Structural Characterization and Ameliorative Effects of Mesona chinensis Benth Polysaccharide Against Deoxynivalenol-Induced Oxidative Stress in Intestinal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction Procedure of MCP
2.3. Separation and Purification of MCP
2.4. Physicochemical Determination of Polysaccharide
2.4.1. Physicochemical Determination
2.4.2. Protein Content
2.4.3. Ultraviolet Absorption Spectrum
2.5. Structural Identification of MCP-3
2.5.1. Congo Red Test
2.5.2. Infrared Spectroscopic Analysis
2.5.3. Molecular Weight Determination
2.5.4. Determination of Monosaccharide Composition
2.5.5. Methylation Analysis
2.5.6. Nuclear Magnetic Resonance Spectroscopy
2.5.7. Scanning Electron Microscope
2.6. In Vitro Antioxidant Ability of MCP-3
2.6.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free-Radical Scavenging Ability
2.6.2. Superoxide Free-Radical Scavenging Ability
2.6.3. Hydroxyl Free-Radical Scavenging Ability
2.6.4. Nitroso Ion Scavenging Ability
2.6.5. 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) Free-Radical Scavenging Ability
2.6.6. 2-Phenyl-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl (PTIO) Free-Radical Scavenging Ability
2.6.7. Ferric Reducing Ability
2.7. Ameliorative Effects of MCP-3 on DON-Induced Oxidative Stress and Inflammation in Caco-2 and NCM460 Cells
2.7.1. Cell Culture
2.7.2. Cell Proliferation Analysis
2.7.3. Oxidative Stress of Intestinal Epithelial Cells Induced by DON
2.7.4. Determination of GSH-Px, CAT, SOD, and MDA
2.7.5. ROS Measurement
2.7.6. Quantitative Analysis of Cytokines
2.8. Statistical Analysis
3. Results and Discussion
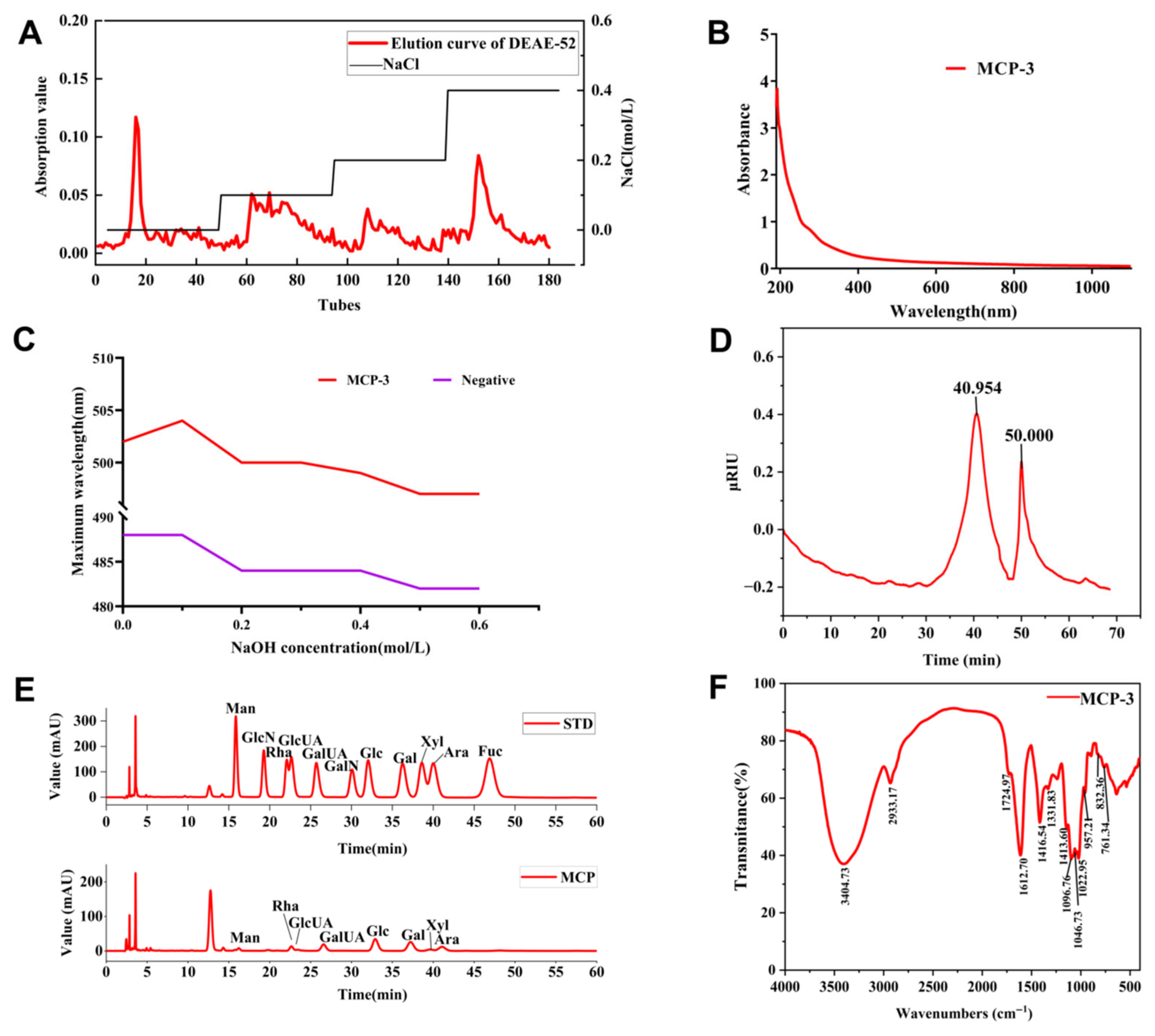
3.1. Extraction, Purification, and Composition of MCP
3.2. Structural Analysis and Characterization
3.2.1. Stereochemical Structure
3.2.2. Molecular Weight
3.2.3. Monosaccharide Composition
3.2.4. FT-IR Spectra
3.2.5. Glycosidic Bond Composition
3.2.6. NMR Characterization
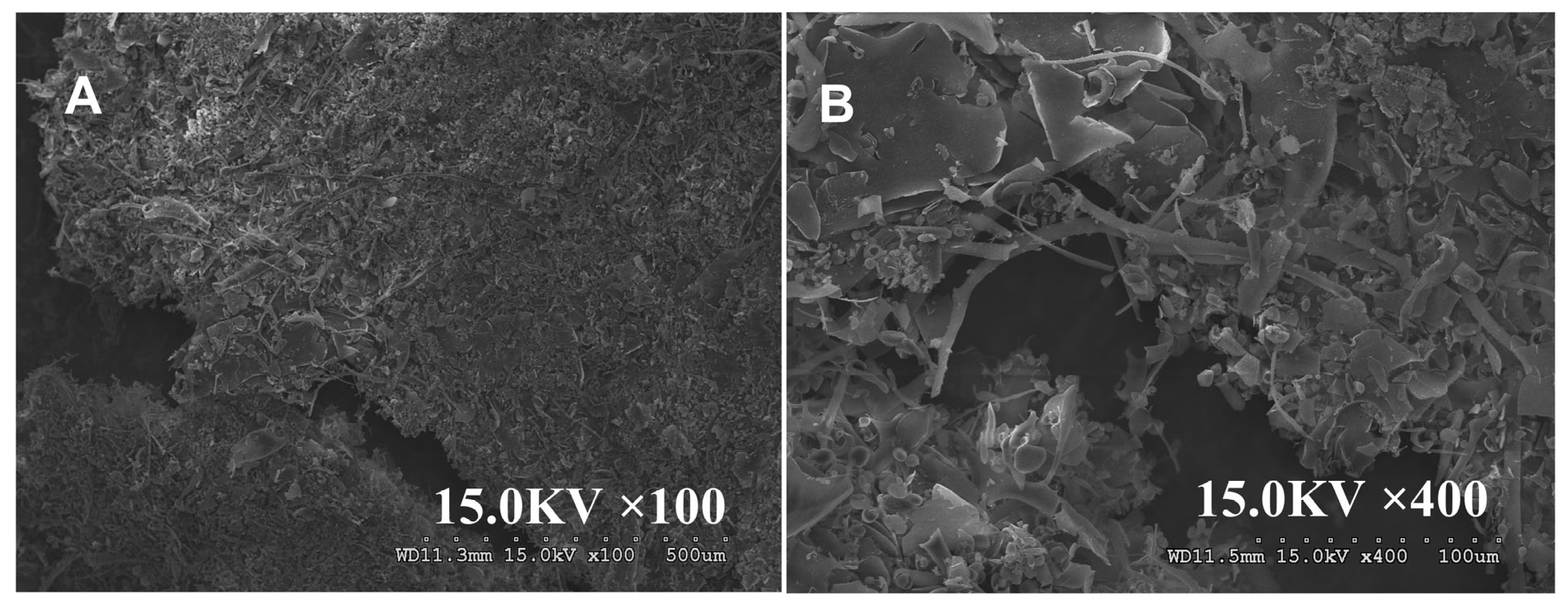
3.2.7. Morphological Characteristics
3.3. In Vitro Antioxidant Ability
3.4. Ameliorative Effects of MCP-3 on DON-Induced Oxidative Stress
3.4.1. Effects of MCP-3 and DON on Cell Proliferation in Caco-2 and NCM460 Cells
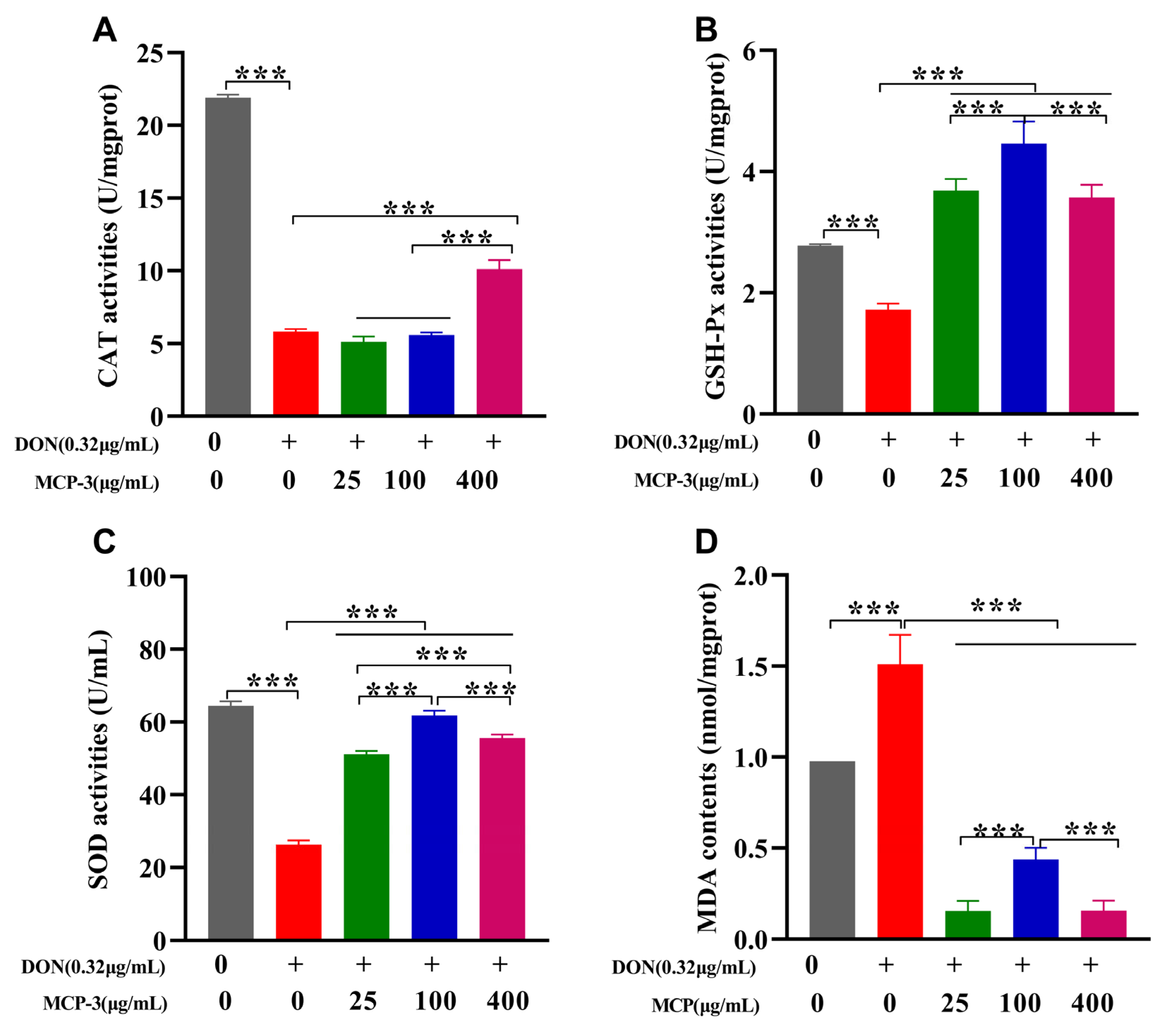
3.4.2. Effects of MCP-3 on DON-Induced Oxidative Stress
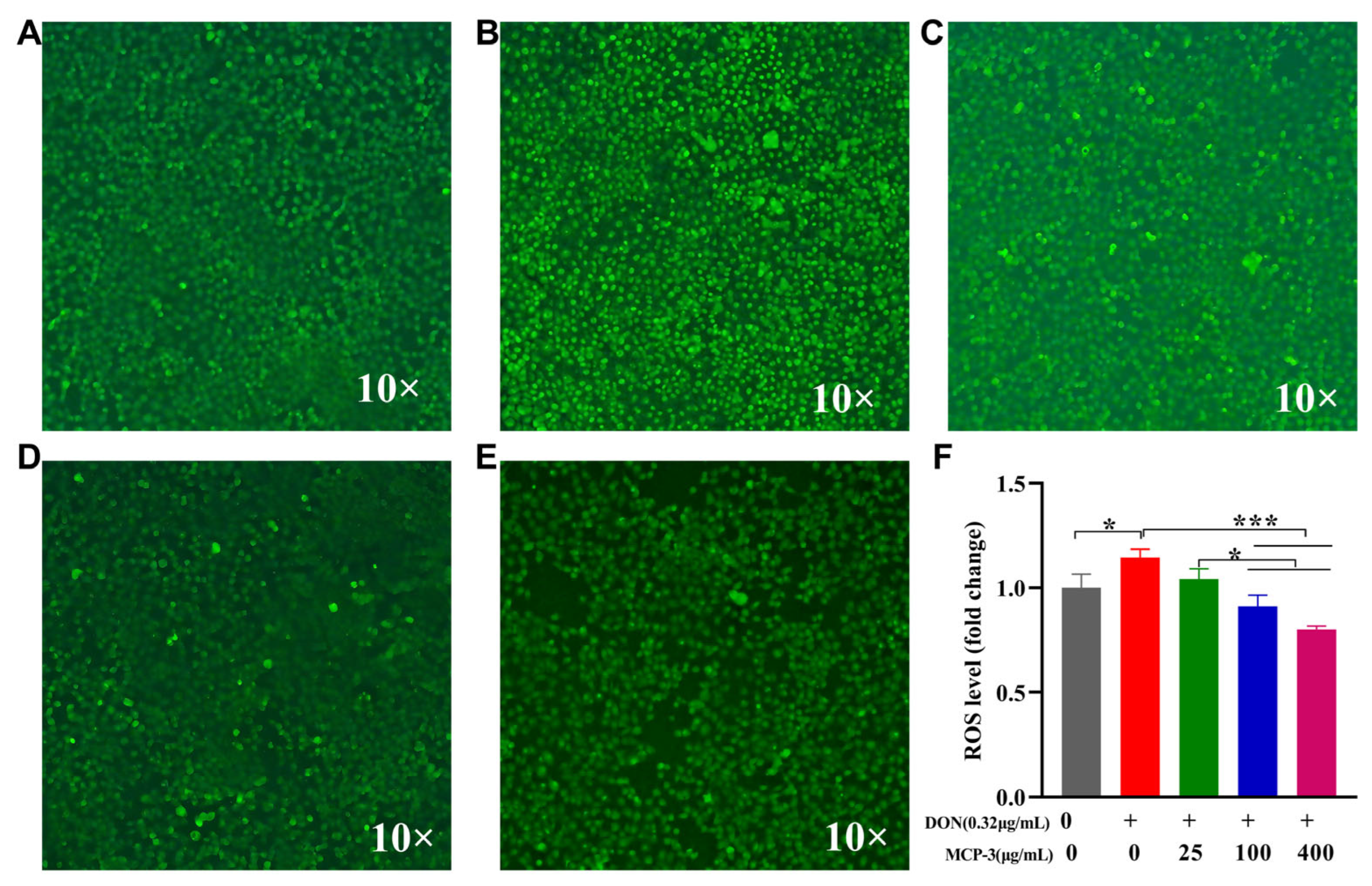
3.4.3. Effects of MCP-3 on ROS Generation Induced by DON
3.5. Effects of MCP-3 on Inflammatory Cytokines Induced by DON
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) |
| ATCC | American Type Culture Collection |
| BCA | Bicinchoninic acid |
| BHT | Butylated hydroxytoluene |
| CAT | Catalase |
| DCFH-DA | Dichlorodihydrofluorescein diacetate |
| DEAE-52 | Diethylaminomethyl cellulose-52 |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DEPT-135 | Distortionless enhancement by polarization transfer 135 |
| DON | Deoxynivalenol |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EMEM | Eagle’s Minimum Essential Medium |
| FBS | Fetal bovine serum |
| FT-IR | Fourier transform infrared spectroscopy |
| GSH-Px | Glutathione peroxidase |
| 1H–1H COSY | Correlated spectroscopy () |
| HMBC | Heteronuclear multiple bond correlation spectroscopy |
| HSQC | Heteronuclear single quantum coherence spectroscopy |
| MCP-3 | Mesona chinensis Benth polysaccharide-3 |
| MDA | Malondialdehyde |
| MTT | 5-Diphenyltetrazolium |
| NMR | Nuclear magnetic resonance |
| NOESY | Nuclear overhauser effect spectroscopy |
| PTIO | 2-Phenyl-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscope |
| SOD | Superoxide dismutase |
| TMSP | 3-(Trimethylsilyl) propionic acid-d4 sodium salt |
References
- Huo, J.; Wu, J.; Zhao, M.; Sun, W.; Sun, J.; Li, H.; Huang, M. Immunomodulatory activity of a novel polysaccharide extracted from Huangshui on THP-1 cells through NO production and increased IL-6 and TNF-alpha expression. Food Chem. 2020, 330, 127257. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Jia, X.; Huang, F.; Zhang, R.; Dong, L.; Liu, L.; Zhang, M. Structural elucidation, anti-inflammatory activity and intestinal barrier protection of longan pulp polysaccharide LPIIa. Carbohydr. Polym. 2020, 246, 116532. [Google Scholar] [CrossRef]
- Elmassry, M.M.; Zayed, A.; Farag, M.A. Gut homeostasis and microbiota under attack: Impact of the different types of food contaminants on gut health. Crit. Rev. Food Sci. Nutr. 2022, 62, 738–763. [Google Scholar] [CrossRef]
- Halmos, E.P.; Mack, A.; Gibson, P.R. Review article: Emulsifiers in the food supply and implications for gastrointestinal disease. Aliment. Pharmacol. Ther. 2019, 49, 41–50. [Google Scholar] [CrossRef]
- He, Y.; Yin, X.; Dong, J.; Yang, Q.; Wu, Y.; Gong, Z. Transcriptome analysis of Caco-2 cells upon the exposure of mycotoxin deoxynivalenol and its acetylated derivatives. Toxins 2021, 13, 167. [Google Scholar] [CrossRef]
- Rajput, S.A.; Liang, S.J.; Wang, X.Q.; Yan, H.C. Lycopene protects intestinal epithelium from deoxynivalenol-induced oxidative damage via regulating Keap1/Nrf2 signaling. Antioxidants 2021, 10, 1493. [Google Scholar] [CrossRef]
- Yin, F.; Huang, X.; Lin, X.; Chan, T.F.; Lai, K.P.; Li, R. Analyzing the synergistic adverse effects of BPA and its substitute, BHPF, on ulcerative colitis through comparative metabolomics. Chemosphere 2022, 287 Pt 2, 132160. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Che, S.; Ruan, Z.; Song, L.; Tang, R.; Zhang, L. Regulatory effects of flavonoids luteolin on BDE-209-induced intestinal epithelial barrier damage in Caco-2 cell monolayer model. Food Chem. Toxicol. 2021, 150, 112098. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, A.; Liu, X.; Ma, Y.; Zhao, F.; Wang, M.; Loor, J.J.; Wang, H. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J. Hazard. Mater. 2021, 407, 124489. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Xie, W.W.; Zan, G.X.; Gao, C.Q.; Yan, H.C.; Zhou, J.Y.; Wang, X.Q. Lauric acid alleviates deoxynivalenol-induced intestinal stem cell damage by potentiating the Akt/mTORC1/S6K1 signaling axis. Chem. Biol. Interact. 2021, 348, 109640. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Xue, D.; Zhang, C.; Rajput, S.A.; Qi, D. Mechanism of deoxynivalenol mediated gastrointestinal toxicity: Insights from mitochondrial dysfunction. Food Chem. Toxicol. 2021, 153, 112214. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Deoxynivalenol, gut microbiota and immunotoxicity: A potential approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, N.; Zhang, C.; Zhou, S.; Zhao, Y.; Wu, Y.; Gong, Y.Y. Comprehensive dietary and internal exposure assessment of deoxynivalenol contamination in a high-risk area in China using duplicate diet studies and urinary biomarkers. Food Control 2021, 124, 107830. [Google Scholar] [CrossRef]
- Mishra, S.; Srivastava, S.; Dewangan, J.; Divakar, A.; Rath, S.K. Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: A survey. Crit. Rev. Food Sci. Nutr. 2020, 60, 1346–1374. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.A.; Shaukat, A.; Rajput, I.R.; Kamboh, A.A.; Iqbal, Z.; Saeed, M.; Akhtar, R.W.; Shah, S.A.H.; Raza, M.A.; Askary, A.E.; et al. Ginsenoside Rb1 prevents deoxynivalenol-induced immune injury via alleviating oxidative stress and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112333. [Google Scholar] [CrossRef]
- Vignal, C.; Djouina, M.; Pichavant, M.; Caboche, S.; Waxin, C.; Beury, D.; Hot, D.; Gower-Rousseau, C.; Body-Malapel, M. Chronic ingestion of deoxynivalenol at human dietary levels impairs intestinal homeostasis and gut microbiota in mice. Arch. Toxicol. 2018, 92, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhang, S.W.; Lin, H.L.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. Hydrolyzed wheat gluten alleviates deoxynivalenol-induced intestinal injury by promoting intestinal stem cell proliferation and differentiation via upregulation of Wnt/beta-catenin signaling in mice. Food Chem. Toxicol. 2019, 131, 110579. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Li, M.; Wei, J.; Wang, Y.; Hao, W.; Sun, W.; Wu, J.; Huang, M. RNA-seq based elucidation of mechanism underlying the protective effect of Huangshui polysaccharide on intestinal barrier injury in Caco-2 cells. Food Res. Int. 2022, 162 Pt B, 112175. [Google Scholar] [CrossRef]
- Tang, Y.; Wei, Z.; Deng, Z.; Dai, T.; Liang, P.; Sun, J.; He, X. Research progress on extraction, structure, functions and mechanism of action of Mesona chinensis Benth polysaccharides. Sci. Technol. Food Ind. 2023, 45, 379–388. (In Chinese) [Google Scholar] [CrossRef]
- Chen, X.; Xiao, W.; Shen, M.; Yu, Q.; Chen, Y.; Yang, J.; Xie, J. Changes in polysaccharides structure and bioactivity during Mesona chinensis Benth storage. Curr. Res. Food Sci. 2022, 5, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. UPLC-Q-TOF/MS-based metabolomics reveals modulatory effects of Mesona chinensis Benth polysaccharide in liver injury mice induced by cyclophosphamide. Food Sci. Hum. Well. 2023, 12, 584–595. [Google Scholar] [CrossRef]
- Shen, Y.; Liang, J.; Guo, Y.L.; Li, Y.; Kuang, H.X.; Xia, Y.G. Ultrafiltration isolation, structures and anti-tumor potentials of two arabinose- and galactose-rich pectins from leaves of Aralia elata. Carbohydr. Polym. 2021, 255, 117326. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shen, M.; Zhang, X.; Jiang, L.; Song, Q.; Xie, J. Effect of high-pressure microfluidization treatment on the physicochemical properties and antioxidant activities of polysaccharide from Mesona chinensis Benth. Carbohydr. Polym. 2018, 200, 191–199. [Google Scholar] [CrossRef]
- Huang, L.; Huang, M.; Shen, M.; Wen, P.; Wu, T.; Hong, Y.; Yu, Q.; Chen, Y.; Xie, J. Sulfated modification enhanced the antioxidant activity of Mesona chinensis Benth polysaccharide and its protective effect on cellular oxidative stress. Int. J. Biol. Macromol. 2019, 136, 1000–1006. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Wu, T.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Mesona chinensis Benth polysaccharides protect against oxidative stress and immunosuppression in cyclophosphamide-treated mice via MAPKs signal transduction pathways. Int. J. Biol. Macromol. 2020, 152, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Ou, N.; Chen, Z.; Guo, X. Study on optimization of extraction process of total flavonoids from Mesona chinensis Benth by orthogonal test. J. China Prescr. Drug 2017, 15, 32–34. (In Chinese) [Google Scholar]
- Hung, C.Y.; Yen, G.C. Antioxidant activity of phenolic compounds isolated from Mesona procumbens Hemsl. J. Agric. Food Chem. 2002, 50, 2993–2997. [Google Scholar] [CrossRef]
- Tang, W.; Shen, M.; Xie, J.; Liu, D.; Du, M.; Lin, L.; Gao, H.; Hamaker, B.R.; Xie, M. Physicochemical characterization, antioxidant activity of polysaccharides from Mesona chinensis Benth and their protective effect on injured NCTC-1469 cells induced by H2O2. Carbohydr Polym. 2017, 175, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Chen, X.; Huang, L.; Yu, Q.; Chen, Y.; Xie, J. Sulfated Mesona chinensis Benth polysaccharide enhance the immunomodulatory activities of cyclophosphamide-treated mice. J. Funct. Foods 2021, 76, 104321. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhou, Y.; Zheng, Z.; Tang, W.; Song, M.; Wang, J.; Wang, K. Lentinan inhibited colon cancer growth by inducing endoplasmic reticulum stress-mediated autophagic cell death and apoptosis. Carbohydr. Polym. 2021, 267, 118154. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Zhang, J.; Zhang, S.; Zhao, W.; Liu, T.; Wang, D. Isolation, purification, structural characteristic and antioxidative property of polysaccharides from A. cepa L. var. agrogatum Don. Food Sci. Hum. Well. 2020, 9, 71–79. [Google Scholar] [CrossRef]
- Yang, R.F.; Geng, L.L.; Lu, H.Q.; Fan, X.D. Ultrasound-synergized electrostatic field extraction of total flavonoids from Hemerocallis citrina baroni. Ultrason. Sonochem. 2017, 34, 571–579. [Google Scholar] [CrossRef]
- Tang, J.; Nie, J.; Li, D.; Zhu, W.; Zhang, S.; Ma, F.; Sun, Q.; Song, J.; Zheng, Y.; Chen, P. Characterization and antioxidant activities of degraded polysaccharides from Poria cocos sclerotium. Carbohydr. Polym. 2014, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.33; Chinese National Food Safety Standard Determination of Nitrite and Nitrate in Food. China Quality and Standard Publishing & Media Co. Ltd.: Beijing, China, 2016.
- Li, X. 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO*) radical scavenging: A new and simple antioxidant assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, W.; Zhang, J.; Guo, Z.; Jiang, A.; Li, Q. Novel coumarin-functionalized inulin derivatives: Chemical modification and antioxidant activity assessment. Carbohydr. Res. 2022, 518, 108597. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Wang, L.; Fu, X.; Li, C. Physicochemical characterization of a polysaccharide from Rosa roxburghii Tratt fruit and its antitumor activity by activating ROS mediated pathways. Curr. Res. Food Sci. 2022, 5, 1581–1589. [Google Scholar] [CrossRef]
- Lin, L.; Xie, J.; Liu, S.; Shen, M.; Tang, W.; Xie, M. Polysaccharide from Mesona chinensis: Extraction optimization, physicochemical characterizations and antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 665–673. [Google Scholar] [CrossRef]
- Niu, G.; You, G.; Zhou, X.; Fan, H.; Liu, X. Physicochemical properties and in vitro hypoglycemic activities of hsian-tsao polysaccharide fractions by gradient ethanol precipitation method. Int. J. Biol. Macromol. 2023, 231, 123274. [Google Scholar] [CrossRef]
- Feng, T.; Gu, Z.B.; Yu, J.Z.; Ning, Z.H. Isolation and characterization of an acidic polysaccharide from Mesona blumes gum. Carbohydr. Polym. 2008, 71, 159–169. [Google Scholar] [CrossRef]
- Lee, Q.; Xue, Z.; Luo, Y.; Lin, Y.; Lai, M.; Xu, H.; Liu, B.; Zheng, M.; Lv, F.; Zeng, F. Low molecular weight polysaccharide of Tremella fuciformis exhibits stronger antioxidant and immunomodulatory activities than high molecular weight polysaccharide. Int. J. Biol. Macromol. 2024, 281, 136097. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zeng, R.; Qi, J.; Ho, C.T.; Li, B.; Chen, Z.; Chen, S.; Xiao, C.; Cai, M.; Xie, Y.; et al. Immunoregulatory activity of a low-molecular-weight heteropolysaccharide from Ganoderma leucocontextum fruiting bodies in vitro and in vivo. Food Chem. X 2022, 14, 100321. [Google Scholar] [CrossRef]
- Yang, K.; Jin, Y.; He, P.; Tian, B.; Guan, R.; Yu, G.; Sun, P. Separation, characterization and hypoglycemic activity in vitro evaluation of a low molecular weight heteropolysaccharide from the fruiting body of Phellinus pini. Food Funct. 2021, 12, 3493–3503. [Google Scholar] [CrossRef]
- Chen, X.; Shen, M.; Yang, J.; Yu, Q.; Chen, Y.; Wang, X.; Lu, H.; Tao, X.; Li, H.; Xie, J. RNA-seq based elucidation of mechanism underlying Mesona chinensis Benth polysaccharide protected H2O2-induced oxidative damage in L02 cells. Food Res. Int. 2022, 157, 111383. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Petersen, B.O.; Meier, S.; Duus, J.O.; Clausen, M.H. Structural characterization of homogalacturonan by NMR spectroscopy-assignment of reference compounds. Carbohydr. Res. 2008, 343, 2830–2833. [Google Scholar] [CrossRef]
- Patova, O.A.; Smirnov, V.V.; Golovchenko, V.V.; Vityazev, F.V.; Shashkov, A.S.; Popov, S.V. Structural, rheological and antioxidant properties of pectins from Equisetum arvense L. and Equisetum sylvaticum L. Carbohydr. Polym. 2019, 209, 239–249. [Google Scholar] [CrossRef]
- Perrone, P.; Hewage, C.M.; Thomson, A.R.; Bailey, K.; Sadler, I.H.; Fry, S.C. Patterns of methyl and O-acetyl esterification in spinach pectins: New complexity. Phytochemistry 2002, 60, 67–77. [Google Scholar] [CrossRef]
- Yang, J.; Wen, L.; Zhao, Y.; Jiang, Y.; Tian, M.; Liu, H.; Liu, J.; Yang, B. Structure identification of an arabinogalacturonan in Citrus reticulata Blanco ‘Chachiensis’ peel. Food Hydrocoll. 2018, 84, 481–488. [Google Scholar] [CrossRef]
- Kostalova, Z.; Hromadkova, Z.; Ebringerova, A. Structural diversity of pectins isolated from the Styrian oil-pumpkin (Cucurbita pepo var. styriaca) fruit. Carbohydr. Polym. 2013, 93, 163–171. [Google Scholar] [CrossRef]
- Lemaire, A.; Garzon, C.D.; Perrin, A.; Habrylo, O.; Treze, P.; Bassard, S.; Lefebvre, V.; Van Wuytswinkel, O.; Guillaume, A.; Pau-Roblot, C.; et al. Three novel rhamnogalacturonan I-pectins degrading enzymes from Aspergillus aculeatinus: Biochemical characterization and application potential. Carbohydr. Polym. 2020, 248, 116752. [Google Scholar] [CrossRef]
- Mikshina, P.V.; Gurjanov, O.P.; Mukhitova, F.K.; Petrova, A.A.; Shashkov, A.S.; Gorshkova, T.A. Structural details of pectic galactan from the secondary cell walls of flax (Linum usitatissimum L.) phloem fibres. Carbohydr. Polym. 2012, 87, 853–861. [Google Scholar] [CrossRef]
- Renard, C.; Jarvis, M. Acetylation and methylation of homogalacturonans 1: Optimisation of the reaction and characterisation of the products. Carbohydr. Polym. 1999, 39, 201–207. [Google Scholar] [CrossRef]
- Roman, L.; Guo, M.; Terekhov, A.; Grossutti, M.; Vidal, N.P.; Reuhs, B.L.; Martinez, M.M. Extraction and isolation of pectin rich in homogalacturonan domains from two cultivars of hawthorn berry (Crataegus pinnatifida). Food Hydrocoll. 2021, 113, 106476. [Google Scholar] [CrossRef]
- Shakhmatov, E.G.; Toukach, P.V.; Makarova, E.N. Structural studies of the pectic polysaccharide from fruits of Punica granatum. Carbohydr. Polym. 2020, 235, 115978. [Google Scholar] [CrossRef]
- Tamaki, Y.; Konishi, T.; Fukuta, M.; Tako, M. Isolation and structural characterisation of pectin from endocarp of Citrus depressa. Food Chem. 2008, 107, 352–361. [Google Scholar] [CrossRef]
- Xia, Y.G.; Liang, J.; Yang, B.Y.; Wang, Q.H.; Kuang, H.X. Structural studies of an arabinan from the stems of Ephedra sinica by methylation analysis and 1D and 2D NMR spectroscopy. Carbohydr. Polym. 2015, 121, 449–556. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, L.; Chen, S.; Deng, Y.; Zhao, J.; Wang, Y.; Li, S. Non-starch polysaccharide from Chinese yam activated RAW 264.7 macrophages through the Toll-like receptor 4 (TLR4)-NF-κB signaling pathway. J. Funct. Foods 2017, 37, 491–500. [Google Scholar] [CrossRef]
- Hosain, N.; Ghosh, R.; Bryant, D.; Arivett, B.; Farone, A.; Kline, P. Isolation, structure elucidation, and immunostimulatory activity of polysaccharide fractions from Boswellia carterii frankincense resin. Int. J. Biol. Macromol. 2019, 133, 76–85. [Google Scholar] [CrossRef]
- Pan, W.J.; Shi, L.L.; Ren, Y.R.; Yao, C.Y.; Lu, Y.M.; Chen, Y. Polysaccharide ORP-1 isolated from Oudemansiella raphanipes ameliorates age-associated intestinal epithelial barrier dysfunction in Caco-2 cells monolayer. Food Res. Int. 2022, 162 Pt A, 112038. [Google Scholar] [CrossRef] [PubMed]






| Retention Time (min) | Methylated Sugar | Mass Fragments (m/z) | Linkage Pattern | Molar Ratio (%) |
|---|---|---|---|---|
| 11.692 | 1,4-di-O-acetyl-2,3,5-tri-O-methyl arabinitol | 32, 43, 60, 71, 87, 102, 113, 118, 129, 161 | Araf-(1→ | 3.22 |
| 12.734 | 1,5-di-O-acetyl-2,3,4-tri-O-methyl xylitol | 32, 43, 59, 72, 89, 102, 118, 131, 162 | Xylp-(1→ | 4.98 |
| 12.734 | 1,5-di-O-acetyl-6-deoxy-2,3,4-tri-O-methyl rhamnitol | 32, 40, 43, 60, 72, 89, 102, 118, 131, 162, 175 | Rhap-(1→ | 2.80 |
| 13.607 | 1,5-di-O-acetyl-6-deoxy-2,3,4-tri-O-methyl fucitol | 32, 40, 43, 60, 74, 87, 101, 118, 129 | Fucp-(1→ | 1.67 |
| 15.464 | 1,3,5-tri-O-acetyl-2,4-di-O-methyl arabinitol | 32, 43, 60, 72, 89, 100, 115, 131, 190 | →3)-Arap-(1→ | 1.57 |
| 15.496 | 1,2,5-tri-O-acetyl-6-deoxy-3,4-di-O-methyl rhamnitol | 32, 40, 43, 60, 87, 102, 118, 129, 189 | →2)-Rhap-(1→ | 3.16 |
| 15.638 | 1,4,5-tri-O-acetyl-2,3-di-O-methyl xylitol | 32, 40, 43, 59, 72, 89, 101, 118, 128, 131, 202, 234 | →4)-Xylp-(1→ | 2.37 |
| 15.910 | 1,3,5-tri-O-acetyl-6-deoxy-2,4-di-O-methyl rhamnitol | 32, 40, 43, 60, 87, 100, 118, 129, 132, 189 | →3)-Rhap-(1→ | 2.11 |
| 16.777 | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl glucitol | 32, 40, 43, 60, 73, 87, 102, 118, 129, 145, 162, 207 | Glcp-UA-(1→ | 0.82 |
| 16.777 | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl glucitol | 32, 43, 73, 87, 102, 118, 131, 147, 162, 207 | Glcp-(1→ | 1.30 |
| 17.469 | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl galactitol | 32, 43, 60, 87, 99, 113, 118, 129, 173, 275 | Galp-UA-(1→ | 5.86 |
| 17.469 | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl galactitol | 32, 40, 43, 60, 88, 101, 117, 130, 143, 190, 203 | Galp-(1→ | 4.37 |
| 17.799 | 1,3,4,5-tetra-O-acetyl-6-deoxy-2-O-methyl fucitol | 32, 40, 43, 60, 88, 100, 130, 163, 190, 207 | →3,4)-Fucp-(1→ | 1.14 |
| 18.394 | 1,2,4,5-tetra-O-acetyl-6-deoxy-3-O-methyl rhamnitol | 32, 40, 43, 60, 87, 99, 118, 129, 157, 171, 231 | →2,4)-Rhap-(1→ | 2.03 |
| 19.688 | 1,3,5-tri-O-acetyl-2,4,6-tri-O-methyl glucitol | 32, 40, 43, 60, 71, 87, 101, 118, 129, 161, 207, 234 | →3)-Glcp-(1→ | 0.79 |
| 19.810 | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl galactitol | 32, 43, 47, 87, 102, 115, 118, 129, 144, 162, 175, 235 | →4)-Galp-UA-(1→ | 43.52 |
| 20.037 | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl glucitol | 32, 43, 59, 71, 87, 102, 113, 118, 129, 142, 162, 173, 233 | →4)-Glcp-(1→ | 8.54 |
| 20.309 | 1,3,5-tri-O-acetyl-2,4,6-tri-O-methyl galactitol | 32, 40, 43, 60, 71, 87, 101, 118, 129, 161, 174, 190, 234 | →3)-Galp-(1→ | 1.38 |
| 21.958 | 1,3,4,5-tetra-O-acetyl-2,6-di-O-methyl galactitol | 32, 43, 47, 59, 87, 118, 131, 145, 160, 186, 307 | →3,4)-Galp-UA-(1→ | 6.09 |
| 22.560 | 1,2,4,5-tetra-O-acetyl-3,6-di-O-methyl mannitol | 32, 40, 43, 60, 88, 99, 113, 130, 140, 190, 207, 233 | →2,4)-Manp-(1→ | 1.78 |
| 23.323 | 1,4,5,6-tetra-O-acetyl-2,3-di-O-methyl glucitol | 32, 40, 43, 60, 69, 85, 102, 118, 127, 159, 207, 261 | →4,6)-Glcp-(1→ | 0.52 |
| Glycosyl Residues | Chemical Shift (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6a | 6b | |||
| A | →4)-α-GalpA-(1→ | H | 4.99 | 3.69 | 3.93 | 4.35 | 4.72 | ns a | |
| C | 100.23 | 69.36 | 69.89 | 79.27 | 72.41 | 176.44 | |||
| A’ | →4)-α-GalpA-(1→ | H | 5.02 | 3.67 | 3.93 | 4.35 | 4.71 | ns | |
| C | 100.51 | 71.28 | 69.85 | 79.27 | 72.52 | 176.44 | |||
| B | →3,4)-α-GalpA-(1→ | H | 4.98 | 3.71 | 4.04 | 4.35 | 4.76 | ns | |
| C | 100.33 | 69.36 | 77.26 | 79.27 | 72.23 | 176.23 | |||
| C | →2)-α-L-Rhap-(1→ | H | 5.27 | 3.95 | 3.77 | 3.43 | 3.67 | 1.20 | |
| C | 101.11 | 79.2 | 72.41 | 72.52 | 69.36 | 17.85 | |||
| D | →2,4)-α-L-Rhap-(1→ | H | 5.27 | 3.95 | 3.78 | 3.59 | 3.84 | 1.20 | |
| C | 101.11 | 79.20 | 72.43 | 79.23 | 70.25 | 17.82 | |||
| E | →3)-α-L-Rhap-(1→ | H | 5.18 | 4.04 | 3.78 | 3.35 | 3.73 | 1.20 | |
| C | 102.12 | 71.23 | 82.27 | 70.61 | ND | 17.82 | |||
| F | →4)-β-D-Xylp-(1→ | H | 4.41 | 3.20 | 3.48 | 3.71 | 3.29 | 4.02 | |
| C | 102.93 | 74.11 | 74.92 | 77.33 | 63.83 | ns | |||
| G | β-D-Xylp-(1→ | H | 4.57 | 3.20 | 3.39 | 3.55 | 3.29 | 4.02 | |
| C | 104.68 | 74.11 | 76.99 | 70.35 | 63.83 | ns | |||
| H | →4)-α-D-Glcp-(1→ | H | 5.33 | 3.57 | 3.89 | 3.59 | 3.67 | 3.77 | 3.69 |
| C | 100.92 | 72.75 | 74.27 | 77.87 | 71.00 | 61.81 | |||
| I | β-D-Galp-(1→ | H | 4.46 | 3.55 | 3.63 | 3.97 | 3.63 | ns | |
| C | 106.20 | ns | 73.79 | 69.47 | 76.21 | ns | |||
| Rα | →4)-α-D-GalpA | H | 5.24 | 3.77 | 3.93 | 4.49 | 4.36 | ns | |
| C | 93.41 | 72.36 | 69.87 | 80.13 | 71.55 | 176.45 | |||
| Rβ | →4)-β-D-GalpA | H | 4.53 | 3.44 | 3.69 | 4.30 | 3.99 | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, A.-H.; Li, Q.-Y.; Su, H.; Huang, L.-J.; Zhou, Q.; Wang, X.-D.; Song, J.; Wu, Y.-N.; Yang, X.-F.; Wu, W.-L. Structural Characterization and Ameliorative Effects of Mesona chinensis Benth Polysaccharide Against Deoxynivalenol-Induced Oxidative Stress in Intestinal Epithelial Cells. Nutrients 2025, 17, 2592. https://doi.org/10.3390/nu17162592
Zhong A-H, Li Q-Y, Su H, Huang L-J, Zhou Q, Wang X-D, Song J, Wu Y-N, Yang X-F, Wu W-L. Structural Characterization and Ameliorative Effects of Mesona chinensis Benth Polysaccharide Against Deoxynivalenol-Induced Oxidative Stress in Intestinal Epithelial Cells. Nutrients. 2025; 17(16):2592. https://doi.org/10.3390/nu17162592
Chicago/Turabian StyleZhong, Ai-Hua, Qiu-Yun Li, Hua Su, Li-Jun Huang, Quan Zhou, Xiao-Dan Wang, Jia Song, Yong-Ning Wu, Xing-Fen Yang, and Wei-Liang Wu. 2025. "Structural Characterization and Ameliorative Effects of Mesona chinensis Benth Polysaccharide Against Deoxynivalenol-Induced Oxidative Stress in Intestinal Epithelial Cells" Nutrients 17, no. 16: 2592. https://doi.org/10.3390/nu17162592
APA StyleZhong, A.-H., Li, Q.-Y., Su, H., Huang, L.-J., Zhou, Q., Wang, X.-D., Song, J., Wu, Y.-N., Yang, X.-F., & Wu, W.-L. (2025). Structural Characterization and Ameliorative Effects of Mesona chinensis Benth Polysaccharide Against Deoxynivalenol-Induced Oxidative Stress in Intestinal Epithelial Cells. Nutrients, 17(16), 2592. https://doi.org/10.3390/nu17162592






