Maternal BMI and Diet Quality Modulate Pregnancy Oxidative and Inflammatory Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
- Normal weight (NW): 18.5 kg/m2 ≤ pregestational BMI < 25 kg/m2;
- Overweight (OW): 25.0 kg/m2 ≤ pregestational BMI < 30 kg/m2;
- Obese (OB): 30.0 kg/m2 ≤ pregestational BMI ≤ 40 kg/m2.
2.2. Clinical Data
2.3. Blood Collection, Placental Biometric Measurements, Inflammatory and Oxidative Markers Assessment
2.4. Dietary Intake Assessment
2.5. Statistical Analysis
3. Results
3.1. Maternal, Placental, and Neonatal Data
3.2. Inflammatory and Oxidative Markers at Third Trimester
3.3. Association of Maternal Markers and Clinical Maternal, Placental, and Neonatal Data
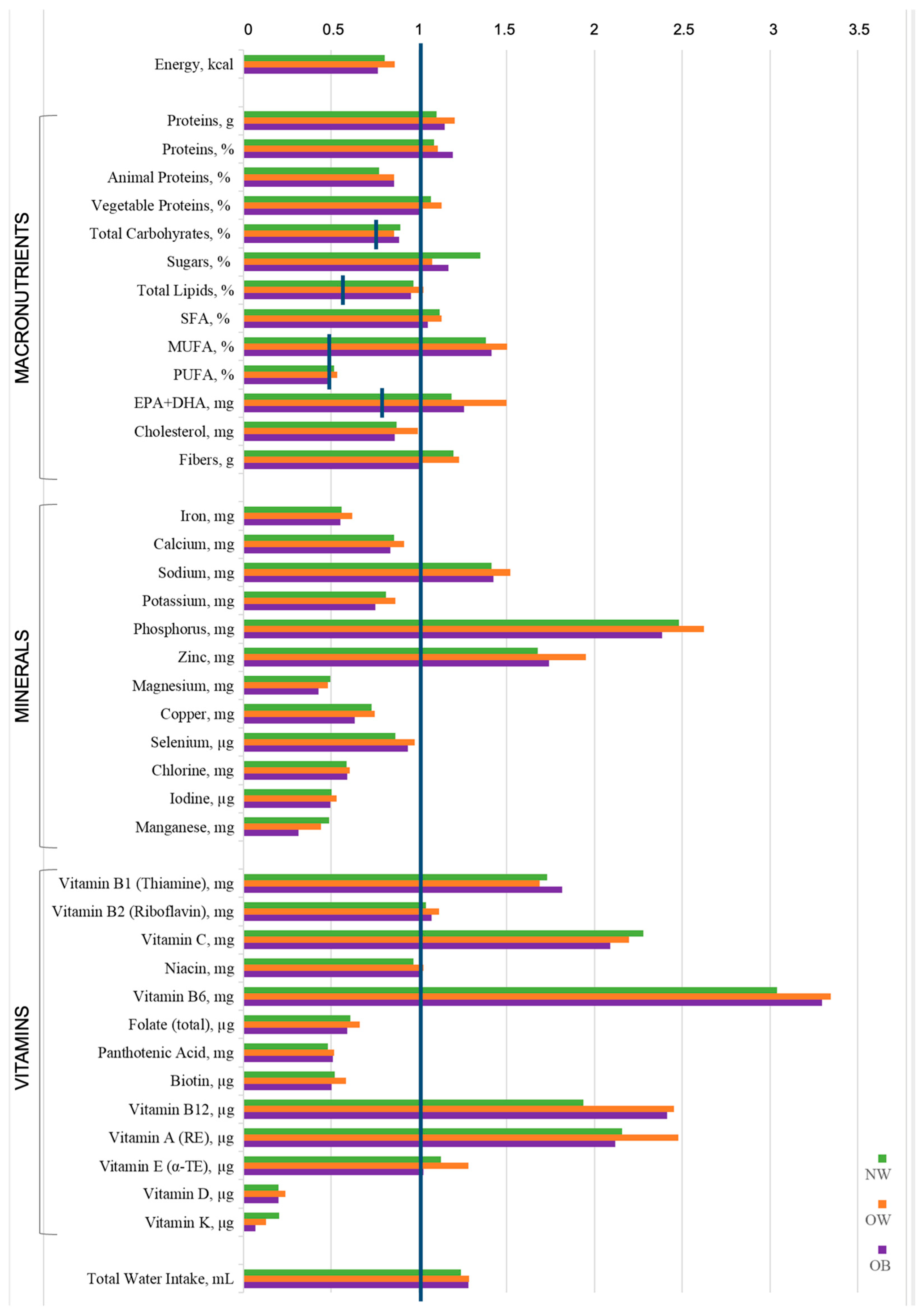
3.4. Energy and Nutrient Daily Intake
3.5. Dietary Patterns
- Pattern 1 (high plant, low animal: “Prudent-style”) was characterized by high adherence to legumes, nuts, vegetables, and fruit, and low adherence to sauces.
- Pattern 2 (ultra-processed food, high animal: “Western-like”) showed high adherence to sugar and snacks, animal fats, cereals, and dairy products.
- Pattern 3 (high protein and carbohydrates: “Moderately beneficial”) featured high fish, meat, and potatoes.
- Pattern 4 (moderate protein, moderate plant, moderate sugar: “Moderate-mixed”) was defined by high adherence to eggs, fruits, vegetable fats, and non-alcoholic beverages (such as sugar-sweetened beverages, artificially sweetened drinks, fruit juices, coffee, tea).
4. Discussion
4.1. Study Population and Maternal Blood Biomarkers
4.2. Dietary Analysis and Association with Biomarkers and Clinical Data
4.3. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 334, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Agosti, M.; Tandoi, F.; Morlacchi, L.; Bossi, A. Nutritional and metabolic programming during the first thousand days of life. Pediatr. Med. Chir. 2017, 39, 157. [Google Scholar] [CrossRef]
- Rojas-Rodriguez, R.; Price, L.L.; Somogie, J.; Hauguel-de Mouzon, S.; Kalhan, S.C.; Catalano, P.M. Maternal Lipid Metabolism Is Associated With Neonatal Adiposity: A Longitudinal Study. J. Clin. Endocrinol. Metab. 2022, 107, e3759–e3768. [Google Scholar] [CrossRef]
- Faa, G.; Fanos, V.; Manchia, M.; Van Eyken, P.; Suri, J.S.; Saba, L. The fascinating theory of fetal programming of adult diseases: A review of the fundamentals of the Barker hypothesis. J. Public Health Res. 2024, 13, 1–10. [Google Scholar] [CrossRef]
- Slade, L.; Syeda, N.; Mistry, H.D.; Bone, J.N.; Wilson, M.; Blackman, M.; Poston, L.; Godfrey, K.M.; von Dadelszen, P.; Magee, L.A.; et al. Do lower antenatal blood pressure cut-offs in pregnant women with obesity identify those at greater risk of adverse maternal and perinatal outcomes? A secondary analysis of data from the UK Pregnancies Better Eating and Activity Trial (UPBEAT). Int. J. Obes. 2025; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Creanga, A.A.; Catalano, P.M.; Bateman, B.T. Obesity in Pregnancy. N. Engl. J. Med. 2022, 387, 248–259. [Google Scholar] [CrossRef]
- Melchor, I.; Burgos, J.; Del Campo, A.; Aiartzaguena, A.; Gutiérrez, J.; Melchor, J.C. Effect of maternal obesity on pregnancy outcomes in women delivering singleton babies: A historical cohort study. J. Perinat. Med. 2019, 47, 625–630. [Google Scholar] [CrossRef]
- Zavatta, A.; Parisi, F.; Mandò, C.; Scaccabarozzi, C.; Savasi, V.M.; Cetin, I. Role of Inflammaging on the Reproductive Function and Pregnancy. Clin. Rev. Allergy Immunol. 2023, 64, 145–160. [Google Scholar] [CrossRef]
- Mandò, C.; Castiglioni, S.; Novielli, C.; Anelli, G.M.; Serati, A.; Parisi, F.; Lubrano, C.; Zocchi, M.; Ottria, R.; Giovarelli, M. Placental Bioenergetics and Antioxidant Homeostasis in Maternal Obesity and Gestational Diabetes. Antioxidants 2024, 13, 858. [Google Scholar] [CrossRef]
- Mandò, C.; Abati, S.; Anelli, G.M.; Favero, C.; Serati, A.; Dioni, L.; Zambon, M.; Albetti, B.; Bollati, V.; Cetin, I. Epigenetic Profiling in the Saliva of Obese Pregnant Women. Nutrients 2022, 14, 2122. [Google Scholar] [CrossRef] [PubMed]
- Serati, A.; Novielli, C.; Anelli, G.M.; Mandalari, M.; Parisi, F.; Cetin, I.; Paleari, R.; Mandò, C. Characterization of Maternal Circulating MicroRNAs in Obese Pregnancies and Gestational Diabetes Mellitus. Antioxidants 2023, 12, 515. [Google Scholar] [CrossRef] [PubMed]
- Montagnoli, C.; Santoro, C.B.; Buzzi, T.; Bortolus, R. Maternal periconceptional nutrition matters. A scoping review of the current literature. J. Matern. Fetal Neonatal Med. 2022, 35, 8123–8140. [Google Scholar] [CrossRef]
- Lisso, F.; Massari, M.; Gentilucci, M.; Novielli, C.; Corti, S.; Nelva Stellio, L.; Milazzo, R.; Troiano, E.; Schaefer, E.; Cetin, I.; et al. Longitudinal Nutritional Intakes in Italian Pregnant Women in Comparison with National Nutritional Guidelines. Nutrients 2022, 14, 1944. [Google Scholar] [CrossRef]
- Massari, M.; Novielli, C.; Mandò, C.; Di Francesco, S.; Della Porta, M.; Cazzola, R.; Panteghini, M.; Savasi, V.; Maggini, S.; Schaefer, E.; et al. Multiple Micronutrients and Docosahexaenoic Acid Supplementation during Pregnancy: A Randomized Controlled Study. Nutrients 2020, 12, 2432. [Google Scholar] [CrossRef]
- Rajendram, R.; Preedy, V.R.; Patel, V.B. (Eds.) Diet, Nutrition, and Fetal Programming; Humana Press, Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Karam, G.; Agarwal, A.; Sadeghirad, B.; Jalink, M.; Hitchcock, C.L.; Ge, L.; Kiflen, R.; Ahmed, W.; Zea, A.M.; Milenkovic, J.; et al. Comparison of seven popular structured dietary programmes and risk of mortality and major cardiovascular events in patients at increased cardiovascular risk: Systematic review and network meta-analysis. BMJ 2023, 380, e072003. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Angelino, D.; Rosi, A.; Dall’Asta, M.; Bresciani, L.; Ferraris, C.; Guglielmetti, M.; Godos, J.; Del Bo’, C.; et al. Effects of Popular Diets on Anthropometric and Cardiometabolic Parameters: An Umbrella Review of Meta-Analyses of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 815–833. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Becerra-Tomás, N.; García-Gavilán, J.F.; Bulló, M.; Barrubés, L. Mediterranean Diet and Cardiovascular Disease Prevention: What Do We Know? Prog. Cardiovasc. Dis. 2018, 61, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Pourrajab, B.; Fotros, D.; Asghari, P.; Shidfar, F. Effect of the Mediterranean Diet Supplemented With Olive Oil Versus the Low-Fat Diet on Serum Inflammatory and Endothelial Indexes Among Adults: A Systematic Review and Meta-analysis of Clinical Controlled Trials. Nutr. Rev. 2025, 83, e1421–e1440. [Google Scholar] [CrossRef]
- González-Palacios, S.; Oncina-Cánovas, A.; García-de-la-Hera, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Schröder, H.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Increased ultra-processed food consumption is associated with worsening of cardiometabolic risk factors in adults with metabolic syndrome: Longitudinal analysis from a randomized trial. Atherosclerosis 2023, 377, 12–23. [Google Scholar] [CrossRef]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef] [PubMed]
- Mazzucca, C.B.; Scotti, L.; Raineri, D.; Cappellano, G.; Chiocchetti, A. Design and Validation of MEDOC, a Tool to Assess the Combined Adherence to Mediterranean and Western Dietary Patterns. Nutrients 2024, 16, 1745. [Google Scholar] [CrossRef]
- Alberti-Fidanza, A.; Fidanza, F. Mediterranean Adequacy Index of Italian diets. Public Health Nutr. 2004, 7, 937–941. [Google Scholar] [CrossRef]
- Vilarnau, C.; Stracker, D.M.; Funtikov, A.; da Silva, R.; Estruch, R.; Bach-Faig, A. Worldwide adherence to Mediterranean Diet between 1960 and 2011. Eur. J. Clin. Nutr. 2019, 72 (Suppl. 1), 83–91. [Google Scholar] [CrossRef]
- Pan, M.; Yin, T.; Yang, Y.; Zhu, F.; Xu, J.; Chen, R.; Zheng, W. The association between four dietary indices and mortality risk in cardiovascular disease patients. Nutr. Metab. 2025, 22, 67. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; A Lear, S.; Wei, L.; Diaz, R.; et al. Diet, cardiovascular disease, and mortality in 80 countries. Eur. Heart J. 2023, 44, 2560–2579. [Google Scholar] [CrossRef] [PubMed]
- Mandò, C.; Savasi, V.M.; Anelli, G.M.; Corti, S.; Serati, A.; Lisso, F.; Tasca, C.; Novielli, C.; Cetin, I. Mitochondrial and Oxidative Unbalance in Placentas from Mothers with SARS-CoV-2 Infection. Antioxidants 2021, 10, 1517. [Google Scholar] [CrossRef]
- Anelli, G.M.; Parisi, F.; Sarno, L.; Fornaciari, O.; Carlea, A.; Coco, C.; Porta, M.D.; Mollo, N.; Villa, P.M.; Guida, M.; et al. Associations between Maternal Dietary Patterns, Biomarkers and Delivery Outcomes in Healthy Singleton Pregnancies: Multicenter Italian GIFt Study. Nutrients 2022, 14, 3631. [Google Scholar] [CrossRef]
- SINU (Società Italiana di Nutrizione Umana—Italian Society of Human Nutrition). LARN—Livelli di Assunzione di Riferimento di Nutrienti ed Energia (Dietary Reference Values of Nutrients and Energy for the Italian Population). V Revision. Available online: https://eng.sinu.it/larn/ (accessed on 27 June 2025).
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. Dietary patterns and their association with food and nutrient intake in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Br. J. Nutr. 2001, 85, 363–373. [Google Scholar] [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Crume, T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes 2011, 60, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Candia, A.A.; Lean, S.C.; Zhang, C.X.W.; McKeating, D.R.; Cochrane, A.; Gulacsi, E.; Herrera, E.A.; Krause, B.J.; Sferruzzi-Perri, A.N. Obesogenic Diet in Mice Leads to Inflammation and Oxidative Stress in the Mother in Association with Sex-Specific Changes in Fetal Development, Inflammatory Markers and Placental Transcriptome. Antioxidants 2024, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Carlini, V.; Mandò, C.; Anelli, G.M.; Pontiroli, A.E.; Trabucchi, E.; Cetin, I.; Abati, S. Maternal Salivary miR-423-5p Is Linked to Neonatal Outcomes and Periodontal Status in Cardiovascular-High-Risk Pregnancies. Int. J. Mol. Sci. 2024, 25, 9087. [Google Scholar] [CrossRef]
- Sureshchandra, S.; Marshall, N.E.; Wilson, R.M.; Barr, T.; Rais, M.; Purnell, J.Q.; Thornburg, K.L.; Messaoudi, I. Inflammatory Determinants of Pregravid Obesity in Placenta and Peripheral Blood. Front. Physiol. 2018, 9, 1089. [Google Scholar] [CrossRef]
- Orisaka, M.; Mizutani, T.; Miyazaki, Y.; Shirafuji, A.; Tamamura, C.; Fujita, M.; Tsuyoshi, H.; Yoshida, Y. Chronic low-grade inflammation and ovarian dysfunction in women with polycystic ovarian syndrome, endometriosis, and aging. Front. Endocrinol. 2023, 14, 1324429. [Google Scholar] [CrossRef]
- Galbarczyk, A.; Klimek, M.; Blukacz, M.; Nenko, I.; Jabłońska, M.; Jasienska, G. Inflammaging: Blame the sons. Relationships between the number of sons and the level of inflammatory mediators among post-reproductive women. Am. J. Phys. Anthropol. 2021, 175, 656–664. [Google Scholar] [CrossRef]
- Rodichkina, V.; Kvetnoy, I.; Polyakova, V.; Arutjunyan, A.; Nasyrov, R.; Ivanov, D. Inflammaging of Female Reproductive System: A Molecular Landscape. Curr. Aging Sci. 2021, 14, 10–18. [Google Scholar] [CrossRef]
- Diceglie, C.; Anelli, G.M.; Martelli, C.; Serati, A.; Lo Dico, A.; Lisso, F.; Parisi, F.; Novielli, C.; Paleari, R.; Cetin, I.; et al. Placental Antioxidant Defenses and Autophagy-Related Genes in Maternal Obesity and Gestational Diabetes Mellitus. Nutrients 2021, 13, 1303. [Google Scholar] [CrossRef]
- Assi, E.; D’Addio, F.; Mandò, C.; Maestroni, A.; Loretelli, C.; Ben Nasr, M.; Usuelli, V.; Abdelsalam, A.; Seelam, A.J.; Pastore, I.; et al. Placental proteome abnormalities in women with gestational diabetes and large-for-gestational-age newborns. BMJ Open Diabetes Res. Care. 2020, 8, e001586. [Google Scholar] [CrossRef]
- Brombach, C.; Tong, W.; Giussani, D.A. Maternal obesity: New placental paradigms unfolded. Trends Mol. Med. 2022, 28, 823–835. [Google Scholar] [CrossRef]
- Musa, E.; Salazar-Petres, E.; Arowolo, A.; Levitt, N.; Matjila, M.; Sferruzzi-Perri, A.N. Obesity and gestational diabetes independently and collectively induce specific effects on placental structure, inflammation and endocrine function in a cohort of South African women. J. Physiol. 2023, 601, 1287–1306. [Google Scholar] [CrossRef]
- Coussons-Read, M.E. Effects of prenatal stress on pregnancy and human development: Mechanisms and pathways. Obstet. Med. 2013, 6, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Dutra, T.A.; Fragoso, M.B.T.; Wanderley, T.M.; Bezerra, A.R.; Bueno, N.B.; de Oliveira, A.C.M. Diet’s total antioxidant capacity and women’s health: Systematic review and meta-analysis. Br. J. Nutr. 2025, 133, 1404–1417. [Google Scholar] [CrossRef]
- Thiele, K.; Diao, L.; Arck, P.C. Immunometabolism, pregnancy, and nutrition. Semin. Immunopathol. 2018, 40, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Zhang, C.X.W.; Candia, A.A.; Sferruzzi-Perri, A.N. Placental inflammation, oxidative stress, and fetal outcomes in maternal obesity. Trends Endocrinol. Metab. 2024, 35, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Z.; Qiao, Y.; Maiti, K.; Smith, R. Involvement of oxidative stress in placental dysfunction, the pathophysiology of fetal death and pregnancy disorders. Reproduction 2023, 166, R25–R38. [Google Scholar] [CrossRef]
- Jansson, N.; Rosario, F.J.; Gaccioli, F.; Lager, S.; Jones, H.N.; Roos, S.; Jansson, T.; Powell, T.L. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J. Clin. Endocrinol. Metab. 2013, 98, 105–113. [Google Scholar] [CrossRef]
- Jansson, T.; Powell, T.L. Role of placental nutrient sensing in developmental programming. Clin. Obstet. Gynecol. 2013, 56, 591–601. [Google Scholar] [CrossRef]
- Gaccioli, F.; Lager, S.; Powell, T.L.; Jansson, T. Placental transport in response to altered maternal nutrition. J. Dev. Orig. Health Dis. 2013, 4, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Poston, L.; Burton, G.J. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum. Reprod. Update 2006, 12, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; Krause, B.; Ebensperger, G.; Reyes, R.V.; Casanello, P.; Parra-Cordero, M.; Llanos, A.J. The placental pursuit for an adequate oxidant balance between the mother and the fetus. Front. Pharmacol. 2014, 5, 149. [Google Scholar] [CrossRef] [PubMed]
- Swain, N.; Moharana, A.K.; Jena, S.R.; Samanta, L. Impact of Oxidative Stress on Embryogenesis and Fetal Development. Adv. Exp. Med. Biol. 2022, 1391, 221–241. [Google Scholar] [CrossRef]
- Vornic, I.; Buciu, V.; Furau, C.G.; Gaje, P.N.; Ceausu, R.A.; Dumitru, C.-S.; Barb, A.C.; Novacescu, D.; Cumpanas, A.A.; Latcu, S.C.; et al. Oxidative Stress and Placental Pathogenesis: A Contemporary Overview of Potential Biomarkers and Emerging Therapeutics. Int. J. Mol. Sci. 2024, 25, 12195. [Google Scholar] [CrossRef]
- Sun, C.; Shen, J.; Fang, R.; Huang, H.; Lai, Y.; Hu, Y.; Zheng, J. The impact of environmental and dietary exposure on gestational diabetes mellitus: A comprehensive review emphasizing the role of oxidative stress. Front. Endocrinol. 2025, 16, 1393883. [Google Scholar] [CrossRef]
- Poston, L.; Igosheva, N.; Mistry, H.D.; Seed, P.T.; Shennan, A.H.; Rana, S.; Karumanchi, S.A.; Chappell, L.C. Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am. J. Clin. Nutr. 2011, 94 (Suppl. 6), 1980S–1985S. [Google Scholar] [CrossRef]
- Sen, S.; Simmons, R.A. Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes 2010, 59, 3058–3065. [Google Scholar] [CrossRef]
- Lin, Y.F.; Tsai, H.L.; Lee, Y.C.; Chang, S.J. Maternal vitamin E supplementation affects the antioxidant capability and oxidative status of hatching chicks. J. Nutr. 2005, 135, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Pressman, E.K.; Cavanaugh, J.L.; Mingione, M.; Norkus, E.P.; Woods, J.R. Effects of maternal antioxidant supplementation on maternal and fetal antioxidant levels: A randomized, double-blind study. Am. J. Obstet. Gynecol. 2003, 189, 1720–1725. [Google Scholar] [CrossRef]
- Hsu, M.H.; Chen, Y.C.; Sheen, J.M.; Huang, L.T. Maternal Obesity Programs Offspring Development and Resveratrol Potentially Reprograms the Effects of Maternal Obesity. Int. J. Environ. Res. Public Health 2020, 17, 1610. [Google Scholar] [CrossRef]
- Díaz, P.; Powell, T.L.; Jansson, T. The role of placental nutrient sensing in maternal-fetal resource allocation. Biol. Reprod. 2014, 91, 82. [Google Scholar] [CrossRef] [PubMed]
- Brett, K.E.; Ferraro, Z.M.; Yockell-Lelievre, J.; Gruslin, A.; Adamo, K.B. Maternal-fetal nutrient transport in pregnancy pathologies: The role of the placenta. Int. J. Mol. Sci. 2014, 15, 16153–16185. [Google Scholar] [CrossRef]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental Responses to Changes in the Maternal Environment Determine Fetal Growth. Front. Physiol. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Divvela, S.S.K.; Gallorini, M.; Gellisch, M.; Patel, G.D.; Saso, L.; Brand-Saberi, B. Navigating redox imbalance: The role of oxidative stress in embryonic development and long-term health outcomes. Front. Cell Dev. Biol. 2025, 13, 1521336. [Google Scholar] [CrossRef]
- Sen, S.; Rifas-Shiman, S.L.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Gold, D.R.; Gillman, M.W.; Oken, E. Dietary Inflammatory Potential during Pregnancy Is Associated with Lower Fetal Growth and Breastfeeding Failure: Results from Project Viva. J. Nutr. 2016, 146, 728–736. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Kamai, E.M.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D.; McElrath, T.F. Associations between repeated ultrasound measures of fetal growth and biomarkers of maternal oxidative stress and inflammation in pregnancy. Am. J. Reprod. Immunol. 2018, 80, e13017. [Google Scholar] [CrossRef]
- Lubrano, C.; Locati, F.; Parisi, F.; Anelli, G.M.; Ossola, M.W.; Cetin, I. Gestational Weight Gain as a Modifiable Risk Factor in Women with Extreme Pregestational BMI. Nutrients 2025, 17, 736. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Johansson, K.; Himes, K.P.; Khodyakov, D.; Abrams, B.; Parisi, S.M.; Hutcheon, J.A. Gestational weight gain below recommendations and adverse maternal and child health outcomes for pregnancies with overweight or obesity: A United States cohort study. Am. J. Clin. Nutr. 2024, 120, 638–647. [Google Scholar] [CrossRef]
- Most, J.; Marlatt, K.L.; Altazan, A.D.; Redman, L.M. Advances in assessing body composition during pregnancy. Eur. J. Clin. Nutr. 2018, 72, 645–656. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee on the Dietary Reference Intakes for Energy. Dietary Reference Intakes for Energy; National Academies Press: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Jhamb, I.; Freeman, A.; Lotfi, M.R.; VanOrmer, M.; Hanson, C.; Anderson-Berry, A.; Thoene, M. Evaluation of Vitamin E Isoforms in Placental Tissue and Their Relationship with Maternal Dietary Intake and Plasma Concentrations in Mother-Infant Dyads. Antioxidants 2023, 12, 1797. [Google Scholar] [CrossRef] [PubMed]
- Bastani, P.; Hamdi, K.; Abasalizadeh, F.; Navali, N. Effects of vitamin E supplementation on some pregnancy health indices: A randomized clinical trial. Int. J. Gen. Med. 2011, 4, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Liu, Y.J.; Tian, L.X.; Niu, J.; Liang, G.Y.; Yang, H.J.; Yuan, Y.; Zhang, Y.Q. Effect of dietary vitamin E and selenium supplementation on growth, body composition, and antioxidant defense mechanism in juvenile largemouth bass (Micropterus salmoides) fed oxidized fish oil. Fish. Physiol. Biochem. 2013, 39, 593–604. [Google Scholar] [CrossRef]
- Lammers, S.; Iovino, N.A.; Pusateri, A.; Snyder, A.; Butnariu, M.; Frey, H.A.; Skeans, J. Maternal and Neonatal Hemorrhage From Vitamin K Deficiency in the Setting of Crohn Disease in Pregnancy. Obstet. Gynecol. 2025, 145, e127–e130. [Google Scholar] [CrossRef]
- Shahrook, S.; Ota, E.; Hanada, N.; Sawada, K.; Mori, R. Vitamin K supplementation during pregnancy for improving outcomes: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 11459. [Google Scholar] [CrossRef]
- Nijsten, K.; van der Minnen, L.; Wiegers, H.M.G.; Koot, M.H.; Middeldorp, S.; Roseboom, T.J.; Grooten, I.J.; Painter, R.C. Hyperemesis gravidarum and vitamin K deficiency: A systematic review. Br. J. Nutr. 2022, 128, 30–42. [Google Scholar] [CrossRef]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W., Jr.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Flor-Alemany, M.; Sandborg, J.; Migueles, J.H.; Söderström, E.; Henström, M.; Marín-Jiménez, N.; Baena-García, L.; Aparicio, V.A.; Löf, M. Mediterranean Diet Adherence and Health-Related Quality of Life during Pregnancy: Is. the Mediterranean Diet. Beneficial in Non-Mediterranean Countries? Nutrients 2024, 16, 718. [Google Scholar] [CrossRef]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef] [PubMed]
- Kouiti, M.; Hernández-Muñiz, C.; Youlyouz-Marfak, I.; Salcedo-Bellido, I.; Mozas-Moreno, J.; Jiménez-Moleón, J.J. Preventing Gestational Diabetes Mellitus by Improving Healthy Diet and/or Physical Activity during Pregnancy: An Umbrella Review. Nutrients 2022, 14, 2066. [Google Scholar] [CrossRef]
- Martín-O’Connor, R.; Ramos-Levi, A.; Melero, V.; Arnoriaga-Rodriguez, M.; Barabash, A.; Valerio, J.; Del Valle, L.; de Miguel, P.; Diaz, A.; Familiar, C.; et al. Early Mediterranean-Based Nutritional Intervention Reduces the Rate of Gestational Diabetes in Overweight and Obese Pregnant Women: A Post-Hoc Analysis of the San Carlos Gestational Prevention Study. Nutrients 2024, 16, 2206. [Google Scholar] [CrossRef]
- Hebert, J.F.; Myatt, L. Placental mitochondrial dysfunction with metabolic diseases: Therapeutic approaches. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 165967. [Google Scholar] [CrossRef]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Flores-Espinosa, P.; Díaz, L.; Velázquez, P.; Ramírez-Isarraraz, C.; Zaga-Clavellina, V. Immunoendocrine Dysregulation during Gestational Diabetes Mellitus: The Central Role of the Placenta. Int. J. Mol. Sci. 2021, 22, 8087. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Gil-Izquierdo, A.; Ferreres, F.; Medina, S. Update on oxidative stress and inflammation in pregnant women, unborn children (nasciturus), and newborns—Nutritional and dietary effects. Free Radic. Biol. Med. 2019, 142, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, S.R.; Mulligan, C.M.; Janssen, R.C.; Baker, P.R., II; Bergman, B.C.; D’Alessandro, A.; Nemkov, T.; Maclean, K.N.; Jiang, H.; Dean, T.A.; et al. Switching obese mothers to a healthy diet improves fetal hypoxemia, hepatic metabolites, and lipotoxicity in non-human primates. Mol. Metab. 2018, 18, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, S.B.; Hegazy, I.S.; Mohamed, D.A.; Abu El Kasem, M.M.A.; Hagag, S.S. Effect of dietary counseling on preventing excessive weight gain during pregnancy. Public Health 2018, 154, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Banafshe, E.; Javadifar, N.; Abbaspoor, Z.; Karandish, M.; Ghanbari, S. Factors Influencing Weight Management in Pregnant Women with Overweight or Obesity: A Meta-Synthesis of Qualitative Studies. J. Acad. Nutr. Diet. 2024, 124, 964–994.e1. [Google Scholar] [CrossRef]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.L.; Wong, Y.P.; Abrams, B.; Gernand, A.D.; Rasmussen, K.M.; Siega-Riz, A.M.; Stang, J.; Casavale, K.O.; et al. Dietary patterns before and during pregnancy and maternal outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109 (Suppl. 7), 705S–728S. [Google Scholar] [CrossRef]
| NW | OW | OB | ||
|---|---|---|---|---|
| Maternal General Data | ||||
| Age 2 [years] | 34 (31–36) | 34.0 (32.0–36.5) | 35.0 (32.0–37.5) | |
| Pre-pregnancy Weight 2 [kg] | 57 (53–61) | 73 (69.3–76.5) °°° | 87 (81–102) °°°,** | |
| Pre-pregnancy BMI 2 [kg/m2] | 20.3 (19.5–22.6) | 26.8 (25.8–28.2) °°° | 32.3 (31.2–35.9) °°°,** | |
| Supplementation: | none (%) | 14.1 | 25.0 | 25.0 |
| only folic acid (%) | 16.7 | 9.1 | 7.1 | |
| iron (%) | 14.1 | 11.4 | 25.0 | |
| multivitamin (%) | 55.1 | 54.5 | 42.9 | |
| Data at Third Trimester (T0) | ||||
| Gestational Age 2 [weeks] | 32.0 (30.5–33.7) | 31.7 (30.9–33.1) | 33.0 (30.9–35.0) | |
| Mat—GWG 2 [kg] | 9.1 (8.0–11.0) | 9.0 (7.0–12.0) | 5.0 (−0.5–9.4) °°°,*** | |
| Mat—Hemoglobin 1 [g/dL] | 11.6 (11.1–12.2) | 11.4 (10.7–11.9) | 12.1 (11.6–12.7) * | |
| Anemia (%) | 20.3 | 27.9 | 18.5 | |
| Mat—Hematocrit 1 [%] | 34.7 (33.2–36.1) | 34.0 (32.0–35.8) | 36.5 (34.2–38.0) *** | |
| Mat—Glycemia 1 [mg/dL] | 75.0 (69.3–79.8) | 79.0 (75.0–83.0) °° | 80.7 (77.0–84.5) °°° | |
| Mat—Vitamin D 2 [ng/mL] | 23.9 (15.9–31.9) | 22.0 (18.0–29.2) | 23.0 (18.0–37.9) | |
| Mat—Ferritin 2 [ng/mL] | 11.0 (7.8–15.3) | 13.0 (8.0–18.0) | 13.7 (9.5–22.0) | |
| Data at Delivery (T1) | ||||
| Gestational Age 2 [weeks] | 39.9 (38.7–40.6) | 39.7 (38.8–40.7) | 39.3 (39.0–40.0) | |
| Mat—Weight 2 [kg] | 69.0 (63.5–75.0) | 85.0 (79.1–92.0) °°° | 96.0 (89.0–107.8) °°°,* | |
| Mat—GWG 2 [kg] | 12.0 (10.0–14.0) | 10.3 (9.0–16.0) | 7.5 (3.0–12.5) °°°,* | |
| Mat—GWG according to IOM recommendations: (Chi-square test: p-value = 0.002) | ||||
| below (%) | 37.3 | 15.4 | 28.6 | |
| within (%) | 50.7 | 43.6 | 32.1 | |
| above (%) | 12.0 | 41.0 | 39.3 | |
| Mat—Hemoglobin 1 [g/dL] | 11.7 (11.1–12.3) | 11.2 (10.3–12.3) | 12.1 (11.2–13.1) * | |
| Anemia (%) (Chi-square test: p-value = 0.038) | 20.0 | 43.8 | 20.7 | |
| Mat—Hematocrit 1 [%] | 34.9 (33.0–36.8) | 33.0 (30.6–35.8) | 36.0 (33.0–38.1) * | |
| Mode of delivery: | ||||
| Spontaneous or Vacuum-Assisted delivery (%) | 84.6 | 77.3 | 79.3 | |
| Cesarean Section (%) | 15.4 | 22.7 | 20.7 | |
| Placental Weight 1 [g] | 480.0 (382.5–560.0) | 520.0 (430.0–609.8) | 500.0 (419.5–640.0) | |
| Placental Area 1 [cm2] | 251.3 (197.9–282.7) | 238.8 (201.5–274.5) | 223.8 (179.5–253.7) | |
| Neonatal/Placental Weight Ratio 2 | 6.9 (6.0–8.4) | 6.6 (5.7–7.4) | 6.5 (5.6–7.6) | |
| Neonatal Weight 2 [g] | 3340 (3135–3530) | 3435.0 (3107.5–3703.8) | 3380 (3190–3725) | |
| Neonatal Head Circumference 2 [cm] | 34.5 (33.5–35.0) | 34.5 (33.5–35.0) | 35.0 (33.6–35.5) | |
| Neonatal Ponderal Index 2 [g/cm3] | 2.6 (2.5–2.8) | 2.6 (2.4–2.8) | 2.8 (2.5–2.9) | |
| Neonatal Sex: | Male (%) | 47.4 | 56.8 | 58.6 |
| Female (%) | 52.6 | 43.2 | 41.4 | |
| Umbilical Artery pH | 7.28 (7.23–7.34) | 7.24 (7.16–7.34) | 7.27 (7.20–7.32) | |
| NW | OW | OB | |
|---|---|---|---|
| Hepcidin 2 [ng/mL] | 71.84 (60.86–79.24) (n = 26) | 81.40 (73.79–92.95) °° (n = 26) | 90.38 (73.66–121.5) °°° (n = 18) |
| C Reactive Protein 1 [mg/L] | 3.009 (1.877–4.743) (n = 27) | 5.682 (3.733–7.090) ° (n = 27) | 6.316 (4.339–9.528) °°° (n = 19) |
| Catalase Activity 2 [nmol/min/mL] | 15.74 (13.27–20.21) (n = 28) | 27.48 (18.13–42.99) °° (n = 25) | 28.44 (23.08–49.45) °°° (n = 15) |
| SOD Activity 1 [U/mL] | 0.600 (0.525–0.770) (n = 32) | 0.580 (0.498–0.665) (n = 26) | 0.620 (0.360–0.750) (n = 17) |
| TAC 1 [mM] | 1.000 (0.963–1.028) (n = 28) | 0.990 (0.960–1.033) (n = 26) | 1.040 (0.985–1.065) (n = 17) |
| DNA/RNA Oxidative Damage 2 [pg/mL] | 8108.7 (6417.3–10,156.9) (n = 76) | 9597.8 (7601.9–13,013.4) ° (n = 40) | 11,213.5 (9640.2–16,888.6) °°° (n = 23) |
| Maternal/General Data | β (95% CI) p-Value | Neonatal/Placental Data | β (95% CI) p-Value | |
|---|---|---|---|---|
| Hepcidin [ng/mL] | Pregestational BMI [kg/m2] | β = 1.419 (0.398; 2.439) p = 0.006 | Neon. ponderal index [g/cm3] | β = 0.006 (0.002; 0.009) p = 0.004 |
| C Reactive Protein [mg/L] | Pregestational BMI [kg/m2] | β = 0.297 (0.159; 0.435) p = 0.000 | Neon. head circumference [cm] | β = −0.135 (−0.240; −0.030) p = 0.012 |
| Catalase activity [nmol/mL] | Pregestational BMI [kg/m2] | β = 1.536 (0.702; 2.369) p = 0.000 | Neonatal weight [g] | β = 5.767 (0.726; 10.81) p = 0.025 |
| Gestational age T0 [weeks] | β = 2.366 (0.562; 4.171) p = 0.010 | Placental weight [g] | β = 2.642 (0.424; 4.861) p = 0.020 | |
| SOD activity [U/mL] | Neon. ponderal index [g/cm3] | β = 0.439 (0.026; 0.851) p = 0.037 | ||
| TAC [mM] | Multivitamin supplementation | β = 0.046 (0.010; 0.082) p = 0.012 | ||
| DNA/RNA oxidative damage [pg/mL] | Maternal age [years] | β = −353.5 (−652.7; −54.2)) p = 0.021 | ||
| Pregestational BMI [kg/m2] | β = 409.9 (227.4; 592.3) p = 0.000 | |||
| Folic acid supplementation | β = 4452.7 (1210.3; 7695.0) p = 0.007 | |||
| Gestational age T0 [weeks] | β = 512.3 (62.5; 962.1) p = 0.026 |
| NW (n = 65) | OW (n = 44) | OB (n = 26) | |
|---|---|---|---|
| Energy (kcal) 2 | 2054.7 (1644.3–2607.1) | 2211.3 (1770.3–2667.4) | 1860.1 (1658.2–2255.1) |
| Total protein (g) 1 | 86.79 (68.35–99.75) | 91.54 (74.27–107.30) | 84.42 (72.73–107.00) |
| Animal protein (g) 1 | 49.77 (38.44–65.51) | 54.92 (41.29–66.70) | 52.48 (42.78–75.75) |
| Vegetable protein (g) 1 | 35.60 (27.50–40.80) | 34.50 (28.80–45.33) | 31.85 (27.55–39.05) |
| Total carbohydrates (g) 2 | 272.8 (207.5–361.4) | 293.5 (228.9–328.2) | 238.8 (215.8–329.2) |
| Glucose (g) 2 | 12.35 (8.17–19.61) | 9.60 (5.68–16.07) | 10.72 (4.84–13.01) |
| Fructose (g) 2 | 15.70 (9.99–23.12) | 11.24 (7.22–20.74) | 11.80 (7.64–15.78) |
| Fiber (g) 2 | 29.40 (22.18–36.92) | 26.69 (22.33–39.31) | 23.15 (18.81–30.45) |
| Total lipids (g) 2 | 78.90 (61.91–99.51) | 83.49 (74.09–107.13) | 66.82 (56.40–91.03) * |
| Animal lipids (g) 2 | 38.98 (29.31–48.00) | 39.34 (31.06–50.27) | 32.01 (24.55–49.25) |
| Vegetable lipids (g) 2 | 36.38 (28.00–47.83) | 43.72 (36.46–54.20) ° | 34.54 (27.95–44.33) * |
| SFA (g) 2 | 24.62 (19.93–32.35) | 25.46 (20.09–33.45) | 21.45 (16.15–30.68) |
| Arachidic acid (g) 2 | 0.17 (0.10–0.20) | 0.20 (0.17–0.22) °° | 0.20 (0.10–0.20) |
| MUFA (g) 2 | 32.17 (24.84–39.26) | 36.69 (31.01–41.71) | 29.80 (24.75–36.58) * |
| Oleic acid (g) 2 | 30.64 (23.69–37.01) | 35.00 (29.66–40.09) | 27.90 (22.80–34.70) * |
| PUFA (g) 2 | 11.42 (9.09–14.85) | 12.04 (8.87–17.25) | 10.25 (7.50–15.25) |
| EPA (g) 2 | 0.20 (0.10–0.26) | 0.20 (0.10–0.30) | 0.16 (0.06–0.30) |
| DHA (g) 2 | 0.30 (0.20–0.43) | 0.30 (0.20–0.50) | 0.30 (0.10–0.50) |
| Cholesterol (mg) 2 | 267.7 (191.3–319.2) | 280.4 (223.4–358.2) | 233.2 (199.7–298.9) |
| Iron (mg) 2 | 14.50 (11.55–18.97) | 16.17 (12.15–19.89) | 13.79 (10.98–19.85) |
| Calcium (mg) 2 | 832.1 (704.3–1105.6) | 946.2 (740.4–1261.8) | 855.2 (643.5–1079.8) |
| Sodium (mg) 2 | 1980.9 (1598.1–2434.9) | 2205.7 (1717.8–2634.2) | 1905.4 (1515.5–2629.0) |
| Potassium (mg) 2 | 3372.1 (2878.8–4268.9) | 3731.9 (2782.4–4892.9) | 3231.5 (2672.1–3842.3) |
| Phosphorus (mg) 2 | 1521.6 (1264.0–1781.7) | 1648.3 (1273.1–1966.9) | 1372.9 (1193.2–1754.5) |
| Zinc (mg) 2 | 17.14 (12.97–22.40) | 20.05 (15.45–24.80) | 16.37 (13.71–21.52) |
| Magnesium (mg) 2 | 163.3 (124.5–220.6) | 167.0 (124.6–190.2) | 141.8 (107.3–173.7) |
| Copper (mg) 2 | 1.00 (0.74–1.35) | 1.06 (0.80–1.30) | 0.85 (0.65–1.13) |
| Selenium (µg) 2 | 47.8 (37.29–61.96) | 49.14 (38.41–78.48) | 49.90 (33.61–69.13) |
| Chlorine (mg) 2 | 1245.2 (926.2–1680.3) | 1241.5 (917.1–1852.2) | 1135.9 (796.3–1712.1) |
| Iodine (µg) 2 | 92.20 (64.61–142.9) | 95.30 (76.69–132.3) | 97.42 (68.91–128.9) |
| Manganese (mg) 2 | 0.99 (0.54–1.61) | 0.80 (0.42–1.78) | 0.69 (0.38–1.05) |
| Sulfur (mg) 1 | 430.5 (342.4–544.3) | 484.6 (334.0–577.5) | 462.1 (315.2–598.1) |
| Vitamin B1 (Thiamine) (mg) 2 | 1.37 (1.15–1.86) | 1.46 (1.19–1.90) | 1.40 (1.15–1.95) |
| Vitamin B2 (Riboflavin) (mg) 2 | 1.81 (1.51–2.22) | 1.95 (1.50–2.49) | 1.80 (1.60–2.42) |
| Vitamin C (mg) 2 | 203.9 (146.0–272.0) | 213.8 (122.9–286.4) | 178.3 (119.3–265.4) |
| Niacin (mg) 2 | 20.29 (16.60–25.08) | 21.85 (16.82–27.37) | 20.11 (16.80–28.98) |
| Vitamin B6 (mg) 2 | 5.25 (3.90–6.67) | 5.60 (3.87–7.33) | 6.19 (5.07–7.83) |
| Folate (total) (µg) 2 | 338.0 (281.7–448.8) | 371.1 (269.7–526.2) | 335.5 (279.6–429.4) |
| Pantothenic acid (mg) 2 | 2.70 (1.94–3.40) | 3.01 (2.21–3.63) | 3.04 (2.00–3.85) |
| Biotin (µg) 2 | 18.80 (14.59–29.39) | 22.80 (14.38–28.25) | 19.50 (13.02–25.53) |
| Vitamin B12 (µg) 2 | 8.09 (6.13–11.34) | 8.74 (6.63–13.21) | 8.55 (5.00–15.08) |
| Vitamin A (RE) (µg) 2 | 1534.3 (1073.0–19.09.5) | 1861.8 (1298.2–2145.7) | 1228.9 (935.9–2092.0) |
| Vitamin E (α-TE) (mg) 2 | 12.75 (10.55–16.15) | 15.03 (11.78–17.66) | 11.48 (9.69–14.64) ** |
| Vitamin D (µg) 2 | 2.86 (1.99–3.75) | 2.62 (2.05–5.08) | 2.50 (1.67–4.08) |
| Vitamin K (µg) 2 | 17.80 (8.81–35.71) | 9.75 (4.48–21.44) ° | 6.55 (3.06–14.03) °°° |
| Water (g) 2 | 1129.7 (944.1–1441.8) | 1117.9 (905.8–1549.3) | 1018.4 (868.9–1251.0) |
| Pattern 1 | Pattern 2 | Pattern 3 | Pattern 4 | |
|---|---|---|---|---|
| Explained variance | 19.1% | 12.3% | 9.5% | 8.1% |
| Adhering women | 54.7% of NW 1 | 50.0% of NW | 32.8% of NW | 48.4% of NW |
| 36.4% of OW 1 | 43.2% of OW | 43.2% of OW | 50.0% of OW | |
| 26.9% of OB 1 | 30.8% of OB | 53.8% of OB | 30.8% of OB | |
| Adherence value 2: | ||||
| NW | 0.495 (0.243–1.054) | 0.700 (0.364–1.346) | 0.442 (0.232–0.794) | 0.340 (0.118–0.990)) |
| OW | 0.726 (0.431–1.477) | 0.657 (0.246–1.214) | 0.746 (0.132–1.920) | 0.805 (0.366–1.603) ° |
| OB | 0.701 (0.326–1.224) | 0.352 (0.067–1.318) | 0.635 (0.280–1.540) | 0.530 (0.382–0.784) |
| Food categories: | ||||
| Dairy | −0.075 | 0.481 | 0.186 | 0.192 |
| Cereals | 0.384 | 0.529 | 0.131 | 0.139 |
| Vegetables | 0.596 | −0.045 | 0.277 | 0.371 |
| Legumes | 0.713 | 0.213 | 0.123 | −0.124 |
| Potatoes | 0.071 | 0.217 | 0.608 | −0.038 |
| Meat | −0.304 | 0.253 | 0.694 | 0.093 |
| Fish | 0.233 | −0.220 | 0.772 | 0.089 |
| Eggs | −0.126 | −0.056 | 0.031 | 0.715 |
| Fruits | 0.395 | 0.214 | 0.064 | 0.545 |
| Nuts | 0.611 | 0.000 | −0.076 | 0.161 |
| Vegetable fats | −0.005 | 0.186 | 0.287 | 0.503 |
| Animal fats | 0.017 | 0.674 | −0.060 | −0.292 |
| Sauces | −0.452 | 0.319 | 0.226 | 0.120 |
| Sugars and snacks | 0.044 | 0.735 | 0.054 | 0.273 |
| Non-alcoholic beverages | 0.215 | 0.089 | −0.115 | 0.494 |
| 3rd Trimester Maternal Blood Markers | β (95% CI) p-Value | Neonatal and Placental Data | β (95% CI) p-Value | |
|---|---|---|---|---|
| Pattern 1 | Gestational age at T1 [weeks] | β = 0.243 (0.019; 0.466) p = 0.033 | ||
| Neon. head circumference [cm] | β = −0.414 (−0.826; −0.001) p = 0.050 | |||
| Pattern 2 | Ferritin [ng/mL] | β = −2.093 (−4050; −0.135) p = 0.036 | Neon. head circumference [cm] | β = 0.403 (0.026; 0.779) p = 0.036 |
| Pattern 3 | TAC [mM] | β = 0.015 (0.000; 0.030) p = 0.046 | ||
| Pattern 4 | Hemoglobin [g/dL] | β = −0.223 (−0.402; −0.043) p = 0.015 | Placental weight [g] | β = 31.479 (7.723; 55.235) p = 0.009 |
| Neonatal/Placental weight ratio | β = −0.384 (−0.717; −0.052) p = 0.023 | |||
| In Pattern 3 Energy [kcal] | Placental area [cm2] | β = −0.031 (−0.058; −0.005) p = 0.020 | ||
| Neonatal/Placental weight ratio | β = 0.001 (0.000; 0.001) p = 0.044 | |||
| In Pattern 4 Energy [kcal] | Placental weight [g] | β = −0.041 (−0.081; −0.000) p = 0.049 | ||
| Placental area [cm2] | β = −0.027 (−0.051; −0.003) p = 0.026 | |||
| Neonatal/Placental weight ratio | β = 0.001 (0.000; 0.001) p = 0.040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandò, C.; Novielli, C.; Nuzzo, A.M.; Parisi, F.; Moretti, L.; Lisso, F.; Revelli, A.; Savasi, V.M.; Laoreti, A.; Anelli, G.M.; et al. Maternal BMI and Diet Quality Modulate Pregnancy Oxidative and Inflammatory Homeostasis. Nutrients 2025, 17, 2590. https://doi.org/10.3390/nu17162590
Mandò C, Novielli C, Nuzzo AM, Parisi F, Moretti L, Lisso F, Revelli A, Savasi VM, Laoreti A, Anelli GM, et al. Maternal BMI and Diet Quality Modulate Pregnancy Oxidative and Inflammatory Homeostasis. Nutrients. 2025; 17(16):2590. https://doi.org/10.3390/nu17162590
Chicago/Turabian StyleMandò, Chiara, Chiara Novielli, Anna Maria Nuzzo, Francesca Parisi, Laura Moretti, Fabrizia Lisso, Alberto Revelli, Valeria M. Savasi, Arianna Laoreti, Gaia M. Anelli, and et al. 2025. "Maternal BMI and Diet Quality Modulate Pregnancy Oxidative and Inflammatory Homeostasis" Nutrients 17, no. 16: 2590. https://doi.org/10.3390/nu17162590
APA StyleMandò, C., Novielli, C., Nuzzo, A. M., Parisi, F., Moretti, L., Lisso, F., Revelli, A., Savasi, V. M., Laoreti, A., Anelli, G. M., Rolfo, A., & Cetin, I. (2025). Maternal BMI and Diet Quality Modulate Pregnancy Oxidative and Inflammatory Homeostasis. Nutrients, 17(16), 2590. https://doi.org/10.3390/nu17162590












