Dose-Dependent Anti-Inflammatory Effects of Live and Heat-Treated Ligilactobacillus salivarius and Bifidobacterium breve via NF-κB and COX-2 Modulation in an In Vitro Model of Airway Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotics
2.2. Materials
2.3. Cell Line and Culture Medium
2.4. Inflammation Induction and Use of Probiotics
2.5. Cell Viability Assay
2.6. ELISAs for Inflammatory Cytokines
2.7. NF-κB Pathway Evaluation
2.8. COX-2 and PGE2 Pathway Evaluation
2.9. Statistical Analysis
3. Results
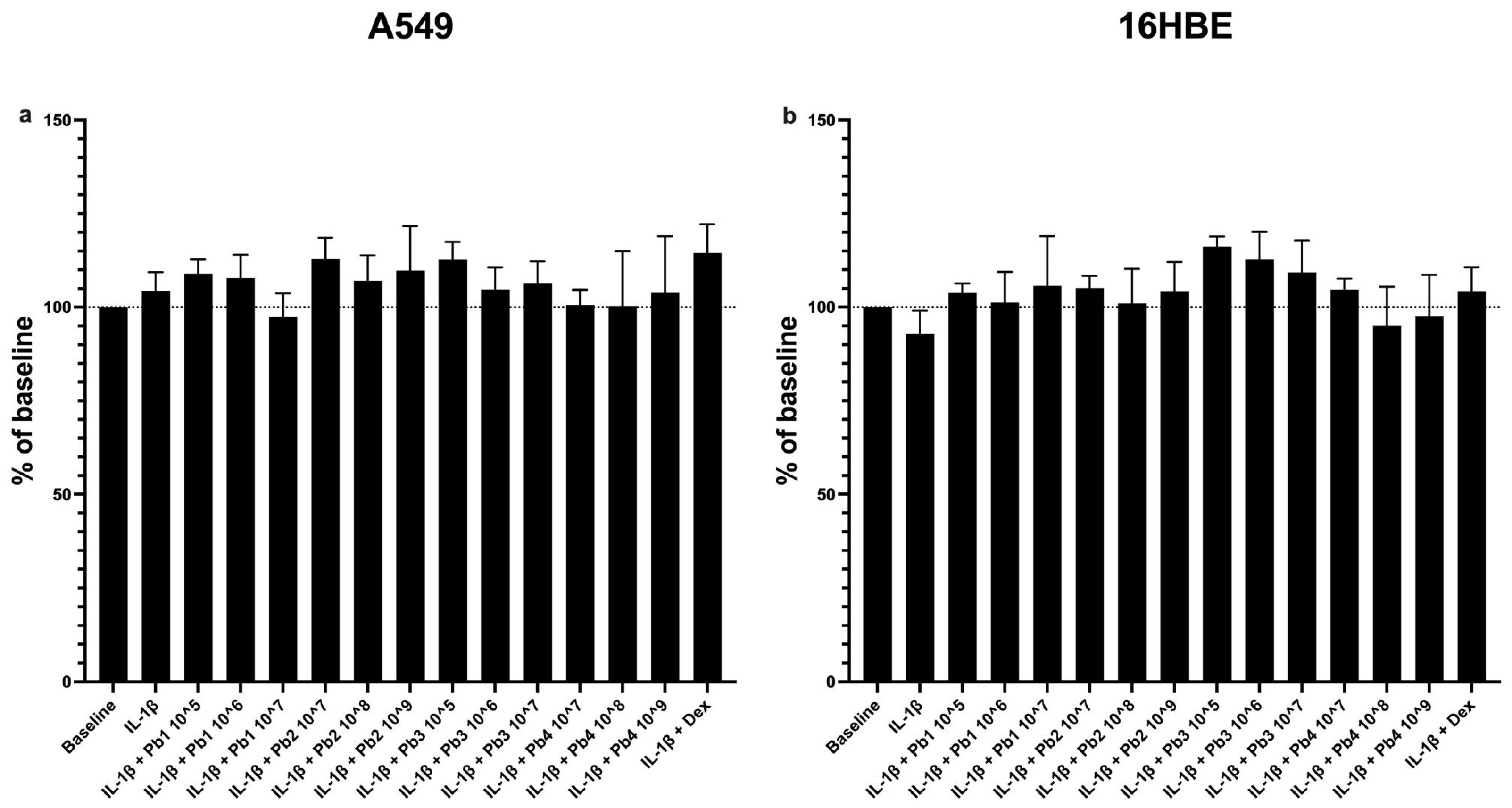
3.1. Effects of Probiotics on Cell Viability
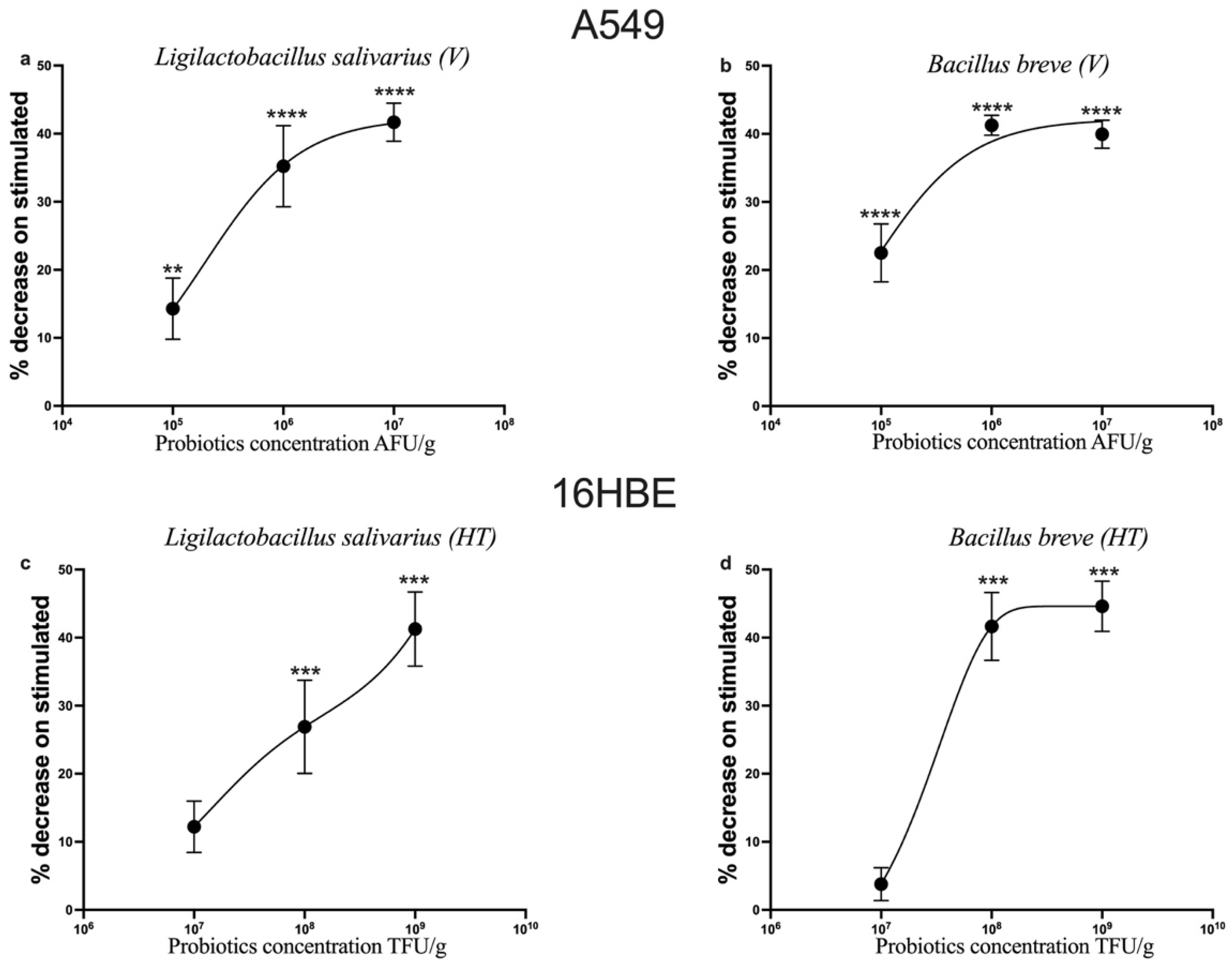
3.2. Dose-Dependent Inhibition of the Proinflammatory Cytokines CCL-2 and TNF-α by Probiotics on IL-1β-Stimulated A549 and 16HBE Cells
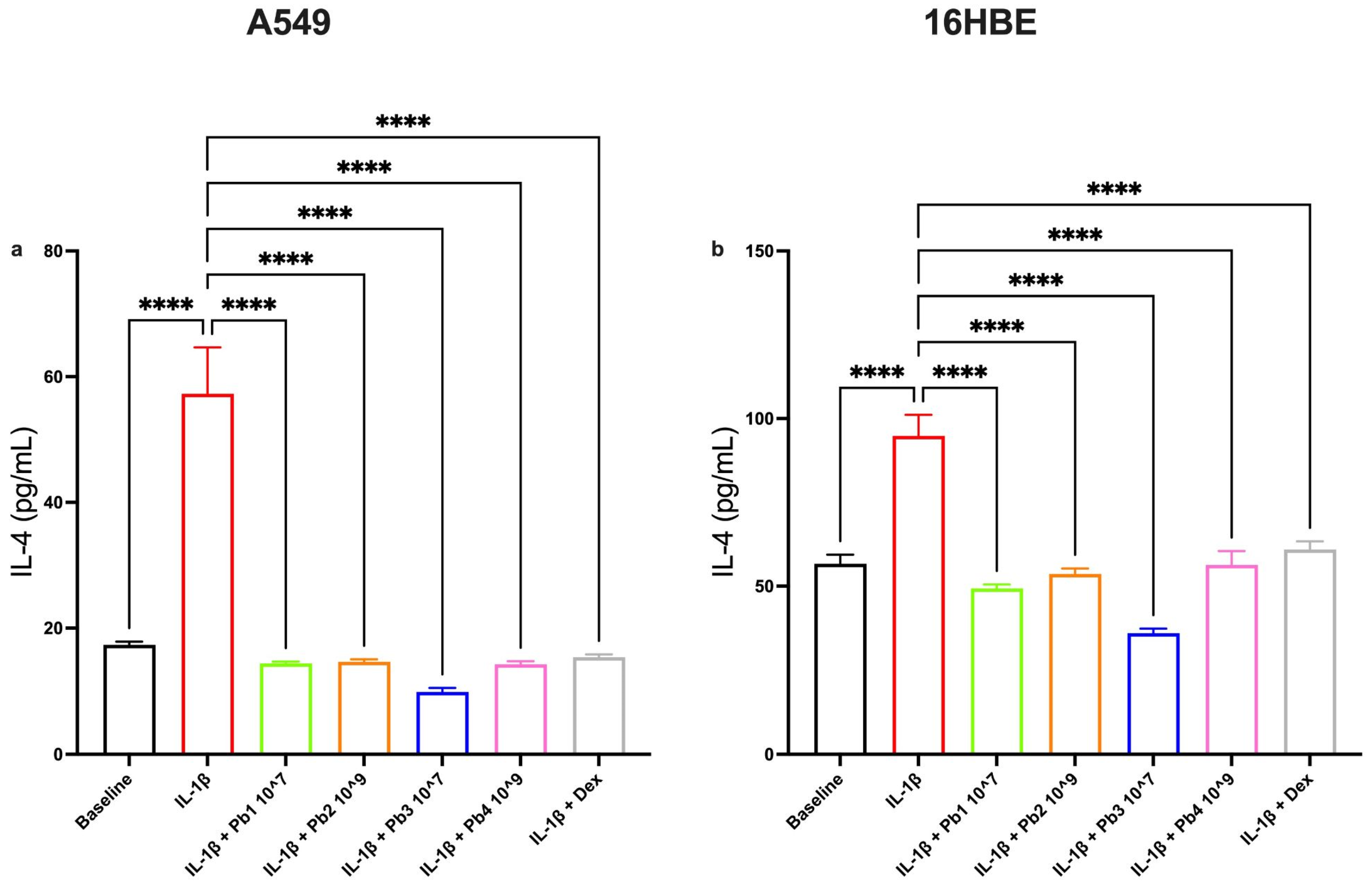
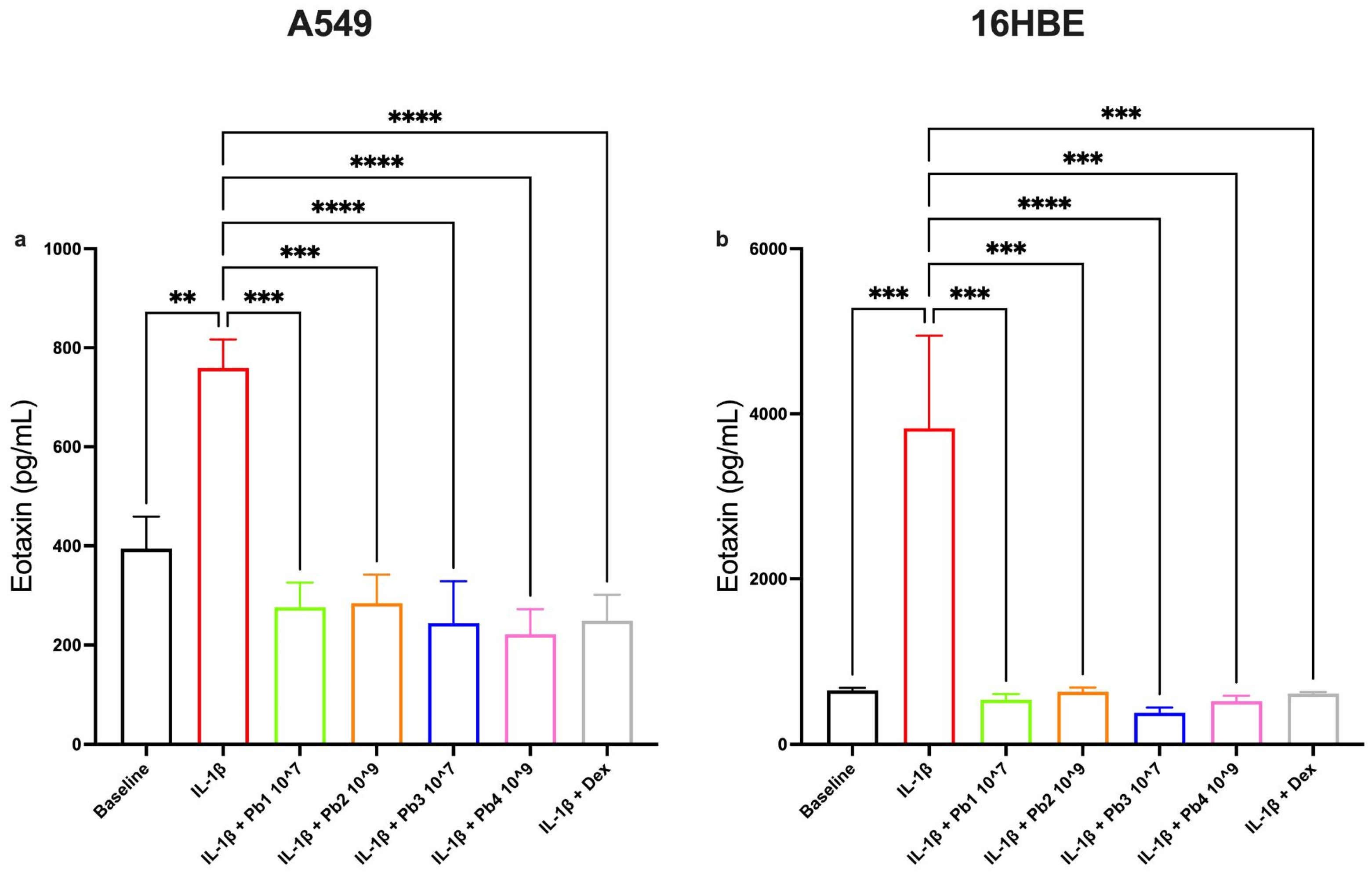
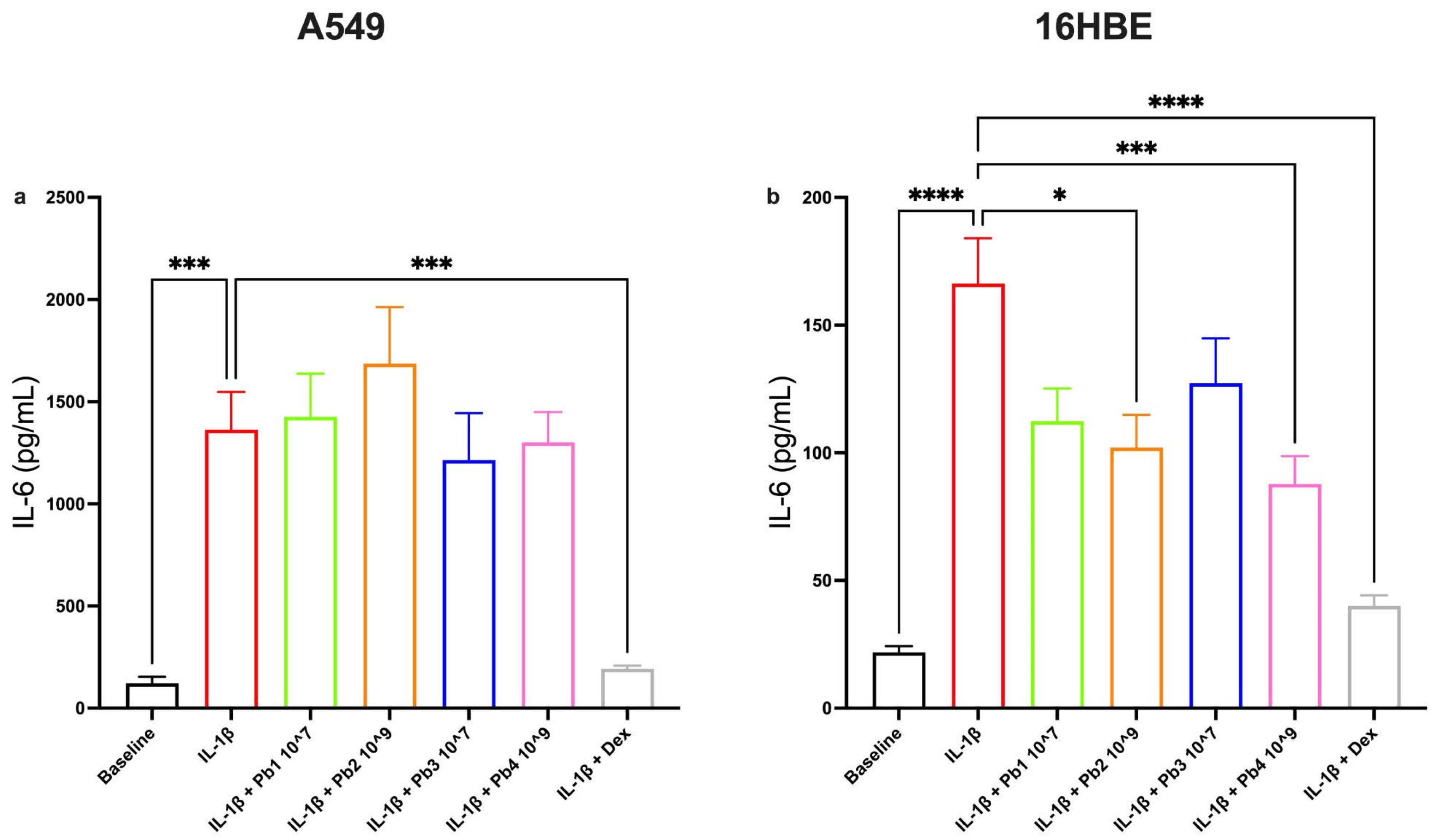
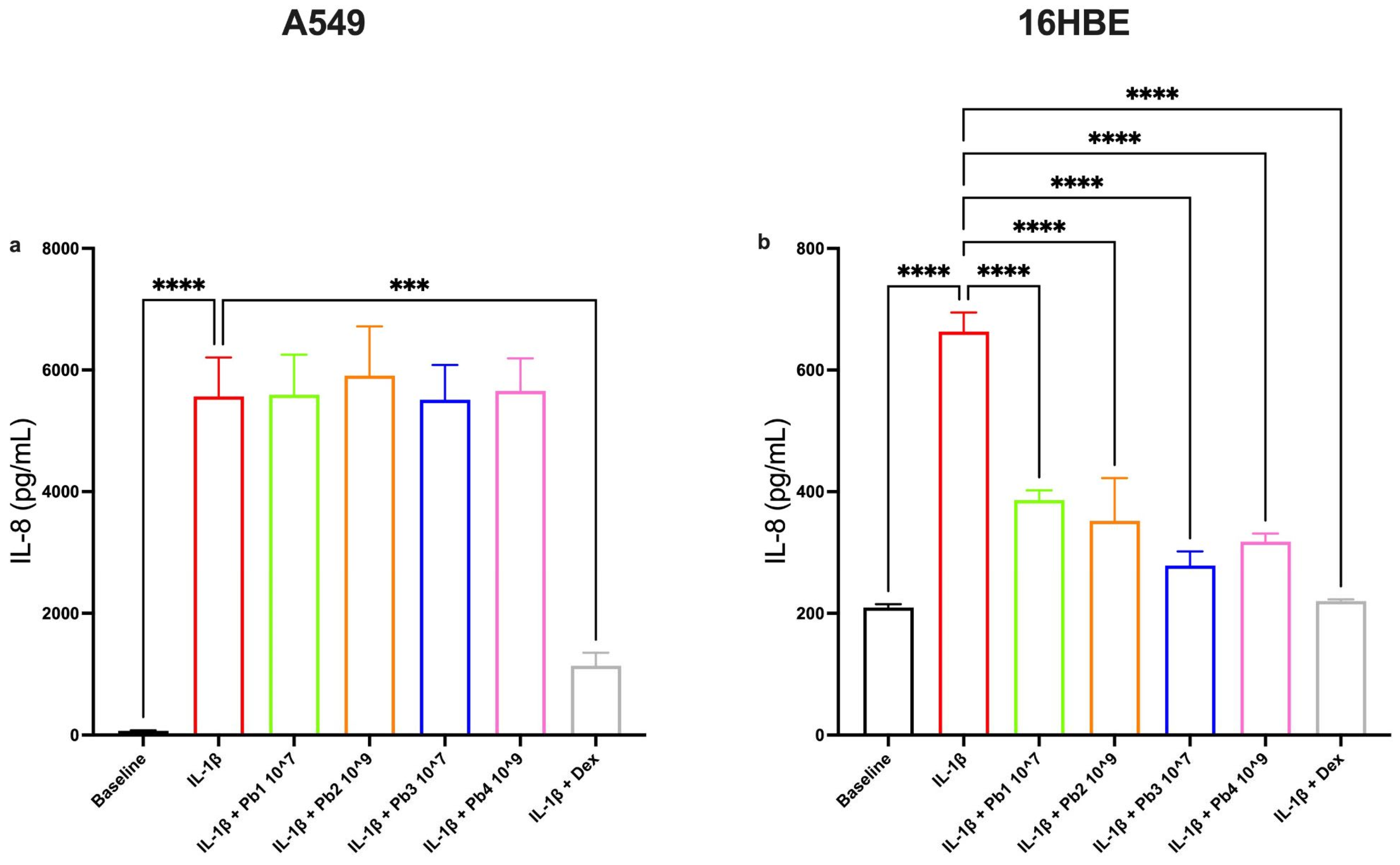
3.3. Effects of Probiotics on IL-1β-Induced IL-4, Eotaxin, IL-6 and IL-8 Production in A549 and 16HBE Cell Lines
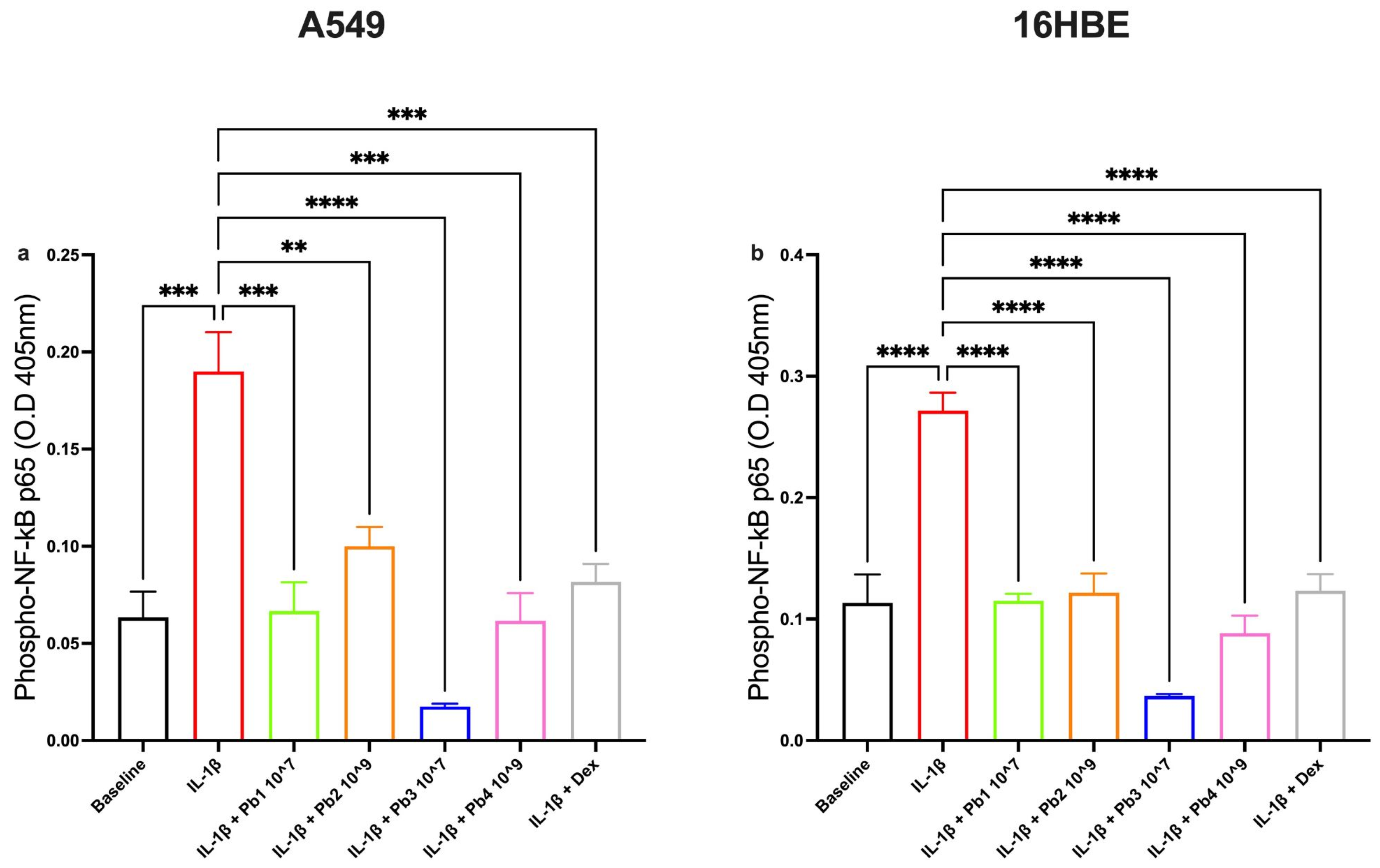
3.4. Effects of Probiotics on the NF-κB Pathway in Inflamed A549 and 16HBE Cells
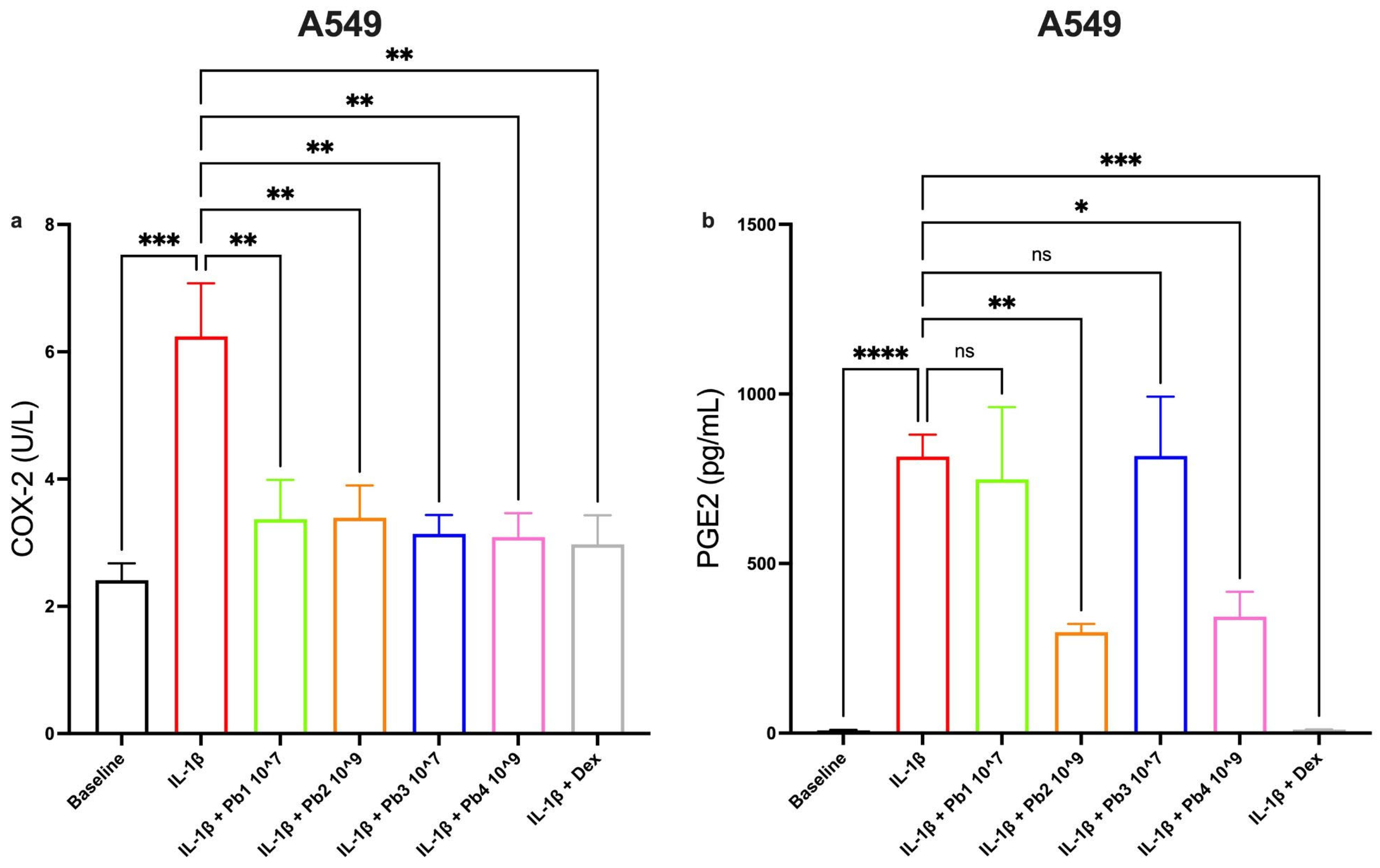
3.5. Effects of Probiotics on COX-2 and PGE2 Pathways in Inflamed A549 and 16HBE Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ichinose, M. Differences of inflammatory mechanisms in asthma and COPD. Allergol. Int. 2009, 58, 307–313. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Zhou, X. Lung microbiome: New insights into the pathogenesis of respiratory diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [CrossRef]
- Semmler, F.; Regis Belisário-Ferrari, M.; Kulosa, M.; Kaysser, L. The Metabolic Potential of the Human Lung Microbiome. Microorganisms 2024, 12, 1448. [Google Scholar] [CrossRef]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell. Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. The gut microbiota: A treasure for human health. Biotechnol. Adv. 2016, 34, 1210–1224. [Google Scholar] [CrossRef]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S2), S150–S156. [Google Scholar] [CrossRef]
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live biotherapeutic products: The importance of a defined regulatory framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef]
- Butel, M.-J. Probiotics, gut microbiota and health. Médecine Et Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Manzoor, S.; Wani, S.M.; Ahmad Mir, S.; Rizwan, D. Role of probiotics and prebiotics in mitigation of different diseases. Nutrition 2022, 96, 111602. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef]
- Tomosada, Y.; Villena, J.; Murata, K.; Chiba, E.; Shimazu, T.; Aso, H.; Iwabuchi, N.; Xiao, J.-Z.; Saito, T.; Kitazawa, H. Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression. PLoS ONE 2013, 8, e59259. [Google Scholar] [CrossRef]
- Haque, M.; Kaminsky, L.; Abdulqadir, R.; Engers, J.; Kovtunov, E.; Rawat, M.; Al-Sadi, R.; Ma, T.Y. Lactobacillus acidophilus inhibits the TNF-α-induced increase in intestinal epithelial tight junction permeability via a TLR-2 and PI3K-dependent inhibition of NF-κB activation. Front. Immunol. 2024, 15, 1348010. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef][Green Version]
- Profita, M.; Sala, A.; Bonanno, A.; Riccobono, L.; Siena, L.; Melis, M.R.; Di Giorgi, R.; Mirabella, F.; Gjomarkaj, M.; Bonsignore, G.; et al. Increased prostaglandin E2 concentrations and cyclooxygenase-2 expression in asthmatic subjects with sputum eosinophilia. J. Allergy Clin. Immunol. 2003, 112, 709–716. [Google Scholar] [CrossRef]
- Rumzhum, N.N.; Ammit, A.J. Cyclooxygenase 2: Its regulation, role and impact in airway inflammation. Clin. Exp. Allergy 2016, 46, 397–410. [Google Scholar] [CrossRef]
- Griet, M.; Zelaya, H.; Mateos, M.V.; Salva, S.; Juarez, G.E.; de Valdez, G.F.; Villena, J.; Salvador, G.A.; Rodriguez, A.V. Soluble factors from Lactobacillus reuteri CRL1098 have anti-inflammatory effects in acute lung injury induced by lipopolysaccharide in mice. PLoS ONE 2014, 9, e110027. [Google Scholar] [CrossRef]
- Lee, J.M.; Hwang, K.-T.; Jun, W.J.; Park, C.-S.; Lee, M.-Y. Antiinflammatory effect of lactic acid bacteria: Inhibition of cyclooxygenase-2 by suppressing nuclear factor-kappaB in Raw264.7 macrophage cells. J. Microbiol. Biotechnol. 2008, 18, 1683–1688. [Google Scholar]
- La Mantia, I.; Gelardi, M.; Drago, L.; Aragona, S.E.; Cupido, G.; Vicini, C.; Berardi, C.; Ciprandi, G.; Italian Study Group on Upper Respiratory Infections. Probiotics in the add-on treatment of rhinosinusitis: A clinical experience. J. Biol. Regul. Homeost. Agents 2020, 34, 27–34. [Google Scholar]
- Ciprandi, G.; Schiavetti, I.; Cioffi, L.; Pane, M.; Drago, L. The Probiotics in Pediatric Asthma Management (PROPAM) study: A Post Hoc analysis in allergic children. Ann. Allergy Asthma Immunol. 2022, 129, 111–113. [Google Scholar] [CrossRef]
- Drago, L.; Cioffi, L.; Giuliano, M.; Pane, M.; Amoruso, A.; Schiavetti, I.; Reid, G.; Ciprandi, G.; Propam, S.G. The Probiotics in Pediatric Asthma Management (PROPAM) Study in the Primary Care Setting: A Randomized, Controlled, Double-Blind Trial with Ligilactobacillus salivarius LS01 (DSM 22775) and Bifidobacterium breve B632 (DSM 24706). J. Immunol. Res. 2022, 2022, 3837418. [Google Scholar] [CrossRef]
- Ciprandi, G.; Tosca, M.A. Probiotics in Children with Asthma. Children 2022, 9, 978. [Google Scholar] [CrossRef]
- D’Agostin, M.; Squillaci, D.; Lazzerini, M.; Barbi, E.; Wijers, L.; Da Lozzo, P. Invasive Infections Associated with the Use of Probiotics in Children: A Systematic Review. Children 2021, 8, 924. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Hu, L.; Wang, X. Advancements related to probiotics for preventing and treating recurrent respiratory tract infections in children. Front. Pediatr. 2025, 13, 1508613. [Google Scholar] [CrossRef]
- Araujo, G.V.D.; Oliveira Junior, M.H.D.; Peixoto, D.M.; Sarinho, E.S.C. Probiotics for the treatment of upper and lower respiratory-tract infections in children: Systematic review based on randomized clinical trials. J. De Pediatr. (Rio J.) 2015, 91, 413–427. [Google Scholar] [CrossRef]
- Yuksel, N.; Gelmez, B.; Yildiz-Pekoz, A. Lung Microbiota: Its Relationship to Respiratory System Diseases and Approaches for Lung-Targeted Probiotic Bacteria Delivery. Mol. Pharm. 2023, 20, 3320–3337. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D. The Current and Future Perspectives of Postbiotics. Probiotics Antimicrob. Proteins 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Miao, Z.; Ran, J.; Mou, D.; Wu, S.; Chen, Y.; Li, C.; Chen, Y.; Yang, M.; Xie, Q. YKL-40 Promotes the Expression of Inflammatory Factors in Type Ⅱ Alveolar Epithelial Cell Model of A549 Cell Line. Sichuan Da Xue Xue Bao Yi Xue Ban 2023, 54, 954–958. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Xiao, C.-X.; Li, X.-D.; Hu, Z.-L.; He, S.-D.; Xiao, X.-J.; Xu, F. Dexamethasone can attenuate the pulmonary inflammatory response via regulation of the lncH19/miR-324-3p cascade. J. Inflamm. 2021, 18, 1. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Liao, T.-W.; Chen, Y.-H.; Chiang, Y.-C.; Tsai, Y.-C. Oral administration of heat-inactivated Lactobacillus plantarum K37 modulated airway hyperresponsiveness in ovalbumin-sensitized BALB/c mice. PLoS ONE 2014, 9, e100105. [Google Scholar] [CrossRef]
- Liu, Y.W.; Fu, T.Y.; Peng, W.S.; Chen, Y.H.; Cao, Y.M.; Chen, C.C.; Hung, W.L.; Tsai, Y.C. Evaluation of the potential anti-allergic effects of heat-inactivated Lactobacillus paracasei V0151 in vitro, ex vivo, and in vivo. Benef. Microbes 2015, 6, 697–705. [Google Scholar] [CrossRef]
- Carmazzi, Y.; Iorio, M.; Armani, C.; Cianchetti, S.; Raggi, F.; Neri, T.; Cordazzo, C.; Petrini, S.; Vanacore, R.; Bogazzi, F.; et al. The mechanisms of nadroparin-mediated inhibition of proliferation of two human lung cancer cell lines. Cell Prolif. 2012, 45, 545–556. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Song, Y.; Kim, S.; Kong, H. Unripe Rubus occidentalis, Ellagic Acid, and Urolithin A Attenuate Inflammatory Responses in IL-1β-Stimulated A549 Cells and PMA-Stimulated Differentiated HL-60 Cells. Nutrients 2023, 15, 3364. [Google Scholar] [CrossRef]
- Duran, G.G.; Küçük, M.U.; Algül, Ö.; Terzi, M.Y. Investigation of New Benzimidazole Derivative Compounds’ Effects on A549 Cell Line. Braz. Arch. Biol. Technol. 2020, 63, 2. [Google Scholar] [CrossRef]
- Peng, H.-L.; Huang, W.-C.; Cheng, S.-C.; Liou, C.-J. Fisetin inhibits the generation of inflammatory mediators in interleukin-1β-induced human lung epithelial cells by suppressing the NF-κB and ERK1/2 pathways. Int. Immunopharmacol. 2018, 60, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Uh, I.; Kim, K.-S.; Kim, K.-H.; Park, J.; Kim, Y.; Jung, J.-H.; Jung, H.-J.; Jang, H.-J. Anti-Inflammatory Effects of Ginsenoside Rg3 via NF-κB Pathway in A549 Cells and Human Asthmatic Lung Tissue. J. Immunol. Res. 2016, 2016, 7521601. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Voll, R.E.; Ghosh, S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1998, 1, 661–671. [Google Scholar] [CrossRef]
- Hegde, P.; Maddur, M.S.; Friboulet, A.; Bayry, J.; Kaveri, S.V. Viscum album exerts anti-inflammatory effect by selectively inhibiting cytokine-induced expression of cyclooxygenase-2. PLoS ONE 2011, 6, e26312. [Google Scholar] [CrossRef]
- Liou, C.-J.; Huang, W.-C. Casticin inhibits interleukin-1β-induced ICAM-1 and MUC5AC expression by blocking NF-κB, PI3K-Akt, and MAPK signaling in human lung epithelial cells. Oncotarget 2017, 8, 101175–101188. [Google Scholar] [CrossRef]
- Newton, R.; Seybold, J.; Kuitert, L.M.; Bergmann, M.; Barnes, P.J. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J. Biol. Chem. 1998, 273, 32312–32321. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Garvin, L.M.; Nickola, T.J.; Watson, A.M.; Colberg-Poley, A.M.; Rose, M.C. IL-1β induction of MUC5AC gene expression is mediated by CREB and NF-κB and repressed by dexamethasone. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 306, L797–L807. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Jedrzkiewicz, S.; Nakamura, H.; Silverman, E.S.; Luster, A.D.; Mansharamani, N.; In, K.H.; Tamura, G.; Lilly, C.M. IL-1beta induces eotaxin gene transcription in A549 airway epithelial cells through NF-kappaB. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 279, L1058–L1065. [Google Scholar] [CrossRef]
- Lee, I.-S.; Cho, D.-H.; Kim, K.-S.; Kim, K.-H.; Park, J.; Kim, Y.; Jung, J.H.; Kim, K.; Jung, H.-J.; Jang, H.-J. Anti-inflammatory effects of embelin in A549 cells and human asthmatic airway epithelial tissues. Immunopharmacol. Immunotoxicol. 2018, 40, 83–90. [Google Scholar] [CrossRef]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Baggiolini, M.; Clark-Lewis, I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992, 307, 97–101. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, Z.; Huang, C.; Liang, B.; Zhang, X.; Sun, W. The gut microbiota modulates airway inflammation in allergic asthma through the gut-lung axis related immune modulation: A review. Biomol. Biomed. 2025, 25, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Atamas, S.P.; Chapoval, S.P.; Keegan, A.D. Cytokines in chronic respiratory diseases. F1000 Biol. Rep. 2013, 5, 3. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Ahmed, N.; Alom, S.; Bhattacharjee, B.; Nalla, L.V.; Rynjah, D.; Gadanec, L.K.; Apostolopoulos, V. Proinflammatory Cytokines in Chronic Respiratory Diseases and Their Management. Cells 2025, 14, 400. [Google Scholar] [CrossRef]
- Swedin, L.; Ellis, R.; Neimert-Andersson, T.; Ryrfeldt, A.; Nilsson, G.; Inman, M.; Dahlén, S.-E.; Adner, M. Prostaglandin modulation of airway inflammation and hyperresponsiveness in mice sensitized without adjuvant. Prostaglandins Other Lipid Mediat. 2010, 92, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.P.; Ayakar, S.; Singhal, R.S. The potential of paraprobiotics and postbiotics to modulate the immune system: A Review. Microbiol. Res. 2023, 275, 127449. [Google Scholar] [CrossRef]
- Baek, J.-Y.; Kim, J.H.; Lee, N.-K.; Paik, H.-D. Heat-killed Lactiplantibacillus plantarum WB3813 and Lactiplantibacillus plantarum WB3814 Alleviate LPS-Induced Inflammatory Damage and Apoptosis in A549 Cells. Probiotics Antimicrob. Proteins 2025, 1–14. [Google Scholar] [CrossRef]
- Kang, C.E.; Kim, J.H.; Lee, N.-K.; Paik, H.-D. Paraprobiotic Levilactobacillus brevis KU15151 exhibits antioxidative and anti-inflammatory activities in LPS-induced A549 cells. Microb. Pathog. 2025, 198, 107143. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, C.E.; Lee, N.-K.; Paik, H.-D. Heat-Killed Lactilactobacillus sakei WB2305 and Lactiplantibacillus plantarum WB2324 Inhibited LPS-Induced Inflammation in Human Airway Epithelial Cells. Probiotics Antimicrob. Proteins 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagnini, M.; Visciglia, A.; Deusebio, G.; Pane, M.; Celi, A.; Amoruso, A.; Neri, T. Dose-Dependent Anti-Inflammatory Effects of Live and Heat-Treated Ligilactobacillus salivarius and Bifidobacterium breve via NF-κB and COX-2 Modulation in an In Vitro Model of Airway Inflammation. Nutrients 2025, 17, 2504. https://doi.org/10.3390/nu17152504
Pagnini M, Visciglia A, Deusebio G, Pane M, Celi A, Amoruso A, Neri T. Dose-Dependent Anti-Inflammatory Effects of Live and Heat-Treated Ligilactobacillus salivarius and Bifidobacterium breve via NF-κB and COX-2 Modulation in an In Vitro Model of Airway Inflammation. Nutrients. 2025; 17(15):2504. https://doi.org/10.3390/nu17152504
Chicago/Turabian StylePagnini, Marta, Annalisa Visciglia, Giovanni Deusebio, Marco Pane, Alessandro Celi, Angela Amoruso, and Tommaso Neri. 2025. "Dose-Dependent Anti-Inflammatory Effects of Live and Heat-Treated Ligilactobacillus salivarius and Bifidobacterium breve via NF-κB and COX-2 Modulation in an In Vitro Model of Airway Inflammation" Nutrients 17, no. 15: 2504. https://doi.org/10.3390/nu17152504
APA StylePagnini, M., Visciglia, A., Deusebio, G., Pane, M., Celi, A., Amoruso, A., & Neri, T. (2025). Dose-Dependent Anti-Inflammatory Effects of Live and Heat-Treated Ligilactobacillus salivarius and Bifidobacterium breve via NF-κB and COX-2 Modulation in an In Vitro Model of Airway Inflammation. Nutrients, 17(15), 2504. https://doi.org/10.3390/nu17152504






