L.; Chou, C.; Lee, T.; Yeh, Y.; Huang, C.; Luo, Y. Heat-Killed Lacticaseibacillus Paracasei GMNL-653 Exerts Antiosteoporotic Effects by Restoring the Gut Microbiota Dysbiosis in Ovariectomized Mice. Front. Nutr. 2022, 9, 804210. [Google Scholar] [CrossRef]
- Lin, W.; Kuo, Y.; Chen, C.; Huang, Y.; Hsu, C.; Lin, J.; Liu, C.; Chen, J.; Hsia, K.; Ho, H. Viable and Heat-Killed Probiotic Strains Improve Oral Immunity by Elevating the IgA Concentration in the Oral Mucosa. Curr. Microbiol. 2021, 78, 3541–3549. [Google Scholar] [CrossRef]
- Furlaneto, F.; Ishikawa, K.H.; Messora, M.R.; Mayer, M.P.A. Probiotics during the Therapeutic Management of Periodontitis. Adv. Exp. Med. Biol. 2022, 1373, 353–375. [Google Scholar]
- Kumar, G.; Tewari, S.; Tagg, J.; Chikindas, M.L.; Popov, I.V.; Tiwari, S.K. Can Probiotics Emerge as Effective Therapeutic Agents in Apical Periodontitis? A Review. Probiotics Antimicrob. Proteins 2021, 13, 299–314. [Google Scholar] [CrossRef]
- Balta, M.G.; Papathanasiou, E.; Blix, I.J.; Van Dyke, T.E. Host Modulation and Treatment of Periodontal Disease. J. Dent. Res. 2021, 100, 798–809. [Google Scholar] [CrossRef]
- Golub, L.M.; Lee, H. Periodontal Therapeutics: Current Host-Modulation Agents and Future Directions. Periodontology 2000 2020, 82, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Deandra, F.A.; Ketherin, K.; Rachmasari, R.; Sulijaya, B.; Takahashi, N. Probiotics and Metabolites Regulate the Oral and Gut Microbiome Composition as Host Modulation Agents in Periodontitis: A Narrative Review. Heliyon 2023, 9, e13475. [Google Scholar] [CrossRef]
- Amato, M.; Di Spirito, F.; D’Ambrosio, F.; Boccia, G.; Moccia, G.; De Caro, F. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms 2022, 10, 2289. [Google Scholar] [CrossRef]
- Ding, Q.; Sun, X.; Cao, S.; Zhao, C.; Wang, Y.; Wang, X. Heat-Killed Lactobacillus Acidophilus Mediates Fusobacterium Nucleatum Induced Pro-Inflammatory Responses in Epithelial Cells. FEMS Microbiol. Lett. 2021, 368, fnaa160. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Twetman, S. Critical Appraisal of Oral Pre- and Probiotics for Caries Prevention and Care. Caries Res. 2019, 53, 514–526. [Google Scholar] [CrossRef]
- Iwasaki, K.; Maeda, K.; Hidaka, K.; Nemoto, K.; Hirose, Y.; Deguchi, S. Daily Intake of Heat-Killed Lactobacillus Plantarum L-137 Decreases the Probing Depth in Patients Undergoing Supportive Periodontal Therapy. Oral Health Prev. Dent. 2016, 14, 207–214. [Google Scholar] [PubMed]
- Zhuang, Z.; Yoshizawa-Smith, S.; Glowacki, A.; Maltos, K.; Pacheco, C.; Shehabeldin, M.; Mulkeen, M.; Myers, N.; Chong, R.; Verdelis, K.; et al. Induction of M2 Macrophages Prevents Bone Loss in Murine Periodontitis Models. J. Dent. Res. 2019, 98, 200–208. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-J.; Kim, M.-K.; Heo, S.C.; Hong, D.K.; Park, S.-D.; Kim, Y.; Bae, S.-K.; Kim, H.J.; Bae, M.-K. Postbiotics Derived from Lactococcus lactis and Streptococcus thermophilus Attenuate Experimental Periodontitis by Modulating Macrophage Polarization and Osteoclastogenesis. Nutrients 2025, 17, 2638. https://doi.org/10.3390/nu17162638
Park H-J, Kim M-K, Heo SC, Hong DK, Park S-D, Kim Y, Bae S-K, Kim HJ, Bae M-K. Postbiotics Derived from Lactococcus lactis and Streptococcus thermophilus Attenuate Experimental Periodontitis by Modulating Macrophage Polarization and Osteoclastogenesis. Nutrients. 2025; 17(16):2638. https://doi.org/10.3390/nu17162638
Chicago/Turabian StylePark, Hyun-Joo, Mi-Kyoung Kim, Soon Chul Heo, Dong Ki Hong, Soo-Dong Park, Yeon Kim, Soo-Kyung Bae, Hyung Joon Kim, and Moon-Kyoung Bae. 2025. "Postbiotics Derived from Lactococcus lactis and Streptococcus thermophilus Attenuate Experimental Periodontitis by Modulating Macrophage Polarization and Osteoclastogenesis" Nutrients 17, no. 16: 2638. https://doi.org/10.3390/nu17162638
APA StylePark, H.-J., Kim, M.-K., Heo, S. C., Hong, D. K., Park, S.-D., Kim, Y., Bae, S.-K., Kim, H. J., & Bae, M.-K. (2025). Postbiotics Derived from Lactococcus lactis and Streptococcus thermophilus Attenuate Experimental Periodontitis by Modulating Macrophage Polarization and Osteoclastogenesis. Nutrients, 17(16), 2638. https://doi.org/10.3390/nu17162638





