Advances in Intestinal-Targeted Release of Phenolic Compounds
Abstract
1. Introduction
| Bioactive Compound | Chemical Structure | Sources | Solubility (mg/mL) | Pharmacological Properties | Limitations | References |
|---|---|---|---|---|---|---|
| Quercetin |  | Apples, onions, buckwheat, green tea | 0.002 | Antioxidant, anti-inflammatory, anticancer, antiviral, antiproliferative, and anti-diabetic in clinical trials | Poor stability under light, heat, and alkaline conditions, poor gastrointestinal stability, and low bioaccessibility (<2%) | [24,25] |
| Epigallocatechin gallate (EGCG) | 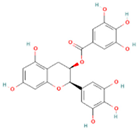 | Green tea, black tea, white tea | 10 | Antioxidant, anti-inflammatory, and anti-colon cancer; efficacy confirmed in vivo in mouse liver injury models | Bitter taste, sensitive to high temperature, oxygen, and pH changes | [26] |
| Gallic acid |  | Tea leaves, grapes, berries, gallnuts, mangoes | 50 | Antioxidant, anti-inflammatory, analgesic, neuroprotective, anticancer, and anti-diabetic in vivo | Astringent taste, large particle size, poor absorption, low bioavailability, and rapid excretion. Unstable at high temperatures | [27,28] |
| Resveratrol |  | Grape skins, peanuts, polygonum cuspidatum | 0.03 | Antioxidant, anti-inflammatory, anti-obesity, and anti-colon cancer in vivo | Low water solubility, low oral bioavailability, sensitive to high temperature, oxygen, and light, prone to rapid degradation and inactivation during intestinal metabolism | [29,30] |
| Ferulic acid | 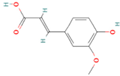 | Whole grains, angelica, oats, tomatoes | 1 | Antioxidant, anti-inflammatory, anti-tumor, and antihyperglycemic activities in vivo | Unstable to oxygen and prone to degradation, poor gastrointestinal stability | [31,32,33,34] |
| Procyanidin | 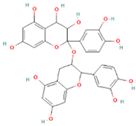 | Grape seeds, cocoa beans, apples, berries | 0.01–0.05 | Anti-obesity, antioxidant, and anti-type 2 diabetes in vivo | Unstable under pH changes, heat, humidity, gastrointestinal environment | [35,36] |
| Chlorogenic acid | 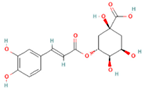 | Coffee beans, peaches, plums, apples, eggplants | 5–10 | Antimicrobial, anti-inflammatory, antioxidant, regulates glucose, lipid metabolism, and antitumor activities in vivo. | Unstable under heat, light, and alkaline conditions | [37,38] |
| Coumaric acid |  | Grapes, olives, coffee beans, tomatoes, carrots, cereals | 0.6 | Anti-inflammatory, antioxidant, and anti-colon cancer; improve cholesterol metabolism and enhance antioxidant capacity in vivo | Sensitive to pH, temperature, oxygen, light, and enzymes | [39,40] |
| Curcumin | 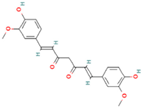 | Turmeric, curcuma zedoaria, curcuma aerugionosa | <0.0004 | Prevents cancer, cardiovascular diseases, and diabetes, neuroprotective in vivo | Hydrophobic compounds, low bioavailability and high chemical transformation rate, sensitive in alkaline pH (>7), high temperature, and light | [41,42,43] |
| Anthocyanins | 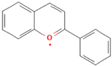 | Blueberries, red cabbage, purple, potatoes | 10 | Antioxidant, anti-inflammatory, anticancer, and anti-diabetic, alleviates chronic intestinal diseases in vivo | Hydrophobic compounds, not resistant to gastric acid, and have low bioavailability | [44,45] |
| Catechin | 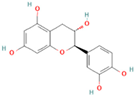 | Green tea, black tea, apples, grapes, | 10 | Antioxidant, antidiabetic, anti-inflammatory anticancer, and antibacterial, inhibit the pathogenesis of colorectal cancers in vivo | Sensitive to pH, temperature, oxygen, and light; instability in the gastrointestinal tract and limited membrane permeability across the intestine | [46,47] |
2. Methods
3. Physiologic Environments of Digestive Tract and Their Effects on Phenol Delivery Systems
3.1. Stomach
3.2. Small Intestine
3.3. Colon
4. Wall Materials for Intestinal-Targeted Release
4.1. Small Intestine Targeted Release
4.1.1. Chitosan
4.1.2. Sodium Alginate
4.1.3. Other Wall Materials
4.2. Colonic Targeted Release
4.2.1. Wall Materials for Microbial Degradation
4.2.2. Reinforcement of Wall Materials to Withstand Small Intestine Digestion
5. Intestinal Release of Phenolic Compounds
5.1. Intestinal Targeted Delivery of Water-Soluble Phenolic Compounds
5.2. Intestinal Targeted Delivery of Water-Insoluble Phenolic Compounds
6. Intestinal Delivery of Phenol-Containing Mixtures
6.1. Intestinal Co-Delivery of Multiple Phenols
6.2. Intestinal Co-Delivery of Phenols and Carotenoids
6.3. Intestinal Co-Delivery of Phenols and Probiotics
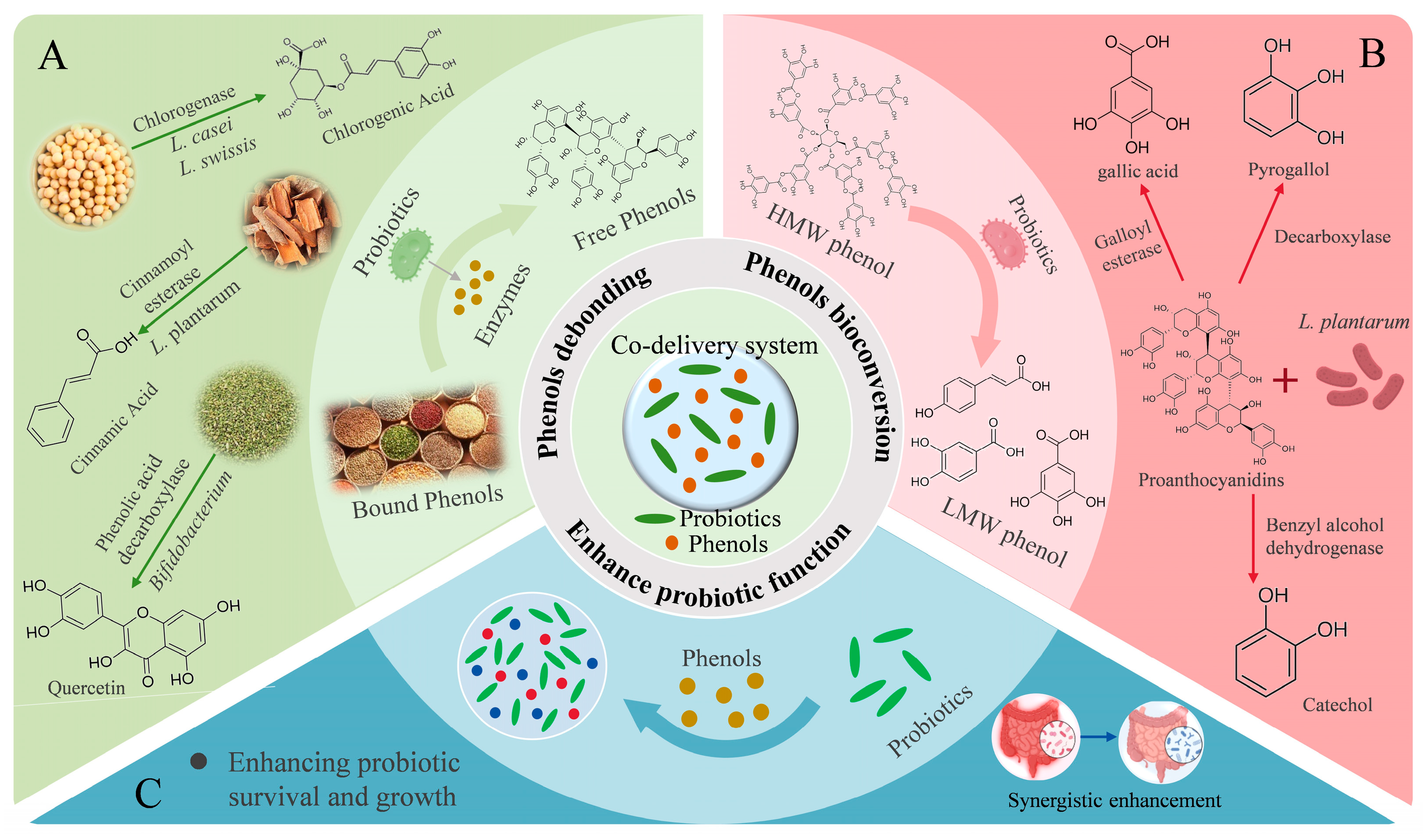
6.3.1. Probiotics Promote Phenols Debonding to Enhance Bioactivity
6.3.2. Probiotics Promote Phenols Bioconversion to Enhance Bioactivity
6.3.3. Phenols Enhance Probiotic Function
| Phenols Name | Wall Material | Preparation Method | Intestinal Targeting Performance | Biological Activity | References | |
|---|---|---|---|---|---|---|
| Single-encapsulation | Quercetin | Chitosan nanoparticles | Coaxial electrospinning technique | Colon-targeted release | Significant improvement in bioavailability, enhanced anticancer activity (induction of cell apoptosis and oxidative stress). | [91] |
| Whey protein isolate, inulin | High-pressure homogenization method | Small intestine- targeted release | Enhanced of the water solubility (389 times), increased of the digestibility (8.59%), enhancing both the stability and bioavailability of quercetin. | [94] | ||
| Resveratrol | Shellac resin ammonium salts | Spray drying technology | Small intestine- targeted release | pH-sensitive, controlled and sustained release of resveratrol in simulated intestinal release experiment, excellent stability, enhanced antioxidant activity (higher radical scavenging activity of DPPH and ABTS compared to pure resveratrol). | [150] | |
| Pectin, chitosan, polyethylene glycol | Layer-by-layer self-assembly technique | Colon-targeted release | Colon-targeted release 49%, improved bioaccessibility. | [74] | ||
| Anthocyanin | Cyclodextrin | Complexation technology | Small intestine- targeted release | Stable in stomach, released in intestine, promoting growth of beneficial bacteria. | [45] | |
| Sodium alginate, hyaluronic acid, foodborne nanoparticles | Electrostatic self-assembly method | Colon-targeted release | Colon-targeted release 35.9%, pH-responsive, enhanced bioavailability, gut microbiota modulation, anti-inflammatory properties and bioavailability. | [96] | ||
| Curcumin | Alginate, whey protein isolate, gum arabic | Layer-by-layer self-assembly technique | Small intestine- targeted release | Small intestine-targeted release 84%, improved bioavailability. | [104] | |
| Epigallocatechin gallate (EGCG) | Chitosan, zein | Antisolvent precipitation method | Colon-targeted release | Higher release efficiency in simulated intestinal fatty environment, the antioxidant activity of EGCG was significantly improved, and the microcapsules with zein had an antioxidant activity four times higher than those without zein. | [151] | |
| Caffeic acid | Chitosan, sodium alginate | Lonic gelation method | Small intestine- targeted release | Resistant to gastric environment, sustained release in small intestine (up to 180 min). | [56] | |
| Ferulic acid | Potato protein, pectin | PH-driven self-assembly | Colon-targeted release | Resistant to gastric environment, sustained release in intestine, antioxidant activity. | [150] | |
| Co-encapsulation | Curcumin and EGCG | Zein, caseinate | Antisolvent method | Small intestine- targeted release | EGCG enhanced the dispersibility, encapsulation properties, and antioxidant activity of curcumin, enhanced the stability and bioaccessibility (87.3 ± 2.8%). | [23] |
| Phenols (quercetin/ rutin/curcumin/tea polyphenols) and Lactobacillus casei | Zein, chitosan | Complex coacervation | Colon-targeted release | Resistant to gastric environment, sustained release in intestine, antioxidant activity, improved bioavailability, and quercetin group probiotic activity significantly increased (1.03 × 1010 CFU). | [144] | |
| Gallic acid and Lactobacillus | Pectin, sodium alginate, fu brick tea polysaccharide | Layer-by-layer complex coacervation | Colon-targeted release | Alleviating colitis, improved probiotic survival rate (1 × 109 CFU). | [90] | |
| Anthocyanins and β-carotene | Chitosan, oxidized konjac glucomannan | Electrostatic adsorption method | Small intestine- targeted release | Improved synergistic antioxidant activity (enhanced thermal stability); reduced various cancers, infectious diseases, obesity, cardiovascular diseases. | [122] | |
| Proanthocyanidins and Bifidobacterium animalis | Chitosan, alginate | Complex coacervation | Colon-targeted release | Enhanced anticancer activity (reduced aberrant crypt foci in mice by 57%). | [137,152] | |
| Chlorogenic acid and lutein | Bovine serum albumin, dextran | Complex coacervation-freeze-drying technology | Small intestine- targeted release | Resisted gastric acid and pepsin, lutein bioaccessibility significantly improved (62.3%). | [126] | |
| Coumaric acid and arbutin | Gelatin, polyglyceryl polyricinoleate, sodium chloride | Drop-wise dispersion | Colon-targeted release | Controlled release of arbutin and coumaric acid, improved bioaccessibility. | [153] | |
| Chlorogenic acid and anthocyanins | Maltodextrin or maltodextrin, carboxymethyl cellulose, gum arabic or xanthan gum | Spray drying technology | Colon-targeted release | Higher retention of anthocyanins in encapsulated wild cherry powder (75%). Encapsulation reduced molecular transformation of anthocyanins and chlorogenic acid during in vitro digestion. | [154] |
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ali, Z.; Ma, H.; Rashid, M.T.; Ayim, I.; Wali, A. Reduction of Body Weight, Body Fat Mass, and Serum Leptin Levels by Addition of New Beverage in Normal Diet of Obese Subjects. J. Food Biochem. 2018, 42, e12554. [Google Scholar] [CrossRef]
- Guo, L.; Guo, Y.; Ping, W.; Feng, L.; Zhu, J.; Ma, H.; Chen, Y.; Zhang, T. Camellia Oil Lowering Blood Pressure in Spontaneously Hypertension Rats. J. Funct. Foods 2020, 70, 103915. [Google Scholar] [CrossRef]
- Wang, M.; Chen, M.; Guo, R.; Ding, Y.; Zhang, H.; He, Y. The Improvement of Sulforaphane in Type 2 Diabetes Mellitus (T2DM) and Related Complications: A Review. Trends Food Sci. Technol. 2022, 129, 397–407. [Google Scholar] [CrossRef]
- Gao, R.; Qi, Z.; Lin, J.; Wang, G.; Chen, G.; Yuan, L.; Sun, Q. Chondroitin Sulfate Alleviated Obesity by Modulating Gut Microbiota and Liver Metabolome in High-Fat-Diet-Induced Obese Mice. J. Agric. Food Chem. 2023, 71, 9419–9428. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent Advances in the Extraction of Bioactive Compounds with Subcritical Water: A Review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, F. The Reciprocal Interaction between Polyphenols and Other Dietary Compounds: Impact on Bioavailability, Antioxidant Capacity and Other Physico-Chemical and Nutritional Parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Zhang, Z.; Li, Z.; Guo, Y.; Zhao, G.; Wu, L. Recent Advances in the Effects of Dietary Polyphenols on Inflammation in Vivo: Potential Molecular Mechanisms, Receptor Targets, Safety Issues, and Uses of Nanodelivery System and Polyphenol Polymers. Curr. Opin. Food Sci. 2022, 48, 100921. [Google Scholar] [CrossRef]
- Lyu, X.; Wang, X.; Wang, Q.; Ma, X.; Chen, S.; Xiao, J. Encapsulation of Sea Buckthorn (Hippophae rhamnoides L.) Leaf Extract via an Electrohydrodynamic Method. Food Chem. 2021, 365, 130481. [Google Scholar] [CrossRef]
- Wang, T.; Xu, H.; Dong, R.; Wu, S.; Guo, Y.; Wang, D. Effectiveness of Targeting the NLRP3 Inflammasome by Using Natural Polyphenols: A Systematic Review of Implications on Health Effects. Food Res. Int. 2023, 165, 112567. [Google Scholar] [CrossRef]
- Xie, F.; Yang, W.; Xing, M.; Zhang, H.; Ai, L. Natural Polyphenols-Gut Microbiota Interactions and Effects on Glycolipid Metabolism via Polyphenols-Gut-Brain Axis: A State-of-the-Art Review. Trends Food Sci. Technol. 2023, 140, 104171. [Google Scholar] [CrossRef]
- Jiang, Q.; Charoensiddhi, S.; Xue, X.; Sun, B.; Liu, Y.; El-Seedi Hesham, R.; Wang, K. A Review on the Gastrointestinal Protective Effects of Tropical Fruit Polyphenols. Crit. Rev. Food Sci. Nutr. 2023, 63, 7197–7223. [Google Scholar] [CrossRef]
- Suo, H.; Shishir, M.R.I.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K.-W. Red Wine High-Molecular-Weight Polyphenolic Complex: An Emerging Modulator of Human Metabolic Disease Risk and Gut Microbiota. J. Agric. Food Chem. 2021, 69, 10907–10919. [Google Scholar] [CrossRef]
- Lei, L.; Tang, Y.; Zheng, J.; Ma, G.; Zhou, Z. Influence of Two Polyphenols on the Structure and Lubrication of Salivary Pellicle: An in Vitro Study on Astringency Mechanism. Friction 2022, 10, 167–178. [Google Scholar] [CrossRef]
- Sahraeian, S.; Rashidinejad, A.; Golmakani, M.-T. Recent Advances in the Conjugation Approaches for Enhancing the Bioavailability of Polyphenols. Food Hydrocoll. 2024, 146, 109221. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Chen, Z.; Liu, W.; Chen, H. Characterization and Storage Properties of a New Microencapsulation of Tea Polyphenols. Ind. Crops Prod. 2016, 89, 152–156. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Fang, Z.; Ng, K. Dietary Fiber-Based Colon-Targeted Delivery Systems for Polyphenols. Trends Food Sci. Technol. 2020, 100, 333–348. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel Approaches for Co-Encapsulation of Probiotic Bacteria with Bioactive Compounds, Their Health Benefits and Functional Food Product Development: A Review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Yu, H.; Zhou, Y. Self-Assembled Polymers for Gastrointestinal Tract Targeted Delivery through the Oral Route: An Update. Polymers 2023, 15, 3538. [Google Scholar] [CrossRef]
- Dehkordi, S.S.; Alemzadeh, I.; Vaziri, A.S.; Vossoughi, A. Optimization of Alginate-Whey Protein Isolate Microcapsules for Survivability and Release Behavior of Probiotic Bacteria. Appl. Biochem. Biotechnol. 2020, 190, 182–196. [Google Scholar] [CrossRef]
- Chu, J.N.; Traverso, G. Foundations of Gastrointestinal-Based Drug Delivery and Future Developments. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 219–238. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Huang, J.; Dai, L.; Du, J.; McClements, D.J.; Mao, L.; Liu, J.; Gao, Y. Fabrication and Characterization of Layer-by-Layer Composite Nanoparticles Based on Zein and Hyaluronic Acid for Codelivery of Curcumin and Quercetagetin. ACS Appl. Mater. Interfaces 2019, 11, 16922–16933. [Google Scholar] [CrossRef]
- Chen, S.; McClements, D.J.; Jian, L.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Core–Shell Biopolymer Nanoparticles for Co-Delivery of Curcumin and Piperine: Sequential Electrostatic Deposition of Hyaluronic Acid and Chitosan Shells on the Zein Core. ACS Appl. Mater. Interfaces 2019, 11, 38103–38115. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, X.; McClements, D.J.; Zou, L.; Liu, X.; Liu, F. Co-Encapsulation of Epigallocatechin Gallate (EGCG) and Curcumin by Two Proteins-Based Nanoparticles: Role of EGCG. J. Agric. Food Chem. 2019, 67, 13228–13236. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.; Nadjarzadeh, A.; Zarshenas, M.M.; Shams, M.; Heydari, M. Efficacy of Cinnamon in Patients with Type II Diabetes Mellitus: A Randomized Controlled Clinical Trial. Clin. Nutr. 2019, 38, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shao, F.; Obadi, M.; Li, H.; Qi, Y.; Xu, B. Enhancing in Vitro Starch Digestion of Tartary Buckwheat: Unveiling the Vital Role of Endogenous Polyphenols in Starch Fine Structure. J. Cereal Sci. 2024, 116, 103870. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Q.; Wang, T.; Kan, Z.; Li, X.; Hu, L.; Peng, C.-Y.; Qian, F.; Wang, Y.; Granato, D. Green Tea Polyphenols and Epigallocatechin-3-Gallate Protect against Perfluorodecanoic Acid Induced Liver Damage and Inflammation in Mice by Inhibiting NLRP3 Inflammasome Activation. Food Res. Int. 2020, 127, 108628. [Google Scholar] [CrossRef]
- Patil, P.; Killedar, S. Chitosan and Glyceryl Monooleate Nanostructures Containing Gallic Acid Isolated from Amla Fruit: Targeted Delivery System. Heliyon 2021, 7, e06526. [Google Scholar] [CrossRef]
- Keyvani-Ghamsari, S.; Rahimi, M.; Khorsandi, K. An Update on the Potential Mechanism of Gallic Acid as an Antibacterial and Anticancer Agent. Food Sci. Nutr. 2023, 11, 5856–5872. [Google Scholar] [CrossRef]
- Ren, X.; Hou, T.; Liang, Q.; Zhang, X.; Hu, D.; Xu, B.; Chen, X.; Chalamaiah, M.; Ma, H. Effects of Frequency Ultrasound on the Properties of Zein-Chitosan Complex Coacervation for Resveratrol Encapsulation. Food Chem. 2019, 279, 223–230. [Google Scholar] [CrossRef]
- Gu, T.; Wang, N.; Wu, T.; Ge, Q.; Chen, L. Antioxidative Stress Mechanisms behind Resveratrol: A Multidimensional Analysis. J. Food Qual. 2021, 2021, 5571733. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Yu, Y.-B.; Cai, W.-D.; Wang, Z.-W.; Yan, J.-K. Emulsifying Properties of a Ferulic Acid-Grafted Curdlan Conjugate and Its Contribution to the Chemical Stability of β-Carotene. Food Chem. 2021, 339, 128053. [Google Scholar] [CrossRef]
- Wang, C.; Cai, W.-D.; Yao, J.; Wu, L.-X.; Li, L.; Zhu, J.; Yan, J.-K. Conjugation of Ferulic Acid onto Pectin Affected the Physicochemical, Functional and Antioxidant Properties. J. Sci. Food Agric. 2020, 100, 5352–5362. [Google Scholar] [CrossRef] [PubMed]
- Daryagasht, M.; Moosavi, M.; Khorsandi, L.; Azadnasab, R.; Khodayar, M.J. Hepatoprotective and Anti-Hyperglycemic Effects of Ferulic Acid in Arsenic-Exposed Mice. Food Chem. Toxicol. 2023, 178, 113924. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, L.; Yang, T.; Wu, Q.; Lv, Z.; Xie, B.; Sun, Z. Increasing Antioxidant Activity of Procyanidin Extracts from the Pericarp of Litchi Chinensis Processing Waste by Two Probiotic Bacteria Bioconversions. J. Agric. Food Chem. 2013, 61, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Cui, J.; Zhang, H.; Dzah, C.S.; He, Y.; Duan, Y. Binding Affinity, Antioxidative Capacity and in Vitro Digestion of Complexes of Grape Seed Procyanidins and Pork, Chicken and Fish Protein. Food Res. Int. 2020, 136, 109530. [Google Scholar] [CrossRef]
- Aree, T. Atomic-Level Understanding on Conformational Flexibility of Neochlorogenic and Chlorogenic Acids and Their Inclusion Complexation with β-Cyclodextrin. Food Hydrocoll. 2023, 141, 108742. [Google Scholar] [CrossRef]
- Akpabli-Tsigbe, N.D.K.; Osabutey, J.; Mintah, B.K.; Tano-Debrah, K.; Ma, Y. Cleavage of Macromolecule (Protein/Polysaccharide)-Phenolic Bond in Soybean Cell Wall through Lactobacillus Casei and Lactobacillus Helviticus Mixed Culture Solid-State Fermentation for Chlorogenic Acid Extraction. Food Biosci. 2023, 55, 102903. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and Anti-Inflammatory Effect of p-Coumaric Acid, a Common Dietary Polyphenol on Experimental Inflammation in Rats. Inflammation 2012, 36, 169–176. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Lee, Y.-T.; Hsieh, H.-S.; Hwang, D.-F. Dietary Caffeic Acid, Ferulic Acid and Coumaric Acid Supplements on Cholesterol Metabolism and Antioxidant Activity in Rats. J. Food Drug Anal. 2020, 17, 4. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.-J.; Kim, H.W.; Park, S.O.; Lee, J.; Ko, S. Curcumin and Catechin Co-Loaded Water-in-Oil-in-Water Emulsion and Its Beverage Application. J. Funct. Foods 2015, 15, 35–43. [Google Scholar] [CrossRef]
- Yixuan, L.; Qaria, M.A.; Sivasamy, S.; Jianzhong, S.; Daochen, Z. Curcumin Production and Bioavailability: A Comprehensive Review of Curcumin Extraction, Synthesis, Biotransformation and Delivery Systems. Ind. Crops Prod. 2021, 172, 114050. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Wang, Y.; Boesch, C.; Zhao, Y.; Sarkar, A. Pickering Emulsions Stabilized by Colloidal Gel Particles Complexed or Conjugated with Biopolymers to Enhance Bioaccessibility and Cellular Uptake of Curcumin. Curr. Res. Food Sci. 2020, 3, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, C.; Lu, J.; Sun, Y.; Cui, Y. Research Progress of Proanthocyanidins and Anthocyanidins. Phytother. Res. 2023, 37, 2552–2577. [Google Scholar] [CrossRef]
- Flores, G.; del Castillo, M.L.R.; Costabile, A.; Klee, A.; Guergoletto, K.B.; Gibson, G.R. In Vitro Fermentation of Anthocyanins Encapsulated with Cyclodextrins: Release, Metabolism and Influence on Gut Microbiota Growth. J. Funct. Foods 2015, 16, 50–57. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Meng, Y.; Lv, G.; Wang, J.; Zhang, D.; Shi, J.; Zhai, X.; Meng, X.; Zou, X. Co-Delivery Mechanism of Curcumin/Catechin Complex by Modified Soy Protein Isolate: Emphasizing Structure, Functionality, and Intermolecular Interaction. Food Hydrocoll. 2024, 152, 109958. [Google Scholar] [CrossRef]
- Ruengdech, A.; Mishra, D.K.; Siripatrawan, U. Multifaceted Roles of Foam-Mat Freeze-Dried Catechins Nanoencapsulation to Enhance Catechins Stability and Bioaccessibility, and Quality of Green Tea Catechins-Fortified Milk. Food Chem. X 2025, 27, 102391. [Google Scholar] [CrossRef]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of Gastrointestinal pH Profiles in Normal Ambulant Human Subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef]
- Roy, B.C.; Das, C.; Hong, H.; Betti, M.; Bruce, H.L. Extraction and Characterization of Gelatin from Bovine Heart. Food Biosci. 2017, 20, 116–124. [Google Scholar] [CrossRef]
- Do, U.T.; Kim, J.; Luu, Q.S.; Nguyen, Q.T.; Jang, T.; Park, Y.; Shin, H.; Whiting, N.; Kang, D.-K.; Kwon, J.-S.; et al. Accurate Detection of Enzymatic Degradation Processes of Gelatin–Alginate Microcapsule by 1H NMR Spectroscopy: Probing Biodegradation Mechanism and Kinetics. Carbohydr. Polym. 2023, 304, 120490. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Santucci, N.R.; Velez, A. Physiology of Lower Gastrointestinal Tract. Aliment. Pharmacol. Ther. 2024, 60, S1–S19. [Google Scholar] [CrossRef]
- Gromova, L.V.; Polozov, A.S.; Savochkina, E.V.; Alekseeva, A.S.; Dmitrieva, Y.V.; Kornyushin, O.V.; Gruzdkov, A.A. Effect of Type 2 Diabetes and Impaired Glucose Tolerance on Digestive Enzymes and Glucose Absorption in the Small Intestine of Young Rats. Nutrients 2022, 14, 385. [Google Scholar] [CrossRef]
- Luan, Q.; Zhang, H.; Chen, C.; Jiang, F.; Yao, Y.; Deng, Q.; Zeng, K.; Tang, H.; Huang, F. Controlled Nutrient Delivery through a pH-Responsive Wood Vehicle. ACS Nano 2022, 16, 2198–2208. [Google Scholar] [CrossRef]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Dietary Polyphenols: A Novel Strategy to Modulate Microbiota-Gut-Brain Axis. Trends Food Sci. Technol. 2018, 78, 224–233. [Google Scholar] [CrossRef]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-Targeted Oral Drug Delivery Systems: Design Trends and Approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The Gastrointestinal Microbiota as a Site for the Biotransformation of Drugs. Int. J. Pharm. 2008, 363, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and Clinical Implications of the Brain–Gut–Enteric Microbiota Axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Backhed, F. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Philip, A.; Philip, B. Colon Targeted Drug Delivery Systems: A Review on Primary and Novel Approaches. Oman Med. J. 2010, 25, 70–78. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa Oil/Chitosan Nanoparticles Embedded Gelatin Nanofibers for Food Packaging against Listeria Monocytogenes and Staphylococcus Aureus on Cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.; Li, J.; Li, M.; Xing, D.; An, H.; Wu, X.; Wu, Y. A Chitosan Composite Film Sprayed before Pathogen Infection Effectively Controls Postharvest Soft Rot in Kiwifruit. Agronomy 2020, 10, 265. [Google Scholar] [CrossRef]
- Edo, G.I.; Yousif, E.; Al-Mashhadani, M.H. Chitosan: An Overview of Biological Activities, Derivatives, Properties, and Current Advancements in Biomedical Applications. Carbohydr. Res. 2024, 542, 109199. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Bai, M.; Rashed, M.M.A.; Lin, L. The Antibacterial Activity of Clove Oil/Chitosan Nanoparticles Embedded Gelatin Nanofibers against Escherichia Coli O157:H7 Biofilms on Cucumber. Int. J. Food Microbiol. 2018, 266, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Gani, A. Alginate-Based pH-Sensitive Hydrogels Encoated with Chitosan as a Bioactive Cargo Carrier with Caffeic Acid as a Model Biomolecule. ACS Food Sci. Technol. 2022, 2, 667–672. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Li, Y.; Huang, X.; Li, Z.; Zhai, X.; Shi, J.; Zou, X.; Xiao, J.; Sun, Y.; et al. Sodium Alginate/Guar Gum Based Nanocomposite Film Incorporating β-Cyclodextrin/Persimmon Pectin-Stabilized Baobab Seed Oil Pickering Emulsion for Mushroom Preservation. Food Chem. 2024, 437, 137891. [Google Scholar] [CrossRef]
- Surendhiran, D.; Cui, H.; Lin, L. Encapsulation of Phlorotannin in Alginate/PEO Blended Nanofibers to Preserve Chicken Meat from Salmonella Contaminations. Food Packag. Shelf Life 2019, 21, 100346. [Google Scholar] [CrossRef]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-Known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Sanchez-Ballester, N.M.; Bataille, B.; Soulairol, I. Sodium Alginate and Alginic Acid as Pharmaceutical Excipients for Tablet Formulation: Structure-Function Relationship. Carbohydr. Polym. 2021, 270, 118399. [Google Scholar] [CrossRef]
- Zhao, B.; Alonso, N.F.; Miras, J.; Vílchez, S.; García-Celma, M.J.; Morral, G.; Esquena, J. Triggered Protein Release from Calcium Alginate/Chitosan Gastro-Resistant Capsules. Colloids Surf. Physicochem. Eng. Asp. 2024, 693, 133998. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, P.; Li, S.; Wang, X.; Zong, W. Sodium Alginate-Based Wall Materials Microencapsulated Lactobacillus Plantarum CICC 20022: Characteristics and Survivability Study. Food Sci. Biotechnol. 2022, 31, 1463–1472. [Google Scholar] [CrossRef]
- González, E.; Gómez-Caravaca, A.M.; Giménez, B.; Cebrián, R.; Maqueda, M.; Martínez-Férez, A.; Segura-Carretero, A.; Robert, P. Evolution of the Phenolic Compounds Profile of Olive Leaf Extract Encapsulated by Spray-Drying during in Vitro Gastrointestinal Digestion. Food Chem. 2019, 279, 40–48. [Google Scholar] [CrossRef]
- Wang, L.; Wu, P.; Hu, Z.; Chen, Y.; Jin, X.; Deng, R.; Kirk, T.V.; Chen, X.D. Curcumin-Loaded Microcapsules with Soy and Whey Protein as Wall Material: In Vitro Release, and Ex Vivo Absorption Based on the Rat Small Intestine. J. Food Eng. 2024, 383, 112254. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol–Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Nakatsu, C.; Jones-Hall, Y.; Jiang, Q. Supplementation of Polyphenol-Rich Grapes Attenuates Colitis, Colitis-Associated Colon Cancer, and Disease-Associated Dysbiosis in Mice, but Fails to Mitigate Colitis in Antibiotic-Treated Mice. J. Nutr. Biochem. 2022, 109, 109124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Feng, H.; Li, C.; Jia, F.; Zhang, X. The Mutual Effect of Dietary Fiber and Polyphenol on Gut Microbiota: Implications for the Metabolic and Microbial Modulation and Associated Health Benefits. Carbohydr. Polym. 2025, 358, 123541. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, B. Chitin Microspheres: A Fascinating Material with High Loading Capacity of Anthocyanins for Colon Specific Delivery. Food Hydrocoll. 2017, 63, 293–300. [Google Scholar] [CrossRef]
- Hurtado, N.H.; Morales, A.L.; González-Miret, M.L.; Escudero-Gilete, M.L.; Heredia, F.J. Colour, pH Stability and Antioxidant Activity of Anthocyanin Rutinosides Isolated from Tamarillo Fruit (Solanum betaceum Cav.). Food Chem. 2009, 117, 88–93. [Google Scholar] [CrossRef]
- Yan, J.-K.; Qiu, W.-Y.; Wang, Y.-Y.; Wu, J.-Y. Biocompatible Polyelectrolyte Complex Nanoparticles from Lactoferrin and Pectin as Potential Vehicles for Antioxidative Curcumin. J. Agric. Food Chem. 2017, 65, 5720–5730. [Google Scholar] [CrossRef]
- Wu, L.-X.; Qiao, Z.-R.; Cai, W.-D.; Qiu, W.-Y.; Yan, J.-K. Quaternized Curdlan/Pectin Polyelectrolyte Complexes as Biocompatible Nanovehicles for Curcumin. Food Chem. 2019, 291, 180–186. [Google Scholar] [CrossRef]
- Dongowski, G.; Lorenz, A.; Anger, H. Degradation of Pectins with Different Degrees of Esterification by Bacteroides Thetaiotaomicron Isolated from Human Gut Flora. Appl. Environ. Microbiol. 2000, 66, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Andishmand, H.; Tabibiazar, M.; Mohammadifar, M.A.; Hamishehkar, H. Pectin-Zinc-Chitosan-Polyethylene Glycol Colloidal Nano-Suspension as a Food Grade Carrier for Colon Targeted Delivery of Resveratrol. Int. J. Biol. Macromol. 2017, 97, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, M.; Li, Z.; Li, Y.; Shi, J.; Huang, X.; Sun, Y.; Zhai, X.; Zou, X.; Xiao, J. Incorporation of Hawthorn Pectin/β-Cyclodextrin-Stabilized Pickering Emulsion and Titanium Dioxide Nanoparticles for Improving the Physical, Biological, and Release Properties of Guar Gum/Agar/Sodium Alginate-Based Bilayer Films. Ind. Crops Prod. 2024, 212, 118302. [Google Scholar] [CrossRef]
- Iftikhar, A.; Rehman, A.; Usman, M.; Ali, A.; Ahmad, M.M.; Shehzad, Q.; Fatim, H.; Mehmood, A.; Moiz, A.; Shabbir, M.A.; et al. Influence of Guar Gum and Chitosan Enriched with Lemon Peel Essential Oil Coatings on the Quality of Pears. Food Sci. Nutr. 2022, 10, 2443–2454. [Google Scholar] [CrossRef]
- Singhal, A.; Jain, H.; Singhal, V.; Elias, E.J.; Showkat, A. Colon-Targeted Quercetin Delivery Using Natural Polymer to Enhance Its Bioavailability. Pharmacogn. Res. 2011, 3, 35. [Google Scholar] [CrossRef]
- Ashames, A.; Ullah, K.; Al-Tabakha, M.; Khan, S.A.; Hassan, N.; Mannan, A.; Ikram, M.; Buabeid, M.; Murtaza, G. Development, Characterization and In-Vitro Evaluation of Guar Gum Based New Polymeric Matrices for Controlled Delivery Using Metformin HCl as Model Drug. PLoS ONE 2022, 17, e0271623. [Google Scholar] [CrossRef]
- Chandel, D.; Uppal, S.; Mehta, S.K.; Shukla, G. Preparation and Characterization of Celecoxib Entrapped Guar Gum Nanoparticles Targeted for Oral Drug Delivery against Colon Cancer: An In-Vitro Study. J. Drug Deliv. Ther. 2020, 10, 14–21. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Jain, S.K. Design and Development of Multiparticulate System for Targeted Drug Delivery to Colon. Drug Deliv. 2004, 11, 201–207. [Google Scholar] [CrossRef]
- Kumar, V.S.; Rijo, J.; Sabitha, M. Guargum and Eudragit ® Coated Curcumin Liquid Solid Tablets for Colon Specific Drug Delivery. Int. J. Biol. Macromol. 2018, 110, 318–327. [Google Scholar] [CrossRef]
- Sun, R.; Lv, Z.; Wang, Y.; Li, M.; Qi, J.; Wang, K.; Yang, H.; Yue, T.; Yuan, Y. Different Polysaccharide-Enhanced Probiotic and Polyphenol Dual-Functional Factor Co-Encapsulated Microcapsules Demonstrate Acute Colitis Alleviation Efficacy and Food Fortification. Carbohydr. Polym. 2024, 345, 122572. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Li, L.; Zong, M.-H.; Wu, H. A Colon-Specific Delivery System for Quercetin with Enhanced Cancer Prevention Based on Co-Axial Electrospinning. Food Funct. 2018, 9, 5999–6009. [Google Scholar] [CrossRef]
- Wang, T.; Xu, H.; Wu, S.; Guo, Y.; Zhao, G.; Wang, D. Mechanisms Underlying the Effects of the Green Tea Polyphenol EGCG in Sarcopenia Prevention and Management. J. Agric. Food Chem. 2023, 71, 9609–9627. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.-Y.; Aheto, J.H.; Bai, J.-W.; Dai, C.; Ren, Y.; Chang, X. Quantitative Analysis and Visualization of Moisture and Anthocyanins Content in Purple Sweet Potato by Vis–NIR Hyperspectral Imaging. J. Food Process. Preserv. 2020, 45, e15128. [Google Scholar] [CrossRef]
- Ding, J.; Liang, T.; Min, Q.; Jiang, L.; Zhu, J.-J. “Stealth and Fully-Laden” Drug Carriers: Self-Assembled Nanogels Encapsulated with Epigallocatechin Gallate and siRNA for Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9938–9948. [Google Scholar] [CrossRef] [PubMed]
- Harlen, W.C.; Prakash, S.; Yuliani, S.; Bhandari, B. Encapsulation of Gallic Acid in Alginate/Lactoferrin Composite Hydrogels: Physical Properties and Gallic Acid Diffusion. Food Hydrocoll. 2025, 160, 110784. [Google Scholar] [CrossRef]
- Zhao, X.; Su, W.; Zhang, X.; Tan, M. Visual Foodborne Nanoparticles for Oral Site-Specific Delivery of Anthocyanins in the Treatment of Inflammatory Bowel Disease. Mater. Today Nano 2023, 24, 100431. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Zou, X.; Arslan, M.; Shi, J.; Zhai, X.; Xiao, J.; Wang, X.; Huang, X.; Li, Z.; et al. A High-Stable and Sensitive Colorimetric Nanofiber Sensor Based on PCL Incorporating Anthocyanins for Shrimp Freshness. Food Chem. 2022, 377, 131909. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, J.; Liu, P.; Cui, B.; El-Aty, A.M.A. Preparation and Characterization of Octenyl Succinylated Starch Microgels via a Water-in-Oil (W/O) Inverse Microemulsion Process for Loading and Releasing Epigallocatechin Gallate. Food Chem. 2021, 355, 129661. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Q.; Liu, X.; Raza, H.; Ma, H.; Ren, X. Treatment with Ultrasound Improves the Encapsulation Efficiency of Resveratrol in Zein-Gum Arabic Complex Coacervates. LWT—Food Sci. Technol. 2022, 153, 112331. [Google Scholar] [CrossRef]
- Baba, W.N.; Mudgil, P.; Regenstein, J.M.; Maqsood, S. Impact of Quercetin Conjugation Using Alkaline and Free Radical Methods with Tandem Ultrasonication on the Functional Properties of Camel Whey and Its Hydrolysates. Food Res. Int. 2024, 190, 114562. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Wang, C.; Hu, J.; Guo, X.; Zhang, D.; Wu, W.; Zhou, F.; Ji, B. Protective Effect of Quercetin and Chlorogenic Acid, Two Polyphenols Widely Present in Edible Plant Varieties, on Visible Light-Induced Retinal Degeneration in Vivo. J. Funct. Foods 2017, 33, 103–111. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, Q.; Chen, Z. Improved Aqueous Solubility, Bioaccessibility and Cellular Uptake of Quercetin Following pH-driven Encapsulation in Whey Protein Isolate. Int. J. Food Sci. Technol. 2022, 57, 2747–2755. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Z.; Zhong, Q. Caseinate Nanoparticles Co-Loaded with Quercetin and Avenanthramide 2c Using a Novel Two-Step pH-Driven Method: Formation, Characterization, and Bioavailability. Food Hydrocoll. 2022, 129, 107669. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Qazi, H.J.; McGillivray, D.J. An Efficient Small Intestine-Targeted Curcumin Delivery System Based on the Positive-Negative-Negative Colloidal Interactions. Food Hydrocoll. 2021, 111, 106375. [Google Scholar] [CrossRef]
- Chen, J.; Gu, Y.; Chen, Z.; Tan, Y. Stability and Digestibility of Quercetin Encapsulated in a Whey Protein Isolate-Inulin Complex Emulsion. LWT—Food Sci. Technol. 2025, 216, 117318. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Xu, G.; Yin, B.; Yao, P. BSA-Dextran Emulsion for Protection and Oral Delivery of Curcumin. Food Hydrocoll. 2016, 61, 11–19. [Google Scholar] [CrossRef]
- Yu, Y.-B.; Wu, M.-Y.; Wang, C.; Wang, Z.-W.; Chen, T.-T.; Yan, J.-K. Constructing Biocompatible Carboxylic Curdlan-Coated Zein Nanoparticles for Curcumin Encapsulation. Food Hydrocoll. 2020, 108, 106028. [Google Scholar] [CrossRef]
- Sebaaly, C.; Haydar, S.; Greige-Gerges, H. Eugenol Encapsulation into Conventional Liposomes and Chitosan-Coated Liposomes: A Comparative Study. J. Drug Deliv. Sci. Technol. 2022, 67, 102942. [Google Scholar] [CrossRef]
- Hamadou, A.H.; Zhang, J.; Chao, C.; Xu, B. Stability of Rutin Using Pectin-Chitosan Dual Coating Nanoliposomes. LWT—Food Sci. Technol. 2022, 170, 114084. [Google Scholar] [CrossRef]
- Shruthi, P.A.; Pushpadass, H.A.; Emerald, F.M.E.; Nath, B.S.; Naik, N.L. Resveratrol-Loaded Proniosomes: Formulation, Characterization and Fortification. LWT—Food Sci. Technol. 2020, 134, 110127. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, W.; Liu, T.; Li, J.; Li, X. Preparation, Characterization, and in Vitro Antioxidant Activity of pH-Sensitive Resveratrol Microcapsule in Simulated Intestinal Fluids. Int. J. Food Prop. 2019, 22, 804–814. [Google Scholar] [CrossRef]
- Xu, G.; Ren, G.; Xu, X.; Yuan, H.; Wang, Z.; Kang, L.; Yu, W.; Tian, K. Combination of Curcumin and Green Tea Catechins Prevents Dimethylhydrazine-Induced Colon Carcinogenesis. Food Chem. Toxicol. 2010, 48, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Eom, D.-W.; Lee, J.H.; Kim, Y.-J.; Hwang, G.S.; Kim, S.-N.; Kwak, J.H.; Cheon, G.J.; Kim, K.H.; Jang, H.-J.; Ham, J.; et al. Synergistic Effect of Curcumin on Epigallocatechin Gallate-Induced Anticancer Action in PC3 Prostate Cancer Cells. BMB Rep. 2015, 48, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, M.; Xiao, Z.; Daglia, M.; Dragan, S.; Delmas, D.; Vong, C.T.; Wang, Y.; Zhao, Y.; Shen, J.; et al. Dietary Polyphenols for Managing Cancers: What Have We Ignored? Trends Food Sci. Technol. 2020, 101, 150–164. [Google Scholar] [CrossRef]
- Liu, F.; Ma, D.; Luo, X.; Zhang, Z.; He, L.; Gao, Y.; McClements, D.J. Fabrication and Characterization of Protein-Phenolic Conjugate Nanoparticles for Co-Delivery of Curcumin and Resveratrol. Food Hydrocoll. 2018, 79, 450–461. [Google Scholar] [CrossRef]
- Jing, H.; Nie, M.; Dai, Z.; Xiao, Y.; Song, J.; Zhang, Z.; Zhou, C.; Li, D. Identification of Carotenoids from Fruits and Vegetables with or without Saponification and Evaluation of Their Antioxidant Activities. J. Food Sci. 2023, 88, 2693–2703. [Google Scholar] [CrossRef]
- Dai, Z.; Li, Z.; Shi, E.; Nie, M.; Feng, L.; Chen, G.; Gao, R.; Zeng, X.; Li, D. Study on the Interaction between Four Typical Carotenoids and Human Gut Microflora Using an in Vitro Fermentation Model. J. Agric. Food Chem. 2022, 70, 13592–13601. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; He, W.; Lu, Y.; Luo, H.; Guo, Q.; Li, D.; Bao, Y.; Zhang, Z. Exogenous Salicylic Acid Promotes Carotenoid Accumulation and Antioxidant Capacity in Germinated Maize Kernels by Regulating Carotenoid Biosynthetic Pathway. Food Biosci. 2024, 59, 103990. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity. Antioxidants 2021, 10, 713. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, L.; Zhou, H.; Zheng, B.; Ji, N.; Xu, X.; He, X.; Xiong, L.; McClements, D.J.; Sun, Q. Comparison of Lutein Bioaccessibility from Dietary Supplement-Excipient Nanoemulsions and Nanoemulsion-Based Delivery Systems. J. Agric. Food Chem. 2021, 69, 13925–13932. [Google Scholar] [CrossRef]
- Tufail, T.; Bader Ul Ain, H.; Noreen, S.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Nutritional Benefits of Lycopene and Beta-carotene: A Comprehensive Overview. Food Sci. Nutr. 2024, 12, 8715–8741. [Google Scholar] [CrossRef]
- Shi, M.; Bai, J.; Zhao, L.; Yu, X.; Liang, J.; Liu, Y.; Nord, W.; Li, Y. Co-Loading and Intestine-Specific Delivery of Multiple Antioxidants in pH-Responsive Microspheres Based on TEMPO-Oxidized Polysaccharides. Carbohydr. Polym. 2017, 157, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Yu, L.; Song, J.; Wu, C.; Li, Y.; Zhang, C. Hybridization of Glucosyl Stevioside and Hydroxypropyl Methylcellulose to Improve the Solubility of Lutein. Food Chem. 2022, 394, 133490. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Nie, M.; Wang, X.; Zhang, Z.; Xu, Y.; Zhang, G.; Li, D.; Dai, Z. Lutein Combined with EGCG Improved Retinitis Pigmentosa against N-Methyl-N Nitrosourea-Induced. Food Funct. 2023, 14, 9554–9566. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Sun, M.; Zhao, M.; Xu, B.; Li, J.; Zheng, T. Enhancement of the Physicochemical and in Vitro Release Properties of Lutein by Gelatin/Octenyl Succinic Anhydride (OSA)-modified Starch Composite as Vehicles. Int. J. Food Sci. Technol. 2022, 57, 738–750. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, Q.; Diao, C.; Wang, J.; Wu, Z.; Wang, H. Enhanced Physicochemical Stability of Lutein-Enriched Emulsions by Polyphenol-Protein-Polysaccharide Conjugates and Fat-Soluble Antioxidant. Food Hydrocoll. 2020, 101, 105447. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Huycke, M.M. Risks Associated with Enterococci as Probiotics. Food Res. Int. 2020, 129, 108788. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; He, Y.; Tufail, T.; Gul, M.; Qayum, A.; Rehman, A.; Rashid, A.; Ekumah, J.-N.; Han, X.; et al. The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs. Nutrients 2024, 16, 546. [Google Scholar] [CrossRef]
- Ji, H.; Yan, X.; Zhang, L.; Yang, L.; Xie, P.; Gu, F.; Bian, S.; Wan, H.; Nie, S. Prebiotics Empower Probiotics with Gastrointestinal Stress Resistance for Colon-Targeted Release to Synergistically Alleviate Colitis. J. Control. Release 2025, 380, 297–316. [Google Scholar] [CrossRef]
- Sultana, K.; Godward, G.; Reynolds, N.; Arumugaswamy, R.; Peiris, P.; Kailasapathy, K. Encapsulation of Probiotic Bacteria with Alginate-Starch and Evaluation of Survival in Simulated Gastrointestinal Conditions and in Yoghurt. Int. J. Food Microbiol. 2000, 62, 47–55. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; Liang, Q.; Sun, Y.; Zhong, M.; Tufail, T.; Rashid, A.; Qayum, A.; Rehman, A.; Ekumah, J.-N.; et al. Enhancing Storage and Gastroprotective Viability of Lactiplantibacillus Plantarum Encapsulated by Sodium Caseinate-Inulin-Soy Protein Isolates Composites Carried within Carboxymethyl Cellulose Hydrogel. Food Res. Int. 2024, 187, 114432. [Google Scholar] [CrossRef]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In Vitro Digestion of Meat- and Cereal-Based Food Matrix Enriched with Grape Extracts: How Are Polyphenol Composition, Bioaccessibility and Antioxidant Activity Affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, T.; Song, Y.; Shu, G.; Chen, H. Effect of Xanthan-Chitosan-Xanthan Double Layer Encapsulation on Survival of Bifidobacterium BB01 in Simulated Gastrointestinal Conditions, Bile Salt Solution and Yogurt. LWT—Food Sci. Technol. 2017, 81, 274–280. [Google Scholar] [CrossRef]
- Filannino, P.; Cavoski, I.; Thlien, N.; Vincentini, O.; Angelis, M.D.; Silano, M.; Gobbetti, M.; Cagno, R.D. Lactic Acid Fermentation of Cactus Cladodes (Opuntia ficus-indica L.) Generates Flavonoid Derivatives with Antioxidant and Anti-Inflammatory Properties. PLoS ONE 2016, 11, e0152575. [Google Scholar] [CrossRef]
- Lee, K.W.; Han, N.S.; Kim, J.H. Purification and Characterization of Beta-Glucosidase from Weissella Cibaria 37. J. Microbiol. Biotechnol. 2012, 22, 1705–1713. [Google Scholar] [CrossRef]
- Doo, H.; Kwak, J.; Keum, G.B.; Ryu, S.; Choi, Y.; Kang, J.; Kim, H.; Chae, Y.; Kim, S.; Kim, H.B.; et al. Lactic Acid Bacteria in Asian Fermented Foods and Their Beneficial Roles in Human Health. Food Sci. Biotechnol. 2024, 33, 2021–2033. [Google Scholar] [CrossRef]
- Holkem, A.T.; Neto, E.J.S.; Nakayama, M.; Souza, C.J.F.; Thomazini, M.; Gallo, F.A.; da Silva, M.P.; de Queiroz Bomdespacho, L.; Luciano, C.G.; Moraes, I.C.F.; et al. Sugarcane Juice with Co-Encapsulated Bifidobacterium animalis subsp. lactis BLC1 and Proanthocyanidin-Rich Cinnamon Extract. Probiotics Antimicrob. Proteins 2019, 12, 1179–1192. [Google Scholar] [CrossRef]
- Akpabli-Tsigbe, N.D.K.; Ma, Y.; Ekumah, J.-N.; Osabutey, J.; Hu, J.; Xu, M.; Johnson, N.A.N.; Quaisie, J. Two-Step Optimization of Solid-State Fermentation Conditions of Heilong48 Soybean Variety for Maximum Chlorogenic Acid Extraction Yield with Improved Antioxidant Activity. Ind. Crops Prod. 2021, 168, 113565. [Google Scholar] [CrossRef]
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; Requena, T. Effect of Grape Polyphenols on Lactic Acid Bacteria and Bifidobacteria Growth: Resistance and Metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Oliver, C.M.; Weerakkody, R.; Singh, T.; Conlon, M.; Borges, G.; Sanguansri, L.; Lockett, T.; Roberts, S.A.; Crozier, A.; et al. Chronic Administration of a Microencapsulated Probiotic Enhances the Bioavailability of Orange Juice Flavanones in Humans. Free Radic. Biol. Med. 2015, 84, 206–214. [Google Scholar] [CrossRef]
- Sharma, P.; Nickerson, M.T.; Korber, D.R. Valorization of Berry Pomace for Extraction of Polyphenol Compounds and Its Co-Encapsulation with Probiotic Bacteria. Food Biosci. 2024, 62, 105124. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Liu, B.; Xiao, J.; Stuart, M.A.C.; Hou, G.; Zhang, H.; Liang, S.; Li, Z.; Wang, Q.; et al. Natural Phenolic-Metal Framework Strengthened Mesona Chinensis Polysaccharides Microgels for Improved Viability of Probiotics to Alleviate the Liver Injury and Gut Microbiota Dysbiosis. Adv. Funct. Mater. 2024, 34, 2401064. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Brandão, L.R.; De Oliveira, M.P.; Da Costa, W.K.A.; Magnani, M. Health Benefits and Technological Effects of Lacticaseibacillus Casei-01: An Overview of the Scientific Literature. Trends Food Sci. Technol. 2021, 114, 722–737. [Google Scholar] [CrossRef]
- Ma, L.; Su, C.; Li, X.; Wang, H.; Luo, M.; Chen, Z.; Zhang, B.; Zhu, J.; Yuan, Y. Preparation and Characterization of Bilayered Microencapsulation for Co-Delivery Lactobacillus Casei and Polyphenols via Zein-Chitosan Complex Coacervation. Food Hydrocoll. 2024, 148, 109410. [Google Scholar] [CrossRef]
- Gaudreau, H.; Champagne, C.P.; Remondetto, G.E.; Gomaa, A.; Subirade, M. Co-Encapsulation of Lactobacillus Helveticus Cells and Green Tea Extract: Influence on Cell Survival in Simulated Gastrointestinal Conditions. J. Funct. Foods 2016, 26, 451–459. [Google Scholar] [CrossRef]
- Colín-Cruz, M.A.; Pimentel-González, D.J.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Guadarrama-Lezama, A.Y. Co-Encapsulation of Bioactive Compounds from Blackberry Juice and Probiotic Bacteria in Biopolymeric Matrices. LWT–Food Sci. Technol. 2019, 110, 94–101. [Google Scholar] [CrossRef]
- Hu, Q.; Li, J.; Wang, T.; Xu, X.; Duan, Y.; Jin, Y. Polyphenolic Nanoparticle-Modified Probiotics for Microenvironment Remodeling and Targeted Therapy of Inflammatory Bowel Disease. ACS Nano 2024, 18, 12917–12932. [Google Scholar] [CrossRef]
- Benito, I.; Encío, I.J.; Milagro, F.I.; Alfaro, M.; Martínez-Peñuela, A.; Barajas, M.; Marzo, F. Microencapsulated Bifidobacterium bifidum and Lactobacillus gasseri in Combination with Quercetin Inhibit Colorectal Cancer Development in Apc−/+ Mice. Int. J. Mol. Sci. 2021, 22, 4906. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, C.; Duan, H.; Narbad, A.; Zhao, J.; Chen, W.; Zhai, Q.; Yu, L.; Tian, F. Cross-Feeding of Bifidobacteria Promotes Intestinal Homeostasis: A Lifelong Perspective on the Host Health. Npj Biofilms Microbiomes 2024, 10, 47. [Google Scholar] [CrossRef]
- Xing, Z.; Han, W.; Dai, T.; Gong, D.; Zhang, G. Encapsulation of Ferulic Acid in Potato Protein-Pectin Nanocomplexes with Improved Stability and Controlled Release Properties. Food Biosci. 2024, 62, 105553. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of Epigallocatechin Gallate in Zein/Chitosan Nanoparticles for Controlled Applications in Food Systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef]
- Holkem, A.T.; Favaro-Trindade, C.S.; Lacroix, M. Study of Anticancer Properties of Proanthocyanidin-Rich Cinnamon Extract in Combination with Bifidobacterium Animalis Subsp. Lactis BLC1 and Resistance of These Free and Co-Encapsulated Materials under in Vitro Simulated Gastrointestinal Conditions. Food Res. Int. 2020, 134, 109274. [Google Scholar] [CrossRef]
- Huang, H.; Belwal, T.; Liu, S.; Duan, Z.; Luo, Z. Novel Multi-Phase Nano-Emulsion Preparation for Co-Loading Hydrophilic Arbutin and Hydrophobic Coumaric Acid Using Hydrocolloids. Food Hydrocoll. 2019, 93, 92–101. [Google Scholar] [CrossRef]
- Jang, Y.; Koh, E. Effect of Encapsulation on Stability of Anthocyanins and Chlorogenic Acid Isomers in Aronia during in Vitro Digestion and Their Transformation in a Model System. Food Chem. 2024, 434, 137443. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Liu, W.; Zhang, J.; Juan, B.; Zhu, Y.; Zhu, L.; Zhao, Y.; Daglia, M.; Xiao, X.; He, Y. Advances in Intestinal-Targeted Release of Phenolic Compounds. Nutrients 2025, 17, 2598. https://doi.org/10.3390/nu17162598
Tang Y, Liu W, Zhang J, Juan B, Zhu Y, Zhu L, Zhao Y, Daglia M, Xiao X, He Y. Advances in Intestinal-Targeted Release of Phenolic Compounds. Nutrients. 2025; 17(16):2598. https://doi.org/10.3390/nu17162598
Chicago/Turabian StyleTang, Yunxuan, Wenjing Liu, Jiayan Zhang, Bai Juan, Ying Zhu, Lin Zhu, Yansheng Zhao, Maria Daglia, Xiang Xiao, and Yufeng He. 2025. "Advances in Intestinal-Targeted Release of Phenolic Compounds" Nutrients 17, no. 16: 2598. https://doi.org/10.3390/nu17162598
APA StyleTang, Y., Liu, W., Zhang, J., Juan, B., Zhu, Y., Zhu, L., Zhao, Y., Daglia, M., Xiao, X., & He, Y. (2025). Advances in Intestinal-Targeted Release of Phenolic Compounds. Nutrients, 17(16), 2598. https://doi.org/10.3390/nu17162598










