Toxigenomic Evaluation of Diallyl Disulfide Effects and Its Association with the Chemotherapeutic Agent 5-Fluorouracil in Colorectal Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagents
2.3. Treatment Protocol
2.4. Cell Viability
2.5. Reactive Species
2.6. Cell Migration
2.7. Genotoxicity
2.8. Apoptosis
2.9. Global DNA Methylation
2.10. Protein Expression
2.11. Statistical Analysis
3. Results
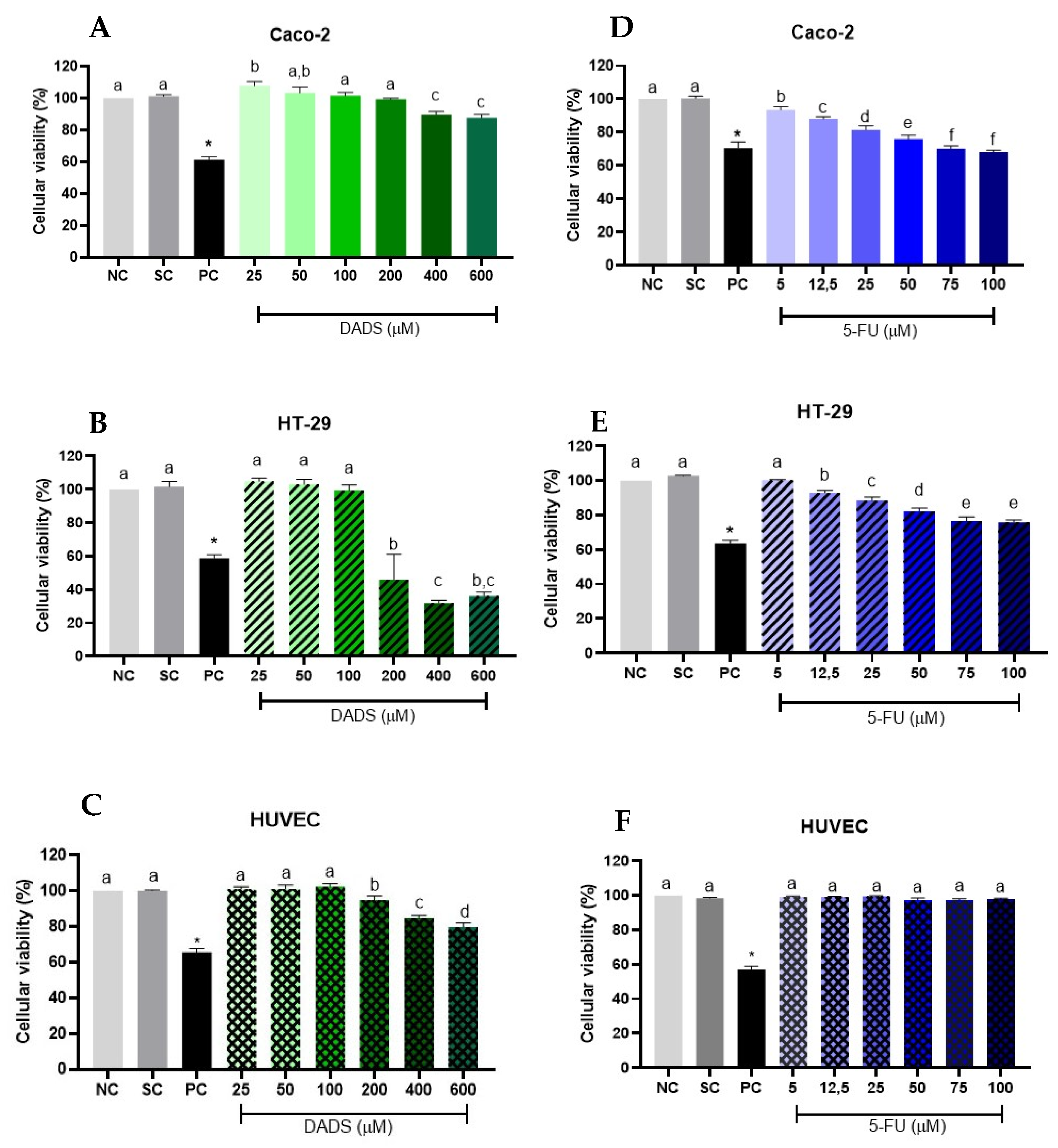
3.1. Cell Viability
3.1.1. Isolate Treatments
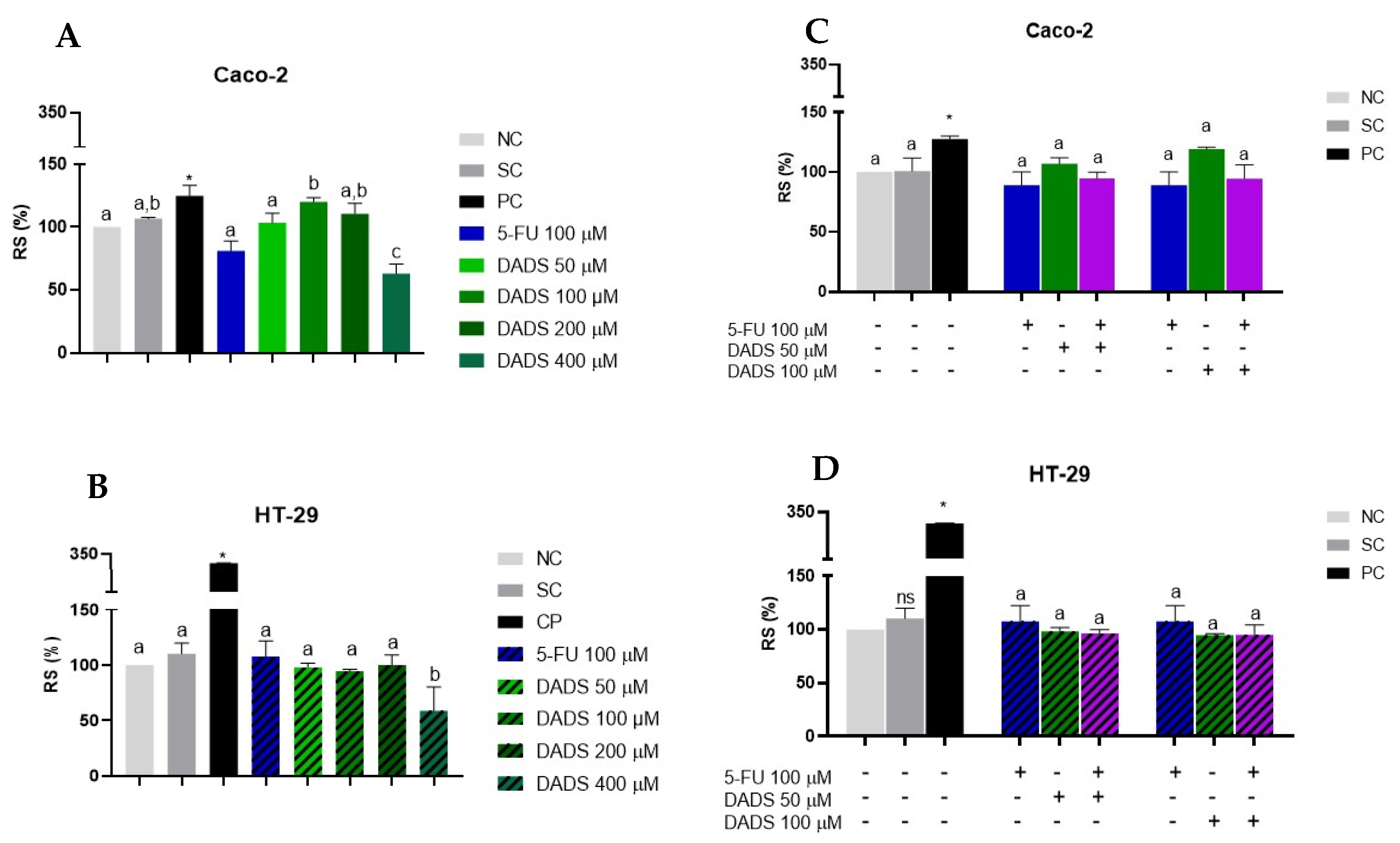
3.1.2. Associated Treatments
3.2. Reactive Species
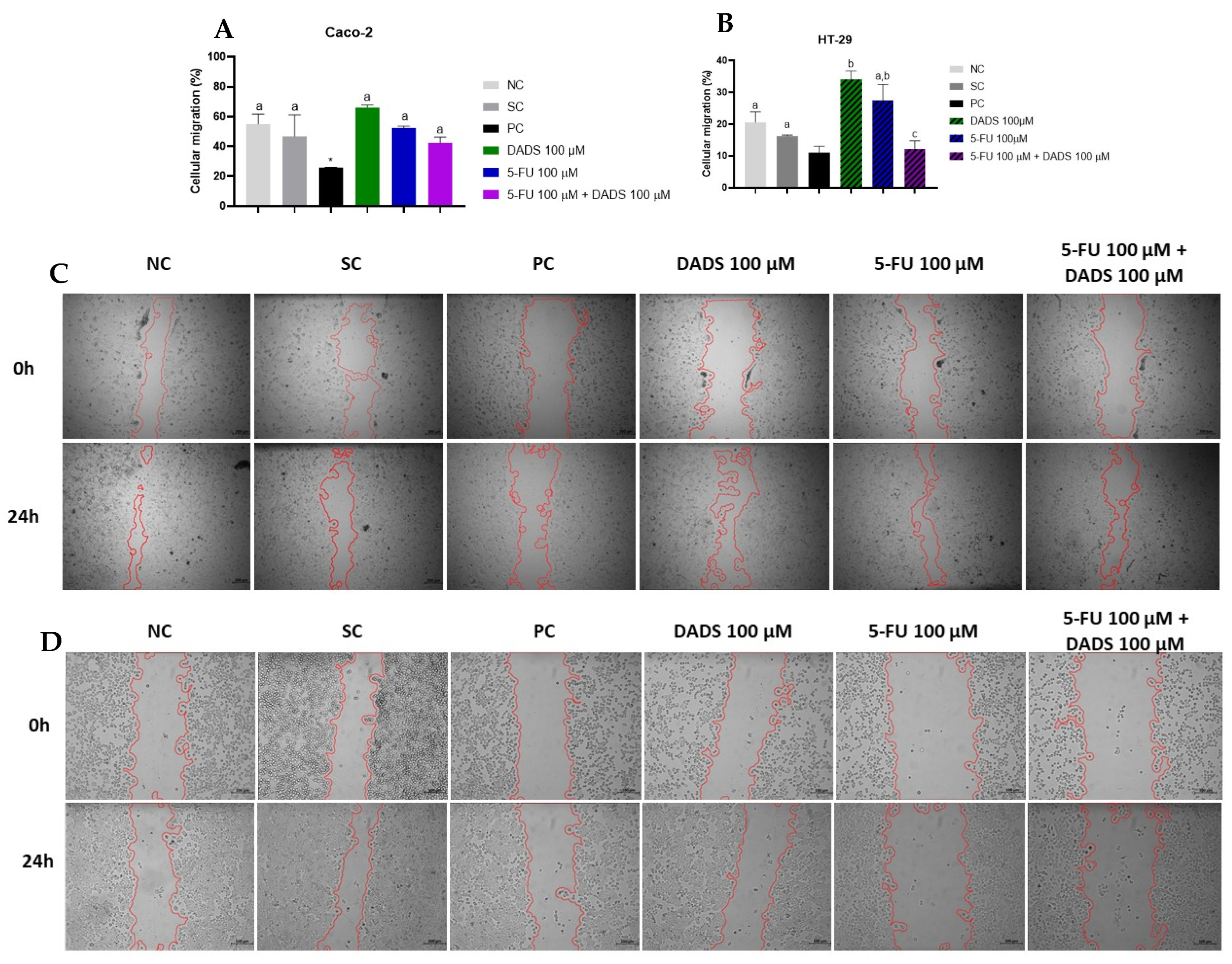
3.3. Cell Migration
3.4. Cell Death and Apoptosis
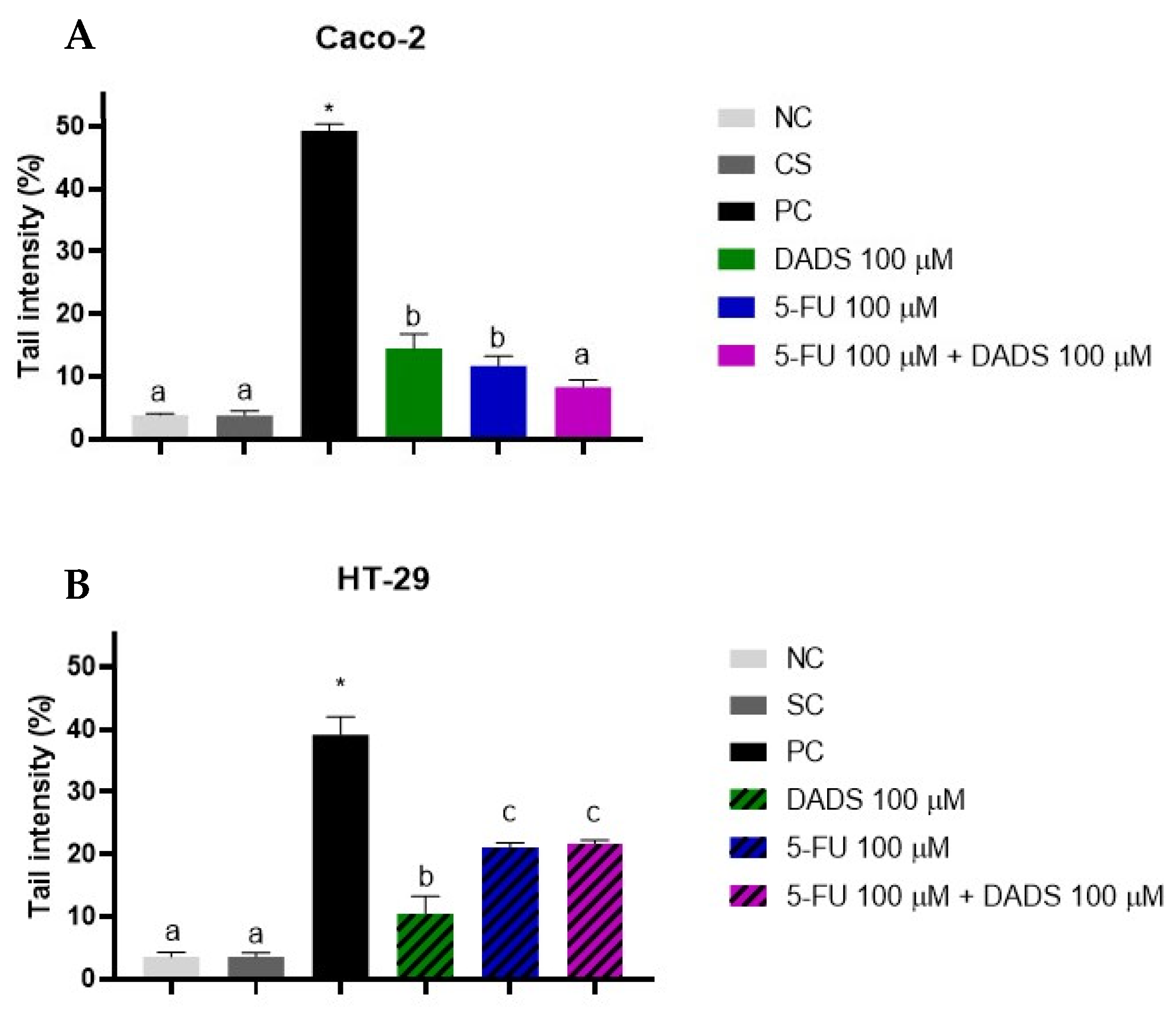
3.5. Genotoxicity
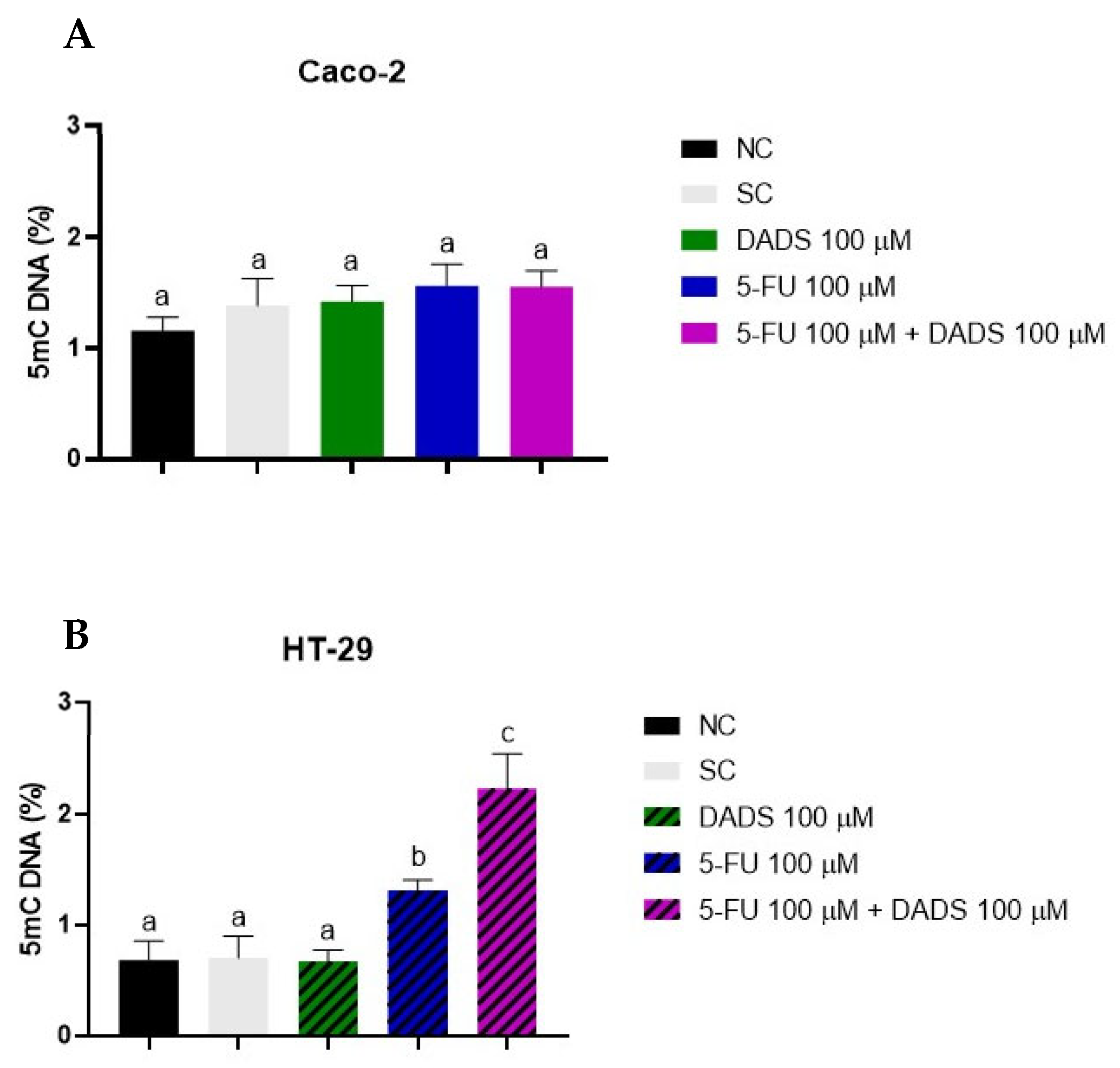
3.6. Global DNA Methylation
3.7. Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| DADS | Diallyl disulfide |
| DNMT1 | DNA Methyltransferase 1 |
| HUVEC | Human umbilical vein endothelial cell |
| MMS | Methyl methanesulfonate |
| PBS | Phosphate-buffered saline |
| RS | Reactive species |
| VEGFA | Vascular endothelial growth factor A |
| 5-FU | 5-Fluorouracil |
References
- Li, Q.; Xia, C.; Li, H.; Yan, X.; Yang, F.; Cao, M.; Zhang, S.; Teng, Y.; He, S.; Cao, M.; et al. Disparities in 36 Cancers across 185 Countries: Secondary Analysis of Global Cancer Statistics. Front. Med. 2024, 18, 911–920. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.M.; Stojanovska, V.; Bornstein, J.C.; Nurgali, K. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr. Med. Chem. 2017, 24, 1537–1557. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Xu, L.; Sun, J.; Guo, J.; Guo, S.; Li, J.; Tang, Y.; Liu, X. Transcriptional Factor KLF9 Overcomes 5-Fluorouracil Resistance in Breast Cancer via PTEN-Dependent Regulation of Aerobic Glycolysis. J. Chemother. 2024, 1–12. [Google Scholar] [CrossRef]
- Pachauri, A.; Chitme, H.; Visht, S.; Chidrawar, V.; Mohammed, N.; Abdel-Wahab, B.A.; Khateeb, M.M.; Habeeb, M.S.; Orabi, M.A.A.; Bakir, M.B. Permeability-Enhanced Liposomal Emulgel Formulation of 5-Fluorouracil for the Treatment of Skin Cancer. Gels 2023, 9, 209. [Google Scholar] [CrossRef]
- Ramaswamy, A.; Bhargava, P.; Dubashi, B.; Gupta, A.; Kapoor, A.; Srinivas, S.; Shetty, O.; Jadhav, P.; Desai, V.; Noronha, V.; et al. Docetaxel-Oxaliplatin-Capecitabine/5-Fluorouracil (DOX/F) Followed by Docetaxel versus Oxaliplatin-Capecitabine/5-Fluorouracil (CAPOX/FOLFOX) in HER2-Negative Advanced Gastric Cancers. JNCI Cancer Spectr. 2024, 8, pkae054. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; De Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-Fluorouracil and Other Fluoropyrimidines in Colorectal Cancer: Past, Present and Future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Wu, H.; Du, J.; Li, C.; Li, H.; Guo, H.; Li, Z. Kaempferol Can Reverse the 5-Fu Resistance of Colorectal Cancer Cells by Inhibiting PKM2-Mediated Glycolysis. Int. J. Mol. Sci. 2022, 23, 3544. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) Resistance and the New Strategy to Enhance the Sensitivity against Cancer: Implication of DNA Repair Inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The Current Use and Evolving Landscape of Nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef] [PubMed]
- Ozalp Unal, D.; Sel, T. Investigation of Antiproliferative Effects of Combinations of White and Black Garlic Extracts with 5-Fluorouracil (5-FU) on Caco-2 Colorectal Adenocarcinoma Cells. Mol. Nutr. Food Res. 2024, 68, 2300820. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Banerjee, S.; Bose, S.; Mazumder, S.; Haber, R.A.; Farzaei, M.H.; Bishayee, A. Garlic Constituents for Cancer Prevention and Therapy: From Phytochemistry to Novel Formulations. Pharmacol. Res. 2022, 175, 105837. [Google Scholar] [CrossRef]
- Machado, A.R.T.; Tuttis, K.; Santos, P.W.d.S.; Aissa, A.F.; Antunes, L.M.G. Diallyl Disulfide Induces Chemosensitization to Sorafenib, Autophagy, and Cell Cycle Arrest and Inhibits Invasion in Hepatocellular Carcinoma. Pharmaceutics 2022, 14, 2582. [Google Scholar] [CrossRef]
- Bal-Price, A.; Coecke, S. Guidance on Good Cell Culture Practice (GCCP). In Cell Culture Techniques; Aschner, M., Suñol, C., Bal-Price, A., Eds.; Neuromethods; Humana Press: Totowa, NJ, USA, 2011; Volume 56, pp. 1–25. ISBN 978-1-61779-076-8. [Google Scholar]
- Page, B.; Page, M.; Noel, C. A New Fluorometric Assay for Cytotoxicity Measurements In-Vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef]
- Bourgine, J.; Billaut-Laden, I.; Happillon, M.; Lo-Guidice, J.-M.; Maunoury, V.; Imbenotte, M.; Broly, F. Gene Expression Profiling of Systems Involved in the Metabolism and the Disposition of Xenobiotics: Comparison between Human Intestinal Biopsy Samples and Colon Cell Lines. Drug Metab. Dispos. 2012, 40, 694–705. [Google Scholar] [CrossRef]
- Mouradov, D.; Sloggett, C.; Jorissen, R.N.; Love, C.G.; Li, S.; Burgess, A.W.; Arango, D.; Strausberg, R.L.; Buchanan, D.; Wormald, S.; et al. Colorectal Cancer Cell Lines Are Representative Models of the Main Molecular Subtypes of Primary Cancer. Cancer Res. 2014, 74, 3238–3247. [Google Scholar] [CrossRef]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-Omics of 34 Colorectal Cancer Cell Lines—A Resource for Biomedical Studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef]
- Altonsy, M.O.; Madkour, H.; Yousef, R.; Andrews, S. Diallyl Disulfide, from Garlic Oil, Synthesizes Human Colonic Adenocarcinoma Cell Line (Caco-2) to TNF-Alpha- Mediated Apoptosis through up-Regulation of Membrane FAS Levels. Am. J. Sci. 2013, 9, 173–180. [Google Scholar]
- Marni, R.; Kundrapu, D.B.; Chakraborti, A.; Malla, R. Insight into Drug Sensitizing Effect of Diallyl Disulfide and Diallyl Trisulfide from Allium sativum L. on Paclitaxel-Resistant Triple-Negative Breast Cancer Cells. J. Ethnopharmacol. 2022, 296, 115452. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Das, R.; Emran, T.B.; Labib, R.K.; Noor-E-Tabassum; Islam, F.; Sharma, R.; Ahmad, I.; Nainu, F.; Chidambaram, K.; et al. Diallyl Disulfide: A Bioactive Garlic Compound with Anticancer Potential. Front. Pharmacol. 2022, 13, 943967. [Google Scholar] [CrossRef] [PubMed]
- Germain, E.; Auger, J.; Ginies, C.; Siess, M.-H.; Teyssier, C. In Vivo Metabolism of Diallyl Disulphide in the Rat: Identification of Two New Metabolites. Xenobiotica 2002, 32, 1127–1138. [Google Scholar] [CrossRef]
- Altonsy, M.O.; Andrews, S.C. Diallyl Disulphide, a Beneficial Component of Garlic Oil, Causes a Redistribution of Cell-Cycle Growth Phases, Induces Apoptosis, and Enhances Butyrate-Induced Apoptosis in Colorectal Adenocarcinoma Cells (HT-29). Nutr. Cancer 2011, 63, 1104–1113. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Xie, N.; Su, Q.; Su, J.; Huang, C.; Liao, Q.-J. Diallyl Disulfide Inhibits the Proliferation of HT-29 Human Colon Cancer Cells by Inducing Differentially Expressed Genes. Mol. Med. Rep. 2011, 4, 553–559. [Google Scholar] [CrossRef]
- Onar, O.; Telkoparan-Akillilar, P.; Yildirim, O. Clitocybe Nebularis Extract and 5-fluorouracil Synergistically Inhibit the Growth of HT-29 Colorectal Cancer Cells by Inducing the S Phase Arrest. 3 Biotech. 2023, 13, 48. [Google Scholar] [CrossRef]
- Yıldız, F.; Eciroğlu, H.; Suat Övey, İ.; Avnioğlu, S. Effect of Combination Treatment of Protocatechuic Acid with 5-Fluorouracil and Oxaliplatin on Colon Cancer Caco-2 Cell Line. IJEB 2023, 61. [Google Scholar] [CrossRef]
- Thejass, P.; Kuttan, G. Inhibition of Angiogenic Differentiation of Human Umbilical Vein Endothelial Cells by Diallyl Disulfide (DADS). Life Sci. 2007, 80, 515–521. [Google Scholar] [CrossRef]
- Rashidi, M.; Mohammadzadeh, G.; Sanaei, A. Quercetin Synergistically Potentiates the Anti-Angiogenic Effect of 5- Fluorouracil on HUVEC Cell Line. Preprints 2021. [Google Scholar]
- Wu, X.-J.; Kassie, F.; Mersch-Sundermann, V. The Role of Reactive Oxygen Species (ROS) Production on Diallyl Disulfide (DADS) Induced Apoptosis and Cell Cycle Arrest in Human A549 Lung Carcinoma Cells. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 579, 115–124. [Google Scholar] [CrossRef]
- Song, J.-D.; Lee, S.K.; Kim, K.M.; Park, S.E.; Park, S.-J.; Kim, K.H.; Ahn, S.C.; Park, Y.C. Molecular Mechanism of Diallyl Disulfide in Cell Cycle Arrest and Apoptosis in HCT-116 Colon Cancer Cells. J. Biochem. Mol. Toxicol. 2009, 23, 71–79. [Google Scholar] [CrossRef]
- Ozturk, R.Y.; Cakir, R. In Vitro Anticancer Efficacy of Calendula Officinalis Extract-Loaded Chitosan Nanoparticles against Gastric and Colon Cancer Cells. Drug Dev. Ind. Pharm. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matuo, R.; Sousa, F.G.; Escargueil, A.E.; Grivicich, I.; Garcia-Santos, D.; Chies, J.A.B.; Saffi, J.; Larsen, A.K.; Henriques, J.A.P. 5-Fluorouracil and Its Active Metabolite FdUMP Cause DNA Damage in Human SW620 Colon Adenocarcinoma Cell Line. J. Appl. Toxicol. 2009, 29, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef] [PubMed]
- Jakubíková, J.; Sedlák, J. Garlic-Derived Organosulfides Induce Cytotoxicity, Apoptosis, Cell Cycle Arrest and Oxidative Stress in Human Colon Carcinoma Cell Lines. Neoplasma 2006, 53, 191–199. [Google Scholar]
- Fouad, M.A.; Salem, S.E.; Hussein, M.M.; Zekri, A.R.N.; Hafez, H.F.; El Desouky, E.D.; Shouman, S.A. Impact of Global DNA Methylation in Treatment Outcome of Colorectal Cancer Patients. Front. Pharmacol. 2018, 9, 1173. [Google Scholar] [CrossRef]
- Akone, S.H.; Ntie-Kang, F.; Stuhldreier, F.; Ewonkem, M.B.; Noah, A.M.; Mouelle, S.E.M.; Müller, R. Natural Products Impacting DNA Methyltransferases and Histone Deacetylases. Front. Pharmacol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Druesne, N.; Pagniez, A.; Mayeur, C.; Thomas, M.; Cherbuy, C.; Duée, P.-H.; Martel, P.; Chaumontet, C. Diallyl Disulfide (DADS) Increases Histone Acetylation and P21 Waf1/Cip1 Expression in Human Colon Tumor Cell Lines. Carcinogenesis 2004, 25, 1227–1236. [Google Scholar] [CrossRef]
- Sarabi, M.M.; Naghibalhossaini, F. Association of DNA Methyltransferases Expression with Global and Gene-Specific DNA Methylation in Colorectal Cancer Cells. Cell Biochem. Funct. 2015, 33, 427–433. [Google Scholar] [CrossRef]
- Scott, A.; Song, J.; Ewing, R.; Wang, Z. Regulation of Protein Stability of DNA Methyltransferase 1 by Post-Translational Modifications. ABBS 2014, 46, 199–203. [Google Scholar] [CrossRef]
- Zhao, S.; Cui, H.; Fang, X.; Xia, W.; Tao, C.; Li, J. Increased DNMT1 Acetylation Leads to Global DNA Methylation Suppression in Follicular Granulosa Cells during Reproductive Aging in Mammals. BMC Genom. 2024, 25, 1030. [Google Scholar] [CrossRef]
- Lee, G.E.; Kim, J.H.; Taylor, M.; Muller, M.T. DNA Methyltransferase 1-Associated Protein (DMAP1) Is a Co-Repressor That Stimulates DNA Methylation Globally and Locally at Sites of Double Strand Break Repair. J. Biol. Chem. 2010, 285, 37630–37640. [Google Scholar] [CrossRef]
- Elliott, E.N.; Sheaffer, K.L.; Kaestner, K.H. The ‘de Novo’ DNA Methyltransferase Dnmt3b Compensates the Dnmt1-Deficient Intestinal Epithelium. eLife 2016, 5, e12975. [Google Scholar] [CrossRef]
- Van Rijnsoever, M.; Elsaleh, H.; Joseph, D.; McCaul, K.; Iacopetta, B. CpG Island Methylator Phenotype Is an Independent Predictor of Survival Benefit from 5-Fluorouracil in Stage III Colorectal Cancer. Clin. Cancer Res. 2003, 9, 2898–2903. [Google Scholar] [PubMed]
- Geißler, A.-L.; Geißler, M.; Kottmann, D.; Lutz, L.; Fichter, C.D.; Fritsch, R.; Weddeling, B.; Makowiec, F.; Werner, M.; Lassmann, S. ATM Mutations and E-Cadherin Expression Define Sensitivity to EGFR-Targeted Therapy in Colorectal Cancer. Oncotarget 2017, 8, 17164–17190. [Google Scholar] [CrossRef] [PubMed]
- Gallois, C.; Laurent-Puig, P.; Taieb, J. Methylator Phenotype in Colorectal Cancer: A Prognostic Factor or Not? Crit. Rev. Oncol./Hematol. 2016, 99, 74–80. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treviso, E.M.; Sanchez, C.A.; Rocha, C.C.S.; Aissa, A.F.; Antunes, L.M.G. Toxigenomic Evaluation of Diallyl Disulfide Effects and Its Association with the Chemotherapeutic Agent 5-Fluorouracil in Colorectal Cancer Cell Lines. Nutrients 2025, 17, 2412. https://doi.org/10.3390/nu17152412
Treviso EM, Sanchez CA, Rocha CCS, Aissa AF, Antunes LMG. Toxigenomic Evaluation of Diallyl Disulfide Effects and Its Association with the Chemotherapeutic Agent 5-Fluorouracil in Colorectal Cancer Cell Lines. Nutrients. 2025; 17(15):2412. https://doi.org/10.3390/nu17152412
Chicago/Turabian StyleTreviso, Estefani Maria, Caroline Andolfato Sanchez, Cecília Cristina Souza Rocha, Alexandre Ferro Aissa, and Lusânia Maria Greggi Antunes. 2025. "Toxigenomic Evaluation of Diallyl Disulfide Effects and Its Association with the Chemotherapeutic Agent 5-Fluorouracil in Colorectal Cancer Cell Lines" Nutrients 17, no. 15: 2412. https://doi.org/10.3390/nu17152412
APA StyleTreviso, E. M., Sanchez, C. A., Rocha, C. C. S., Aissa, A. F., & Antunes, L. M. G. (2025). Toxigenomic Evaluation of Diallyl Disulfide Effects and Its Association with the Chemotherapeutic Agent 5-Fluorouracil in Colorectal Cancer Cell Lines. Nutrients, 17(15), 2412. https://doi.org/10.3390/nu17152412







