Enhancing Human Health Through Nutrient and Bioactive Compound Recovery from Agri-Food By-Products: A Decade of Progress
Abstract
1. Introduction
2. Valorization of Agri-Food Waste and By-Products in the Context of Sustainable Development Goals (SDGs)
3. Novel Sustainable Extraction Processes/Methods for Agri-Food Bio-Products and Chemical Characterization of Bioactive Components
3.1. Ultrasound-Assisted Extraction (UAE)
3.2. Microwave-Assisted Extraction (MAE)
3.3. Hot Pressurized Liquid Extraction (HPLE) and Subcritical Water Extraction (SWE)
3.4. High-Hydrostatic-Pressure Extraction (HHPE)
3.5. Supercritical Fluid Extraction (SFE)
3.6. Pulsed Electric Field Extraction (PEF) and High-Voltage Electrical Discharge Extraction (HVED)
3.6.1. PEF Application
3.6.2. HVED Application
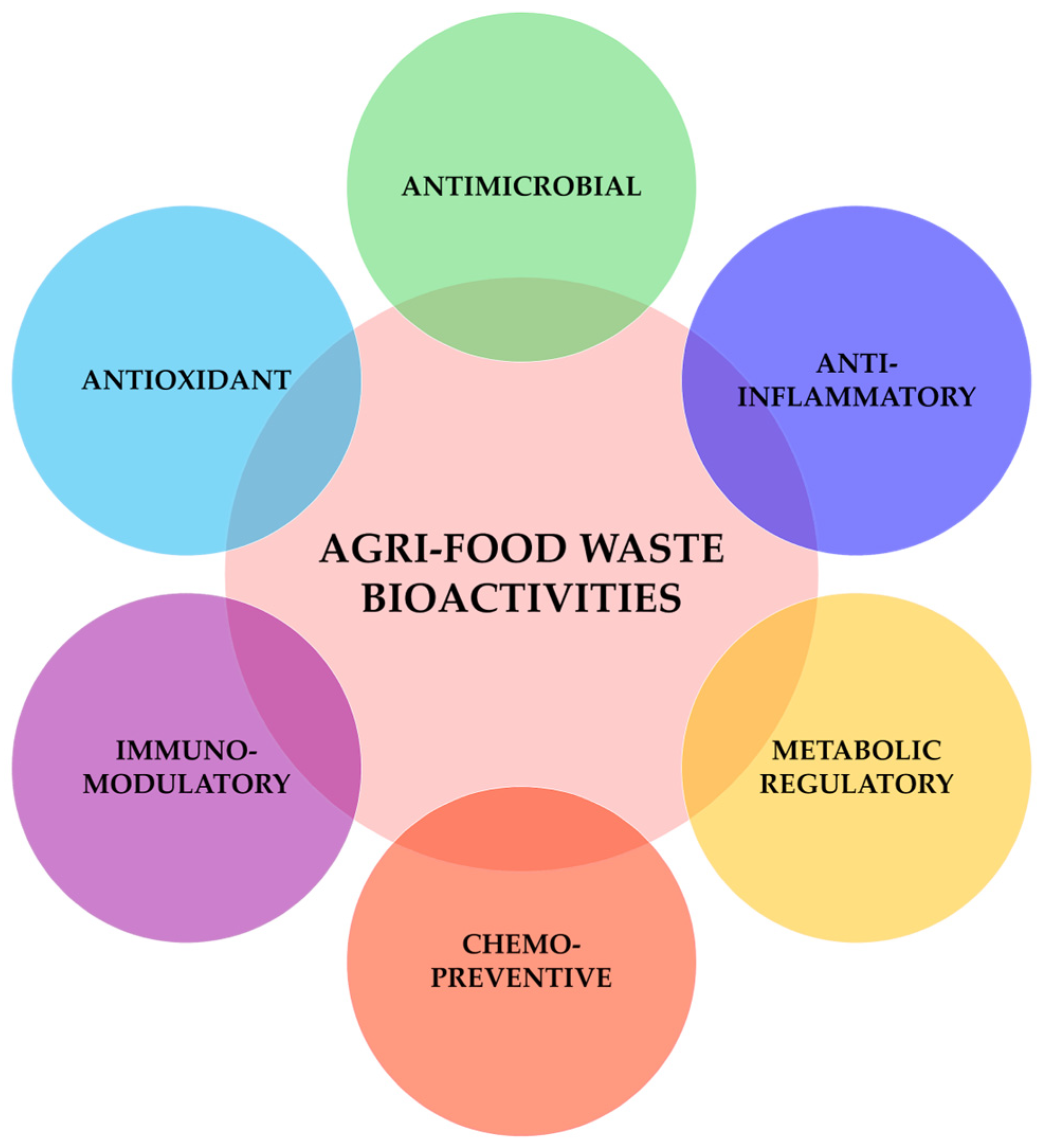
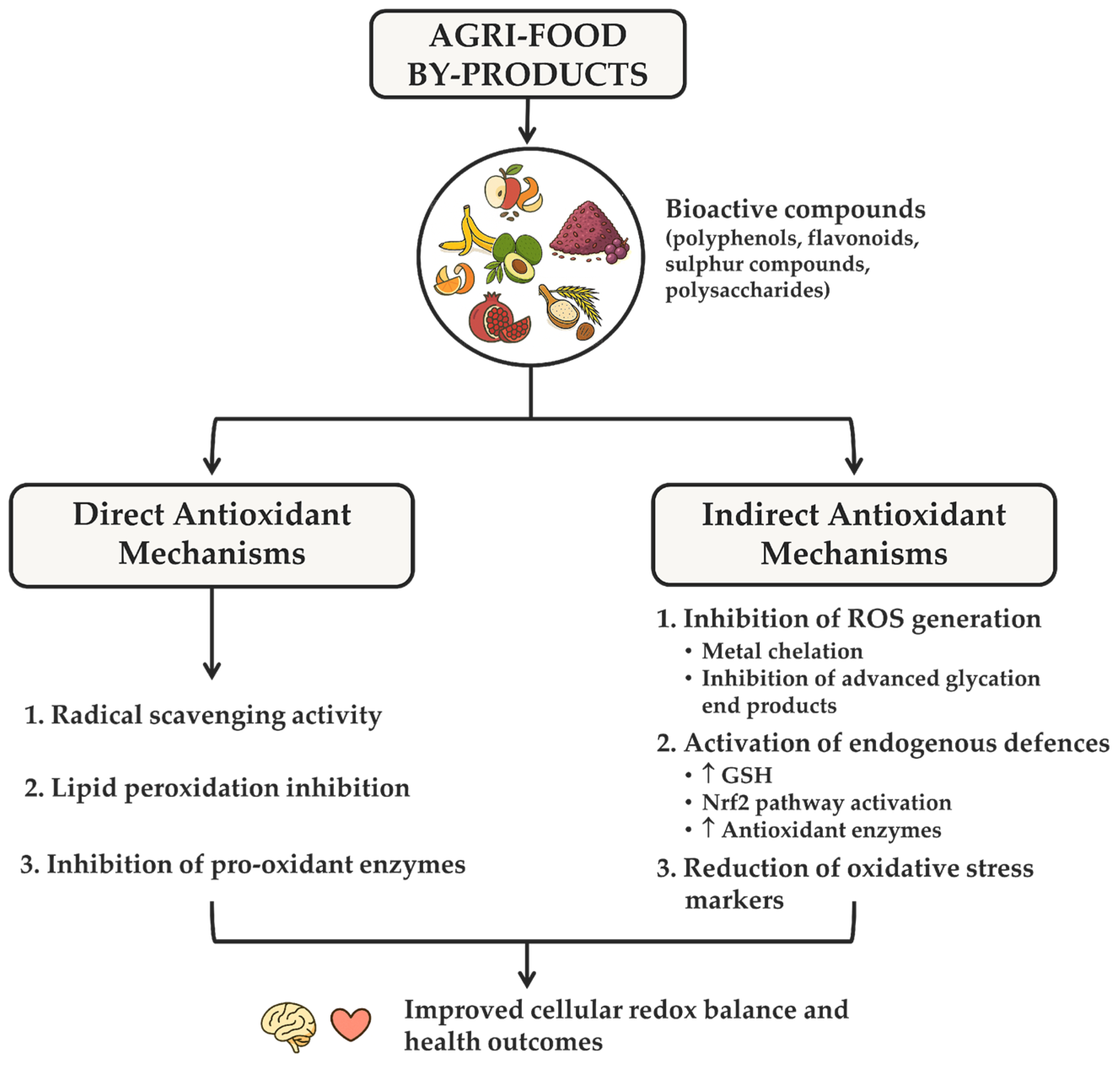
4. Biological Activities of Agri-Food Waste and By-Products and Mechanistic Insights
4.1. Antimicrobial
4.2. Antioxidant Activity
4.3. Anti-Inflammatory
4.4. Immunomodulatory
4.5. Chemopreventive
4.6. Metabolic Regulatory
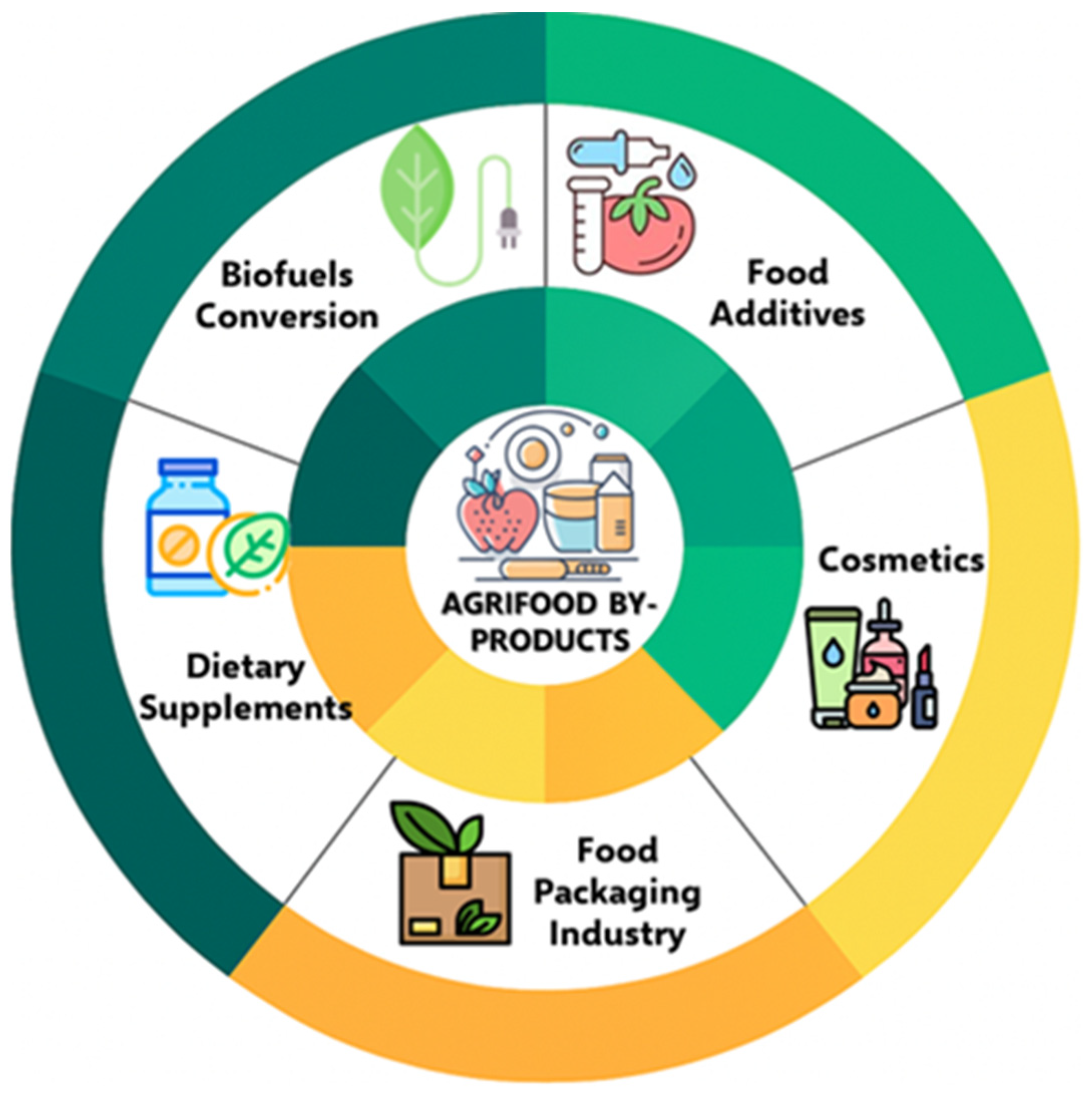
5. Application of Bioactive Compounds Extracted from Agri-Food Waste/By-Products in Novel Functional Food Formulation, in the Pharmaceutical and Cosmetic Sectors
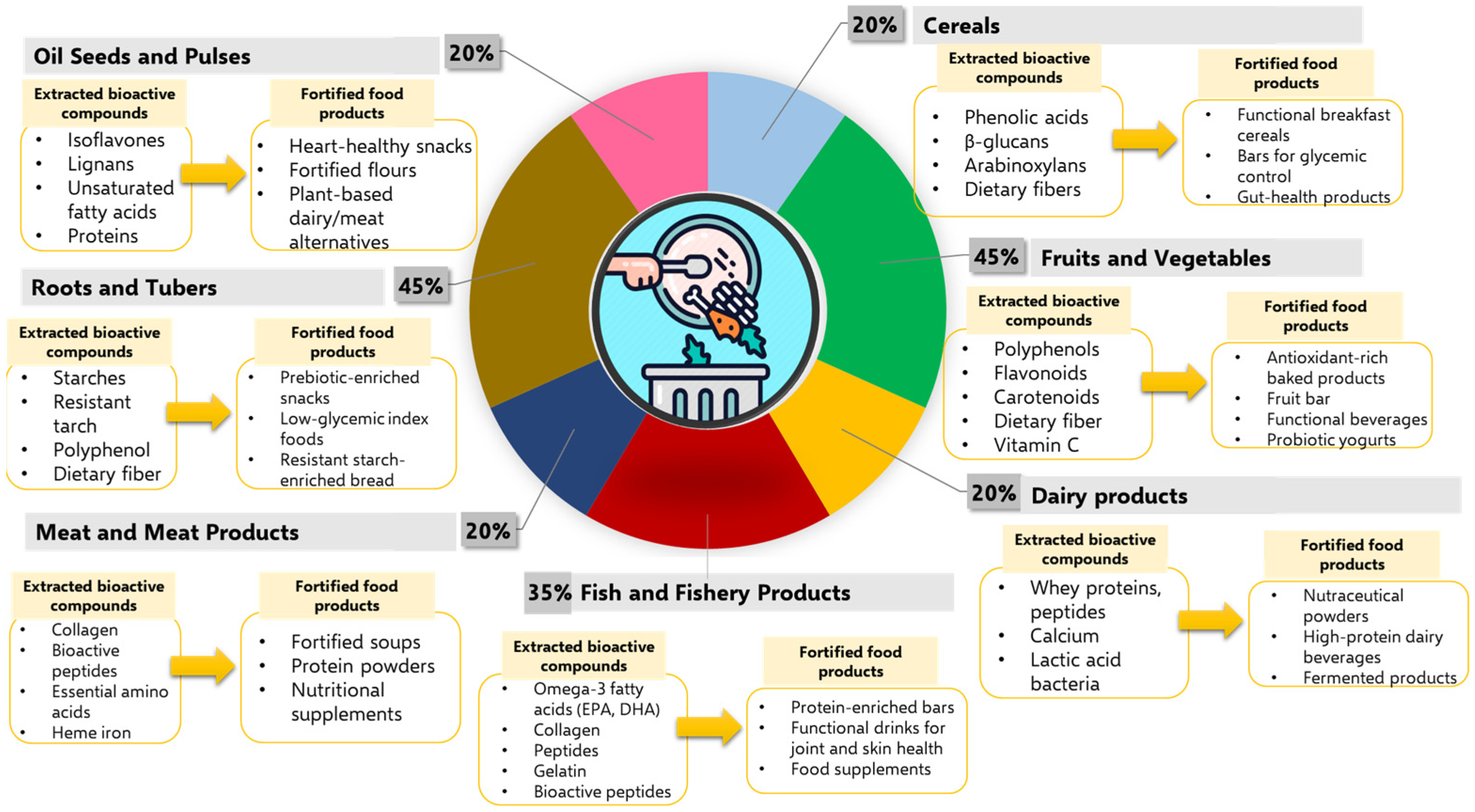
5.1. Valorization of Plant-Based Agri-Food By-Products for Novel Functional Food Development
| Article Title | Authors | Year | Source/Journal | Key Topics | Reference |
|---|---|---|---|---|---|
| Section A: in vitro/laboratory studies | |||||
| High-Value Compounds in Fruit, Vegetable and Cereal By-products: An Overview of Potential Sustainable Reuse and Exploitation | Tlais, A.Z.A. et al. | 2020 | Molecules | In vitro study; analysis of bioactive compounds in food by-products, without product development | [157] |
| Functional Ingredients from Agri-Food Waste: Effect on Phenolic Content and Bioaccessibility in Bakery Products | Melini, V. et al. | 2020 | Antioxidants | Laboratory-based study; bakery fortification, focused on phenolics and bioaccessibility; not an effective product formulation | [26] |
| The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process | Comunian, T.A. et al. | 2021 | Trends in Food Science & Technology | In vitro/technological approach; emphasizes encapsulation processes; functional food innovation | [24] |
| A Rational Definition for Functional Foods: A Perspective | Temple, N.J. | 2022 | Frontiers in Nutrition | Conceptual paper; functional food definition; health benefits; no product application | [158] |
| A Sustainable Waste-to-Protein System for Developing Food- and Feed-Grade Protein | Piercy & Verstraete | 2022 | Green Chemistry | Technological study on waste valorization; waste to protein; sustainable proteins; no effective food products | [159] |
| Application of Agri-Food By-Products in the Food Industry | Rațu R.N. et al. | 2023 | Agriculture | Narrative review; agri-food by-products; bioactive compounds; value-added foods; no product development | [155] |
| Innovative Foods: The Future Food Supply, Nutrition and Health | Hussain & Bekhit | 2023 | Foods | Conceptual data: novel food innovation; sustainable nutrition; alternative proteins; no experimental data or food applications | [160] |
| Food Waste Upcycling and Functional Foods: Innovations for Health and Sustainability | Ullagaddi, R. | 2025 | African Journal of Biomedical Research | General discussion; upcycling; food waste; functional ingredients; sustainability; not focused on food testing | [161] |
| Section B: application in prototypes or product development | |||||
| Food By-products as Sustainable Ingredients for Innovative and Healthy Dairy Foods | Iriondo-DeHond, M. et al. | 2018 | Nutrients | Development of dairy-based food prototypes using food by-products | [150] |
| Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects | Di Nunzio, M. et al. | 2020 | Food Research International | Functional bakery products developed and tested with olive pomace; anti-inflammatory effect | [162] |
| Antioxidant Properties of Gluten-Free Pasta Enriched with Vegetable By-Product | Betrouche, A. et al. | 2022 | Molecules | Gluten-free pasta prototypes enriched with vegetable by-products, experimentally tested; polyphenols; antioxidants | [156] |
| Can a fraction of Flour and Sugar Be Replaced with Fruit By-Product Extracts in Gluten-Free and Vegan Cookie Recipe? | Breschi, C. et al. | 2024 | Molecules | Vegan cookie formulation created and tested for nutritional properties; gluten-free | [163] |
| Hazelnut skin polyphenolic green extract as a promising natural antioxidant in pork burgers: Assessment of quality parameters and consumer acceptance | D’Ambra, K. et al. | 2025 | Food Research International | Prototype burgers enriched with hazelnut skin; pork burger; lipid oxidation and sensory quality evaluated | [164] |
| Surface application and impact of Yarrowia lipolytica grown in cheese whey as adjunct culture for innovative and fast-ripening Caciotta-like cheeses | Gottardi, D. et al. | 2025 | International Journal of Food Microbiology | Cheese whey prototype; by-product valorization; Yarrowia; fast cheese ripening; tested for microbial and textural changes | [165] |
| Food Category | Incorporated Agri-Food By-Products | Obtained Functional Food | Key Functional/ Nutritional Benefits | Reference |
|---|---|---|---|---|
| Bakery Products | Banana peel | Low-glycemic cookies. Prototype tested. | Decreased glycemic index Increased phenolic content and antioxidant activity | [166] |
| Sunflower oil by-products, nut residues, cereal by-products, fruit pomace (apple, carrot, etc.) | Protein snack bars. Prototype and nutritional analysis | High protein and fiber content, enriched with antioxidants and vitamins Rich in thiamin, Ca, Mg, Zn | [167] | |
| Grape pomace | High-fiber muffins. Prototype tested in human crossover trial | Increased total phenolic content High protein and fiber content Decreased glycemic index Increase satiety Improved texture | [168] | |
| Powdered mango peel, green banana flour, pea-based powder, chickpea flour, powdered banana peel | Fiber-enriched bread Functional bread prototype developed and analyzed |
Increased protein, resistant starch | [154] | |
| Dairy Products | Grape seed extract and skin flour | Plant-based yogurts. Prototypes tested for texture and antioxidant properties | Texturizing agent Enhances phenolic compound content Natural colorant Improved textural integrity and gel-forming ability | [152] |
| Peer/apple stones, orange by-products, pomegranate peel, tomato peel, grape seeds, grape pomace, wine pomace, skin, and seed extract | Enhanced probiotic viability and antioxidant content | Enhanced phenolic compound content and antioxidant activity Improved probiotic viability Texturizing agent Increased antimicrobial properties | [151] | |
| Olive oil or fruit processing by-products | Milk alternatives. Fermented milk enriched with antioxidants | Source of protein Probiotic protection Texturizing agent Source of fiber Source of phenols Increased antioxidant capacity Colorant agent | [148] | |
| Meat Analogs | Mosambi peel powder | Chicken meat, patties, chicken’s thigh. Meat prototype tested for antioxidant and antimicrobial activity | Enhanced antioxidant activity Antibacterial agent Increased growth of beneficial microflora | [169] |
| Soymilk pulp | Okara burgers, pea patties. Texture and nutritional enhancement in meat alternatives |
| [170] | |
| Pomegranate peel, orange peel | Beef meatballs, sausages. Prototypes tested for antioxidant and antibacterial properties | Increased antioxidant capacity Antibacterial agent | [171] | |
| Beverages | Citrus peels | Smoothies. Functional beverage with enhanced vitamin C and phenolic content |
| [172] |
| Rice bran, pomegranate peel, orange pulp, and peel | Juice prototypes tested for antioxidant and lipid-lowering effects |
| [173,174] | |
| Grape skins | Grape-based kombucha. Probiotic kombucha with enhanced fiber and antioxidant capacity |
| [175] |
5.2. Valorization of Plant-Based Agri-Food By-Products for Nutraceutical Formulation
5.3. Application of Bioactive Compounds Extracted from Agri-Food Waste/By-Products in Pharmaceutical Applications for Therapeutic Use
5.4. Plant-Derived Extracellular Vesicles in Biomedical Applications
6. Agri-Food Waste/By-Product Valorization for Sustainable Bio-Based Packaging
6.1. Conventional Plastic Packaging and Bio-Based Alternatives
6.2. Biopolymers Derived from Agro-Industrial By-Products
6.2.1. Polysaccharides
6.2.2. Proteins
6.3. Incorporation of Agro-Food Waste into Biopolymeric Matrices
6.3.1. Citrus Peel
6.3.2. Spent Coffee Grounds
6.3.3. Grape Pomace
6.3.4. Pomegranate Peel
6.3.5. Olive Waste
7. Strategic Research Priorities and Innovation Roadmap
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Food Loss and Waste Database. Available online: https://www.fao.org/platform-food-loss-waste/flw-data/en/ (accessed on 20 May 2025).
- Celeiro, M.; Lončarić, A. Editorial: Plant Bioactive Compounds from Agro-Industrial by-Products for Improvement of Nutritional Quality of Foods. Front. Nutr. 2024, 11, 1448549. [Google Scholar] [CrossRef]
- UN Environment Programme. Emissions Gap Report 2024. Available online: https://www.unep.org/resources/emissions-gap-report-2024 (accessed on 5 June 2025).
- Galanakis, C.M. Sustainable Food Systems from Agriculture to Industry: Improving Production and Processing; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128119358. [Google Scholar]
- Mirabella, N.; Castellani, V.; Sala, S. Current Options for the Valorization of Food Manufacturing Waste: A Review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Shawky, E.; Gibbons, S.; Selim, D.A. Bio-Sourcing from Byproducts: A Comprehensive Review of Bioactive Molecules in Agri-Food Waste (AFW) Streams for Valorization and Sustainable Applications. Bioresour. Technol. 2025, 431, 132640. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Ambroselli, D.; Carradori, S.; Gallorini, M.; Giusti, A.M.; Salvo, A.; Grosso, M.; Mannina, L. Modulatory Properties of Food and Nutraceutical Components Targeting NLRP3 Inflammasome Activation. Nutrients 2022, 14, 490. [Google Scholar] [CrossRef]
- Graziani, G.; Gaspari, A.; Di Vaio, C.; Cirillo, A.; Ronca, C.L.; Grosso, M.; Ritieni, A. Assessment of In Vitro Bioaccessibility of Polyphenols from Annurca, Limoncella, Red Delicious, and Golden Delicious Apples Using a Sequential Enzymatic Digestion Model. Antioxidants 2021, 10, 541. [Google Scholar] [CrossRef]
- Foti, P.; Romeo, F.V.; Caggia, C. Olive Mill Wastewater as Booster to Produce a Blood Orange Juice with High Healthy Value. Food Saf. Health 2024, 2, 524–525. [Google Scholar] [CrossRef]
- Kurek, M.; Debeaufort, F.; Voilley, A. Achievements in Applications of Antioxidants and Bioactive Compounds in Food: From Agriculture to Health Benefits. Antioxidants 2024, 13, 1247. [Google Scholar] [CrossRef]
- Abbaspour, N. Fermentation’s Pivotal Role in Shaping the Future of Plant-Based Foods: An Integrative Review of Fermentation Processes and Their Impact on Sensory and Health Benefits. Appl. Food Res. 2024, 4, 100468. [Google Scholar] [CrossRef]
- Boboua, S.Y.B.; Wen, Q.; Zhang, L.; Chen, Y.; Yu, J.; Chen, P.; Sun, Y.; Zheng, T. Valorization of Animal Waste Proteins for Agricultural, Food Production, and Medicinal Applications. Front. Sustain. Food Syst. 2024, 8, 1366333. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M. Global Diets Link Environmental Sustainability and Human Health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Cappelli, K.; Ferlisi, F.; Mecocci, S.; Maranesi, M.; Trabalza-Marinucci, M.; Zerani, M.; Dal Bosco, A.; Acuti, G. Dietary Supplementation of Olive Mill Waste Water Polyphenols in Rabbits: Evaluation of the Potential Effects on Hepatic Apoptosis, Inflammation and Metabolism through Rt-Qpcr Approach. Animals 2021, 11, 2932. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.E.; Farhan, A.; Qamar, M.A. Global Impact of COVID-19 on Food Safety and Environmental Sustainability: Pathways to Face the Pandemic Crisis. Heliyon 2024, 10, e35154. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Khan, M.I.; Kumar, V.; Shang, X.; Lee, J.; Ko, E. Bioactive Compounds of Agro-Industrial By-Products: Current Trends, Recovery, and Possible Utilization. Antioxidants 2025, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- Cristofoli, N.L.; Lima, A.R.; Tchonkouang, R.D.N.; Quintino, A.C.; Vieira, M.C. Advances in the Food Packaging Production from Agri-Food Waste and By-Products: Market Trends for a Sustainable Development. Sustainability 2023, 15, 6153. [Google Scholar] [CrossRef]
- Lazaridis, D.G.; Andritsos, N.D.; Giannakas, A.E.; Karabagias, I.K. Development and Valuation of Novel PLA-Based Biodegradable Packaging Materials Complemented with Food Waste of Plant and Animal Origin for Shelf-Life Extension of Selected Foods: Trends and Challenges. Sustainability 2025, 17, 720. [Google Scholar] [CrossRef]
- Salvo, A.; Masciulli, F.; Ambroselli, D.; Romano, E.; Ingallina, C.; Spano, M.; Di Matteo, G.; Giusti, A.M.; Di Sotto, A.; Percaccio, E.; et al. Hydrolysates from Cauliflower and Artichoke Industrial Wastes as Biostimulants on Seed Germination and Seedling Growth: A Chemical and Biological Characterization. J. Sci. Food Agric. 2024, 105, 151–161. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Nutritional and Environmental Losses Embedded in Global Food Waste. Resour. Conserv. Recycl. 2020, 160, 104912. [Google Scholar] [CrossRef]
- Delgado, L.; Schuster, M.; Torero, M. On the Origins of Food Loss. Appl. Econ. Perspect. Policy 2021, 43, 750–780. [Google Scholar] [CrossRef]
- Machado, M.; Silva, S.; Costa, E.M. Byproducts as a Sustainable Source of Cosmetic Ingredients. Appl. Sci. 2024, 14, 10241. [Google Scholar] [CrossRef]
- Ahmad, T.; Esposito, F.; Cirillo, T. Valorization of Agro-Food by-Products: Advancing Sustainability and Sustainable Development Goals 2030 through Functional Compounds Recovery. Food Biosci. 2024, 62, 105194. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The Use of Food By-Products as a Novel for Functional Foods: Their Use as Ingredients and for the Encapsulation Process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Upadhyay, S.; Tiwari, R.; Kumar, S.; Gupta, S.M.; Kumar, V.; Rautela, I.; Kohli, D.; Rawat, B.S.; Kaushik, R. Utilization of Food Waste for the Development of Composite Bread. Sustainability 2023, 15, 13079. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Luziatelli, F.; Ruzzi, M. Functional Ingredients from Agri-Food Waste: Effect of Inclusion Thereof on Phenolic Compound Content and Bioaccessibility in Bakery Products. Antioxidants 2020, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Klojdova, I.; Ngasakul, N.; Kozlu, A.; Baigts Allende, D.K. Apple Pomace as a Functional Component of Sustainable Set-Type Yogurts. LWT 2024, 211, 116909. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; López-Hernández, L.H. Food Vegetable and Fruit Waste Used in Meat Products. Food Rev. Int. 2022, 38, 628–654. [Google Scholar] [CrossRef]
- Torres-León, C.; Vicente, A.A.; Flores-López, M.L.; Rojas, R.; Serna-Cock, L.; Alvarez-Pérez, O.B.; Aguilar, C.N. Edible Films and Coatings Based on Mango (Var. Ataulfo) by-Products to Improve Gas Transfer Rate of Peach. LWT 2018, 97, 624–631. [Google Scholar] [CrossRef]
- Ortiz, L.; Dorta, E.; Gloria Lobo, M.; Antonio González-Mendoza, L.; Díaz, C.; González, M. Use of Banana Peel Extract to Stabilise Antioxidant Capacity and Sensory Properties of Orange Juice During Pasteurisation and Refrigerated Storage. Food Bioprocess Technol. 2017, 10, 1883–1891. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Liceaga, A.M. Cellular Antioxidant Effect of Bioactive Peptides and Molecular Mechanisms Underlying: Beyond Chemical Properties. Int. J. Food Sci. Technol. 2021, 56, 2193–2204. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green Ultrasound-Assisted Extraction of Carotenoids from Pomegranate Wastes Using Vegetable Oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Veríssimo, L.; Finimundy, T.; Rodrigues, J.; Oliveira, I.; Gonçalves, J.; Fernandes, I.P.; Barros, L.; Heleno, S.A.; Calhelha, R.C. Chemical and Bioactive Screening of Green Polyphenol-Rich Extracts from Chestnut By-Products: An Approach to Guide the Sustainable Production of High-Added Value Ingredients. Foods 2023, 12, 2596. [Google Scholar] [CrossRef]
- Sachs, J.D.; Lafortune, G.; Fuller, G.; Iablonovski, G. Financing Sustainable Development to 2030 and Mid-Century. Sustainable Development Report 2025; Dublin University Press: Dublin, Ireland, 2025; ISBN 978-0-903200-26-4. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gómez-Mejía, E.; Morante-Zarcero, S.; Rosales-Conrado, N.; Sierra, I. Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues. Molecules 2025, 30, 1326. [Google Scholar] [CrossRef]
- Sonawane, A.; Pathak, S.; Pradhan, R.C. Bioactive Compounds in Bael Fruit Pulp Waste: Ultrasound-Assisted Extraction, Characterization, Modeling, and Optimization Approaches. Biointerface Res. Appl. Chem. 2021, 11, 9318–9334. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-Assisted Heating Extraction of Pectin from Grapefruit Peel: Optimization and Comparison with the Conventional Method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Khalfi, A.; Garrigós, M.C.; Ramos, M.; Jiménez, A. Optimization of the Microwave-Assisted Extraction Conditions for Phenolic Compounds from Date Seeds. Foods 2024, 13, 3771. [Google Scholar] [CrossRef] [PubMed]
- Solaberrieta, I.; Mellinas, C.; Jiménez, A.; Garrigós, M.C. Recovery of Antioxidants from Tomato Seed Industrial Wastes by Microwave-Assisted and Ultrasound-Assisted Extraction. Foods 2022, 11, 3068. [Google Scholar] [CrossRef]
- Varadharajan, V.; Shanmugam, S.; Ramaswamy, A. Model Generation and Process Optimization of Microwave-Assisted Aqueous Extraction of Anthocyanins from Grape Juice Waste. J. Food Process Eng. 2017, 40, e12486. [Google Scholar] [CrossRef]
- Alchera, F.; Ginepro, M.; Giacalone, G. Microwave-Assisted Extraction (MAE) of Bioactive Compounds from Blueberry By-Products Using a Sugar-Based NADES: A Novelty in Green Chemistry. LWT 2024, 192, 115642. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Optimization of Microwave-Assisted Extraction of Phenolic Compounds from Chestnut Processing Waste Using Response Surface Methodology. J. Clean. Prod. 2023, 395, 136452. [Google Scholar] [CrossRef]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorisation of Grape Stems as a Source of Phenolic Antioxidants by Using a Sustainable Extraction Methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized Liquid Extraction of Bioactive Compounds from Grape Marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- Ruiz-Aceituno, L.; García-Sarrió, M.J.; Alonso-Rodriguez, B.; Ramos, L.; Sanz, M.L. Extraction of Bioactive Carbohydrates from Artichoke (Cynara scolymus L.) External Bracts Using Microwave Assisted Extraction and Pressurized Liquid Extraction. Food Chem. 2016, 196, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Solana, M.; Boschiero, I.; Dall’Acqua, S.; Bertucco, A. A Comparison between Supercritical Fluid and Pressurized Liquid Extraction Methods for Obtaining Phenolic Compounds from Asparagus officinalis L. J. Supercrit. Fluids 2015, 100, 201–208. [Google Scholar] [CrossRef]
- Çam, M.; Hişil, Y. Pressurised Water Extraction of Polyphenols from Pomegranate Peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- de Andrade Maia, F.; Fasolin, L.H. Recovery of Bioactive Compounds from Pineapple Waste through High-Pressure Technologies. J. Supercrit. Fluids 2025, 218, 106455. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Quirantes-Piné, R.; Segura-Carretero, A. Optimization of Drying Process and Pressurized Liquid Extraction for Recovery of Bioactive Compounds from Avocado Peel By-Product. Electrophoresis 2018, 39, 1908–1916. [Google Scholar] [CrossRef]
- Katsinas, N.; Bento Da Silva, A.; Enríquez-De-Salamanca, A.; Fernández, N.; Bronze, M.R.; Rodríguez-Rojo, S. Pressurized Liquid Extraction Optimization from Supercritical Defatted Olive Pomace: A Green and Selective Phenolic Extraction Process. ACS Sustain. Chem. Eng. 2021, 9, 5590–5602. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Maróstica, M.R.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Subcritical Water Extraction of Flavanones from Defatted Orange Peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Modifiers Based on Natural Deep Eutectic Mixtures to Enhance Anthocyanins Isolation from Grape Pomace by Pressurized Hot Water Extraction. LWT 2021, 149, 111889. [Google Scholar] [CrossRef]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Acid and Deep Eutectic Solvent (DES) Extraction of Pectin from Pomelo (Citrus grandis (L.) Osbeck) Peels. Biocatal. Agric. Biotechnol. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Implementation of Subcritical Water Extraction with Natural Deep Eutectic Solvents for Sustainable Extraction of Phenolic Compounds from Winemaking By-Products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef]
- Grassino, A.N.; Pedisić, S.; Dragović-Uzelac, V.; Karlović, S.; Ježek, D.; Bosiljkov, T. Insight into High-Hydrostatic Pressure Extraction of Polyphenols from Tomato Peel Waste. Plant Foods Hum. Nutr. 2020, 75, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Okur, I.; Namlı, S.; Oztop, M.H.; Alpas, H. High-Pressure-Assisted Extraction of Phenolic Compounds from Olive Leaves: Optimization and Comparison with Conventional Extraction. ACS Food Sci. Technol. 2023, 3, 161–169. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of High Hydrostatic Pressure and High Pressure Homogenization Processing on Characteristics of Potato Peel Waste Pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef]
- Scaglia, B.; D’Incecco, P.; Squillace, P.; Dell’Orto, M.; De Nisi, P.; Pellegrino, L.; Botto, A.; Cavicchi, C.; Adani, F. Development of a Tomato Pomace Biorefinery Based on a CO2-Supercritical Extraction Process for the Production of a High Value Lycopene Product, Bioenergy and Digestate. J. Clean. Prod. 2020, 243, 118650. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Mellinas, A.C.; Espagnol, J.; Hamzaoui, M.; Jiménez, A.; Garrigós, M.C. Valorization of Tomato Seed By-Products as a Source of Fatty Acids and Bioactive Compounds by Using Advanced Extraction Techniques. Foods 2022, 11, 2408. [Google Scholar] [CrossRef]
- Trabelsi, D.; Aydi, A.; Zibetti, A.W.; Della Porta, G.; Scognamiglio, M.; Cricchio, V.; Langa, E.; Abderrabba, M.; Mainar, A.M. Supercritical Extraction from Citrus Aurantium Amara Peels Using CO2 with Ethanol as Co-Solvent. J. Supercrit. Fluids 2016, 117, 33–39. [Google Scholar] [CrossRef]
- Jerkovic, I.; Druzic, J.; Marijanovic, Z.; Gugic, M.; Jokić, S.D.; Roje, M. GC-FID/MS Profiling of Supercritical CO2 extracts of Peels from Citrus aurantium, C. sinensis Cv. Washington Navel, C. sinensis Cv. Tarocco and C. sinensis Cv. Doppio Sanguigno from Dubrovnik Area (Croatia). Nat. Prod. Commun. 2015, 10, 1315–1318. [Google Scholar] [CrossRef]
- Mora, J.J.; Tavares, H.M.; Curbelo, R.; Dellacassa, E.; Cassel, E.; Apel, M.A.; von Poser, G.L.; Vargas, R.M.F. Supercritical Fluid Extraction of Coumarins and Flavonoids from Citrus Peel. J. Supercrit. Fluids 2025, 215, 106396. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Rossi, D.; Pizzolongo, F.; Masi, P. Bioactive Compounds Extracted by Liquid and Supercritical Carbon Dioxide from Citrus Peels. Int. J. Food Sci. Technol. 2022, 57, 3826–3837. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Agudelo-Patiño, T.; Cardona Alzate, C.A. Maximizing the Hesperidin Extraction Using Supercritical Carbon Dioxide and Ethanol: Theoretical Prediction and Experimental Results. Processes 2024, 12, 2457. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving Carotenoid Extraction from Tomato Waste by Pulsed Electric Fields. Front. Nutr. 2014, 1, 12. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of Pulsed Electric Fields to Improve Product Yield and Waste Valorization in Industrial Tomato Processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Brianceau, S.; Turk, M.; Vitrac, X.; Vorobiev, E. Combined Densification and Pulsed Electric Field Treatment for Selective Polyphenols Recovery from Fermented Grape Pomace. Innov. Food Sci. Emerg. Technol. 2015, 29, 2–8. [Google Scholar] [CrossRef]
- Carpentieri, S.; Ferrari, G.; Pataro, G. Pulsed Electric Fields-Assisted Extraction of Valuable Compounds from Red Grape Pomace: Process Optimization Using Response Surface Methodology. Front. Nutr. 2023, 10, 1158019. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ju, T.; Xi, J. Circulating Polyphenols Extraction System with High-Voltage Electrical Discharge: Design and Performance Evaluation. ACS Sustain. Chem. Eng. 2018, 6, 15402–15410. [Google Scholar] [CrossRef]
- Xi, J.; He, L.; Yan, L.-g. Continuous Extraction of Phenolic Compounds from Pomegranate Peel Using High Voltage Electrical Discharge. Food Chem. 2017, 230, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Mellinas, C.; Solaberrieta, I.; Pelegrín, C.J.; Jiménez, A.; Garrigós, M.C. Valorization of Agro-Industrial Wastes by Ultrasound-Assisted Extraction as a Source of Proteins, Antioxidants and Cutin: A Cascade Approach. Antioxidants 2022, 11, 1739. [Google Scholar] [CrossRef]
- Kewlani, P.; Singh, L.; Singh, B.; Bhatt, I.D. Sustainable Extraction of Phenolics and Antioxidant Activities from Prinsepia Utilis Byproducts for Alleviating Aging and Oxidative Stress. Sustain. Chem. Pharm. 2022, 29, 100791. [Google Scholar] [CrossRef]
- Mármol, I.; Quero, J.; Ibarz, R.; Ferreira-Santos, P.; Teixeira, J.A.; Rocha, C.M.R.; Pérez-Fernández, M.; García-Juiz, S.; Osada, J.; Martín-Belloso, O.; et al. Valorization of Agro-Food by-Products and Their Potential Therapeutic Applications. Food Bioprod. Process. 2021, 128, 247–258. [Google Scholar] [CrossRef]
- Boateng, I.D.; Clark, K. Trends in Extracting Agro-Byproducts’ Phenolics Using Non-Thermal Technologies and Their Combinative Effect: Mechanisms, Potentials, Drawbacks, and Safety Evaluation. Food Chem. 2024, 437, 137841. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Maccelli, A.; Spano, M.; Di Matteo, G.; Di Sotto, A.; Giusti, A.M.; Vinci, G.; Di Giacomo, S.; Rapa, M.; Ciano, S.; et al. Chemico-Biological Characterization of Torpedino Di Fondi® Tomato Fruits: A Comparison with San Marzano Cultivar at Two Ripeness Stages. Antioxidants 2020, 9, 1027. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Fernandez Retamozo, C.A.; Lasalvia, A.; Ruggeri, M.; Sandri, G.; Cordeiro, C.; Sousa Silva, M.; Totaro Fila, C.; Garzoli, S.; et al. A Multimethodological Approach for the Chemical Characterization of Edible Insects: The Case Study of Acheta domesticus. Foods 2023, 12, 2331. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Arenas Ocampo, M.L.; Jiménez-Aparicio, A.R. Microwave-Assisted Extraction of Functional Compounds from Plants: A Review. BioResources 2023, 18, 6614–6638. [Google Scholar] [CrossRef]
- Sparr Eskilsson, C.; Björklund, E. Analytical-Scale Microwave-Assisted Extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ferdosh, S.; Haque Akanda, M.J.; Ghafoor, K.; Rukshana, A.H.; Ali, M.E.; Kamaruzzaman, B.Y.; Fauzi, M.B.; Hadijah, S.; Shaarani, S.; et al. Techniques for the Extraction of Phytosterols and Their Benefits in Human Health: A Review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent Extraction Techniques for Natural Products: Microwave-Assisted Extraction and Pressurised Solvent Extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Haffizi, M.; Sulaiman, S.; Noraini Jimat, D.; Amid, A. Review: A Comparison of Conditions for the Extraction of Vegetable and Essential Oils Via Microwave-Assisted Extraction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012172. [Google Scholar] [CrossRef]
- Perino, S.; Petitcolas, E.; de la Guardia, M.; Chemat, F. Portable Microwave Assisted Extraction: An Original Concept for Green Analytical Chemistry. J. Chromatogr. A 2013, 1315, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.Y.; Lai, T.L.; Lin, H.S.; Yang, T.C.; Chang, C.P. Study of Factors Affecting on the Extraction Efficiency of Polycyclic Aromatic Hydrocarbons from Soils Using Open-Vessel Focused Microwave-Assisted Extraction. Chemosphere 2003, 52, 1667–1676. [Google Scholar] [CrossRef]
- Ferrara, D.; Beccaria, M.; Cordero, C.E.; Purcaro, G. Microwave-Assisted Extraction in Closed Vessel in Food Analysis. J. Sep. Sci. 2023, 46, 2300390. [Google Scholar] [CrossRef]
- Rhazi, N.; Hannache, H.; Oumam, M.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier-El Bouhtoury, F. Green Extraction Process of Tannins Obtained from Moroccan Acacia Mollissima Barks by Microwave: Modeling and Optimization of the Process Using the Response Surface Methodology RSM. Arab. J. Chem. 2019, 12, 2668–2684. [Google Scholar] [CrossRef]
- Taghian Dinani, S.; van der Goot, A.J. Challenges and Solutions of Extracting Value-Added Ingredients from Fruit and Vegetable by-Products: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7749–7771. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Esparza, I.; Bimbela, F.; Gandía, L.M.; Ancín-Azpilicueta, C. Valorization of Selected Fruit and Vegetable Wastes as Bioactive Compounds: Opportunities and Challenges. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2061–2108. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Herrero, M.; Cifuentes, A.; Ibañez, E. Use of Compressed Fluids for Sample Preparation: Food Applications. J. Chromatogr. A 2007, 1152, 234–246. [Google Scholar] [CrossRef]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; Da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pre-Treatment and Extraction Techniques for Recovery of Added Value Compounds from Wastes throughout the Agri-Food Chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef]
- Liew, S.Q.; Teoh, W.H.; Tan, C.K.; Yusoff, R.; Ngoh, G.C. Subcritical Water Extraction of Low Methoxyl Pectin from Pomelo (Citrus grandis (L.) Osbeck) Peels. Int. J. Biol. Macromol. 2018, 116, 128–135. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, A.S.; Bakar, M.F.A.; Alomar, M.; Sabran, S.F.; Hanafi, A.F.M.; Mohamad, A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations 2021, 8, 176. [Google Scholar] [CrossRef]
- Balaraman, H.; Selvasembian, R.; Rangarajan, V.; Rathnasamy, S. Sustainable and Green Engineering Insights on Deep Eutectic Solvents toward the Extraction of Nutraceuticals. ACS Sustain. Chem. Eng. 2021, 9, 11290–11313. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed Electric Field (PEF): Avant-Garde Extraction Escalation Technology in Food Industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Xi, J. Recent Advances in High Voltage Electric Discharge Extraction of Bioactive Ingredients from Plant Materials. Food Chem. 2019, 277, 246–260. [Google Scholar] [CrossRef]
- Moro, K.I.B.; Bender, A.B.B.; da Silva, L.P.; Penna, N.G. Green Extraction Methods and Microencapsulation Technologies of Phenolic Compounds from Grape Pomace: A Review. Food Bioprocess Technol. 2021, 14, 1407–1431. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. (Sophia) Spent Coffee Grounds: A Review on Current Utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Shaban, N.Z.; El-Kersh, M.A.L.; El-Rashidy, F.H.; Habashy, N.H. Protective Role of Punica granatum (Pomegranate) Peel and Seed Oil Extracts on Diethylnitrosamine and Phenobarbital-Induced Hepatic Injury in Male Rats. Food Chem. 2013, 141, 1587–1596. [Google Scholar] [CrossRef]
- Sha, S.P.; Modak, D.; Sarkar, S.; Roy, S.K.; Sah, S.P.; Ghatani, K.; Bhattacharjee, S. Fruit Waste: A Current Perspective for the Sustainable Production of Pharmacological, Nutraceutical, and Bioactive Resources. Front. Microbiol. 2023, 14, 1260071. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, H.; Yu, Y.; Peng, S.; Zhu, S. Role of Curcuma Longae Rhizoma in Medical Applications: Research Challenges and Opportunities. Front. Pharmacol. 2024, 15, 1430284. [Google Scholar] [CrossRef]
- Liczbiński, P.; Michałowicz, J.; Bukowska, B. Molecular Mechanism of Curcumin Action in Signaling Pathways: Review of the Latest Research. Phytother. Res. 2020, 34, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Di Giacomo, S.; Amatore, D.; Locatelli, M.; Vitalone, A.; Toniolo, C.; Rotino, G.L.; Lo Scalzo, R.; Palamara, A.T.; Marcocci, M.E.; et al. A Polyphenol Rich Extract from Solanum melongena L. DR2 Peel Exhibits Antioxidant Properties and Anti-Herpes Simplex Virus Type 1 Activity In Vitro. Molecules 2018, 23, 2066. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Sargent, L.J.; Chatzidiakou, Y.; Saunders, C.; Harkness, L.; Bordenave, N.; Rowland, I.; Spencer, J.P.E.; Lovegrove, J.A. Orange Pomace Fibre Increases a Composite Scoring of Subjective Ratings of Hunger and Fullness in Healthy Adults. Appetite 2016, 107, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Choleva, M.; Matalliotaki, E.; Antoniou, S.; Asimomyti, E.; Drouka, A.; Stefani, M.; Yannakoulia, M.; Fragopoulou, E. Postprandial Metabolic and Oxidative Stress Responses to Grape Pomace Extract in Healthy Normal and Overweight/Obese Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2023, 15, 156. [Google Scholar] [CrossRef]
- López-Yerena, A.; Domínguez-López, I.; Abuhabib, M.M.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Pérez, M. Tomato Wastes and By-Products: Upcoming Sources of Polyphenols and Carotenoids for Food, Nutraceutical, and Pharma Applications. Crit. Rev. Food Sci. Nutr. 2023, 64, 2226211. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Weng, Y.X.; Wang, H.C.; Chu, Y.L.; Wu, Y.Z.; Liao, J.A.; Su, Z.Y. Essential Oil from Citrus Depressa Peel Exhibits Antimicrobial, Antioxidant and Cancer Chemopreventive Effects. J. Sci. Food Agric. 2024, 104, 3982–3991. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, M.; Wen, J.; Ren, F.; Yang, Z.; Jiang, X.; Chen, Y. The Bioactivity and Applications of Pomegranate Peel Extract: A Review. J. Food Biochem. 2022, 46, e14105. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Ruíz-Delgado, A.; Roccamante, M.A.; Oller, I.; Agüera, A.; Malato, S. Natural Chelating Agents from Olive Mill Wastewater to Enable Photo-Fenton-like Reactions at Natural PH. Catal. Today 2019, 328, 281–285. [Google Scholar] [CrossRef]
- Khanam, A.; Ahmad, S.; Husain, A.; Rehman, S.; Farooqui, A.; Yusuf, M.A. Glycation and Antioxidants: Hand in the Glove of Antiglycation and Natural Antioxidants. Curr. Protein Pept. Sci. 2020, 21, 899–915. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. A Review on Mechanism of Inhibition of Advanced Glycation End Products Formation by Plant Derived Polyphenolic Compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef]
- Coelho, O.G.L.; Ribeiro, P.V.M.; Alfenas, R.d.C.G. Can Grape Polyphenols Affect Glycation Markers? A Systematic Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1208–1218. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, S.; Patial, V.; Gupta, M.; Bhushan, S.; Padwad, Y.S. Antioxidant and Hepatoprotective Effect of Polyphenols from Apple Pomace Extract via Apoptosis Inhibition and Nrf2 Activation in Mice. Hum. Exp. Toxicol. 2016, 35, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Alves, I.; Araújo, E.M.Q.; Dalgaard, L.T.; Singh, S.; Børsheim, E.; Carvalho, E. Protective Effects of Sulforaphane Preventing Inflammation and Oxidative Stress to Enhance Metabolic Health: A Narrative Review. Nutrients 2025, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Daniloski, D.; Pratibha; Neeraj; D’Cunha, N.M.; Naumovski, N.; Petkoska, A.T. Pomegranate Peel Extract—A Natural Bioactive Addition to Novel Active Edible Packaging. Food Res. Int. 2022, 156, 111378. [Google Scholar] [CrossRef]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A.H.; et al. Polyphenols Inhibiting MAPK Signalling Pathway Mediated Oxidative Stress and Inflammation in Depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef]
- Treasure, K.; Harris, J.; Williamson, G. Exploring the Anti-Inflammatory Activity of Sulforaphane. Immunol. Cell Biol. 2023, 101, 805–828. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-ΚB Signaling in Inflammation and Cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- He, Y.; Sun, Z.; Bai, J.Y.; Zhang, Y.; Qian, Y.; Zhao, X.; Chen, S. Citrus Peel Polyphenols Alleviate Intestinal Inflammation in Mice with Dextran Sulfate Sodium-Induced Acute Colitis. Heliyon 2023, 9, e18137. [Google Scholar] [CrossRef] [PubMed]
- Fadel, A.; Mahmoud, A.M.; Ashworth, J.J.; Li, W.; Ng, Y.L.; Plunkett, A. Health-Related Effects and Improving Extractability of Cereal Arabinoxylans. Int. J. Biol. Macromol. 2018, 109, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Franco-Robles, E.; López, M.G. Implication of Fructans in Health: Immunomodulatory and Antioxidant Mechanisms. Sci. World J. 2015, 2015, 289267. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short Chain Fatty Acids: Key Regulators of the Local and Systemic Immune Response in Inflammatory Diseases and Infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Albini, A.; Albini, F.; Corradino, P.; Dugo, L.; Calabrone, L.; Noonan, D.M. From Antiquity to Contemporary Times: How Olive Oil By-Products and Waste Water Can Contribute to Health. Front. Nutr. 2023, 10, 1254947. [Google Scholar] [CrossRef]
- Azmat, F.; Safdar, M.; Ahmad, H.; Khan, M.R.J.; Abid, J.; Naseer, M.S.; Aggarwal, S.; Imran, A.; Khalid, U.; Zahra, S.M.; et al. Phytochemical Profile, Nutritional Composition of Pomegranate Peel and Peel Extract as a Potential Source of Nutraceutical: A Comprehensive Review. Food Sci. Nutr. 2024, 12, 661–674. [Google Scholar] [CrossRef]
- Coelho, M.C.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Integral Valorisation of Tomato By-Products towards Bioactive Compounds Recovery: Human Health Benefits. Food Chem. 2023, 410, 135319. [Google Scholar] [CrossRef]
- Albini, A.; Festa, M.M.G.; Ring, N.; Baci, D.; Rehman, M.; Finzi, G.; Sessa, F.; Zacchigna, S.; Bruno, A.; Noonan, D.M. A Polyphenol-Rich Extract of Olive Mill Wastewater Enhances Cancer Chemotherapy Effects, While Mitigating Cardiac Toxicity. Front. Pharmacol. 2021, 12, 694762. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Fakharany, E.M.; Kheadr, E.; Ibrahim, W.H. Grape Seed Proanthocyanidin Extract Inhibits DNA and Protein Damage and Labile Iron, Enzyme, and Cancer Cell Activities. Sci. Rep. 2022, 12, 12393. [Google Scholar] [CrossRef]
- Di Sotto, A.; Vecchiato, M.; Abete, L.; Toniolo, C.; Giusti, A.M.; Mannina, L.; Locatelli, M.; Nicoletti, M.; Di Giacomo, S. Capsicum annuum L. Var. Cornetto Di Pontecorvo PDO: Polyphenolic Profile and In Vitro Biological Activities. J. Funct. Foods 2018, 40, 679–691. [Google Scholar] [CrossRef]
- Durmus, N.; Kilic-Akyilmaz, M. Bioactivity of Non-Extractable Phenolics from Lemon Peel Obtained by Enzyme and Ultrasound Assisted Extractions. Food Biosci. 2023, 53, 102571. [Google Scholar] [CrossRef]
- Bai, R.; Yuan, C.; Wang, T.; Liu, L.; Li, J.; Lai, Y.; Li, H.; Chen, Z.; Li, C.; Ke, D.; et al. Apple Pomace and Rosemary Extract Ameliorates Hepatic Steatosis in Fructose-Fed Rats: Association with Enhancing Fatty Acid Oxidation and Suppressing Inflammation. Exp. Ther. Med. 2020, 20, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Nagar, S.; Goyal, S.; Maan, S.; Chugh, V.; Kumar, V.; Kharor, N. Xylooligosaccharide Production from Lignocellulosic Biomass and Their Health Benefits as Prebiotics. Biochem. Res. Int. 2024, 62, 105482. [Google Scholar] [CrossRef]
- Zurbau, A.; Noronha, J.C.; Khan, T.A.; Sievenpiper, J.L.; Wolever, T.M.S. The Effect of Oat β-Glucan on Postprandial Blood Glucose and Insulin Responses: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2021, 75, 1540–1554. [Google Scholar] [CrossRef]
- Blasi, F.; Ianni, F.; Mangiapelo, L.; Pinna, N.; Cossignani, L. In Vitro Anti-Obesity Activity by Pancreatic Lipase Inhibition—Simple HPLC Approach Using EVOO as Natural Substrate. J. Sci. Food Agric. 2023, 103, 2786–2793. [Google Scholar] [CrossRef]
- Liu, T.T.; Liu, X.T.; Chen, Q.X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Binou, P.; Yanni, A.E.; Stergiou, A.; Karavasilis, K.; Konstantopoulos, P.; Perrea, D.; Tentolouris, N.; Karathanos, V.T. Enrichment of Bread with Beta-Glucans or Resistant Starch Induces Similar Glucose, Insulin and Appetite Hormone Responses in Healthy Adults. Eur. J. Nutr. 2021, 60, 455–464. [Google Scholar] [CrossRef]
- Warrilow, A.; Mellor, D.; McKune, A.; Pumpa, K. Dietary Fat, Fibre, Satiation, and Satiety—A Systematic Review of Acute Studies. Eur. J. Clin. Nutr. 2019, 73, 333–344. [Google Scholar] [CrossRef]
- Mah, E.; Liska, D.A.J.; Goltz, S.; Chu, Y.F. The Effect of Extracted and Isolated Fibers on Appetite and Energy Intake: A Comprehensive Review of Human Intervention Studies. Appetite 2023, 180, 106340. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health Effects of Trans-Fatty Acids: Experimental and Observational Evidence. Eur. J. Clin. Nutr. 2009, 63, S5–S21. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of Vegetable and Fruit By-Products as Functional Ingredient and Food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarian, B.; Casale, M.; Paini, M.; Casazza, A.A.; Lanteri, S.; Perego, P. Production of a Novel Fermented Milk Fortified with Natural Antioxidants and Its Analysis by NIR Spectroscopy. LWT 2015, 62, 376–383. [Google Scholar] [CrossRef]
- Haque, A.; Ahmad, S.; Azad, Z.R.A.A.; Adnan, M.; Ashraf, S.A. Incorporating Dietary Fiber from Fruit and Vegetable Waste in Meat Products: A Systematic Approach for Sustainable Meat Processing and Improving the Functional, Nutritional and Health Attributes. PeerJ 2023, 11, e14977. [Google Scholar] [CrossRef]
- Iriondo-Dehond, M.; Miguel, E.; Del Castillo, M.D. Food Byproducts as Sustainable Ingredients for Innovative and Healthy Dairy Foods. Nutrients 2018, 10, 1358. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Marinelli, V.; Saccotelli, M.A.; Del Nobile, M.A.; Conte, A. Fruit and Vegetable By-Products to Fortify Spreadable Cheese. Antioxidants 2018, 7, 61. [Google Scholar] [CrossRef]
- Issar, K.; Sharma, P.C.; Gupta, A. Utilization of Apple Pomace in the Preparation of Fiber-Enriched Acidophilus Yoghurt. J. Food Process. Preserv. 2017, 41, 13098. [Google Scholar] [CrossRef]
- Shinali, T.S.; Zhang, Y.; Altaf, M.; Nsabiyeze, A.; Han, Z.; Shi, S.; Shang, N. The Valorization of Wastes and Byproducts from Cruciferous Vegetables: A Review on the Potential Utilization of Cabbage, Cauliflower, and Broccoli Byproducts. Foods 2024, 13, 1163. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.F.; Vodnar, D.C. Bioactive Potential of Fruit and Vegetable Wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar] [CrossRef]
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipșa, F.D.; Filipov, F.; Florea, A.M.; et al. Application of Agri-Food By-Products in the Food Industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Betrouche, A.; Estivi, L.; Colombo, D.; Pasini, G.; Benatallah, L.; Brandolini, A.; Hidalgo, A. Antioxidant Properties of Gluten-Free Pasta Enriched with Vegetable By-Products. Molecules 2022, 27, 8993. [Google Scholar] [CrossRef] [PubMed]
- Tlais, A.Z.A.; Maria Fiorino, G.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal By-products: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J. A Rational Definition for Functional Foods: A Perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef]
- Piercy, E.; Verstraete, W.; Ellis, P.R.; Banks, M.; Rockström, J.; Smith, P.; Witard, O.C.; Hallett, J.; Hogstrand, C.; Knott, G.; et al. A Sustainable Waste-to-Protein System to Maximise Waste Resource Utilisation for Developing Food- and Feed-Grade Protein Solutions. Green Chem. 2022, 25, 808–832. [Google Scholar] [CrossRef]
- Hussain, M.A.; Bekhit, A.E.D.A. Innovative Foods: The Future Food Supply, Nutrition and Health. Foods 2023, 12, 1359. [Google Scholar] [CrossRef]
- Ullagaddi, R. Food Waste Upcycling and Functional Foods: Innovations for Health and Sustainability. Afr. J. Biomed. Res. 2025, 27, 2260–2266. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive Oil By-Product as Functional Ingredient in Bakery Products. Influence of Processing and Evaluation of Biological Effects. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef]
- Breschi, C.; D’Agostino, S.; Meneguzzo, F.; Zabini, F.; Chini, J.; Lovatti, L.; Tagliavento, L.; Guerrini, L.; Bellumori, M.; Cecchi, L.; et al. Can a Fraction of Flour and Sugar Be Replaced with Fruit By-Product Extracts in a Gluten-Free and Vegan Cookie Recipe? Molecules 2024, 29, 1102. [Google Scholar] [CrossRef]
- D’Ambra, K.; Trovato, R.; Minelli, G.; Cattivelli, A.; Zannini, M.; Tagliazucchi, D.; Tabasso, S.; Lo Fiego, D. Pietro Hazelnut Skin Polyphenolic Green Extract as a Promising Natural Antioxidant in Pork Burgers: Assessment of Quality Parameters and Consumer Acceptance. Food Res. Int. 2025, 202, 115764. [Google Scholar] [CrossRef]
- Gottardi, D.; Siroli, L.; Braschi, G.; D’Alessandro, M.; Vannini, L.; Patrignani, F.; Lanciotti, R. Surface Application and Impact of Yarrowia Lipolytica Grown in Cheese Whey as Adjunct Culture for Innovative and Fast-Ripening Caciotta-like Cheeses. Int. J. Food Microbiol. 2025, 432, 111112. [Google Scholar] [CrossRef]
- González-Montelongo, R.; Gloria Lobo, M.; González, M. Antioxidant Activity in Banana Peel Extracts: Testing Extraction Conditions and Related Bioactive Compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Shahar, F.S.; Hameed Sultan, M.T.; Md Shah, A.U.; Azrie Safri, S.N.; Mat Yazik, M.H. Overview of Bioplastic Introduction and Its Applications in Product Packaging. Coatings 2021, 11, 1423. [Google Scholar] [CrossRef]
- Bajerska, J.; Mildner-Szkudlarz, S.; Górnaś, P.; Seglina, D. The Effects of Muffins Enriched with Sour Cherry Pomace on Acceptability, Glycemic Response, Satiety and Energy Intake: A Randomized Crossover Trial. J. Sci. Food Agric. 2016, 96, 2486–2493. [Google Scholar] [CrossRef] [PubMed]
- Younis, K.; Ahmad, S.; Malik, M.A. Mosambi Peel Powder Incorporation in Meat Products: Effect on Physicochemical Properties and Shelf Life Stability. Appl. Food Res. 2021, 1, 100015. [Google Scholar] [CrossRef]
- Nam, J.K.; Lee, J.Y.; Jang, H.W. Quality Characteristics and Volatile Compounds of Plant-Based Patties Supplemented with Biji Powder. Food Chem. X 2024, 23, 101576. [Google Scholar] [CrossRef]
- Olufunso, A.E.; Cyril, N.C.; Grace, T.O.; Olajide, A.A.; Kehinde, O.O.; Martha, O.D.; Gibson, C.O.; Olusola, A.O. Sensory Evaluation of Meat of Broiler Poultry Birds Fed with Tomato-Supplemented Feed. Technol. Sci. Am. Sci. Res. J. Eng. 2018, 47, 145–150. [Google Scholar]
- Rafiq, S.; Singh, B.; Gat, Y. Effect of Different Drying Techniques on Chemical Composition, Color and Antioxidant Properties of Kinnow (Citrus reticulata) Peel. J. Food Sci. Technol. 2019, 56, 2458–2466. [Google Scholar] [CrossRef]
- Amofa-Diatuo, T.; Anang, D.M.; Barba, F.J.; Tiwari, B.K. Development of New Apple Beverages Rich in Isothiocyanates by Using Extracts Obtained from Ultrasound-Treated Cauliflower by-Products: Evaluation of Physical Properties and Consumer Acceptance. J. Food Compos. Anal. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Wedamulla, N.E.; Fan, M.; Choi, Y.J.; Kim, E.K. Citrus Peel as a Renewable Bioresource: Transforming Waste to Food Additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Morales, D.; Gutiérrez-Pensado, R.; Bravo, F.I.; Muguerza, B. Novel Kombucha Beverages with Antioxidant Activity Based on Fruits as Alternative Substrates. LWT 2023, 189, 115482. [Google Scholar] [CrossRef]
- Lauro, M.R.; Crasci, L.; Carbone, C.; Aquino, R.P.; Panico, A.M.; Puglisi, G. Encapsulation of a Citrus By-Product Extract: Development, Characterization and Stability Studies of a Nutraceutical with Antioxidant and Metalloproteinases Inhibitory Activity. LWT 2015, 62, 169–176. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, M.G.; Centonze, G.; Maggiore, R.; D’antona, N. Polyphenolic Fraction from Olive Mill Wastewater: Scale-up and in Vitro Studies for Ophthalmic Nutraceutical Applications. Antioxidants 2019, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, N.; Sao, K.; Kaushik, A. Bioactive Compounds, Antioxidant Properties, and Metal Content Studies of Guava Fruit By-Products for Value Added Processing. Braz. J. Anal. Chem. 2018, 5, 8–18. [Google Scholar] [CrossRef]
- Tapal, A.; Vegarud, G.E.; Sreedhara, A.; Kaul Tiku, P. Nutraceutical Protein Isolate from Pigeon Pea (Cajanus cajan) Milling Waste by-Product: Functional Aspects and Digestibility. Food Funct. 2019, 10, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; D’avino, M.; Buonomo, G.; Caruso, G.; Ciampaglia, R.; Schiano, E.; Maisto, M.; Annunziata, G.; Novellino, E. A Pilot Screening of Agro-Food Waste Products as Sources of Nutraceutical Formulations to Improve Simulated Postprandial Glycaemia and Insulinaemia in Healthy Subjects. Nutrients 2020, 12, 1292. [Google Scholar] [CrossRef]
- Bellumori, M.; De Marchi, L.; Mainente, F.; Zanoni, F.; Cecchi, L.; Innocenti, M.; Mulinacci, N.; Zoccatelli, G. A By-Product from Virgin Olive Oil Production (Pâté) Encapsulated by Fluid Bed Coating: Evaluation of the Phenolic Profile after Shelf-Life Test and in Vitro Gastrointestinal Digestion. Int. J. Food Sci. Technol. 2021, 56, 3773–3783. [Google Scholar] [CrossRef]
- Buzzi, R.; Gugel, I.; Costa, S.; Molesini, S.; Boreale, S.; Baldini, E.; Marchetti, N.; Vertuani, S.; Pinelli, P.; Urciuoli, S.; et al. Up-Cycling of Olea europaea L. Ancient Cultivars Side Products: Study of a Combined Cosmetic–Food Supplement Treatment Based on Leaves and Olive Mill Wastewater Extracts. Life 2023, 13, 1509. [Google Scholar] [CrossRef]
- Picerno, P.; Crascì, L.; Iannece, P.; Esposito, T.; Franceschelli, S.; Pecoraro, M.; Giannone, V.; Panico, A.M.; Aquino, R.P.; Lauro, M.R. A Green Bioactive By-Product Almond Skin Functional Extract for Developing Nutraceutical Formulations with Potential Antimetabolic Activity. Molecules 2023, 28, 7913. [Google Scholar] [CrossRef]
- Sánchez-Quezada, V.; Gaytán-Martínez, M.; Recio, I.; Loarca-Piña, G. Avocado Seed By-Product Uses in Emulsion-Type Ingredients with Nutraceutical Value: Stability, Cytotoxicity, Nutraceutical Properties, and Assessment of In Vitro Oral-Gastric Digestion. Food Chem. 2023, 421, 136118. [Google Scholar] [CrossRef]
- Castangia, I.; Corrias, F.; Leyva Jiménez, F.J.; Aroffu, M.; Fulgheri, F.; Perra, M.; Atzei, A.; del Giudice, A.; Zengin, G.; Ak, G.; et al. Formulation and Testing of Cutting-Edge Food Supplements Tailored for Glucose and Oxidation Controlling, Converting Artichoke By-Products in Inulin-Rich Antioxidant Phytocomplex Loaded into Zein Liposomes. Food Biosci. 2024, 62, 105482. [Google Scholar] [CrossRef]
- Grabauskaitė, R.; Jūrienė, L.; Pukalskienė, M.; Šipailienė, A.; Skurkienė, R.; Venskutonis, P.R. Isolation of Valuable Substances from Berry Seeds and Pomace by the Green High-Pressure Methods, Their Evaluation and Application in Cosmetic Creams. Ind. Crops Prod. 2024, 222, 119729. [Google Scholar] [CrossRef]
- Maccarronello, A.E.; Cardullo, N.; Silva, A.M.; Di Francesco, A.; Costa, P.C.; Rodrigues, F.; Muccilli, V. Unlocking the Nutraceutical Potential of Corylus avellana L. Shells: Microwave-Assisted Extraction of Phytochemicals with Antiradical and Anti-Diabetic Properties. J. Sci. Food Agric. 2024, 2024, 9472–9485. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Jafari, S.; Shiekh, K.A.; Gulzar, S.; Assatarakul, K. Sustainable Ultrasound-Assisted Extraction and Encapsulation of Phenolic Compounds from Sacha Inchi Shell for Future Application. Sustainability 2024, 16, 1820. [Google Scholar] [CrossRef]
- Ilgaz, C.; Casula, L.; Sarais, G.; Schlich, M.; Dessì, D.; Cardia, M.C.; Sinico, C.; Kadiroglu, P.; Lai, F. Proniosomal Encapsulation of Olive Leaf Extract for Improved Delivery of Oleuropein: Towards the Valorization of an Agro-Industrial Byproduct. Food Chem. 2025, 479, 143877. [Google Scholar] [CrossRef]
- Mello, V.C.; de Brito, G.O.; Radicchi, M.A.; Florêncio, I.; Piau, T.B.; Ferreira, E.A.; de Azevedo Chang, L.F.; Silveira, A.P.; Simões, M.M.; de Paiva, K.L.R.; et al. Advanced Solubilization of Brazilian Cerrado Byproduct Extracts Using Green Nanostructured Lipid Carriers and NaDESs for Enhanced Antioxidant Potentials. Antioxidants 2025, 14, 290. [Google Scholar] [CrossRef]
- Schiano, E.; Piccolo, V.; Novellino, E.; Maisto, M.; Iannuzzo, F.; Summa, V.; Tenore, G.C. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods 2022, 11, 1010. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Castaldo, L.; Sessa, R.; Ricci, L.; Vardaro, E.; Izzo, L.; Grosso, M.; Ritieni, A.; Laneri, S. Chemical Profile and Promising Applications of Cucurbita pepo L. Flowers. Antioxidants 2024, 13, 1476. [Google Scholar] [CrossRef]
- Chauhan, S.; Pandit, N.K.; Mohanty, A.; Meena, S.S. Resource Recovery of Bioactive Compounds from Food Waste and Their Diverse Industrial Applications. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef]
- Narita, K.; Hisamoto, M.; Okuda, T.; Takeda, S. Differential Neuroprotective Activity of Two Different Grape Seed Extracts. PLoS ONE 2011, 6, e14575. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M.; Ksiezak-Reding, H.; Santa-Maria, I.; Wang, J.; Ho, L. Development of a Grape Seed Polyphenolic Extract with Anti-Oligomeric Activity as a Novel Treatment in Progressive Supranuclear Palsy and Other Tauopathies. J. Neurochem. 2010, 114, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.N.; et al. Resveratrol and Grape Extract-Loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; Abbas, H.; Zewail, M.; Noureldin, M.H.; Ali, M.M.; Shamaa, M.M.; Khattab, M.A.; Ibrahim, N. Neuroprotective Effect of Artichoke-Based Nanoformulation in Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceuticals 2022, 15, 1202. [Google Scholar] [CrossRef]
- Mounir, R.; Alshareef, W.A.; El Gebaly, E.A.; El-Haddad, A.E.; Ahmed, A.M.S.; Mohamed, O.G.; Enan, E.T.; Mosallam, S.; Tripathi, A.; Selim, H.M.R.M.; et al. Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals 2023, 16, 1379. [Google Scholar] [CrossRef]
- Panda, J.; Mishra, A.K.; Mohanta, Y.K.; Patowary, K.; Rauta, P.R.; Mishra, B. Exploring Biopolymer for Food and Pharmaceuticals Application in the Circular Bioeconomy: An Agro-Food Waste-to-Wealth Approach. Waste Biomass Valorization 2024, 15, 5607–5637. [Google Scholar] [CrossRef]
- Kučuk, N.; Primožič, M.; Kotnik, P.; Knez, Ž.; Leitgeb, M. Mango Peels as an Industrial By-Product: A Sustainable Source of Compounds with Antioxidant, Enzymatic, and Antimicrobial Activity. Foods 2024, 13, 553. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Miyamoto, A.; Hoang, H.T.; Vu, T.T.T.; Pothinuch, P.; Nguyen, H.T.T. Effects of Maturation on Antibacterial Properties of Vietnamese Mango (Mangifera indica) Leaves. Molecules 2024, 29, 1443. [Google Scholar] [CrossRef]
- Al-Naymi, H.A.S.; Mahmoudi, E.; Kamil, M.M.; Almajidi, Y.Q.; Al-Musawi, M.H.; Mohammadzadeh, V.; Ghorbani, M.; Mortazavi Moghadam, F. A Novel Designed Nanofibrous Mat Based on Hydroxypropyl Methyl Cellulose Incorporating Mango Peel Extract for Potential Use in Wound Care System. Int. J. Biol. Macromol. 2024, 259, 129159. [Google Scholar] [CrossRef]
- Veeruraj, A.; Liu, L.; Zheng, J.; Wu, J.; Arumugam, M. Evaluation of Astaxanthin Incorporated Collagen Film Developed from the Outer Skin Waste of Squid Doryteuthis Singhalensis for Wound Healing and Tissue Regenerative Applications. Mater. Sci. Eng. C 2019, 95, 29–42. [Google Scholar] [CrossRef]
- Ferreira, D.F.; da Silva, T.M.; de Melo, R.C.G.; Bastos, K.A.; Ucella-Filho, J.G.M.; Severi, J.A.; Villanova, J.C.O.; Resende, J.A. Development of a Gel Formulation with Pomegranate Peel Extract (Punica granatum L.) for Antimicrobial and Wound Healing Action. S. Afr. J. Bot. 2024, 173, 284–294. [Google Scholar] [CrossRef]
- Mahabeer, G.; Jin, S. Upcycling Food Waste into Biomaterials Applicable to Medical Products. Sustainability 2024, 16, 4473. [Google Scholar] [CrossRef]
- Shahzad, A.; Khan, A.; Afzal, Z.; Umer, M.F.; Khan, J.; Khan, G.M. Formulation Development and Characterization of Cefazolin Nanoparticles-Loaded Cross-Linked Films of Sodium Alginate and Pectin as Wound Dressings. Int. J. Biol. Macromol. 2019, 124, 255–269. [Google Scholar] [CrossRef]
- Cui, X.; Lee, J.; Ng, K.R.; Chen, W.N. Food Waste Durian Rind-Derived Cellulose Organohydrogels: Toward Anti-Freezing and Antimicrobial Wound Dressing. ACS Sustain. Chem. Eng. 2021, 9, 1304–1312. [Google Scholar] [CrossRef]
- Yu, N.; Wang, X.; Ning, F.; Jiang, C.; Li, Y.; Peng, H.; Xiong, H. Development of Antibacterial Pectin from Akebia trifoliata Var. Australis Waste for Accelerated Wound Healing. Carbohydr. Polym. 2019, 217, 58–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Pham, H.M.; Tran, S.D. The Chicken Egg: An Advanced Material for Tissue Engineering. Biomolecules 2024, 14, 439. [Google Scholar] [CrossRef]
- Mensah, R.A.; Jo, S.B.; Kim, H.; Park, S.M.; Patel, K.D.; Cho, K.J.; Cook, M.T.; Kirton, S.B.; Hutter, V.; Sidney, L.E.; et al. The Eggshell Membrane: A Potential Biomaterial for Corneal Wound Healing. J. Biomater. Appl. 2021, 36, 912–929. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Nooeaid, P.; Thanyacharoen, T.; Techasakul, S.; Pavasant, P.; Kanjanamekanant, K. Injectable Eggshell-Derived Hydroxyapatite-Incorporated Fibroin-Alginate Composite Hydrogel for Bone Tissue Engineering. Int. J. Biol. Macromol. 2021, 193, 799–808. [Google Scholar] [CrossRef]
- Nayak, S.K.; Baliyarsingh, B.; Singh, A.; Mannazzu, I.; Mishra, B.B. Advances in Agricultural and Industrial Microbiology: Applications of Microbes for Sustainable Agriculture and In-Silico Strategies; Springer: Singapore, 2022; Volume 2, ISBN 9789811696824. [Google Scholar]
- Perwez, M.; Al Asheh, S. Valorization of Agro-Industrial Waste through Solid-State Fermentation: Mini Review. Biotechnol. Rep. 2025, 45, e00873. [Google Scholar] [CrossRef]
- Asagbra, A.E.; Sanni, A.I.; Oyewole, O.B. Solid-State Fermentation Production of Tetracycline by Streptomyces Strains Using Some Agricultural Wastes as Substrate. World J. Microbiol. Biotechnol. 2005, 21, 107–114. [Google Scholar] [CrossRef]
- Mahalaxmi, Y.; Sathish, T.; Subba Rao, C.; Prakasham, R.S. Corn Husk as a Novel Substrate for the Production of Rifamycin B by Isolated Amycolatopsis sp. RSP 3 under SSF. Process Biochem. 2010, 45, 47–53. [Google Scholar] [CrossRef]
- El-Housseiny, G.S.; Ibrahim, A.A.; Yassien, M.A.; Aboshanab, K.M. Production and Statistical Optimization of Paromomycin by Streptomyces Rimosus NRRL 2455 in Solid State Fermentation. BMC Microbiol. 2021, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Kalaiyarasi, M.; Ahmad, P.; Vijayaraghavan, P. Enhanced Production Antibiotics Using Green Gram Husk Medium by Streptomyces sp. SD1 Using Response Surface Methodology. J. King Saud Univ.—Sci. 2020, 32, 2134–2141. [Google Scholar] [CrossRef]
- Espro, C.; Paone, E.; Mauriello, F.; Gotti, R.; Uliassi, E.; Bolognesi, M.L.; Rodríguez-Padrón, D.; Luque, R. Sustainable Production of Pharmaceutical, Nutraceutical and Bioactive Compounds from Biomass and Waste. Chem. Soc. Rev. 2021, 50, 11191–11207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cao, Y.; Shan, F.; Huang, P.; Yang, Y.; Liu, S. Analyses of Chemical Components and Their Functions in Single Species Plant-Derived Exosome like Vesicle. TrAC—Trends Anal. Chem. 2023, 167, 117274. [Google Scholar] [CrossRef]
- Buratta, S.; Latella, R.; Chiaradia, E.; Salzano, A.M.; Tancini, B.; Pellegrino, R.M.; Urbanelli, L.; Cerrotti, G.; Calzoni, E.; Alabed, H.B.R.; et al. Characterization of Nanovesicles Isolated from Olive Vegetation Water. Foods 2024, 13, 835. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-Derived Extracellular Vesicles: A Novel Nanomedicine Approach with Advantages and Challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef]
- Veerman, R.E.; Teeuwen, L.; Czarnewski, P.; Güclüler Akpinar, G.; Sandberg, A.S.; Cao, X.; Pernemalm, M.; Orre, L.M.; Gabrielsson, S.; Eldh, M. Molecular Evaluation of Five Different Isolation Methods for Extracellular Vesicles Reveals Different Clinical Applicability and Subcellular Origin. J. Extracell. Vesicles 2021, 10, e12128. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Álvarez-Chávez, C.R.; Edwards, S.; Moure-Eraso, R.; Geiser, K. Sustainability of Bio-Based Plastics: General Comparative Analysis and Recommendations for Improvement. J. Clean. Prod. 2012, 23, 47–56. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endres, H.J. Bio-Based Plastics—A Review of Environmental, Social and Economic Impact Assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- Jõgi, K.; Bhat, R. Valorization of Food Processing Wastes and By-Products for Bioplastic Production. Sustain. Chem. Pharm. 2020, 18, 100326. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Ilyas, R.A.; Hawanis, H.S.N.; Khalina, A.; Jumaidin, R.; Asyraf, M.R.M.; Nurazzi, N.M.; Norrrahim, M.N.F.; Rajeshkumar, L.; et al. Introduction to Bio-Based Packaging Materials. Phys. Sci. Rev. 2023. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Yuvaraj, D.; Iyyappan, J.; Gnanasekaran, R.; Ishwarya, G.; Harshini, R.P.; Dhithya, V.; Chandran, M.; Kanishka, V.; Gomathi, K. Advances in Bio Food Packaging—An Overview. Heliyon 2021, 7, e07998. [Google Scholar] [CrossRef]
- Shen, Y.; Seidi, F.; Ahmad, M.; Liu, Y.; Saeb, M.R.; Akbari, A.; Xiao, H. Recent Advances in Functional Cellulose-Based Films with Antimicrobial and Antioxidant Properties for Food Packaging. J. Agric. Food Chem. 2023, 71, 16469–16487. [Google Scholar] [CrossRef]
- García-Guzmán, L.; Cabrera-Barjas, G.; Soria-Hernández, C.G.; Castaño, J.; Guadarrama-Lezama, A.Y.; Rodríguez Llamazares, S. Progress in Starch-Based Materials for Food Packaging Applications. Polysaccharides 2022, 3, 136–177. [Google Scholar] [CrossRef]
- Fatima, S.; Khan, M.R.; Ahmad, I.; Sadiq, M.B. Recent Advances in Modified Starch Based Biodegradable Food Packaging: A Review. Heliyon 2024, 10, e27453. [Google Scholar] [CrossRef]
- Santhosh, R.; Ahmed, J.; Thakur, R.; Sarkar, P. Starch-Based Edible Packaging: Rheological, Thermal, Mechanical, Microstructural, and Barrier Properties—A Review. Sustain. Food Technol. 2024, 2, 307–330. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Zhang, Y.; Xu, E.; Yan, S.; Xu, H.; Li, M. Recent Advances in the Fabrication, Characterization and Application of Starch-Based Materials for Active Food Packaging: Hydrogels and Aerogels. Sustain. Food Technol. 2024, 2, 615–634. [Google Scholar] [CrossRef]
- Forte, J.; Hanieh, P.N.; Poerio, N.; Olimpieri, T.; Ammendolia, M.G.; Fraziano, M.; Fabiano, M.G.; Marianecci, C.; Carafa, M.; Bordi, F.; et al. Mucoadhesive Rifampicin-Liposomes for the Treatment of Pulmonary Infection by Mycobacterium abscessus: Chitosan or ε-Poly-L-Lysine Decoration. Biomolecules 2023, 13, 924. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Wrońska, N.; Katir, N.; Nowak-Lange, M.; El Kadib, A.; Lisowska, K. Biodegradable Chitosan-Based Films as an Alternative to Plastic Packaging. Foods 2023, 12, 3519. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-Based Composite Films and Their Application in Food Packaging: A Review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- de Vargas, V.H.; Marczak, L.D.F.; Flôres, S.H.; Mercali, G.D. Morphology and Functional Properties of Gelatin-Based Films Modified by UV Radiation and Bacterial Cellulose Nanofibers. J. Food Process Eng. 2023, 46, e14399. [Google Scholar] [CrossRef]
- Yu, M.; Hou, Y.; Zheng, L.; Han, Y.; Wang, D. Soy Protein Isolate-Based Active Films Functionalized with Zanthoxylum Bungeanum by-Products: Effects on Barrier, Mechanical, Antioxidant and Cherry Tomato Preservation Performance. Int. J. Biol. Macromol. 2023, 253, 127539. [Google Scholar] [CrossRef]
- Abang, S.; Wong, F.; Sarbatly, R.; Sariau, J.; Baini, R.; Besar, N.A. Bioplastic Classifications and Innovations in Antibacterial, Antifungal, and Antioxidant Applications. J. Bioresour. Bioprod. 2023, 8, 361–387. [Google Scholar] [CrossRef]
- García-Juárez, A.; Garzón-García, A.M.; Ramos-Enríquez, J.R.; Tapia-Hernández, J.A.; Ruiz-Cruz, S.; Canizales-Rodríguez, D.F.; Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Ocaño-Higuera, V.M.; Ornelas-Paz, J.d.J. Evaluation of Antioxidant and Antibacterial Activity of Gelatin Nanoparticles with Bitter Orange Peel Extract for Food Applications. Foods 2024, 13, 3838. [Google Scholar] [CrossRef]
- Arafat, Y.; Altemimi, A.; Pratap-Singh, A.; Badwaik, L.S. Active Biodegradable Films Based on Sweet Lime Peel Residue and Its Effect on Quality of Fish Fillets. Polymers 2021, 13, 1240. [Google Scholar] [CrossRef]
- Fiorentini, C.; Garrido, G.D.; Bassani, A.; Cortimiglia, C.; Zaccone, M.; Montalbano, L.; Martinez-Nogues, V.; Cocconcelli, P.S.; Spigno, G. Citrus Peel Extracts for Industrial-Scale Production of Bio-Based Active Food Packaging. Foods 2022, 11, 30. [Google Scholar] [CrossRef]
- Arslan, D.; Tuccitto, N.; Auditore, A.; Licciardello, A.; Marletta, G.; Riolo, M.; La Spada, F.; Conti Taguali, S.; Calpe, J.; Meca, G.; et al. Chitosan-Based Films Grafted with Citrus Waste-Derived Antifungal Agents: An Innovative and Sustainable Approach to Enhance Post-Harvest Preservation of Citrus Fruit. Int. J. Biol. Macromol. 2024, 264, 130514. [Google Scholar] [CrossRef]
- Masssijaya, S.Y.; Lubis, M.A.R.; Nissa, R.C.; Nurhamiyah, Y.; Nugroho, P.; Antov, P.; Lee, S.H.; Papadopoulos, A.N.; Kusumah, S.S.; Karlinasari, L. Utilization of Spent Coffee Grounds as a Sustainable Resource for the Synthesis of Bioplastic Composites with Polylactic Acid, Starch, and Sucrose. J. Compos. Sci. 2023, 7, 512. [Google Scholar] [CrossRef]
- Mendes, J.F.; Martins, J.T.; Manrich, A.; Sena Neto, A.R.; Pinheiro, A.C.M.; Mattoso, L.H.C.; Martins, M.A. Development and Physical-Chemical Properties of Pectin Film Reinforced with Spent Coffee Grounds by Continuous Casting. Carbohydr. Polym. 2019, 210, 92–99. [Google Scholar] [CrossRef]
- Dordevic, D.; Dordevic, S.; Abdullah, F.A.A.; Mader, T.; Medimorec, N.; Tremlova, B.; Kushkevych, I. Edible/Biodegradable Packaging with the Addition of Spent Coffee Grounds Oil. Foods 2023, 12, 2626. [Google Scholar] [CrossRef]
- Amiri Samani, S.; PourvatanDoust, S.; Savarolyia, M.; Aboutalebzadeh, S.; Khezri, M.; Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Valorization of Red Grape Pomace for Sustainable Food Packaging: Development of Pectin/Kidney Bean Protein Based Biocomposite Films Enriched with Grape Pomace Polyphenols. Food Hydrocoll. 2025, 160, 110806. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Marasciulo, C.; Maggi, F.; Caprioli, G.; Mustafa, A.M.; Fini, P.; De Vietro, N.; Aresta, A.M.; Cosma, P. Realizing Eco-Friendly Water-Resistant Sodium-Alginate-Based Films Blended with a Polyphenolic Aqueous Extract from Grape Pomace Waste for Potential Food Packaging Applications. Int. J. Mol. Sci. 2023, 24, 11462. [Google Scholar] [CrossRef]
- Gowman, A.; Wang, T.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Bio-Poly(Butylene Succinate) and Its Composites with Grape Pomace: Mechanical Performance and Thermal Properties. ACS Omega 2018, 3, 15205–15216. [Google Scholar] [CrossRef]
- Diaz-Herrera, R.; Alvarez-Pérez, O.B.; Ventura-Sobrevilla, J.; Ascacio-Valdés, A.; Aguilar-Gonzalez, M.A.; Buenrostro-Figueroa, J.; Aguilar, C.N. Pomegranate Peel Polyphenols as an Antioxidant Additive for the Development and Characterization of a New Active Pectin Edible Film. eFood 2023, 4, e115. [Google Scholar] [CrossRef]
- Nabeel Ahmad, H.; Yong, Y.; Wang, S.; Munawar, N.; Zhu, J. Development of Novel Carboxymethyl Cellulose/Gelatin-Based Edible Films with Pomegranate Peel Extract as Antibacterial/Antioxidant Agents for Beef Preservation. Food Chem. 2024, 443, 138511. [Google Scholar] [CrossRef]
- Lammi, S.; Le Moigne, N.; Djenane, D.; Gontard, N.; Angellier-Coussy, H. Dry Fractionation of Olive Pomace for the Development of Food Packaging Biocomposites. Ind. Crops Prod. 2018, 120, 250–261. [Google Scholar] [CrossRef]
- Fiorentini, C.; Leni, G.; de Apodaca, E.D.; Fernández-de-Castro, L.; Rocchetti, G.; Cortimiglia, C.; Spigno, G.; Bassani, A. Development of Coated PLA Films Containing a Commercial Olive Leaf Extract for the Food Packaging Sector. Antioxidants 2024, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Chabni, A.; Bañares, C.; Sanchez-Rey, I.; Torres, C.F. Active Biodegradable Packaging Films Based on the Revalorization of Food-Grade Olive Oil Mill By-Products. Appl. Sci. 2025, 15, 312. [Google Scholar] [CrossRef]
- Apicella, A.; Adiletta, G.; Di Matteo, M.; Incarnato, L. Valorization of Olive Industry Waste Products for Development of New Eco-Sustainable, Multilayer Antioxidant Packaging for Food Preservation. Chem. Eng. Trans. 2019, 75, 85–90. [Google Scholar] [CrossRef]
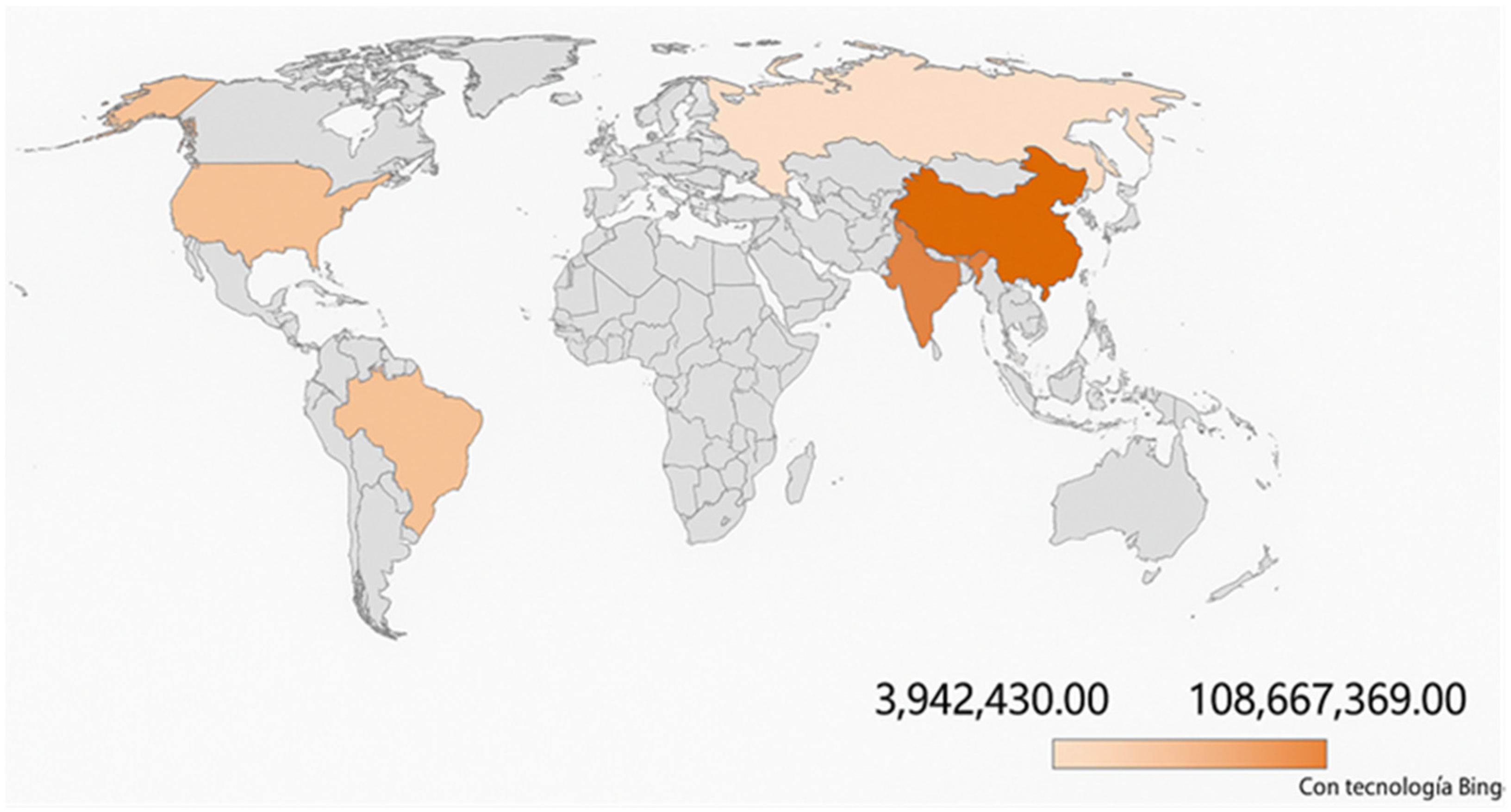
| Country | 2024 SDG Index Score | SDG2: No Hunger | SDG3: Good Health and Well-Being | SDG12: Responsible Consumption and Production | SDG13: Climate Action | ||||
|---|---|---|---|---|---|---|---|---|---|
| Italy | 79.29 | → | ➚ | → | ➚ | ||||
| BRICS members * | 67.89 | → | ➚ | → | → | ||||
| BRICS Plus members † | 67.01 | → | ➚ | → | → | ||||
| East and South Asia | 66.53 | → | ➚ | → | → | ||||
| Eastern Europe and Central Asia | 70.56 | → | ➚ | → | → | ||||
| Latin America and the Caribbean | 70.15 | → | → | ➚ | ↑ | ||||
| Middle East and North Africa | 65.60 | ↓ | → | ➚ | ➚ | ||||
| OECD members | 77.25 | → | ➚ | → | → | ||||
| Small island developing states | 64.62 | → | → | → | |||||
| Sub-Saharan Africa | 53.73 | → | → | ↑ | ↑ | ||||
| Low-income countries | 51.02 | → | → | ↑ | ➚ | ||||
| Lower-middle-income countries | 63.18 | → | ➚ | ↓ | → | ||||
| Upper-middle-income countries | 71.04 | → | ➚ | → | → | ||||
| High-income countries | 77.61 | → | ➚ | → | → | ||||
| World | 66.30 | → | ➚ | → | → | ||||
| Green Extraction Methods | Extraction Conditions | Agri-Food By-Products | Main Extracted Metabolites/Bioactive Metabolites | Analytical Methods | Ref. |
|---|---|---|---|---|---|
| UAE | T 1: 45 °C S 2: ethanol (50%) t 3: 4–10 min | Bael fruit pulp | Polyphenols, flavonoids, carotenoids | UV-Vis | [36] |
| T: 20–60 °C S: soy oil t: 30 min | Pomegranate peel | Carotenoids | HPLC-DAD | [32] | |
| T: 0 °C S: ethanol 80% t: 27 min | Chestnut burs, shells, and leaves | Polyphenols | HPLC-DAD, LC-MS | [33] | |
| T: 67 °C S: water (pH 1.5) t: 28 min | Grapefruit | Pectin | FTIR, SEM | [37] | |
| MAE | T: 62 °C S: ethanol (46%) t: 27 min | Date seeds | Polyphenols | HPLC-DAD | [38] |
| T: 80 °C S: ethanol (63%) t: 15 min | Tomato seeds | TPC, polyphenols | UV-Vis, HPLC-DAD-MS | [39] | |
| PW 4: 428.02 W S: water S/M 5: 18.43 mL/g t: 2.23 min | Grape juice waste | TMA | UV-Vis | [40] | |
| T: 60 °C S: NADES (glucose/glycerol/lactic acid, 1:2:5) S/M: 20 mL/g t: 30 min | Blueberry by-products | TPC, anthocyanins | UV-Vis | [41] | |
| T: 107 °C S: water S/M: 50 mL/g t: 5 min | Chestnut shells | TPC, TAC | UV-Vis | [42] | |
| PW: 1000 W S: ethanol (50%) t: 4.5 min | Pistachio shells | TPC, TFC, polyphenols, flavonoids | UV-Vis, HPLC/ESI-MS/MS, NMR | [43] | |
| PLE | T: 120 °C S: ethanol (30%) P 6: 10.3 MPa t: 10 min | Grape stems | Polyphenols | RP-HPLC-PAD-MS | [44] |
| T: 40 °C (anthocyanins) T: 100 °C (phenolics) S: ethanol–water | Grape stems | anthocyanins, phenolic compounds | UHPLC-QToF-MS, UHPLC-UV-Vis | [45] | |
| T: 75 °C S: water P: 10 MPa t: 27 min | Artichoke and cardoon wastes | Inulin | GC-MS | [46] | |
| T: 65 °C S: ethanol (50%) t: 30 min | Asparagus wastes and by-products | Flavonols, phenylpropanoids | HPLC-MS | [47] | |
| T: 40 °C S: water P: 10.3 MPa t: 5 min | Pomegranate wastes | TPC, flavonoids, tannins | HPLC-DAD-MS | [48] | |
| T: 100 °C S: ethanol (50%; 75%) | Pineapple residues | Phenolics, flavonoids, carotenoids | HPLC-DAD-MS, UV–Vis | [49] | |
| T: 200 °C S: ethanol (50%) | Avocado peel | Phenolic compounds | HPLC-DAD-ESI-TOF-MS | [50] | |
| S: ethanol (different %) P: 10 MPa t: 20 min | Olive pomace | Hydroxytyrosol, tyrosol, oleuropein | HPLC-DAD- MS/MS | [51] | |
| SWE | T: 150 °C P: 10 MPa F 7: 10 mL/min | Defatted orange peels | Flavanones | HPLC-UV | [52] |
| T: 60 °C S: NADES 30% (w/w) of choline chloride and oxalic acid | Grape pomace | Anthocyanins | HPLC-DAD-ESI-MS/MS | [53] | |
| T: 120 °C S: ethanol (95%) P: 3 MPa | Pomelo (Citrus grandis (L.) Osbeck) peels | Low-methoxyl (LM) pectin | FT-IR | [54] | |
| T: 100 °C S: choline chloride with urea (30%) P: 10 MPa t: 10 min (two cycles) | Winery by-products | Catechin, epicatechin | HPLC-DAD | [55] | |
| HHPE | T: 45 °C; 55 °C S: methanol (50%;70%) P: 600 MPa | Tomato peels | Polyphenols | HPLC-DAD | [56] |
| P: 300–500 MPa t: 5–10 min | Olive leaves | TPC | HPLC-DAD, FT-IR | [57] | |
| P: 200 MPa t: 5 min | Potato peels | Pectin | FT-IR, NMR | [58] | |
| SFE | T: 801 °C P: 38 MPaS/M: 1034 Kg CO2 Kg−1 d.m. | Tomato skins and seeds | Lycopene | HPLC- UV-Vis | [59] |
| T: 60.2 °C P: 40 MPa F (CO2): 64.6 g min−1 | Tomato seeds | Tocoferols, fatty acids | HPLC-DAD, GC-MS, GC-FID | [60] | |
| S: ethanol P: 17 MPa F (CO2): 2.7 Kgh−1 t: 120 min | Bitter orange (C. aurantium) peels | Coumarin (osthole) | GM-MS | [61] | |
| T: 40 °C P: 10 MPa F (CO2): 1.76 Kgh−1 | C. aurantium and C. sinensis peels | Terpenes, coumarins | GC-FID/MS | [62] | |
| T: 40 °C P: 8 MPa F (CO2): 1.2 kgh−1 | Peels of C. limonia, C. deliciosa, C. latifolia, and C. sinensis | Coumarins, polymethoxyflavonoids | LC-MS, GC-MS | [63] | |
| T: 60 °C S: ethanol (20%) P: 30 MPa | Orange, tangerine, and lemon peels | TPC, polyphenols, terpenes | UV-Vis, HPLC-DAD, GC-MS | [64] | |
| T: 25 °C S: ethanol (10%) P: 8 MPa | Citrus peel | TPC, hesperidin | UV-Vis | [65] | |
| PEF | S: hexane/acetone/hanol (50:25:25) E 8: 5 kV/cm tp 9: 90 µs | Tomato peels | Carotenoids | UV-Vis, HPLC/DAD | [66] |
| S: acetone E: 1,2,3,4, and 5 kV/cm tp: 15 µs | Tomato peels and seeds | Carotenoids | UV-Vis, HPLC/DAD | [67] | |
| S: ethanol/water E: 1.2, 1.8, and 3.0 kV/cm tp: 100 µs | Grape pomace | TPC | UV-Vis, HPLC/DAD | [68] | |
| S: ethanol/water E: 4.6 kV/cm tp: 20 µs | Grape pomace | TPC, flavonoids, anthocyanins, tannins | UV-Vis, HPLC/DAD | [69] | |
| HVED | S: ethanol (24%) LSR: 50 mL/g E: 11 kV/cm F: 200 mL/min t: 20 min | Spent coffee grounds | TPC | UV-Vis | [70] |
| S: water S/M: 35 mL/g E: 29 kV/cm F: 12 mL/min | Pomegranate peels | Total polyphenols | UV-Vis | [71] |
| Source | Biological Activity | Source | Biological Activity |
|---|---|---|---|
| Apple pomace | Antimicrobial [105], antioxidant [118,120], metabolic regulatory [105,137,143] | Grape seeds | Antimicrobial [105] |
| Broccoli by-products | Antioxidant [120], metabolic regulatory [105] | Olive by-products | Antioxidant [116], chemopreventive [130,133] |
| Celery by-products | Chemopreventive [134] | Pepper peel | Metabolic regulatory [135] |
| Cereal brans | Immunomodulatory [127], metabolic regulatory [127,139] | Pomegranate peel | Antimicrobial [105,114], antioxidant [116,118,121], anti-inflammatory [126], chemopreventive [131], metabolic regulatory [105] |
| Citrus peel | Antimicrobial [105], chemopreventive [113] | Rice husk | Antioxidant [118] |
| Coffee silverskin | Antioxidant [118] | Tomato pomace | Chemopreventive [132] |
| Grape pomace | Antimicrobial [105], antioxidant [118,120], metabolic regulatory [105] |
| Food Matrix | By-Product | Main Bioactive Compounds | Functional Effects | Potential Administration Way | References |
|---|---|---|---|---|---|
| Citrus sinensis (L.) Osbeck | Pomace | Cyanide-3-glucoside Narirutin Hesperidin | Antioxidant Anti-inflammatory | Oral | [176] |
| Olea europaea L. | Mill wastewater | Hydroxytyrosol Tyrosol | Anti-inflammatory Antioxidant | Ophthalmic | [177] |
| Psidium guajava | Peel Pulp Seed | Apigenin Chlorogenic acid Myricetin | Antioxidant | Oral | [178] |
| Cajanus cajan (L.) Huth | Protein from mill waste | Protein hydrolysate | Antioxidant | Oral | [179] |
| Prunus persica (L.) Batsch Solanum lycopersicum L. Olea europaea L. | Thinning waste (nectarine) Peel (tomato) Leaves (olive) | Abscisic acid (nectarine) Carotenoids (tomato) Oleuropein (olive) | Antidiabetic | Oral | [180] |
| Olea europaea L. | Extruded olive paste (“Pâté”) | Hydroxytyrosol Tyrosol Verbascoside Luteolin | Antioxidant | Oral | [181] |
| Prunus persica (L.) Batsch | Thinning waste | Chlorogenic acid Neochlorogenic acid | Antioxidant Antidiabetic | Oral | [181] |
| Olea europaea L. | Mill wastewater | Hydroxytyrosol glycol Hydroxytyrosol Tyrosol Verbascoside Oleuropein | Antioxidant Improved skin hydration Reduced skin photo-irritation | Topical Oral | [182] |
| Prunus dulcis (Mill.) D. A, Webb | Peel | Catechin Procyanidin B3 | Antioxidant Anti-inflammatory Antidiabetic | Oral | [183] |
| Persea americana Mill. | Seed | Catechin Hydroxybenzoic acid Rutin | Antioxidant | Oral | [184] |
| Cynara cardunculus L. | External bracts and stems | Caffeoylquinic acids Luteolin-7-glucoside | Antidiabetic | Oral | [185] |
| Fragaria x ananassa Duchesne Rubus fruticosus L. Sambucus nigra L. Sorbus aucuparia L. | Seeds (strawberry, blackberry, elderberry) Pomace (rowanberry) | β-sitosterol Proanthocyanidins Chlorophylls | Antioxidant UV radiation absorption Antimicrobial | Topical | [186] |
| Corylus avellana L. | Shell | Shikimic acid Ellagic acid Dihydroxy stearic acid Gallagic acid dilactone | Antioxidant Antidiabetic | Oral | [187] |
| Plukenetia volubilis L. | Seed shell | Phenolic compounds | Antimicrobial | Oral | [188] |
| Olea europaea L. | Leaves | Oleuropein | Antioxidant | Oral | [189] |
| Spondias mombin L. | Peel | Quercetin Ellagic acid Chlorogenic acid | Antioxidant | Oral | [190] |
| By-Product | Polymer Matrix | Key Features | Reference |
|---|---|---|---|
| Citrus peel | Gelatin, glycerol, PLA/PHB, chitosan | Improved antioxidant and antimicrobial activity; enhanced UV barrier and mechanical properties | [246,247,248,249] |
| Spent coffee grounds | PLA, PHBV, pectin, κ-carrageenan | Improved thermal stability, antioxidant activity, enhanced mechanical strength | [250,251,252] |
| Grape pomace | Pectin, BioPBS, sodium alginate | Antioxidant and antimicrobial activity; UV screening; improved mechanical properties | [253,254,255] |
| Pomegranate peel | Chitosan, pectin, CMC | Antioxidant and antimicrobial activity; reduced water vapor permeability | [122,256,257] |
| Olive pomace/leaf | PHBV, PLA, CMC, chitosan | Antioxidant activity; mechanical reinforcement; antimicrobial activity | [258,259,260,261] |
| Pillar | Key Barrier | Strategic Action | TRL Stage | TRL |
|---|---|---|---|---|
| Mechanistic Research | Limited understanding of in vivo effects | Identify bioactive compounds, mechanisms, and clinical studies | Basic/applied | 2–4 |
| Extraction and Formulation | Lack of scalable, standardized processes | Optimize green extraction and delivery systems | Pilot scale | 4–6 |
| Industrial Integration | High-cost variability of feedstock | Develop a modular biorefinery system to ensure raw material traceability | Scale-up | 5–7 |
| Regulatory Framework | Fragmented policies, absence of standards | Harmonize regulations, promote certifications | Regulatory | All TRLs |
| Cross-Sector Collaboration | Poor communication among disciplines | Launch educational campaign, enhance transparency, and sustainability storytelling | Deployment | 8 |
| Social Engagement | Law, consumer awareness, and potential skepticism | Deployment | 8–9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingallina, C.; Spano, M.; Prencipe, S.A.; Vinci, G.; Di Sotto, A.; Ambroselli, D.; Vergine, V.; Crestoni, M.E.; Di Meo, C.; Zoratto, N.; et al. Enhancing Human Health Through Nutrient and Bioactive Compound Recovery from Agri-Food By-Products: A Decade of Progress. Nutrients 2025, 17, 2528. https://doi.org/10.3390/nu17152528
Ingallina C, Spano M, Prencipe SA, Vinci G, Di Sotto A, Ambroselli D, Vergine V, Crestoni ME, Di Meo C, Zoratto N, et al. Enhancing Human Health Through Nutrient and Bioactive Compound Recovery from Agri-Food By-Products: A Decade of Progress. Nutrients. 2025; 17(15):2528. https://doi.org/10.3390/nu17152528
Chicago/Turabian StyleIngallina, Cinzia, Mattia Spano, Sabrina Antonia Prencipe, Giuliana Vinci, Antonella Di Sotto, Donatella Ambroselli, Valeria Vergine, Maria Elisa Crestoni, Chiara Di Meo, Nicole Zoratto, and et al. 2025. "Enhancing Human Health Through Nutrient and Bioactive Compound Recovery from Agri-Food By-Products: A Decade of Progress" Nutrients 17, no. 15: 2528. https://doi.org/10.3390/nu17152528
APA StyleIngallina, C., Spano, M., Prencipe, S. A., Vinci, G., Di Sotto, A., Ambroselli, D., Vergine, V., Crestoni, M. E., Di Meo, C., Zoratto, N., Izzo, L., Navarré, A., Adiletta, G., Russo, P., Di Matteo, G., Mannina, L., & Giusti, A. M. (2025). Enhancing Human Health Through Nutrient and Bioactive Compound Recovery from Agri-Food By-Products: A Decade of Progress. Nutrients, 17(15), 2528. https://doi.org/10.3390/nu17152528















