Assessment of Sarcopenia in Patients with Liver Cirrhosis—A Literature Review
Abstract
1. Introduction
2. Pathophysiology of Sarcopenia in Liver Cirrhosis
3. Definition and Screening
4. Assessment of Muscle Mass
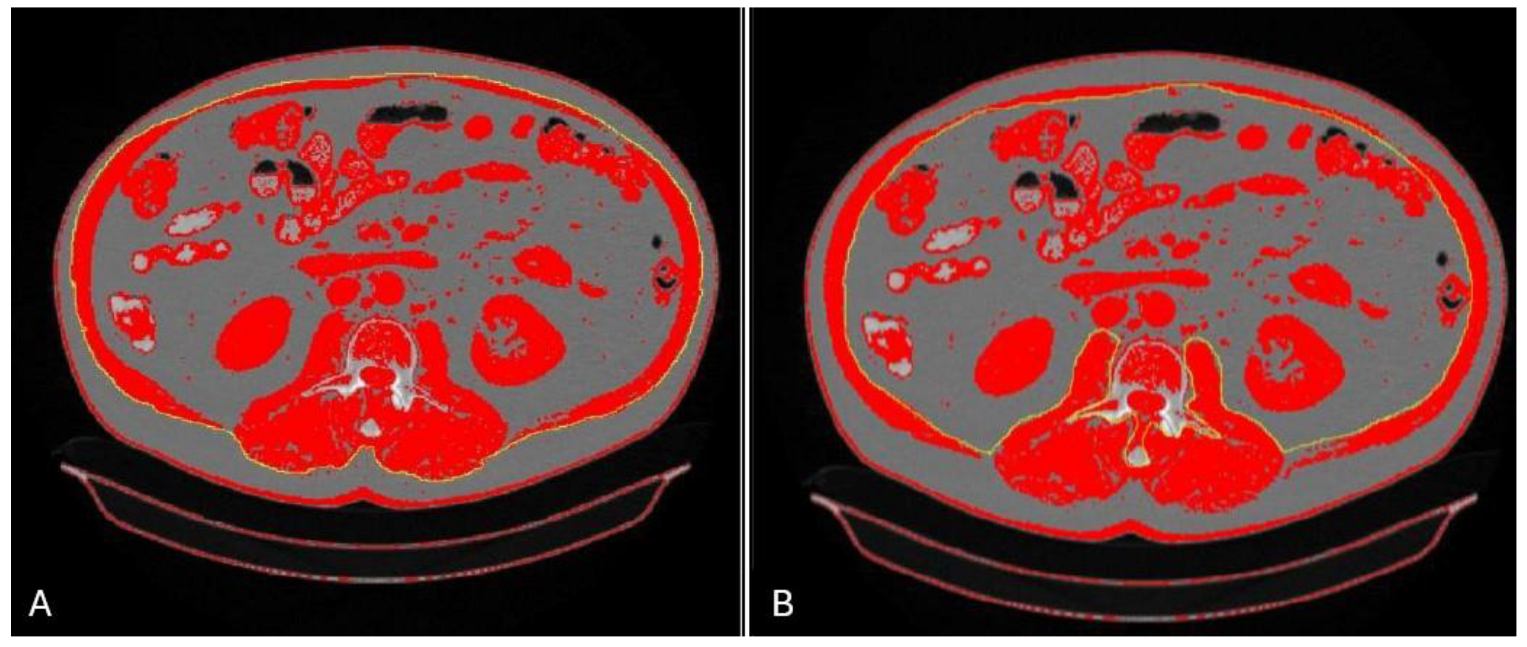
4.1. Multislice Computed Tomography: L3 SMI
4.2. Multislice Computed Tomography: Psoas and Paraspinal Muscles
4.3. Magnetic Resonance Imaging
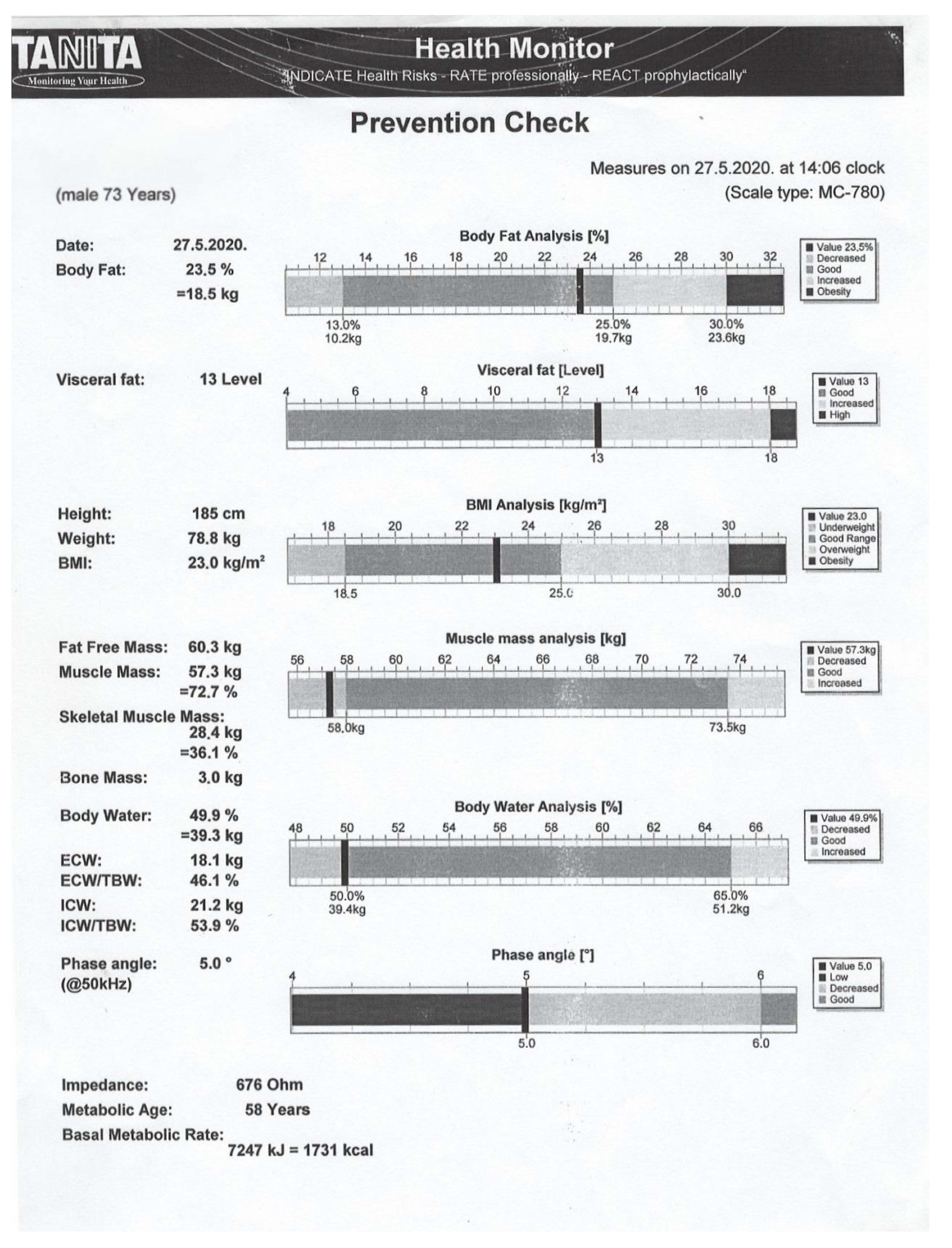
4.4. Bioelectrical Impedance Analysis
4.5. Dual X-Ray Absorptiometry
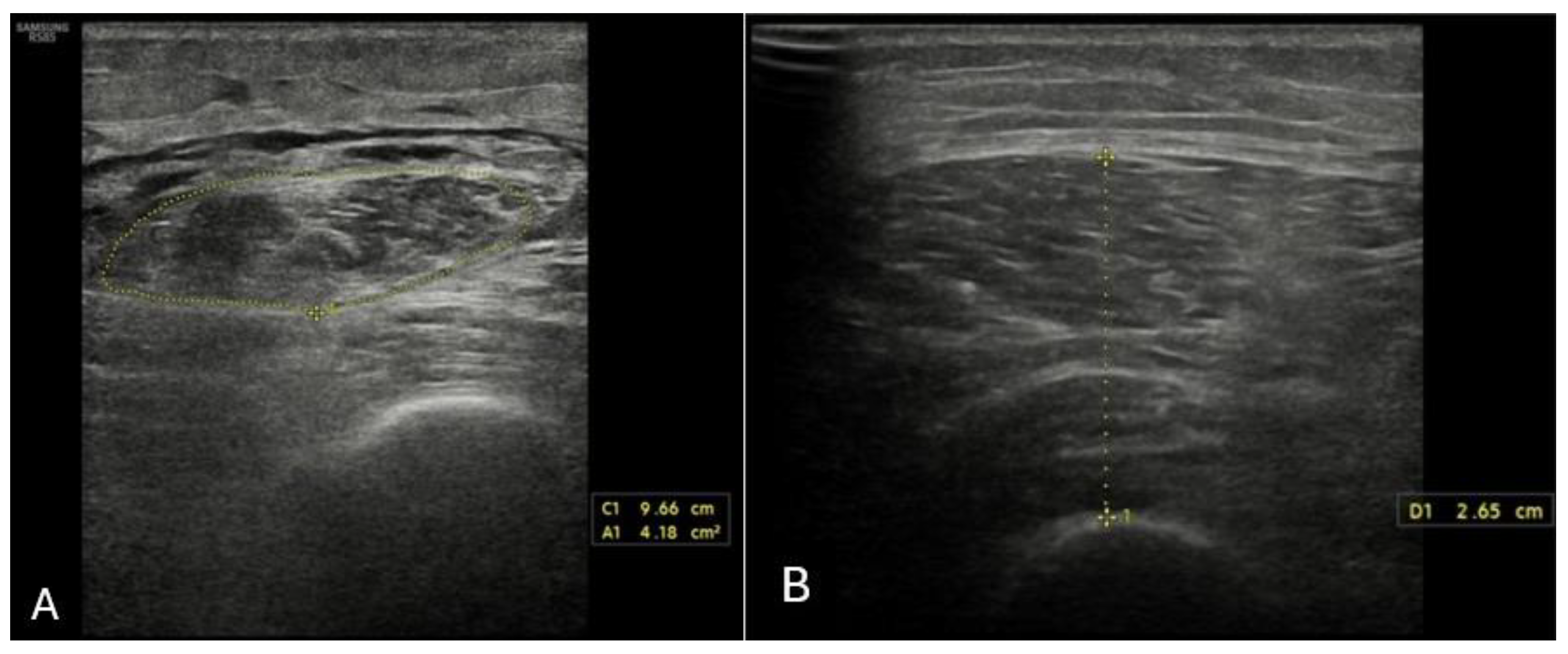
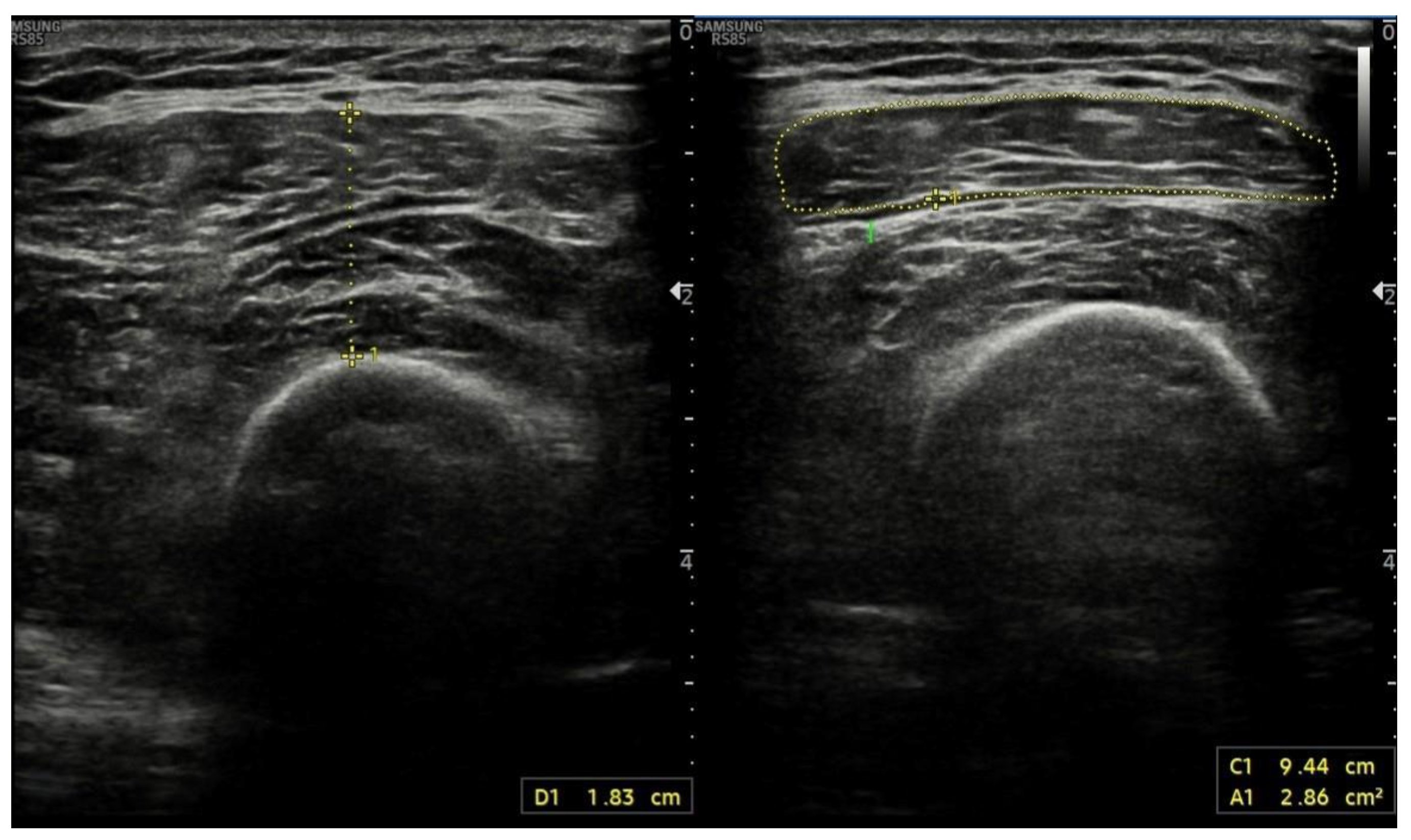
4.6. Ultrasound Assessment of Skeletal Muscle Tissues
4.7. Anthropometric Methods
| Method | Advantages | Disadvantages | Cut-Off |
|---|---|---|---|
| MSCT (L3-SMI) | Accuracy Reproducibility High resolution Different tissues on an anatomical level | Long learning curve Requires experience of the examiner Time consuming Expensive Radiation exposure Contraindicated in pregnancy | L3-SMI: <50 cm2/m2 (M) and <39 cm2/m2 (W) [32] PMA: 1561 mm2 (M) and 1464 mm2 (W) [39] TPMI: <10.7 mm/m (M) and <7.8 mm/m (W) [40] PSMI: <26.3 cm2/m2 (M) and <20.8 cm2/m2 (W) [40] |
| MRI | Accuracy Reproducibility High resolution Different tissues on an anatomical level | Long learning curve Requires experience of the examiner Time consuming Expensive Not widely available | L1-SMI: 43.24 cm2/m2 (M) and 33.73 cm2/m2 (W) [44] |
| DXA | Fast and simple Minimal radiation exposure Low cost | Requires experience of the examiner Contraindicated in pregnancy Does not differentiate between subcutaneous, visceral, and intramuscular fat | ULLMI: 2.014 kg/m2 (M) and 1.506 kg/m2 (W) [64] ASMI: <7 kg/m2 [65] |
| BIA | Fast and very simple No radiation exposure Low cost Widely available | Impact of ascites and edema | BIA-SMI: <11.1 kg/m2 [51] PA: ≤4.9–5.6° [51,53,54,55,56,57] |
| US | US used routinely by hepatologists No radiation exposure Low cost Widely available | QFTI: ≤1.83 cm/m [67] | |
| Anthropometric methods | Fast and simple Low cost | High interobserver variability Low reproducibility |
5. Assessment of Muscle Function
5.1. Muscle Strength
5.2. Physical Performance
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASLD | American Association for the Study of Liver Disease |
| AI | Artificial intelligence |
| ALD | Alcoholic liver disease |
| ALM | Appendicular lean mass |
| ALMI | Appendicular lean mass index |
| APMT | Axial psoas muscle thickness |
| APTI | Axial psoas thickness index |
| ASM | Appendicular skeletal muscle mass |
| ASMI | Appendicular skeletal mass index |
| BCAA | Branched-chain amino acids |
| BCM | Body cell mass |
| BIA | Bioelectrical impedance analysis |
| BMC | Bone mineral content |
| BMI | Body mass index |
| CAGR | compound annual growth rate |
| CC | Calf circumference |
| CSA | Cross-sectional area |
| CTP | Child–Turcotte–Pugh |
| DAMPs | Damage-associated molecular patterns |
| DXA | Dual X-ray absorptiometry |
| EASL | European Association for the Study of the Liver |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| FFMI | Fat-free mass index |
| FM | Fat mass |
| GI | Gastrointestinal |
| GLIS | Global Leadership Initiative in Sarcopenia |
| HCC | Hepatocellular cancer |
| HGF | α-hepatocyte growth factor alpha |
| HGS | Hand-grip strength |
| HRS | Hepatorenal syndrome |
| HU | Hounsfield units |
| ICC | Intraclass correlation coefficient |
| IGF1 | Insulin growth factor 1 |
| IκB | IkappaB kinase |
| L3-SMI | Skeletal muscle index at the level of the third lumbar vertebra |
| LH | Luteinizing hormone |
| LM | Lean mass |
| LVP | Large volume paracentesis |
| NF-κB | Nuclear factor kappa B |
| MAC | Mid-arm circumference |
| MAMC | Mid-arm muscle circumference |
| MAsS | Muscle assessment score |
| MELD | Model for End-Stage Liver Disease |
| MRI | Magnetic resonance imaging |
| MSCT | Multislice computed tomography |
| mTOR | Mammalian target of rapamycin |
| OLT | Orthotopic liver transplantation |
| PA | Phase angle |
| PAMPs | Pathogen-associated molecular patterns |
| PMA | Psoas muscle area |
| PMI | Psoas muscle index |
| PSMI | Paraspinal muscle index |
| RFH-NPT | Royal Free Hospital Nutritional Prioritizing Tool |
| SGA | Subjective Global Assessment |
| SMI | Skeletal muscle index |
| SMM | Skeletal muscle mass |
| SPPB | Short physical performance battery |
| tPMT | Transversal psoas muscle thickness |
| TPTI | Transverse psoas thickness index |
| TSF | Triceps skinfold thickness |
| TUG | Timed up and go |
| ULLMI | Upper limb lean mass index |
| U-P | Ubiquitin–proteasome |
| WC | Waist circumference |
References
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Kirk, B.; Cawthon, P.M.; Arai, H.; Ávila-Funes, J.A.; Barazzoni, R.; Bhasin, S.; Binder, E.F.; Bruyere, O.; Cederholm, T.; Chen, L.-K.; et al. The Conceptual Definition of Sarcopenia: Delphi Consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing 2024, 53, afae052. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Cooper, R.; Arai, H.; Cawthon, P.M.; Essomba, M.-J.N.; Fielding, R.A.; Grounds, M.D.; Witham, M.D.; Cruz-Jentoft, A.J. Sarcopenia. Nat. Rev. Dis. Primers 2024, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Tantai, X.; Liu, Y.; Yeo, Y.H.; Praktiknjo, M.; Mauro, E.; Hamaguchi, Y.; Engelmann, C.; Zhang, P.; Jeong, J.Y.; van Vugt, J.L.A.; et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 76, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Gow, P.J.; Grossmann, M.; Angus, P.W. Review article: Sarcopenia in cirrhosis—Aetiology, implications and potential therapeutic interventions. Aliment. Pharmacol. Ther. 2016, 43, 765–777. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Meyer, F.; Bannert, K.; Wiese, M.; Esau, S.; Sautter, L.F.; Ehlers, L.; Aghdassi, A.A.; Metges, C.C.; Garbe, L.A.; Jaster, R.; et al. Molecular Mechanism Contributing to Malnutrition and Sarcopenia in Patients with Liver Cirrhosis. Int. J. Mol. Sci. 2020, 21, 5357. [Google Scholar] [CrossRef]
- Fox, R.; Stenning, K.; Slee, A.; Macnaughtan, J.; Davies, N. Sarcopenia in liver cirrhosis: Prevalence, pathophysiology and therapeutic strategies. Anal. Biochem. 2022, 647, 114581. [Google Scholar] [CrossRef]
- Del Cioppo, S.; Faccioli, J.; Ridola, L. Hepatic Cirrhosis and Decompensation: Key Indicators for Predicting Mortality Risk. World J. Hepatol. 2025, 17, 104580. [Google Scholar] [CrossRef]
- Emanuele, N.V.; LaPaglia, N.; Benefield, J.; Emanuele, M.A. Ethanol-induced hypogonadism is not dependent on activation of the hypothalamic-pituitary-adrenal axis. Endocr. Res. 2001, 27, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Angeli, P.; Moreau, R.; Jalan, R.; Clària, J.; Trebicka, J.; Fernández, J.; Gustot, T.; Caraceni, P.; Bernardi, M. The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J. Hepatol. 2021, 74, 670–685. [Google Scholar] [CrossRef]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar] [CrossRef]
- Espina, S.; Sanz-Paris, A.; Bernal-Monterde, V.; Casas-Deza, D.; Arbonés-Mainar, J.M. Role of Branched-Chain Amino Acids and Their Derivative β-Hydroxy-β-Methylbutyrate in Liver Cirrhosis. J. Clin. Med. 2022, 11, 7337. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef]
- Topan, M.M.; Sporea, I.; Dănilă, M.; Popescu, A.; Ghiuchici, A.-M.; Lupușoru, R.; Șirli, R. Comparison of Different Nutritional Assessment Tools in Detecting Malnutrition and Sarcopenia among Cirrhotic Patients. Diagnostics 2022, 12, 893. [Google Scholar] [CrossRef]
- Borhofen, S.M.; Gerner, C.; Lehmann, J.; Fimmers, R.; Görtzen, J.; Hey, B.; Geiser, F.; Strassburg, C.P.; Trebicka, J. The Royal Free Hospital-Nutritional Prioritizing Tool Is an Independent Predictor of Deterioration of Liver Function and Survival in Cirrhosis. Dig. Dis. Sci. 2016, 61, 1735–1743. [Google Scholar] [CrossRef]
- Tan, J.Y.T.; Cheah, C.C.M.; Wang, Y.T.; Chang, P.E.; Krishnamoorthy, T.L.; Tan, H.K.; Salazar, E. Outpatient screening with the Royal Free Hospital-Nutrition Prioritizing Tool for patients with cirrhosis at risk of malnutrition. Nutrition 2023, 114, 112139. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Inavolu, P.; Kumar, B.R.; Macherla, R.; Reddy, D.N. SARC-F Score: A Quick Bedside Tool to Screen Sarcopenia in Patients With Cirrhosis. J. Clin. Exp. Hepatol. 2024, 14, 101318. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef]
- Beaudart, C.; McCloskey, E.; Bruyere, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo De Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St.-Onge, M.-P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Meza-Junco, J.; Prado, C.M.; Lieffers, J.R.; Baracos, V.E.; Bain, V.G.; Sawyer, M.B. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2012, 10, 166–173. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, J.L.A.; Alferink, L.J.M.; Buettner, S.; Gaspersz, M.P.; Bot, D.; Murad, S.D.; Feshtali, S.; van Ooijen, P.M.A.; Polak, W.G.; Porte, R.J.; et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort. J. Hepatol. 2018, 68, 707–714. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Duarte-Rojo, A.; Meza-Junco, J.; Baracos, V.E.; Sawyer, M.B.; Pang, J.X.Q.; Beaumont, C.; Esfandiari, N.; Myers, R.P. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin. Transl. Gastroenterol. 2015, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Lai, J.C.; Wang, C.W.; Dasarathy, S.; Lobach, I.; Montano-Loza, A.J.; Dunn, M.A.; for the Fitness, Life Enhancement, and Exercise in Liver Transplantation Consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017, 23, 625–633. [Google Scholar] [CrossRef]
- Resorlu, H.; Savas, Y.; Aylanc, N.; Gokmen, F. Evaluation of Paravertebral Muscle Atrophy and Fatty Degeneration in Ankylosing Spondylitis. Mod. Rheumatol. 2017, 27, 683–687. [Google Scholar] [CrossRef]
- Ebadi, M.; Wang, C.W.; Lai, J.C.; Dasarathy, S.; Kappus, M.R.; Dunn, M.A.; Carey, E.J.; Montano-Loza, A.J.; From the Fitness, Life Enhancement, and Exercise in Liver Transplantation (FLEXIT) Consortium. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J. Cachexia Sarcopenia Muscle 2018, 9, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Buyse, S.; Francoz, C.; Laouénan, C.; Bruno, O.; Belghiti, J.; Moreau, R.; Vilgrain, V.; Valla, D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J. Hepatol. 2014, 60, 1151–1157. [Google Scholar] [CrossRef]
- Huguet, A.; Latournerie, M.; Debry, P.H.; Jezequel, C.; Legros, L.; Rayar, M.; Boudjema, K.; Guyader, D.; Jacquet, E.B.; Thibault, R. The psoas muscle transversal diameter predicts mortality in patients with cirrhosis on a waiting list for liver transplantation: A retrospective cohort study. Nutrition 2018, 51–52, 73–79. [Google Scholar] [CrossRef]
- Golse, N.; Bucur, P.O.; Ciacio, O.; Pittau, G.; Cunha, A.S.; Adam, R.; Castaing, D.; Antonini, T.; Coilly, A.; Samuel, D.; et al. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl. 2017, 23, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, R.; Lampichler, K.; Bardach, C.; Asenbaum, U.; Landler, C.; Bauer, D.; Mandorfer, M.; Schwarzer, R.; Trauner, M.; Reiberger, T.; et al. The value of different CT-based methods for diagnosing low muscle mass and predicting mortality in patients with cirrhosis. Liver Int. 2019, 39, 2374–2385. [Google Scholar] [CrossRef]
- Wang, N.C.; Zhang, P.; Tapper, E.B.; Saini, S.; Wang, S.C.; Su, G.L. Automated Measurements of Muscle Mass Using Deep Learning Can Predict Clinical Outcomes in Patients With Liver Disease. Am. J. Gastroenterol. 2020, 115, 1210–1216. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Gitto, S.; Ruffo, G.; Guarino, S.; Del Grande, F.; Sconfienza, L.M. Sarcopenia: Imaging assessment and clinical application. Abdom. Radiol. 2022, 47, 3205–3216. [Google Scholar] [CrossRef]
- Tandon, P.; Mourtzakis, M.; Low, G.; Zenith, L.; Ney, M.; Carbonneau, M.; Alaboudy, A.; Mann, S.; Esfandiari, N.; Ma, M. Comparing the Variability Between Measurements for Sarcopenia Using Magnetic Resonance Imaging and Computed Tomography Imaging. Am. J. Transplant. 2016, 16, 2766–2767. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, D.; Luo, J.; Xu, H.; Jia, J.; Yang, Z. Diagnosis of Sarcopenia Using the L3 Skeletal Muscle Index Estimated from the L1 Skeletal Muscle Index on MR Images in Patients With Cirrhosis. J. Magn. Reson. Imaging 2023, 58, 1569–1578. [Google Scholar] [CrossRef]
- Beer, L.; Bastati, N.; Ba-Ssalamah, A.; Pötter-Lang, S.; Lampichler, K.; Bican, Y.; Lauber, D.; Hodge, J.; Binter, T.; Pomej, K.; et al. MRI-defined sarcopenia predicts mortality in patients with chronic livmer disease. Liver Int. 2020, 40, 2797–2807. [Google Scholar] [CrossRef]
- Thuluvath, A.J.; Forsgren, M.F.; Ladner, D.P.; Tevar, A.D.; Duarte-Rojo, A. Utilizing a novel MRI technique to identify adverse muscle composition in end-stage liver disease: A pilot study. Ann. Hepatol. 2024, 29, 101508. [Google Scholar] [CrossRef] [PubMed]
- NIH. Bioelectrical Impedance Analysis (BIA) Procedure [Internet]. USA: NIH. Available online: https://repository.niddk.nih.gov/media/studies/fhn_nocturnal/MOP/Chapter15_BIA.pdf (accessed on 28 May 2024).
- Jo, M.H.; Lim, T.S.; Jeon, M.Y.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.-H.; Kim, S.U. Predictors of Discordance in the Assessment of Skeletal Muscle Mass between Computed Tomography and Bioimpedance Analysis. J. Clin. Med. 2019, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. (1985) 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Bozic, D.; Grgurevic, I.; Mamic, B.; Capkun, V.; Bilandzic-Ivisic, J.; Ivanovic, T.; Bozic, I.; Zaja, I.; Podrug, K.; Puljiz, Z.; et al. Detection of Sarcopenia in Patients with Liver Cirrhosis Using the Bioelectrical Impedance Analysis. Nutrients 2023, 15, 3335. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef]
- Ruiz-Margáin, A.; Xie, J.J.; Román-Calleja, B.M.; Pauly, M.; White, M.G.; Chapa-Ibargüengoitia, M.; Campos-Murguía, A.; González-Regueiro, J.A.; Macias-Rodríguez, R.U.; Duarte-Rojo, A. Phase Angle from Bioelectrical Impedance for the Assessment of Sarcopenia in Cirrhosis with or Without Ascites. Clin. Gastroenterol. Hepatol. 2021, 19, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Margáin, A.; Macías-Rodríguez, R.U.; Duarte-Rojo, A.; Ríos-Torres, S.L.; Espinosa-Cuevas, Á.; Torre, A. Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: A prospective cohort study. Dig. Liver Dis. 2015, 47, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Belarmino, G.; Gonzalez, M.C.; Torrinhas, R.S.; Sala, P.; Andraus, W.; D’aLbuquerque, L.A.C.; Pereira, R.M.R.; Caparbo, V.F.; Ravacci, G.R.; Damiani, L.; et al. Phase angle obtained by bioelectrical impedance analysis independently predicts mortality in patients with cirrhosis. World J. Hepatol. 2017, 9, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.P.; Sicchieri, J.M.F.; Schiavoni, I.L.; Barbeiro, D.; Manca, C.S.; da Silva, B.R.; Bezerra, A.E.; Pinto, L.C.M.; Araújo, R.C.; Teixeira, A.C.; et al. Phase angle as a severity indicator for liver diseases. Nutrition 2020, 70, 110607. [Google Scholar] [CrossRef]
- Saueressig, C.; Glasenapp, J.H.; Luft, V.C.; Alves, F.D.; Ferreira, P.K.; Hammes, T.O.; Dall’ALba, V. Phase Angle Is an Independent Predictor of 6-Month Mortality in Patients with Decompensated Cirrhosis: A Prospective Cohort Study. Nutr. Clin. Pract. 2020, 35, 1061–1069. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Ng, B.K.; Sommer, M.J.; Heymsfield, S.B. Body composition by DXA. Bone 2017, 104, 101–105. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Barbosa-Silva, T.G.; Heymsfield, S.B. Bioelectrical impedance analysis in the assessment of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 366–374. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Alberino, F.; Gatta, A.; Amodio, P.; Merkel, C.; Di Pascoli, L.; Boffo, G.; Caregaro, L. Nutrition and survival in patients with liver cirrhosis. Nutrition 2001, 17, 445–450. [Google Scholar] [CrossRef]
- Sinclair, M.; Hoermann, R.; Peterson, A.; Testro, A.; Angus, P.W.; Hey, P.; Chapman, B.; Gow, P.J. Use of Dual X-ray Absorptiometry in men with advanced cirrhosis to predict sarcopenia-associated mortality risk. Liver Int. 2019, 39, 1089–1097. [Google Scholar] [CrossRef]
- Eriksen, C.S.; Kimer, N.; Suetta, C.; Møller, S. Arm lean mass determined by dual-energy X-ray absorptiometry is superior to characterize skeletal muscle and predict sarcopenia-related mortality in cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G729–G740. [Google Scholar] [CrossRef]
- Santos, L.A.A.; Lima, T.B.; Qi, X.; de Paiva, S.A.R.; Romeiro, F.G. Refining dual-energy x-ray absorptiometry data to predict mortality among cirrhotic outpatients: A retrospective study. Nutrition 2021, 85, 111132. [Google Scholar] [CrossRef]
- Belarmino, G.; Gonzalez, M.C.; Sala, P.; Torrinhas, R.S.; Andraus, W.; D’aLbuquerque, L.A.C.; Pereira, R.M.R.; Caparbo, V.F.; Ferrioli, E.; Pfrimer, K.; et al. Diagnosing Sarcopenia in Male Patients with Cirrhosis by Dual-Energy X-Ray Absorptiometry Estimates of Appendicular Skeletal Muscle Mass. JPEN J. Parenter. Enteral Nutr. 2018, 42, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Mirón Mombiela, R.; Vucetic, J.; Rossi, F.; Tagliafico, A.S. Ultrasound Biomarkers for Sarcopenia: What Can We Tell So Far? Semin. Musculoskelet. Radiol. 2020, 24, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Gödiker, J.; Schwind, L.; Jacob, T.; Böhling, N.; Groba, S.N.R.; Kimmann, M.; Meier, J.A.; Peiffer, K.; Trebicka, J.; Chang, J.; et al. Ultrasound-Defined Sarcopenia Independently Predicts Acute Decompensation in Advanced Chronic Liver Disease. J. Cachexia Sarcopenia Muscle 2024, 15, 2792–2802. [Google Scholar] [CrossRef] [PubMed]
- Becchetti, C.; Berzigotti, A. Ultrasonography as a diagnostic tool for sarcopenia in patients with cirrhosis: Examining the pros and cons. Eur. J. Intern. Med. 2023, 116, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Low, G.; Mourtzakis, M.; Zenith, L.; Myers, R.P.; Abraldes, J.G.; Shaheen, A.A.M.; Qamar, H.; Mansoor, N.; Carbonneau, M.; et al. A Model to Identify Sarcopenia in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1473–1480. [Google Scholar] [CrossRef]
- de Luis Roman, D.; García Almeida, J.M.; Bellido Guerrero, D.; Rolo, G.G.; Martín, A.; Martín, D.P.; García-Delgado, Y.; Guirado-Peláez, P.; Palmas, F.; Pérez, C.T.; et al. Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk. Nutrients 2024, 16, 1552. [Google Scholar] [CrossRef] [PubMed]
- Ciocîrlan, M.; Mănuc, M.; Diculescu, M.; Ciocîrlan, M. Is rectus abdominis thickness associated with survival among patients with liver cirrhosis? A prospective cohort study. Sao Paulo Med. J. 2019, 137, 401–406. [Google Scholar] [CrossRef]
- Hari, A.; Berzigotti, A.; Štabuc, B.; Caglevič, N. Muscle psoas indices measured by ultrasound in cirrhosis—Preliminary evaluation of sarcopenia assessment and prediction of liver decompensation and mortality. Dig. Liver Dis. 2019, 51, 1502–1507. [Google Scholar] [CrossRef]
- Buchard, B.; Boirie, Y.; Cassagnes, L.; Lamblin, G.; Coilly, A.; Abergel, A. Assessment of Malnutrition, Sarcopenia and Frailty 318 in Patients with Cirrhosis: Which Tools Should We Use in Clinical Practice? Nutrients 2020, 12, 186. [Google Scholar] [CrossRef]
- Morgan, M.Y.; Madden, A.M.; Soulsby, C.T.; Morris, R.W. Derivation and validation of a new global method for assessing nutritional status in patients with cirrhosis. Hepatology 2006, 44, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Ponti, F.; Agostini, M.; Amadori, M.; Battista, G.; Bazzocchi, A. The role of DXA in sarcopenia. Aging Clin. Exp. Res. 2016, 28, 1047–1060. [Google Scholar] [CrossRef]
- Saueressig, C.; Alves, B.C.; Luft, V.C.; Anastácio, L.R.; Santos, B.C.; Ferreira, L.G.; Fonseca, A.L.F.; de Jesus, R.P.; de Oliveira, L.P.M.; Boulhosa, R.S.d.S.B.; et al. Mid-arm muscle circumference cut-off points in patients with cirrhosis: Low muscle mass related to malnutrition predicts mortality. Nutrition 2024, 125, 112471. [Google Scholar] [CrossRef] [PubMed]
- Giusto, M.; Lattanzi, B.; Albanese, C.; Galtieri, A.; Farcomeni, A.; Giannelli, V.; Lucidi, C.; Di Martino, M.; Catalano, C.; Merli, M. Sarcopenia in liver cirrhosis: The role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur. J. Gastroenterol. Hepatol. 2015, 27, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Iwata, Y.; Sakai, Y.; Kishino, K.; Shimono, Y.; Ikeda, N.; Takashima, T.; Aizawa, N.; et al. Calf Circumference as a Useful Predictor of Sarcopenia in Patients With Liver Diseases. In Vivo 2020, 34, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Riggio, O.; Dally, L. Does malnutrition affect survival in cirrhosis? PINC (Policentrica Italiana Nutrizione Cirrosi). Hepatology 1996, 23, 1041–1046. [Google Scholar] [CrossRef]
- Tapper, E.B.; Derstine, B.; Baki, J.; Su, G.L. Bedside Measures of Frailty and Cognitive Function Correlate with Sarcopenia in Patients with Cirrhosis. Dig. Dis. Sci. 2019, 64, 3652–3659. [Google Scholar] [CrossRef]
- Luengpradidgun, L.; Chamroonkul, N.; Sripongpun, P.; Kaewdech, A.; Tanutit, P.; Ina, N.; Piratvisuth, T. Utility of handgrip strength (HGS) and bioelectrical impedance analysis (BIA) in the diagnosis of sarcopenia in cirrhotic patients. BMC Gastroenterol. 2022, 22, 159. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; Shimizu, M. Reduced handgrip strength is predictive of poor survival among patients with liver cirrhosis: A sex-stratified analysis. Hepatol. Res. 2019, 49, 1414–1426. [Google Scholar] [CrossRef]
- Short Physical Performance Battery (SPPB) [Internet]. USA: NIH. Available online: https://www.nia.nih.gov/research/labs/leps/short-physical-performance-battery-sppb (accessed on 7 August 2025).
- Podsiadlo, D.; Richardson, S. The timed ‘Up & Go’: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Soto, R.; Díaz, L.A.; Rivas, V.; Fuentes-López, E.; Zalaquett, M.; Bruera, M.J.; González, C.; Mezzano, G.; Benítez, C. Frailty and reduced gait speed are independently related to mortality of cirrhotic patients in long-term follow-up. Ann. Hepatol. 2021, 25, 100327. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186990. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Hua, X.; Wu, M.; Wu, J.; Xu, X.; Li, J.; Meng, Q. Longitudinal Changes in Sarcopenia Was Associated with Survival Among Cirrhotic Patients. Front. Nutr. 2024, 11, 1375994. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Jung, Y.K.; Yim, H.J.; Baik, J.W.; Yim, S.Y.; Lee, Y.-S.; Seo, Y.S.; Kim, J.H.; Yeon, J.E.; Byun, K.S. Impacts of muscle mass dynamics on prognosis of outpatients with cirrhosis. Clin. Mol. Hepatol. 2022, 28, 876–889. [Google Scholar] [CrossRef]
- Mauro, E.; Diaz, J.M.; Garcia-Olveira, L.; Spina, J.C.; Savluk, L.; Zalazar, F.; Saidman, J.; De Santibañes, M.; Pekolj, J.; De Santibañes, E.; et al. Sarcopenia HIBA Score Predicts Sarcopenia and Mortality in Patients on the Liver Transplant Waiting List. Hepatol. Commun. 2022, 6, 1699–1710. [Google Scholar] [CrossRef]
- Ravaioli, F.; De Maria, N.; Di Marco, L.; Pivetti, A.; Casciola, R.; Ceraso, C.; Frassanito, G.; Pambianco, M.; Pecchini, M.; Sicuro, C.; et al. From Listing to Recovery: A Review of Nutritional Status Assessment and Management in Liver Transplant Patients. Nutrients 2023, 15, 2778. [Google Scholar] [CrossRef]
- Christodoulidis, G.; Tsagkidou, K.; Bartzi, D.; Prisacariu, I.A.; Agko, E.S.; Koumarelas, K.E.; Zacharoulis, D. Sarcopenia and Frailty: An In-Depth Analysis of the Pathophysiology and Effect on Liver Transplant Candidates. World J. Hepatol. 2025, 17, 106182. [Google Scholar] [CrossRef]
- Markakis, G.E.; Lai, J.C.; Karakousis, N.D.; Papatheodoridis, G.V.; Psaltopoulou, T.; Merli, M.; Sergentanis, T.N.; Cholongitas, E. Sarcopenia As a Predictor of Survival and Complications of Patients With Cirrhosis After Liver Transplantation: A Systematic Review and Meta-Analysis. Clin. Transplant. 2025, 39, e70088. [Google Scholar] [CrossRef]
- Ferreira, A.P.; Machado, M.V. Impact of Pretransplant Frailty and Sarcopenia on the Post-Transplant Prognosis of Patients with Liver Cirrhosis: A Systematic Review. Eur. J. Gastroenterol. Hepatol. 2021, 33 (Suppl. S1), e883–e897. [Google Scholar] [CrossRef]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The Healthcare Costs of Sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef]
- Goates, S.; Du, K.; Arensberg, M.B.; Gaillard, T.; Guralnik, J.; Pereira, S.L. Economic Impact of Hospitalizations in US Adults with Sarcopenia. J. Frailty Aging 2019, 8, 93–99. [Google Scholar] [CrossRef]
- Verified Market Research. Sarcopenia Treatment Market Size and Forecast; Verified Market Research: Washington, DC, USA. Available online: https://www.verifiedmarketresearch.com/product/sarcopenia-treatment-market/ (accessed on 15 June 2024).
- Tapper, E.B.; Zhang, P.; Garg, R.; Nault, T.; Leary, K.; Krishnamurthy, V.; Su, G.L. Body Composition Predicts Mortality and Decompensation in Compensated Cirrhosis Patients: A Prospective Cohort Study. JHEP Rep. 2020, 2, 100061. [Google Scholar] [CrossRef]
- Sconfienza, L.M. Sarcopenia: Ultrasound today, smartphones tomorrow? Eur. Radiol. 2019, 29, 1–2. [Google Scholar] [CrossRef]
- Polan, D.F.; Brady, S.L.; Kaufman, R.A. Tissue segmentation of computed tomography images using a Random Forest algorithm: A feasibility study. Phys. Med. Biol. 2016, 61, 6553–6569. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Messina, C.; Vitale, J.; Sconfienza, L.M. Imaging of sarcopenia: Old evidence and new insights. Eur. Radiol. 2020, 30, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, M.; Kucybała, I.; Urbanik, A.; Wojciechowski, W. Use of Artificial Intelligence in the Imaging of Sarcopenia: A Narrative Review of Current Status and Perspectives. Nutrition 2021, 89, 111227. [Google Scholar] [CrossRef] [PubMed]
- Bedrikovetski, S.; Seow, W.; Kroon, H.M.; Traeger, L.; Moore, J.W.; Sammour, T. Artificial Intelligence for Body Composition and Sarcopenia Evaluation on Computed Tomography: A Systematic Review and Meta-Analysis. Eur. J. Radiol. 2022, 149, 110218. [Google Scholar] [CrossRef]

| Guidelines | Definition | Cut-Offs |
|---|---|---|
| EWGSOP2 (2019) [10] | Reduced muscle strength and muscle mass (or quality) Severe sarcopenia: when accompanied by reduced physical performance | Muscle strength HGS: <27 kg (M) and <16 kg (F) Stand-up test: >15 s for 5 stands Muscle mass ASM < 20 kg (M) or <15 kg (F) ASMI < 7 kg/m2 (M) or <5.5 kg/m2 (F) Physical performance Walking speed < 0.8 m/s SPPB < 8 TUG > 20 s 400 m walk test: >6 min or failure |
| EASL (2019) [8] | Reduced muscle mass and function | Not clearly defined. |
| GLIS (2024) [4] | Concurrent combination of reduced muscle mass and muscle strength | Not clearly defined |
| Questionnaires | |
|---|---|
| SGA | SARC-F |
| Weight loss | Muscle strength |
| Dietary intake | Assistance in walking |
| GI symptoms | Rise from a chair |
| Functional status | Climbing stairs |
| Disease and its relation to nutritional requirements | Falls |
| Physical findings | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozic, D.; Mamic, B.; Peric, I.; Bozic, I.; Zaja, I.; Ivanovic, T.; Gugic Ratkovic, A.; Grgurevic, I. Assessment of Sarcopenia in Patients with Liver Cirrhosis—A Literature Review. Nutrients 2025, 17, 2589. https://doi.org/10.3390/nu17162589
Bozic D, Mamic B, Peric I, Bozic I, Zaja I, Ivanovic T, Gugic Ratkovic A, Grgurevic I. Assessment of Sarcopenia in Patients with Liver Cirrhosis—A Literature Review. Nutrients. 2025; 17(16):2589. https://doi.org/10.3390/nu17162589
Chicago/Turabian StyleBozic, Dorotea, Bisera Mamic, Iva Peric, Ivona Bozic, Ivan Zaja, Tomislav Ivanovic, Ana Gugic Ratkovic, and Ivica Grgurevic. 2025. "Assessment of Sarcopenia in Patients with Liver Cirrhosis—A Literature Review" Nutrients 17, no. 16: 2589. https://doi.org/10.3390/nu17162589
APA StyleBozic, D., Mamic, B., Peric, I., Bozic, I., Zaja, I., Ivanovic, T., Gugic Ratkovic, A., & Grgurevic, I. (2025). Assessment of Sarcopenia in Patients with Liver Cirrhosis—A Literature Review. Nutrients, 17(16), 2589. https://doi.org/10.3390/nu17162589






