Effects of Leucine Supplementation in Older Adults with Sarcopenia: A Meta-Analysis
Abstract
1. Introduction
1.1. Definition of Sarcopenia
1.2. Current Management of Sarcopenic Patients
1.3. Effects of Leucine Administration in Sarcopenic Patients
1.4. Aim of This Meta-Analysis
2. Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
2.3. Inclusion and Exclusion Criteria
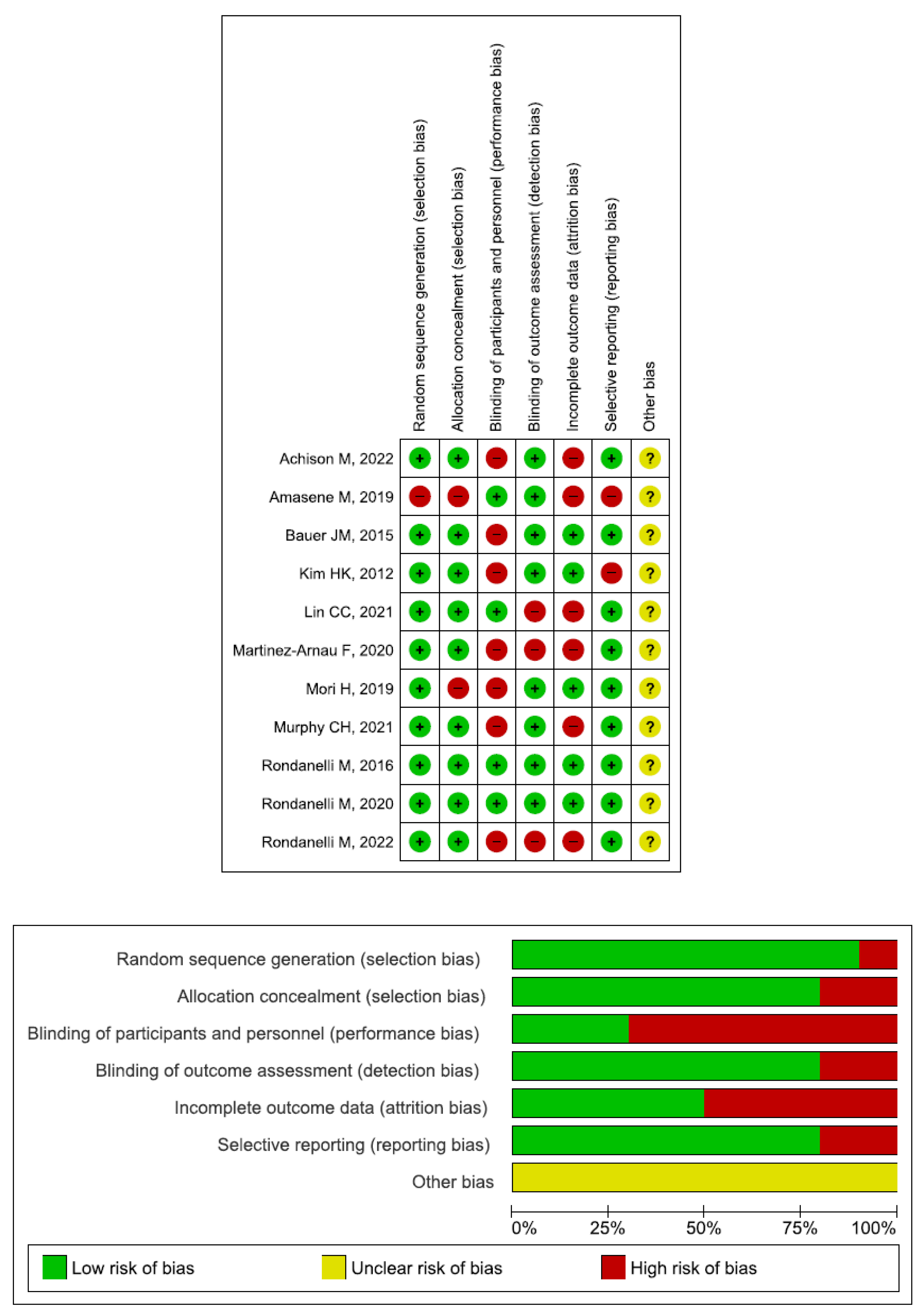
2.4. Quality Assessment and Statistical Analysis
3. Results
4. Discussion
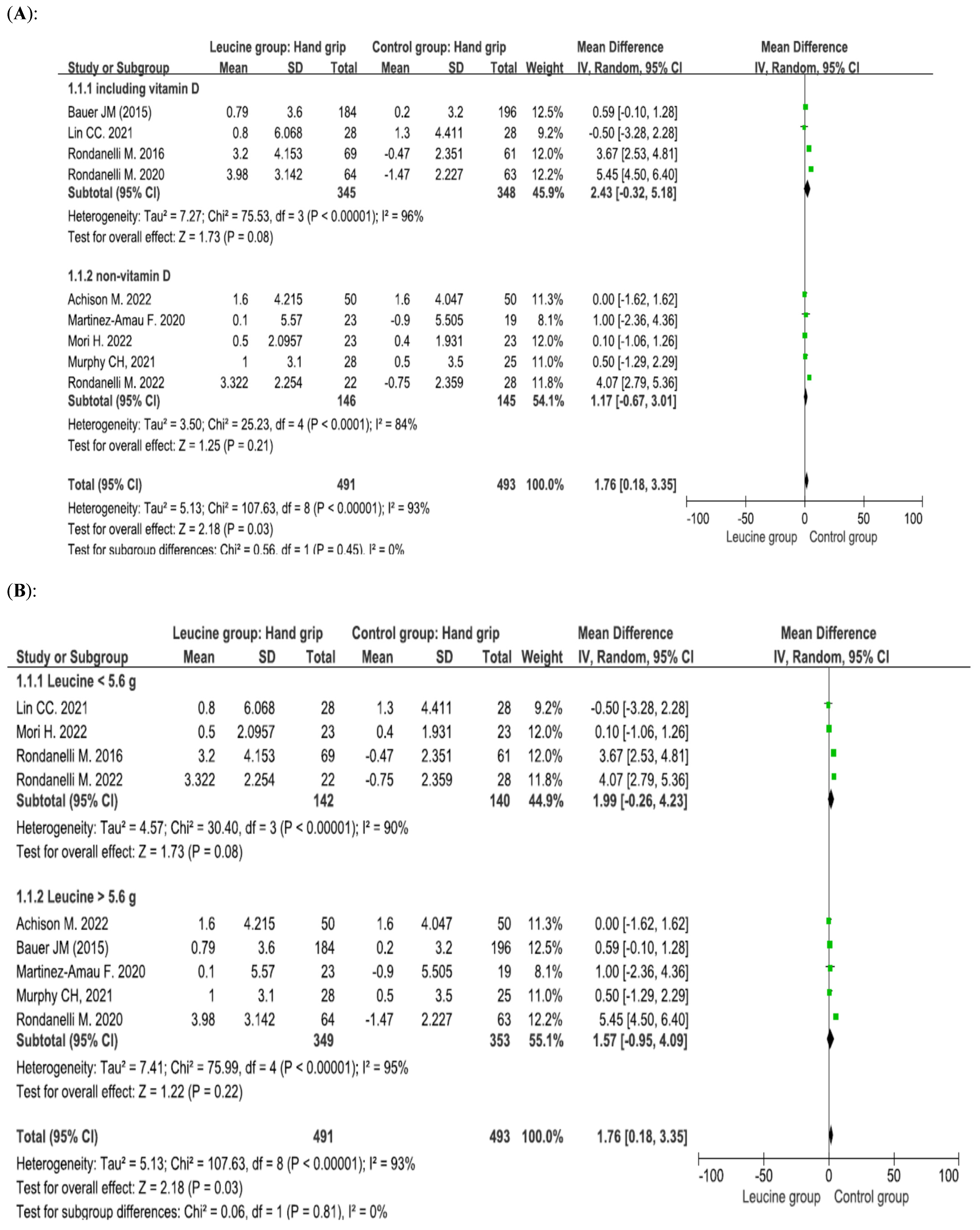
4.1. Effect of Leucine Supplementation on Handgrip Strength
4.2. Effect of Leucine Supplementation on the ASMM/Height2
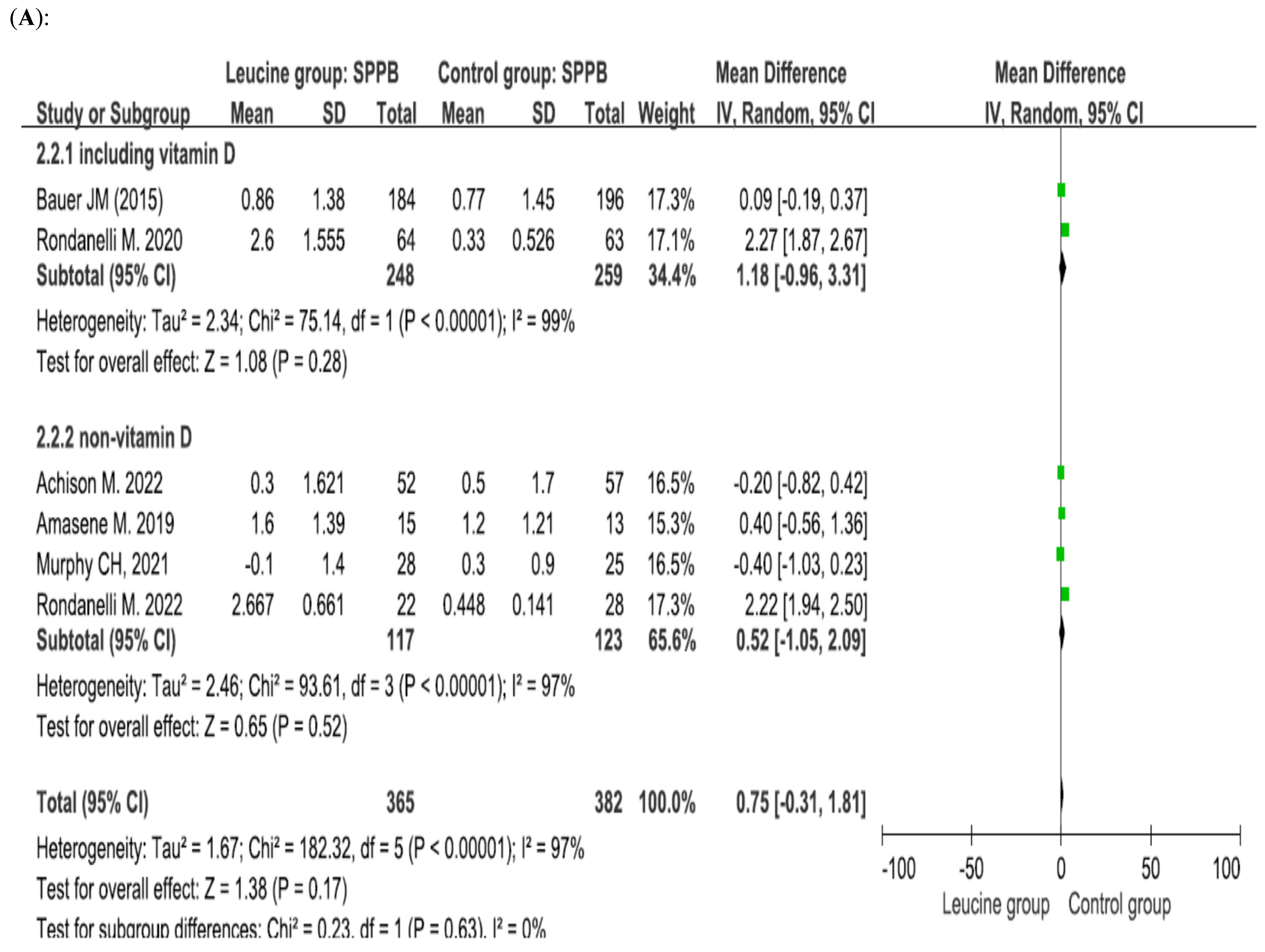
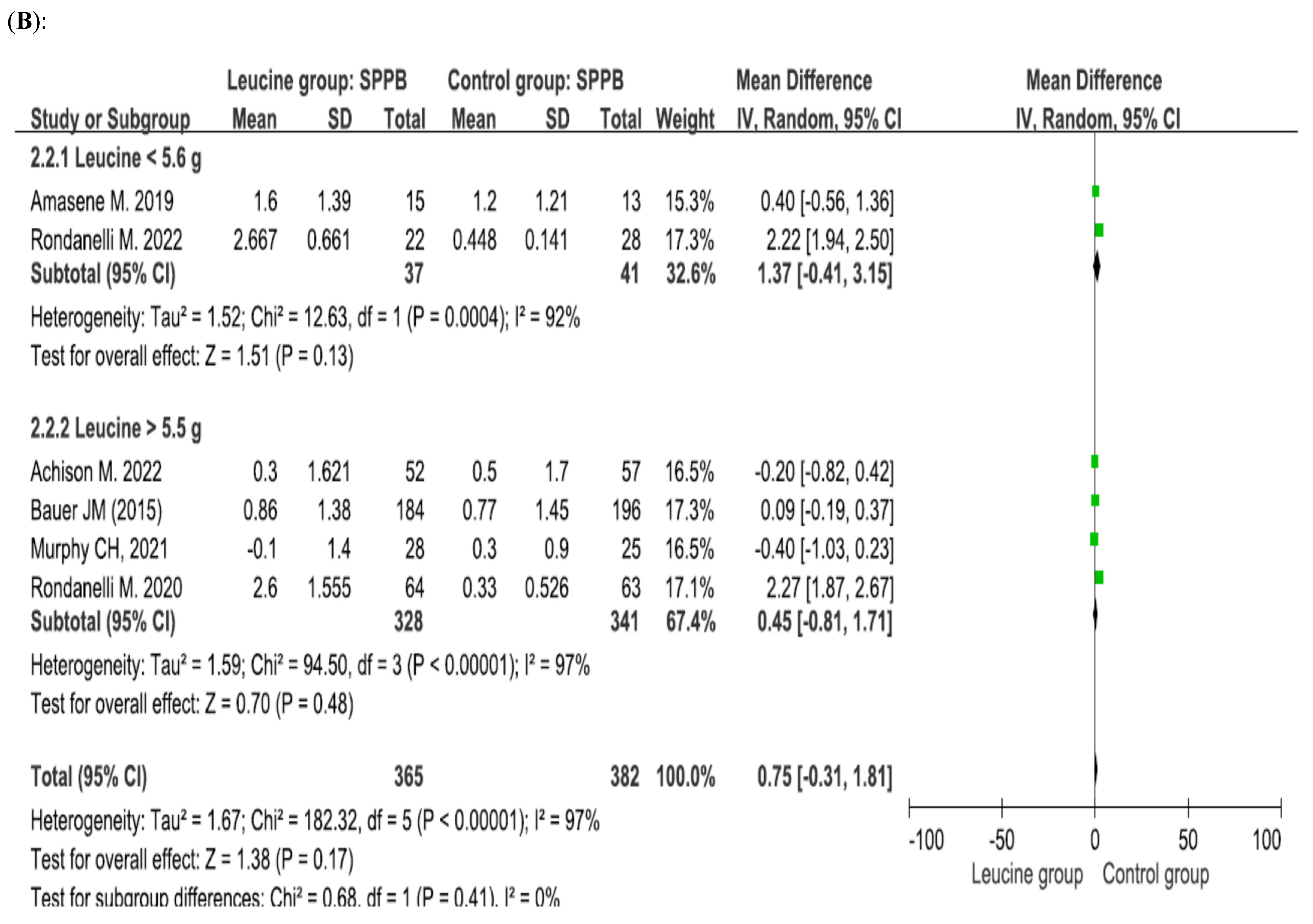
4.3. Effect of Leucine Supplementation on the SPPB Score
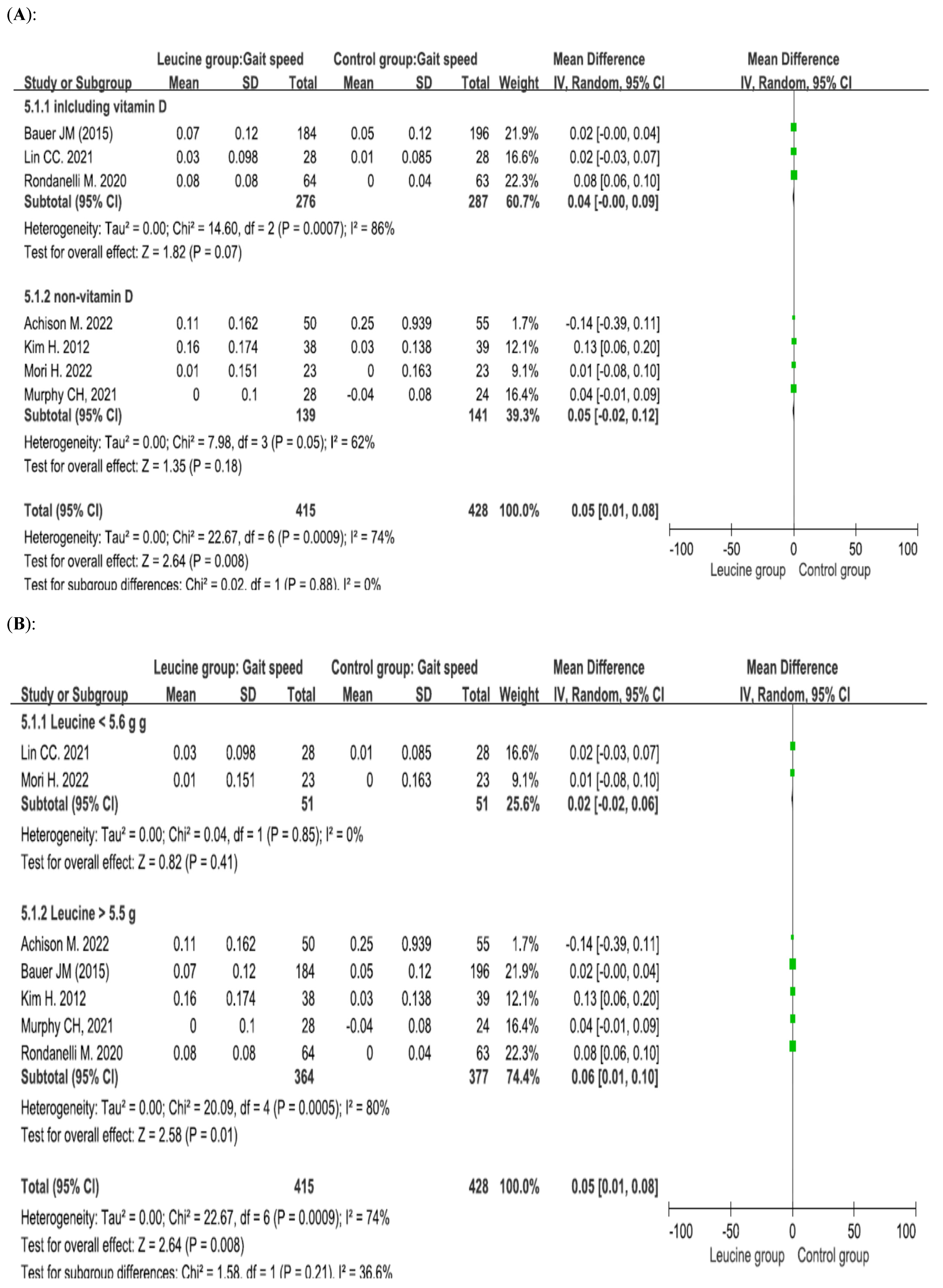
4.4. Effect of Leucine Supplementation on Gait Speed
4.5. Leucine Dosage
4.6. Vitamin D Supplementation
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Schaap, L.A.; van Schoor, N.M.; Lips, P.; Visser, M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.; May, C.; Patel, H.P.; Baxter, M.; Sayer, A.A.; Roberts, H. A feasibility study of implementing grip strength measurement into routine hospital practice (GRImP): Study protocol. Pilot Feasibility Stud. 2016, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Koster, A.; Visser, M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol. Rev. 2013, 35, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Lunt, E.; Ong, T.; Gordon, A.L.; Greenhaff, P.L.; Gladman, J.R.F. The clinical usefulness of muscle mass and strength measures in older people: A systematic review. Age Ageing 2021, 50, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hao, Q.; Hai, S.; Wang, H.; Cao, L.; Dong, B. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: A systematic review and meta-analysis. Maturitas 2017, 103, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.P.; Syddall, H.E.; Jameson, K.; Robinson, S.; Denison, H.; Roberts, H.C.; Edwards, M.; Dennison, E.; Cooper, C.; Sayer, A.A. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: Findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013, 42, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Kwon, K.S. Pharmacological interventions for treatment of sarcopenia: Current status of drug development for sarcopenia. Ann. Geriatr. Med. Res. 2019, 23, 98–104. [Google Scholar] [CrossRef]
- Moore, S.A.; Hrisos, N.; Errington, L.; Rochester, L.; Rodgers, H.; Witham, M.; Sayer, A.A. Exercise as a treatment for sarcopenia: An umbrella review of systematic review evidence. Physiotherapy 2020, 107, 189–201. [Google Scholar] [CrossRef]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyère, O.; de Saint-Hubert, M.; Bautmans, I. Exercise interventions for the prevention and treatment of sarcopenia: A systematic umbrella review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Offord, N.J.; Clegg, A.; Turner, G.; Dodds, R.M.; Sayer, A.A.; Witham, M.D. Current practice in the diagnosis and management of sarcopenia and frailty—Results from a UK-wide survey. J. Frailty Sarcopenia Falls 2019, 4, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Bae, C.H.; Han, S.J.; Bae, H. Association between length of stay in the intensive care unit and sarcopenia among hemiplegic stroke patients. Ann. Rehabil. Med. 2021, 45, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.X.; Yeo, A.; Tan, C.N.; Yew, S.; Tay, L.; Ding, Y.Y.; Lim, W.S. Combined impact of positive screen for sarcopenia and frailty on physical function, cognition and nutrition in the community-dwelling older adult. Ann. Geriatr. Med. Res. 2021, 25, 210–216. [Google Scholar] [CrossRef]
- Prior, S.J.; Ryan, A.S.; Blumenthal, J.B.; Watson, J.M.; Katzel, L.I.; Goldberg, A.P. Sarcopenia is associated with lower skeletal muscle capillarization and exercise capacity in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Dupuy, C.; Abellan van Kan, G.; Gillette, S.; Vellas, B. Treatment strategies for sarcopenia and frailty. Med. Clin. N. Am. 2011, 95, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.; Motti, M.L.; Meccariello, R.; Mazzeo, F. Resveratrol and physical activity: A winning combination for maintaining health and well-being? Nutrients 2025, 17, 837. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Casperson, S.L.; Sheffield-Moore, M.; Paddon-Jones, D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin. Nutr. 2012, 31, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; van Loon, L.J. Nutritional strategies to attenuate muscle disuse atrophy. Nutr. Rev. 2013, 71, 195–208. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.J.; Kruijshoop, M.; Menheere, P.P.; Wagenmakers, A.J.; Saris, W.H.; Keizer, H.A. Amino acid ingestion strongly enhances insulin secretion in patients with long-term type 2 diabetes. Diabetes Care 2003, 26, 625–630. [Google Scholar] [CrossRef]
- De Bandt, J.P. Leucine and mammalian target of rapamycin-dependent activation of muscle protein synthesis in aging. J. Nutr. 2016, 146, 2616S–2624S. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Cauli, O. Beneficial effects of leucine supplementation on criteria for sarcopenia: A systematic review. Nutrients 2019, 11, 2504. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of leucine administration in sarcopenia: A randomized and placebo-controlled clinical trial. Nutrients 2020, 12, 932. [Google Scholar] [CrossRef]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Amasene, M.; Besga, A.; Echeverria, I.; Urquiza, M.; Ruiz, J.R.; Rodriguez-Larrad, A.; Aldamiz, M.; Anaut, P.; Irazusta, J.; Labayen, I. Effects of leucine-enriched whey protein supplementation on physical function in post-hospitalized older adults participating in 12-weeks of resistance training program: A randomized controlled trial. Nutrients 2019, 11, 2337. [Google Scholar] [CrossRef] [PubMed]
- LACE Study Group; Achison, M.; Adamson, S.; Akpan, A.; Aspray, T.; Avenell, A.; Band, M.M.; Bashir, T.; Burton, L.A.; Cvoro, V.; et al. Effect of perindopril or leucine on physical performance in older people with sarcopenia: The LACE randomized controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Tokuda, Y. De-training effects following leucine-enriched whey protein supplementation and resistance training in older adults with sarcopenia: A randomized controlled trial with 24 weeks of follow-up. J. Nutr. Health Aging 2022, 26, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chae, M.; Park, H.; Park, K. Higher branched-chain amino acid intake is associated with handgrip strength among Korean older adults. Nutrients 2021, 13, 1522. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H., Jr. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Ebeling, P.R.; Sanders, K.M.; Aitken, D.; Winzenberg, T.; Jones, G. Vitamin D and physical activity status: Associations with five-year changes in body composition and muscle function in community-dwelling older adults. J. Clin. Endocrinol. Metab. 2015, 100, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and sarcopenia: Potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Fu, X.; Hu, Q.; Chen, L.; Zuo, H. The effect of leucine supplementation on sarcopenia-related measures in older adults: A systematic review and meta-analysis of 17 randomized controlled trials. Front. Nutr. 2022, 9, 929891. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Eccles, M.; Flottorp, S.; Guyatt, G.H.; Henry, D.; Hill, S.; Liberati, A.; O’Connell, D.; Oxman, A.D.; Phillips, B.; et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv. Res. 2004, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, S.; Maier, A.B.; Bauer, J.M.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults—The PROVIDE study. Clin. Nutr. 2018, 37, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Suzuki, T.; Saito, K.; Yoshida, H.; Kobayashi, H.; Kato, H.; Katayama, M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.H.; Flanagan, E.M.; De Vito, G.; Susta, D.; Mitchelson, K.A.J.; de Marco Castro, E.; Senden, J.M.G.; Goessens, J.P.B.; Mikłosz, A.; Chabowski, A.; et al. Does supplementation with leucine-enriched protein alone and in combination with fish-oil-derived n-3 PUFA affect muscle mass, strength, physical performance, and muscle protein synthesis in well-nourished older adults? A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2021, 113, 1411–1427. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H.; et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Cereda, E.; Klersy, C.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Gasparri, C.; Iannello, G.; Spadaccini, D.; Infantino, V.; et al. Improving rehabilitation in sarcopenia: A randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J. Cachexia Sarcopenia Muscle 2020, 11, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Gasparri, C.; Barrile, G.C.; Battaglia, S.; Cavioni, A.; Giusti, R.; Mansueto, F.; Moroni, A.; Nannipieri, F.; Patelli, Z.; et al. Effectiveness of a novel food composed of leucine, omega-3 fatty acids and probiotic Lactobacillus paracasei PS23 for the treatment of sarcopenia in elderly subjects: A 2-month randomized double-blind placebo-controlled trial. Nutrients 2022, 14, 4566. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Shih, M.H.; Chen, C.D.; Yeh, S.L. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: An open-label, parallel-group study. Clin. Nutr. 2021, 40, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Choo, Y.J. Effects of whey protein, leucine, and vitamin D supplementation in patients with sarcopenia: A systematic review and meta-analysis. Nutrients 2023, 15, 521. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, H.J.; Lim, J.Y. Effects of leucine-rich protein supplements in older adults with sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Arch. Gerontol. Geriatr. 2022, 102, 104758. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Nichetti, M.; Peroni, G.; Faliva, M.A.; Naso, M.; Gasparri, C.; Perna, S.; Oberto, L.; Di Paolo, E.; Riva, A.; et al. Where to find leucine in food and how to feed elderly with sarcopenia in order to counteract loss of muscle mass: Practical advice. Front. Nutr. 2021, 7, 622391. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.; Doehner, W.; Fearon, K.C.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Szwiega, S.; Pencharz, P.B.; Rafii, M.; Lebarron, M.; Chang, J.; Ball, R.O.; Kong, D.; Xu, L.; Elango, R.; Courtney-Martin, G. Dietary leucine requirement of older men and women is higher than current recommendations. Am. J. Clin. Nutr. 2021, 113, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Gkekas, N.K.; Anagnostis, P.; Paraschou, V.; Stamiris, D.; Dellis, S.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. The effect of vitamin D plus protein supplementation on sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2021, 145, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Giannos, P.; Katsikas Triantafyllidis, K.; Kechagias, K.S.; Mesinovic, J.; Witard, O.C.; Scott, D. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 1642–1652. [Google Scholar] [CrossRef] [PubMed]


| Author/ Year | Region | Subject Characteristics | Leucine Dose (per Day) | Common Treatment in Both Groups | Duration | Outcomes | No. of Participants (Leucine) | No. of Participants (Control) |
|---|---|---|---|---|---|---|---|---|
| Achison M, 2022 [34] | UK | ≥70 Y/O | 7.5 g | NIL | 12 months | Handgrip strength, SPPB, Gait speed | 72 | 72 |
| Amasene M, 2019 [33] | Spain | ≥70 Y/O (Post-hospitalized) | 3.0 g | Both: RTP | 12 weeks | SPPB | 15 | 13 |
| Bauer JM, 2015 [32] | EURO | ≥65 Y/O | 6.0 g | Leucine: Vitamin D 1600 IU Control: NIL | 13 weeks | Handgrip strength, SPPB, Gait speed | 184 | 196 |
| Kim HK, 2012 [45] | Japan | ≥75 Y/O (Women) | 6.0 g | Both: PMTP | 3 months | Gait speed | 38 | 39 |
| Lin CC, 2021 [50] | Taiwan | ≥65 Y/O | 3.6 g | Leucine: Vitamin D 360 IU Control: NIL | 12 weeks | Handgrip strength, ASMM, Gait speed | 28 | 28 |
| Martinez-Arnau F, 2020 [31] | EURO | ≥65 Y/O | 6 g | NIL | 13 weeks | Handgrip strength | 23 | 19 |
| Mori H, 2022 [35] | Japan | ≥65 Y/O | 2.3 g | Both: RTP | 24 weeks | Handgrip strength, ASMM, Gait speed | 23 | 23 |
| Murphy CH, 2021 [46] | EURO | ≥65 Y/O | 6.2 g | NIL | 24 weeks | Handgrip strength, SPPB, Gait speed | 28 | 25 |
| Rondanelli M, 2016 [47] | Italy | ≥65 Y/O | 4 g | Leucine: vitamin D 100 IU and PMTP. Control: PMTP | 12 weeks | Handgrip strength, ASMM, | 69 | 61 |
| Rondanelli M, 2020 [48] | Italy | ≥65 Y/O | 5.6 g | Leucine: vitamin D 1600 IU Control: NIL | 4–8 weeks | Handgrip strength, SPPB, ASMM, Gait speed | 64 | 63 |
| Rondanelli M, 2022 [49] | Italy | ≥65 Y/O | 2.5 g | Leucine: Lactobacillus paracasei PS23; omega-3 fatty acids 500 mg Control: NIL | 2 months | Handgrip strength, SPPB, ASMM | 22 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Hsieh, M.-H. Effects of Leucine Supplementation in Older Adults with Sarcopenia: A Meta-Analysis. Nutrients 2025, 17, 2413. https://doi.org/10.3390/nu17152413
Huang C, Hsieh M-H. Effects of Leucine Supplementation in Older Adults with Sarcopenia: A Meta-Analysis. Nutrients. 2025; 17(15):2413. https://doi.org/10.3390/nu17152413
Chicago/Turabian StyleHuang, Chienhsiu, and Min-Hong Hsieh. 2025. "Effects of Leucine Supplementation in Older Adults with Sarcopenia: A Meta-Analysis" Nutrients 17, no. 15: 2413. https://doi.org/10.3390/nu17152413
APA StyleHuang, C., & Hsieh, M.-H. (2025). Effects of Leucine Supplementation in Older Adults with Sarcopenia: A Meta-Analysis. Nutrients, 17(15), 2413. https://doi.org/10.3390/nu17152413







