The Potential of Nutraceutical Supplementation in Counteracting Cancer Development and Progression: A Pathophysiological Perspective
Abstract
1. Introduction
1.1. Complexities of Cancer Pathogenesis
1.2. Nutrition and Nutraceutical Compounds in Cancer Risk Modulation
1.2.1. Bioactive Compounds from Citrus bergamia Risso & Poiteau and Cynara cardunculus L.
1.2.2. Bioactive Polyphenolic Compounds in Olive Oil
1.2.3. The Key Bioactive Role of Quercetin and Resveratrol
1.2.4. Bioactive Compounds from Ferula communis L.
2. Biological Functions and Pathological Implications of Reactive Oxygen and Nitrogen Species
2.1. Oxidative Stress in Cancer Development: The Role of Scavenger Enzymes
2.2. Pathways Involved in Oxidative Stress-Induced Tumorigenesis
The Dual Action of Nutraceutical Compounds and Their Therapeutic Role
3. Highlights of the Inflammatory Process
3.1. Hallmarks of Cancer Inflammation
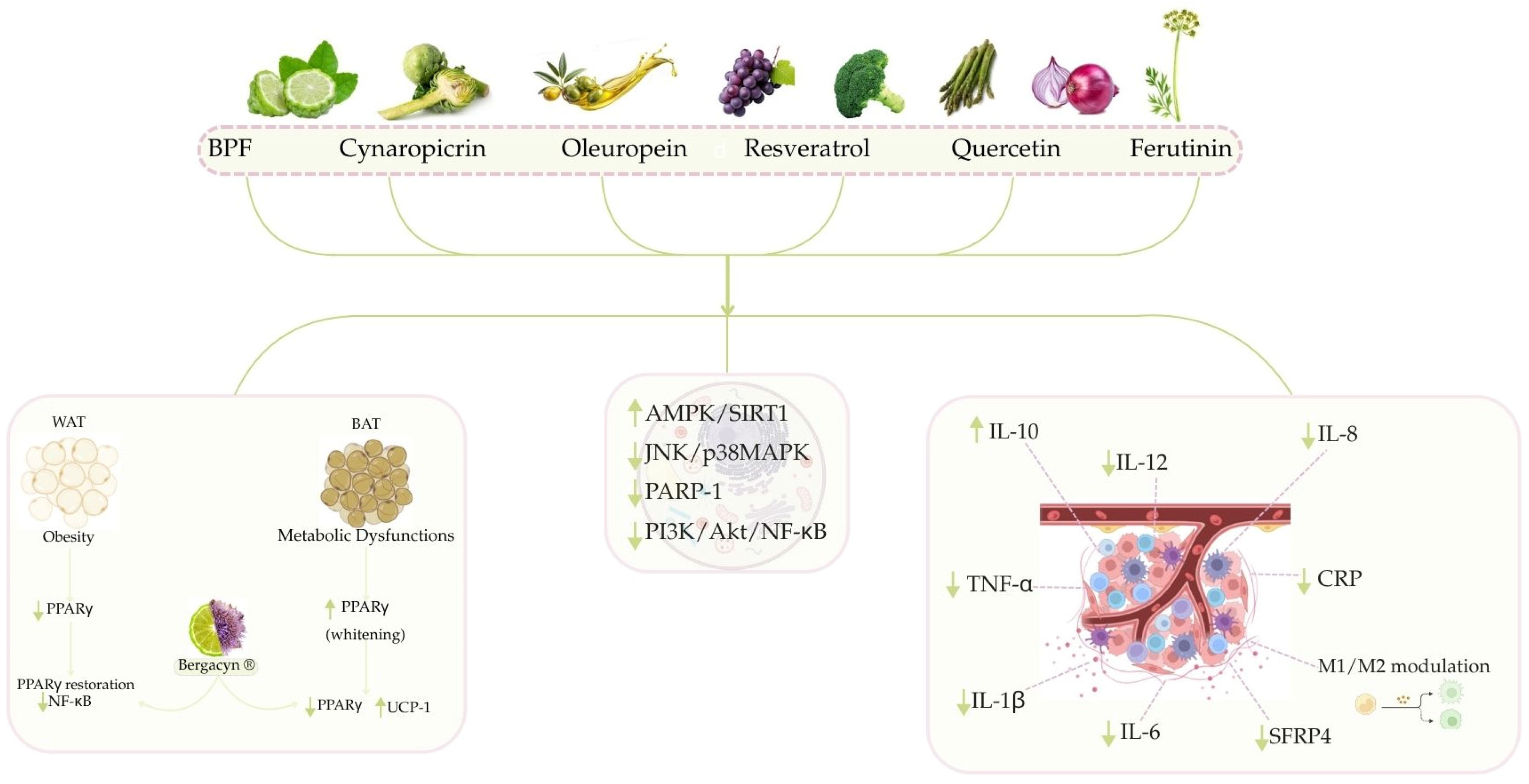
3.2. Modulation of Inflammatory Pathways by Nutraceutical Compounds from the Mediterranean Diet
3.2.1. Anti-Inflammatory Activity of Citrus Bergamia
3.2.2. The Beneficial Effects of Cynaropicrin and Bergacyn® to Counteract Inflammation
3.2.3. Anti-Inflammatory Activity of Oleuropein
3.2.4. The Beneficial Role of Resveratrol and Quercetin to Counteract Inflammation
3.2.5. Preliminary Evidence of Anti-Inflammatory Activity of Ferutinin
4. Cell Cycle Regulation: Key Mechanisms and Links to Tumorigenesis
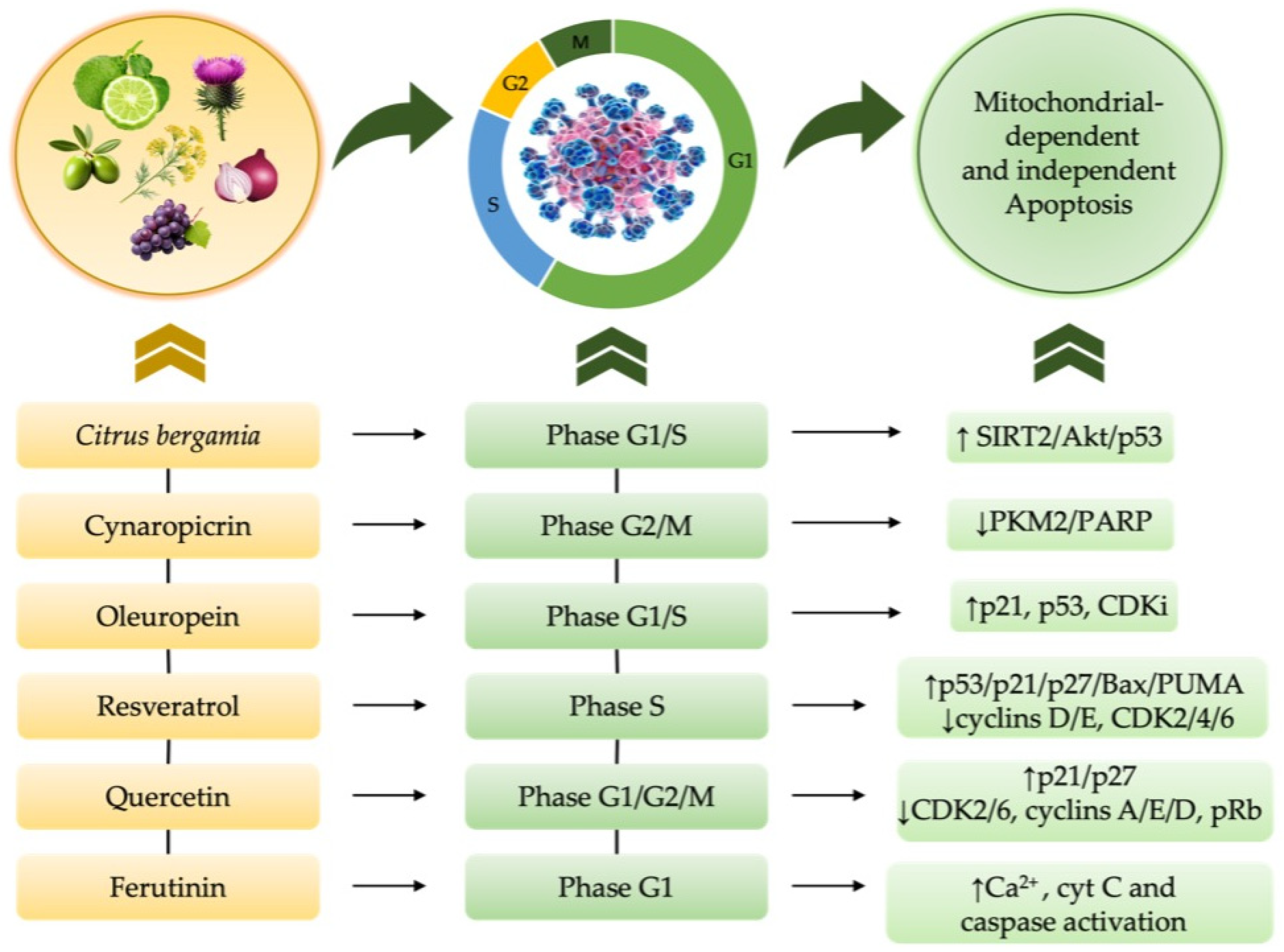
4.1. Mechanistic Insights into Cell Cycle Alterations in Cancer Development
4.2. Nutraceutical-Induced Cell Cycle Modulation in Cancer and Chemotherapy—Induced Cytotoxicity
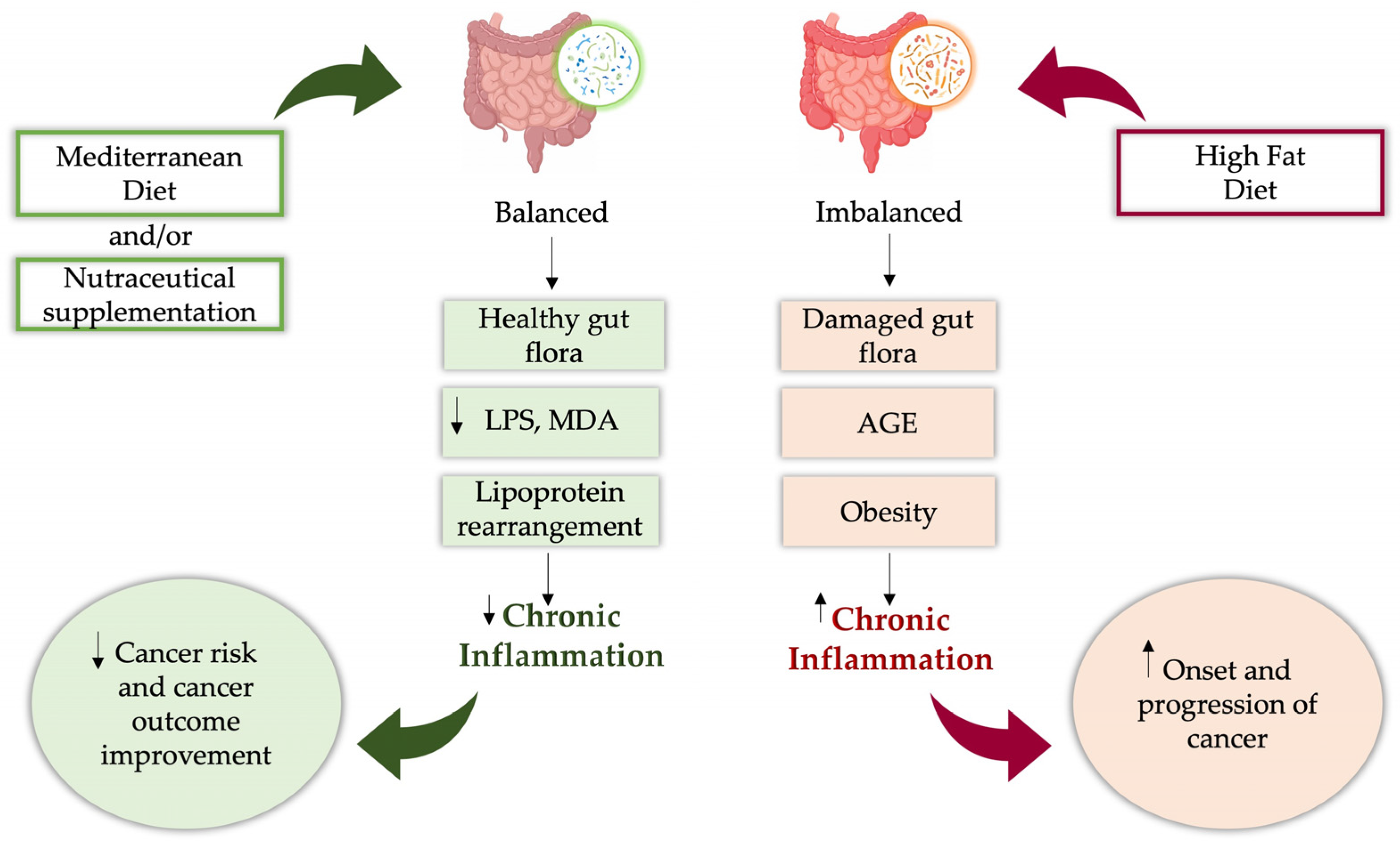
5. The Human Microbial Landscape in Cancer Development
Emerging Connections and Therapeutic Perspectives of Gut Microbiota and Nutraceuticals in Cancer
6. Limitations of Nutraceutical Supplementation: Current Challenges and Emerging Evidence-Based Strategies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 8-oxodG | 8-oxo-2′-deoxyguanosine |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| 3-NT | 3-nitrotyrosine |
| AGEs | advanced glycation end products |
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| APAF1 | apoptotic protease-activating factor 1 |
| ARE | antioxidant response element |
| ATM | ataxia-telangiectasia mutated |
| ATP | Adenosine Triphosphate |
| BAD | BCL2 associated agonist of cell death |
| BAT | brown adipose tissue |
| Bax | BCL2 Associated X, Apoptosis Regulator |
| BC | breast cancer |
| BCL2L11 | B-cell lymphoma 2 (Bcl-2)-like protein 11 |
| BEO | bergamot essential oil |
| Birc5 | Baculoviral Inhibitor of Apoptosis Repeat-containing 5 |
| BMF | albedo and pulp-derived micronized fibers |
| BJ | bergamot juice |
| BPE | bergamot polyphenol extract |
| BPF | bergamot polyphenolic fraction |
| BRAF | B-Raf proto-oncogene serine/threonine kinase |
| c-Met | mesenchymal–epithelial transition factor |
| c-myc | cellular myelocytomatosis oncogene |
| CAFs | cancer-associated fibroblasts |
| CAT | catalase |
| CDK | cyclin-dependent kinase |
| CDKs | cyclin-dependent kinases |
| Chk1 | checkpoint kinases 1 |
| Chk2 | checkpoint kinases 2 |
| CIC | chemotherapy-induced cardiac damage |
| CIN | chromosomal instability |
| CKIs | CDK inhibitors |
| CLRs | C-type lectin receptors |
| COX-1 | cyclooxygenases 1 |
| COX-2 | cyclooxygenases 2 |
| CRC | colorectal carcinoma |
| CRP | C reactive protein |
| CSCs | cancer stem cells |
| CVD | cardiovascular disease |
| Cyn | cynaropicrin |
| Cyt C | cytochrome C |
| Daun | daunorubicin |
| DCs | dendritic cells |
| DISCs | death-inducing signaling complexes |
| DM | diabetes mellitus |
| DNA | DeoxyriboNucleic Acid |
| DNMTs | DNA methyltransferases |
| DSBs | double-strand breaks |
| Dox | doxorubicin |
| E. coli | Escherichia coli |
| EC | endometrial cancer |
| ECM | extracellular matrix |
| ECs | endothelial cells |
| eCSCs | endogenous cardiac stem cells |
| EFSA | The European Food Safety Authority |
| EGFR | epidermal growth factor receptor |
| EMT | Epithelial–Mesenchymal Transition |
| eNOS | endothelial nitric oxide synthase |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| ERK1/2 | extracellular signal-regulated kinase ½ |
| ERα | Estrogen receptor alpha |
| ERβ | Estrogen receptor beta |
| EVOO | extra virgin olive oil |
| FADD | Fas-Associated Death Domain Protein |
| FOXO | Forkhead box transcription factors |
| FOXO3a | Forkhead Transcription Factor O Subfamily Member 3a |
| FRE | Flavonoid-Rich Extract |
| GCL | glutamate-cysteine ligase |
| GGT | gamma-glutamyl transferase |
| GPX | glutathione peroxidase |
| GRX | glutathione reductase |
| GSH-PX | Glutathione Peroxidase |
| GSH | glutathione |
| GSK-3β | glycogen synthase kinase 3 beta |
| GST | glutathione transferase |
| GSTs | glutathione S-transferases |
| H. pylori | Helicobacter pylori |
| H2O2 | Hydrogen Peroxide |
| H9c2 | rat embryonic cardiac myoblast |
| HBV | Hepatitis B Virus |
| HCC | hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HDACs | histone deacetylases |
| HDL | high density lipoprotein |
| HFD | high-fat diets |
| HIF1-α | hypoxia-inducible factor 1-alpha |
| HLD | hyperlipidemic diet |
| HMOX-1 | heme oxygenase |
| HO-1 | heme oxygenase-1 |
| HOCl | hypochlorous acid |
| HPV | Human Papillomavirus |
| HRAS | Harvey Rat sarcoma virus |
| HRT | hormone therapy |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| I/R | ischemia–reperfusion |
| IARC | International Agency for Research on Cancer |
| ICAM-1 | intercellular adhesion molecule 1 |
| ICAM-2 | intercellular adhesion molecule 2 |
| IFN-γ | interferon-gamma |
| IL-10 | Interleukin-10 |
| IL-10R2 | Interleukin-10 Receptor Subunit 2 |
| IL-12 | Interleukin-12 |
| IL-13 | interleukin-13 |
| IL-1R | interleukin-1 receptor |
| IL-1α | Interleukin-1 alpha |
| IL-1β | Interleukin-1 beta |
| IL-22R1 | Interleukin-22 Receptor Subunit 1 |
| IL-23 | interleukin-23 |
| IL-4 | interleukin-4 |
| IL-6 | interleukin-6 |
| IL-6R | interleukin-6 receptor |
| IL-8 | interleukin-8 |
| iNOs | Inducible nitric oxide synthase |
| ITM | intratumoral microbiota |
| JAK-STAT | Janus kinase (JAK)/signal transducer and activator of transcription (STAT) |
| JAK1/TYK2 | Janus kinase 1 and Tyrosine kinase 2 |
| JNK-1 | c-Jun N-terminal kinase 1 |
| JNK | c-Jun amino-terminal kinase |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LDL | low density lipoprotein |
| LOX-1 | Lectin-like oxidized low-density lipoprotein receptor-1 |
| LOX | lipoxygenase |
| LPS | lipopolysaccharide |
| LTB4 | leukotriene B4 |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA | Malondialdehyde |
| MDSCs | myeloid-derived suppressor cells |
| MedDiet | Mediterranean Diet |
| MIP-2 | Macrophage-inflammatory protein 2 |
| MKP-1 | Mitogen-activated protein kinase phosphatase 1 |
| MOMP | mitochondrial outer membrane permeabilization |
| MS | metabolic syndrome |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mammalian target of rapamycin complex 1 |
| MUFAs | monounsaturated fatty acids |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NAFLD | Non-alcoholic fatty liver disease |
| NF-κβ | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGS | next-generation sequencing |
| NK | natural killer |
| NLRs | NOD-like receptors |
| NOTCH | Notch signaling pathway |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| NRAS | Neuroblastoma RAS viral oncogene homolog |
| Nrf2 | nuclear erythroid 2-related factor |
| O2− | superoxide anion |
| O2 | dioxygen |
| OMWW | Olive Mill Wastewater |
| OxLDL | oxidized low-density lipoprotein |
| P53 | tumor protein p53 |
| PAF | platelet-activating factor |
| PAMPs | pathogen-associated molecular patterns |
| PARP-1 | poly (ADP-ribose) polymerase-1. |
| PARP | poly-ADP-ribose-polymerase |
| PC | pancreatic cancer |
| PI3K | phosphatidylinositol 3-kinase |
| PKB | protein-kinase B |
| PKC | protein kinase C |
| PKD1 | Polycystin 1, Transient Receptor Potential Channel Interacting |
| PKM2 | Pyruvate kinase M2 |
| PLA2 | phospholipase A2 |
| PLK1 | polo-like kinase 1 |
| PMN-MDSC | polymorphonuclear myeloid-derived suppressor cells |
| PPARγ | peroxisome proliferator-activated receptor-gamma |
| PRX | peroxiredoxin |
| PTEN | Phosphatase and tensin homolog |
| PTMs | post-translational modifications |
| PTP1B | Tyrosine-protein phosphatase non-receptor type 1 |
| PTPs | Protein Tyrosine Phosphatases |
| PUFAs | polyunsaturated fatty acids |
| PUMA | p53 upregulated modulator of apoptosis |
| RAGE | Receptor for advanced glycation end products |
| RAS | Rat sarcoma virus |
| Rb | Retinoblastoma protein |
| Ref-1 | Redox Effector Factor-1 |
| RLRs | RIG-I-like receptors |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RV | Resveratrol |
| SAC | spindle assembly checkpoint |
| SERM | selective estrogen receptor modulator |
| SIRT1 | silent mating type information regulation 2 homolog 1 |
| SIRT2 | Silent mating type information regulation 2 homolog 2 |
| SFRP4 | secreted frizzled-related protein 4 |
| SOD | superoxide dismutase |
| STAT | Signal Transducer and Activator of Transcription |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| T2DM | type 2 diabetes mellitus |
| TAMs | tumor-associated macrophages |
| TEER | trans-epithelial electrical resistance |
| TFs | transcription factors |
| TGF-β | Transforming growth factor beta |
| Th2 | helper T 2 cells |
| TLR | Toll-like receptor |
| TME | tumor microenvironment |
| TNF-α | Tumor Necrosis Factor Alpha |
| TNFR-1 | tumor necrosis factor receptor 1 |
| Topo II | topoisomerase II |
| TRAIL-R1/2 | tumor necrosis factor-related apoptosis-inducing ligand receptors |
| Tregs | regulatory T-cells |
| TRX | thyroxine |
| TXNRD1 | thioredoxin reductase-1 |
| TxR | thioredoxin reductase |
| UV | Ultraviolet radiation |
| VEGF | Vascular Endothelial Growth Factor |
| VOO | virgin olive oil |
| WAT | white adipose tissue |
| WD SW | High-fat Western diet-fed |
| Wnt | Wingless-related integration site |
| XO | xanthine oxidase |
| ZEB1/2 | Zinc finger E-box binding homeobox 1 and Zinc finger E-box binding homeobox 2 |
| ZFP57 | zinc finger protein 57 |
References
- WHO. Cancer; World Health Organization: Geneva, Switzerland, 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 13 June 2025).
- Hausman, D.M. What Is Cancer? Perspect. Biol. Med. 2019, 62, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Akhtar, N.; Ahmed, A.; Qazi, A.S. Dietary Pattern and Cancer. Cancer Treat. Res. 2024, 191, 191–216. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Levatić, J.; Salvadores, M.; Fuster-Tormo, F.; Supek, F. Mutational signatures are markers of drug sensitivity of cancer cells. Nat. Commun. 2022, 25, 2926. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Zheng, C.C.; Huang, Y.N.; He, M.L.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2021, 10, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 20, 710304. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xia, X.; Huang, L.B.; An, H.; Cao, M.; Kim, G.D.; Chen, H.N.; Zhang, W.H.; Shu, Y.; Kong, X.; et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 2022, 4, 6619. [Google Scholar] [CrossRef] [PubMed]
- Musella, M.; Guarracino, A.; Manduca, N.; Galassi, C.; Ruggiero, E.; Potenza, A.; Maccafeo, E.; Manic, G.; Mattiello, L.; Soliman Abdel Rehim, S. Type I IFNs promote cancer cell stemness by triggering the epigenetic regulator KDM1B. Nat. Immunol. 2022, 23, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.N.; Naccarato, A.G.; Scatena, C. Recent Advances in Cancer Plasticity: Cellular Mechanisms, Surveillance Strategies, and Therapeutic Optimization. Front. Oncol. 2020, 22, 569. [Google Scholar] [CrossRef] [PubMed]
- Nyga, A.; Ganguli, S.; Matthews, H.K.; Baum, B. The role of RAS oncogenes in controlling epithelial mechanics. Trends Cell Biol. 2023, 33, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 18, 455. [Google Scholar] [CrossRef] [PubMed]
- Cordani, M.; Dando, I.; Ambrosini, G.; González-Menéndez, P. Signaling, cancer cell plasticity, and intratumor heterogeneity. Cell Commun. Signal. 2024, 3, 255. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Somarelli, J.A.; Sheth, M.; Biddle, A.; Tripathi, S.C.; Armstrong, A.J.; Hanash, S.M.; Bapat, S.A.; Rangarajan, A.; Levine, H. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol. Ther. 2019, 194, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.R.; Kim, J.L.; Kim, Y.; Kang, S.; Oh, S.C.; Park, J.K. A novel RIP1-mediated canonical WNT signaling pathway that promotes colorectal cancer metastasis via β -catenin stabilization-induced EMT. Cancer Gene Ther. 2023, 30, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Deshmukh, A.P.; den Hollander, P.; Addanki, S.; Kuburich, N.A.; Kudaravalli, S.; Joseph, R.; Chang, J.T.; Soundararajan, R.; Mani, S.A. EMTome: A resource for pan-cancer analysis of epithelial-mesenchymal transition genes and signatures. Br. J. Cancer 2021, 124, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, J.; Zhao, G.; Huang, K.C.; Cardenas, H.; Wang, Y.; Matei, D.; Cheng, J.X. Metabolic reprogramming from glycolysis to fatty acid uptake and beta-oxidation in platinum-resistant cancer cells. Nat. Commun. 2022, 5, 4554. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.D.; Chorawala, M.R.; Raghani, N.R.; Patel, R.; Fareed, M.; Kashid, V.A.; Prajapati, B.G. Tumor microenvironment: Recent advances in understanding and its role in modulating cancer therapies. Med. Oncol. 2025, 42, 117. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Asbaghi, O.; Hooshmand, F.; Aghayan, A.H.; Shariati, A.A.; Kazemi, K.; Amirpour, M.; Davoodi, S.H.; Larijani, B. Adherence to Mediterranean Diet and Breast Cancer Risk: A Meta-Analysis of Prospective Observational Studies. Health Sci. Rep. 2025, 8, 70736. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Mastrantoni, L.; Terranova, R.; Colloca, G.F.; Zuccalà, G.; Landi, F. The role of Mediterranean diet in cancer incidence and mortality in the older adults. NPJ Aging 2024, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Wu, Y.; Yang, Q.; Ge, L.; Gao, C.; Xun, Y.; Tian, J.; Ding, G. The impact of major dietary patterns on glycemic control, cardiovascular risk factors, and weight loss in patients with type 2 diabetes: A network meta-analysis. J. Evid. Based Med. 2019, 12, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Macrì, R.; Carresi, C.; Maiuolo, J.; Serra, M.; Cardamone, A.; et al. PUFA Supplementation and Heart Failure: Effects on Fibrosis and Cardiac Remodeling. Nutrients 2021, 13, 2965. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Casalino, E.; D’Alessandro, A.G.; Laudadio, V. Dietary Phenolic Compounds: Biochemistry, Metabolism and Significance in Animal and Human Health. Curr. Drug Metab. 2017, 18, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, 14264. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. Bioavailability of Food Polyphenols: Current State of Knowledge. Annu. Rev. Food Sci. Technol. 2025, 16, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Bobrysheva, T.N.; Anisimov, G.S.; Zolotoreva, M.S.; Bobryshev, D.V.; Budkevich, R.O.; Moskalev, A.A. [Polyphenols as promising bioactive compounds]. Vopr. Pitan. 2023, 92, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Luo, J.; Zhu, Y.; An, P.; Luo, Y.; Xing, Q. The Effect of Antioxidant Polyphenol Supplementation on Cardiometabolic Risk Factors: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 4206. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Du, Q.; Meng, X.; Zhang, Y. Natural polyphenols: A potential prevention and treatment strategy for metabolic syndrome. Food Funct. 2022, 13, 9734–9753. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem. Biol. Interact. 2025, 25, 111489. [Google Scholar] [CrossRef] [PubMed]
- González Mosquera, D.M.; Hernández Ortega, Y.; Fernández, P.L.; González, Y.; Doens, D.; Vander Heyden, Y.; Foubert, K.; Pieters, L. Flavonoids from Boldoa purpurascens inhibit proinflammatory cytokines (TNF-α and IL-6) and the expression of COX-2. Phytother. Res. 2018, 32, 1750–1754. [Google Scholar] [CrossRef] [PubMed]
- Singaravelan, N.; Tollefsbol, T.O. Polyphenol-Based Prevention and Treatment of Cancer Through Epigenetic and Combinatorial Mechanisms. Nutrients 2025, 17, 616. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, M.; Albonici, L.; Focaccetti, C.; Ciuffa, S.; Fazi, S.; Cifaldi, L.; Miele, M.T.; De Maio, F.; Tresoldi, I.; Manzari, V.; et al. Polyphenol-Mediated Autophagy in Cancer: Evidence of In Vitro and in vivo Studies. Int. J. Mol. Sci. 2020, 21, 6635. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Khalil, M.; Di Luca, A.; Abdallah, H.; D’Alessandro, A.G. Comprehensive Strategies for Metabolic Syndrome: How Nutrition, Dietary Polyphenols, Physical Activity, and Lifestyle Modifications Address Diabesity, Cardiovascular Diseases, and Neurodegenerative Conditions. Metabolites 2024, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, M. Multilevel Mechanisms of Cancer Drug Resistance. Int. J. Mol. Sci. 2024, 25, 12402. [Google Scholar] [CrossRef] [PubMed]
- Auti, A.; Tathode, M.; Marino, M.M.; Vitiello, A.; Ballini, A.; Miele, F.; Mazzone, V.; Ambrosino, A.; Boccellino, M. Nature’s weapons: Bioactive compounds as anti-cancer agents. AIMS Public Health 2024, 11, 747–772. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, M.; Cicero, A.F.G.; Veronesi, M.; Fogacci, F.; Riccioni, C.; Benassi, B. Effect of Citrus bergamia extract on lipid profile: A combined in vitro and human study. Phytother. Res. 2023, 37, 4185–4195. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, K.C.; Scaffo, J.; Flexa, B.N.; Gama, C.C.A.; Ferreira, M.A.; Cruz, R.A.S.; Aguiar-Alves, F.; Rocha, L.; Machado, F.P.; Fernandes, C.P. Characterization of bergamot essential oil: Chemical, microbiological and colloidal aspects. Braz. J. Biol. 2024, 26, 275622. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo- Controlled Study. Endocr. Metab. Immune Disord.-Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, A.R.; Tarantini, A.; Bruno, A.; Logrieco, A.F.; Gallo, A.; Mita, G.; Valerio, F.; Bleve, G.; Cardinali, A. Functional foods in Mediterranean diet: Exploring the functional features of vegetable case-studies obtained also by biotechnological approaches. Aging Clin. Exp. Res. 2024, 36, 208. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Chen, Y.; Du, L.; Li, J.; Meng, X.; Lv, H.; Tong, B.; Niu, G.; Jian, T.; Chen, J. Benefits of inulin and fructo-oligosaccharides on high fat diet-induced type 2 diabetes mellitus by regulating the gut microbiota in mice. J. Nutr. Biochem. 2025, 141, 109908. [Google Scholar] [CrossRef] [PubMed]
- Tsimihodimos, V.; Psoma, O. Extra Virgin Olive Oil and Metabolic Diseases. Int. J. Mol. Sci. 2024, 25, 8117. [Google Scholar] [CrossRef] [PubMed]
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A.; et al. The Mediterranean Diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur. J. Med. Chem. 2019, 181, 111579. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Bellumori, M.; Cecchi, L.; Bartolomei, M.; Bollati, C.; Clodoveo, M.L.; Corbo, F.; Arnoldi, A.; Mulinacci, N. Extra Virgin Olive Oil Phenol Extracts Exert Hypocholesterolemic Effects through the Modulation of the LDLR Pathway: In Vitro and Cellular Mechanism of Action Elucidation. Nutrients 2020, 12, 1723. [Google Scholar] [CrossRef] [PubMed]
- Rishmawi, S.; Haddad, F.; Dokmak, G.; Karaman, R. A Comprehensive Review on the Anti-Cancer Effects of Oleuropein. Life 2022, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef] [PubMed]
- Ileriturk, M.; Kandemir, O.; Kandemir, F.M. Evaluation of protective effects of quercetin against cypermethrin-induced lung toxicity in rats via oxidative stress, inflammation, apoptosis, autophagy, and endoplasmic reticulum stress pathway. Environ. Toxicol. 2022, 37, 2639–2650. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.X.; Xiong, R.G.; Huang, S.Y.; Zhou, D.D.; Saimaiti, A.; Zhao, C.N.; Shang, A.; Zhang, Y.J.; Gan, R.Y.; Li, H.B. Effects and mechanisms of resveratrol for prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2023, 63, 12422–12440. [Google Scholar] [CrossRef] [PubMed]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Zhou, Y.; Tan, Y.; Feng, W.; Peng, C. Interactions between gut microbiota and polyphenols: A mechanistic and metabolomic review. Phytomedicine 2023, 119, 154979. [Google Scholar] [CrossRef] [PubMed]
- Ed-Dahmani, I.; El Fadili, M.; Nouioura, G.; Kandsi, F.; Atki, Y.E.; Abuelizz, H.A.; Conte, R.; Zahra Lafdil, F.; Taleb, A.; Abdellaoui, A.; et al. Ferula communis leaf extract: Antioxidant capacity, UHPLC-MS/MS analysis, and in vivo and In Silico toxicity investigations. Front. Chem. 2025, 12, 1485463. [Google Scholar] [CrossRef] [PubMed]
- Macrì, R.; Maiuolo, J.; Scarano, F.; Musolino, V.; Fregola, A.; Gliozzi, M.; Carresi, C.; Nucera, S.; Serra, M.; Caminiti, R.; et al. Evaluation of the Potential Beneficial Effects of Ferula communis L. Extract Supplementation in Postmenopausal Discomfort. Nutrients 2024, 16, 2651. [Google Scholar] [CrossRef] [PubMed]
- Salerno, R.; Casale, F.; Calandruccio, C.; Procopio, A. Characterization of flavonoids in Citrus bergamia (Bergamot) polyphenolic fraction by liquid chromatography–high resolution mass spectrometry (LC/HRMS). PharmaNutrition 2016, 4, 2213–4344. [Google Scholar] [CrossRef]
- Buzzanca, C.; Di Stefano, V.; D’Amico, A.; Gallina, A.; Melilli, M.G. A systematic review on Cynara cardunculus L.: Bioactive compounds, nutritional properties and food-industry applications of a sustainable food. Nat. Prod. Res. 2024, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Muñoz-Díez, C.; Miho, H.; Zhang, L.; Li, P.; Priego, F.; Oulbi, S.; Uyanik, E.B.; Koubouris, G.; Perri, E.; et al. Evaluation of phenolics in the analysis of virgin olive oil using near infrared spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 326, 125262. [Google Scholar] [CrossRef] [PubMed]
- Ussia, S.; Ritorto, G.; Mollace, R.; Serra, M.; Tavernese, A.; Altomare, C.; Muscoli, C.; Fini, M.; Barillà, F.; Indolfi, C.; et al. Exploring the Benefits of Extra Virgin Olive Oil on Cardiovascular Health Enhancement and Disease Prevention: A Systematic Review. Nutrients 2025, 17, 1843. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants-Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Leischner, C.; Burkard, M.; Michel, A.; Berchtold, S.; Niessner, H.; Marongiu, L.; Busch, C.; Frank, J.; Lauer, U.M.; Venturelli, S. Comparative Analysis of the Antitumor Activity of Cis- and Trans-Resveratrol in Human Cancer Cells with Different p53 Status. Molecules 2021, 26, 5586. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Nouioura, G.; El Fadili, M.; El Barnossi, A.; Loukili, E.H.; Laaroussi, H.; Bouhrim, M.; Giesy, J.P.; Aboul-Soud, M.A.M.; Al-Sheikh, Y.A.; Lyoussi, B.; et al. Comprehensive analysis of different solvent extracts of Ferula communis L. fruit reveals phenolic compounds and their biological properties via in vitro and in silico assays. Sci. Rep. 2024, 14, 8325. [Google Scholar] [CrossRef] [PubMed]
- Sonigra, P.; Meena, M. Metabolic Profile, Bioactivities, and Variations in the Chemical Constituents of Essential Oils of the Ferula Genus (Apiaceae). Front. Pharmacol. 2021, 11, 608649. [Google Scholar] [CrossRef] [PubMed]
- Louvet, M.S.; Gault, G.; Lefebvre, S.; Popowycz, F.; Boulven, M.; Besse, S.; Benoit, E.; Lattard, V.; Grancher, D. Comparative inhibitory effect of prenylated coumarins, ferulenol and ferprenin, contained in the ‘poisonous chemotype’ of Ferula communis on mammal liver microsomal VKORC1 activity. Phytochemistry 2015, 118, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Naji Reyhani Garmroudi, S.; Karimi, E.; Oskoueian, E.; Homayouni-Tabrizi, M.; Iranshahi, M. Ferutinin: A phytoestrogen from ferula and its anticancer, antioxidant, and toxicity properties. J. Biochem. Mol. Toxicol. 2021, 35, 22713. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yao, J.; Xiao, M.; Zhang, X.; Zhang, M.; Xi, X. Targeting Nrf2 signaling pathway: New therapeutic strategy for cardiovascular diseases. J. Drug Target. 2024, 32, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Sciaccotta, R.; Gangemi, S.; Penna, G.; Giordano, L.; Pioggia, G.; Allegra, A. Potential New Therapies “ROS-Based” in CLL: An Innovative Paradigm in the Induction of Tumor Cell Apoptosis. Antioxidants 2024, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Baron, G.; Gianazza, E.; Banfi, C.; Carini, M.; Aldini, G. Lipid peroxidation derived reactive carbonyl species in free and conjugated forms as an index of lipid peroxidation: Limits and perspectives. Redox Biol. 2021, 42, 101899. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yang, Y.; Lou, Y.; Zuo, Z.; Cui, H.; Deng, H.; Zhu, Y.; Fang, J. Apoptosis and DNA damage mediated by ROS involved in male reproductive toxicity in mice induced by Nickel. Ecotoxicol. Environ. Saf. 2023, 268, 115679. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lu, M.; Chen, Q.; Zou, E.; Bo, Z.; Li, J.; Zhao, R.; Zhao, J.; Yu, Z.; Chen, G.; et al. Systematic profiling of mitochondria-related transcriptome in tumorigenesis, prognosis, and tumor immune microenvironment of intrahepatic cholangiocarcinoma: A multi-center cohort study. Front. Genet. 2024, 15, 1430885. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Escalante, M.L.; Coop-Gamas, F.; Cervantes-Rodríguez, M.; Méndez-Iturbide, D.; Aranda-González, I.I. The effect of diet on oxidative stress and metabolic diseases-Clinically controlled trials. J. Food Biochem. 2020, 44, 13191. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.P.; Teng, T.; Yan, L.; Tang, Q.Z. Underlying the Mechanisms of Doxorubicin-Induced Acute Cardiotoxicity: Oxidative Stress and Cell Death. Int. J. Biol. Sci. 2022, 18, 760–770. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 19, 1225578. [Google Scholar] [CrossRef] [PubMed]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-Related Enzymes and Proteins: A Review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Erbasan, H.; Riso, P.; Perna, S. Impact of the Mediterranean Diet on Athletic Performance, Muscle Strength, Body Composition, and Antioxidant Markers in Both Athletes and Non-Professional Athletes: A Systematic Review of Intervention Trials. Nutrients 2024, 16, 3454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Fogelholm, M.; Poppitt, S.D.; Silvestre, M.P.; Møller, G.; Huttunen-Lenz, M.; Stratton, G.; Sundvall, J.; Råman, L.; Jalo, E.; et al. Adherence to a Plant-Based Diet and Consumption of Specific Plant Foods-Associations with 3-Year Weight-Loss Maintenance and Cardiometabolic Risk Factors: A Secondary Analysis of the PREVIEW Intervention Study. Nutrients 2021, 13, 3916. [Google Scholar] [CrossRef] [PubMed]
- La Scala, S.; Naselli, F.; Quatrini, P.; Gallo, G.; Caradonna, F. Drought-Adapted Mediterranean Diet Plants: A Source of Bioactive Molecules Able to Give Nutrigenomic Effects per sè or to Obtain Functional Foods. Int. J. Mol. Sci. 2024, 25, 2235. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Osati, P.; Seifollahy Fakhr, S.; Faghihkhorasani, F.; Ghanaatian, M.; Faghihkhorasani, F.; Rezaei-Tazangi, F.; Pazhouhesh Far, N.; Shourideh, A.; Ebrahimi, N.; et al. New Emerging Therapeutic Strategies Based on Manipulation of the Redox Regulation Against Therapy Resistance in Cancer. Antioxid. Redox Signal. 2024. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhang, G.; Bai, W.; Han, X.; Li, C.; Bian, S. The Role of Bioactive Compounds in Natural Products Extracted from Plants in Cancer Treatment and Their Mechanisms Related to Anticancer Effects. Oxid. Med. Cell Longev. 2022, 15, 1429869. [Google Scholar] [CrossRef] [PubMed]
- Trinh, V.H.; Nguyen Huu, T.; Sah, D.K.; Choi, J.M.; Yoon, H.J.; Park, S.C.; Jung, Y.S.; Lee, S.R. Redox Regulation of PTEN by Reactive Oxygen Species: Its Role in Physiological Processes. Antioxidants 2024, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani-Shahpar, M.; Pakravan, K.; Razmara, E.; Amooie, F.; Mahmoudian, M.; Heshmati, M.; Babashah, S. Cancer-associated fibroblasts drive colorectal cancer cell progression through exosomal miR-20a-5p-mediated targeting of PTEN and stimulating interleukin-6 production. BMC Cancer 2024, 24, 400. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer. 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Mohideen, K.; Chandrasekaran, K.; Kareema, M.; Jeyanthikumari, T.; Dhungel, S.; Ghosh, S. Assessment of Antioxidant Enzyme Superoxide Dismutase (SOD) in Oral Cancer: Systematic Review and Meta-Analysis. Dis. Markers 2024, 16, 2264251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell. 2024, 42, 946–967. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells. 2023, 46, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tülüce, Y.; Keleş, A.Y.; Köstekci, S. Assessment of redox homeostasis via genotoxicity, cytotoxicity, apoptosis and NRF-2 in colorectal cancer cell lines after treatment with Ganoderma lucidum extract. Drug Chem. Toxicol. 2024, 47, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, S.; Patinen, T.; Jawahar Deen, A.; Pitkänen, S.; Härkönen, J.; Kansanen, E.; Küblbeck, J.; Levonen, A.L. The KEAP1-NRF2 pathway: Targets for therapy and role in cancer. Redox Biol. 2023, 63, 102726. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Ro, S.W. MAPK/ERK Signaling Pathway in Hepatocellular Carcinoma. Cancers 2021, 13, 3026. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Yang, Y.J.; Meng, X.Y.; Lin, R.H.; Tian, X.Y.; Zhang, Y.; Lai, W.F.; Yang, C.; Ma, X.Q.; Huang, M.Q. Oxysophoridine inhibits oxidative stress and inflammation in hepatic fibrosis via regulating Nrf2 and NF-κB pathways. Phytomedicine 2024, 132, 155585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; An, B.; Lin, Y.; Ni, Y.; Zhao, X.; Liang, X. Molecular mechanisms of ROS-modulated cancer chemoresistance and therapeutic strategies. Biomed. Pharmacother. 2023, 165, 115036. [Google Scholar] [CrossRef] [PubMed]
- Fleming Martinez, A.K.; Storz, P. Protein kinase D1—A targetable mediator of pancreatic cancer development. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119646. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, B.; Peng, J.; Tang, H.; Wang, S.; Peng, S.; Ye, F.; Wang, J.; Ouyang, K.; Li, J.; et al. Inhibition of NF-κB signaling unveils novel strategies to overcome drug resistance in cancers. Drug Resist. Updat. 2024, 73, 101042. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Johnson, S.A.; Opresko, P.L. Roles for the 8-Oxoguanine DNA Repair System in Protecting Telomeres From Oxidative Stress. Front. Cell Dev. Biol. 2021, 9, 758402. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Rishi, B.; George, N.G.; Kushwaha, N.; Dhandha, H.; Kaur, M.; Jain, A.; Jain, A.; Chaudhry, S.; Singh, A.; et al. Recent advances and future directions in etiopathogenesis and mechanisms of reactive oxygen species in cancer treatment. Pathol. Oncol. Res. 2023, 29, 1611415. [Google Scholar] [CrossRef] [PubMed]
- Morse, P.T.; Arroum, T.; Wan, J.; Pham, L.; Vaishnav, A.; Bell, J.; Pavelich, L.; Malek, M.H.; Sanderson, T.H.; Edwards, B.F.P.; et al. Phosphorylations and Acetylations of Cytochrome c Control Mitochondrial Respiration, Mitochondrial Membrane Potential, Energy, ROS, and Apoptosis. Cells 2024, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Bernardini, C.; Oppedisano, F.; La Mantia, D.; Trombetti, F.; Palma, E.; Forni, M.; Mollace, V.; Romeo, G.; Nesci, S. Mitochondria Bioenergetic Functions and Cell Metabolism Are Modulated by the Bergamot Polyphenolic Fraction. Cells 2022, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Mollace, R.; Macrì, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Fini, M.; Volterrani, M.; Mollace, V. Comparative Effect of Bergamot Polyphenolic Fraction and Red Yeast Rice Extract in Rats Fed a Hyperlipidemic Diet: Role of Antioxidant Properties and PCSK9 Expression. Nutrients 2022, 14, 477. [Google Scholar] [CrossRef] [PubMed]
- Mollace, R.; Macrì, R.; Nicita, M.; Musolino, V.; Gliozzi, M.; Carresi, C.; Bava, I.; Maiuolo, J.; Tavernese, A.; Cardamone, A.; et al. Bergamot Polyphenolic Extract Combined with Albedo and Pulp Fibres Counteracts Changes in Gut Microbiota Associated with High-Fat Diet: Implications for Lipoprotein Size Re-Arrangement. Int. J. Mol. Sci. 2023, 24, 12967. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S.; et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Masala, V.; Jokić, S.; Aladić, K.; Molnar, M.; Casula, M.; Tuberoso, C.I.G. Chemical Profiling and Evaluation of Antioxidant Activity of Artichoke (Cynara cardunculus var. scolymus) Leaf By-Products’ Extracts Obtained with Green Extraction Techniques. Molecules 2024, 29, 4816. [Google Scholar] [CrossRef]
- Acquaviva, R.; Malfa, G.A.; Santangelo, R.; Bianchi, S.; Pappalardo, F.; Taviano, M.F.; Miceli, N.; Di Giacomo, C.; Tomasello, B. Wild Artichoke (Cynara cardunculus subsp. sylvestris, Asteraceae) Leaf Extract: Phenolic Profile and Oxidative Stress Inhibitory Effects on HepG2 Cells. Molecules 2023, 28, 2475. [Google Scholar] [CrossRef]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268-77. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kim, A.R.; Jung, J.H.; Chun, T.; Rhee, M.H.; Yoo, E.S. Cytotoxic and pro-apoptotic activities of cynaropicrin, a sesquiterpene lactone, on the viability of leukocyte cancer cell lines. Eur. J. Pharmacol. 2004, 492, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, J.; Han, X.; Xu, J.; Wu, Y.; Fang, J. Promotion of HeLa cells apoptosis by cynaropicrin involving inhibition of thioredoxin reductase and induction of oxidative stress. Free Radic. Biol. Med. 2019, 135, 216–226. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Busà, R.; Ercolano, G.; Formisano, C.; Allegra, M.; Taglialatela-Scafati, O.; Ianaro, A. Inhibitory effects of cynaropicrin on human melanoma progression by targeting MAPK, NF-κB, and Nrf-2 signaling pathways in vitro. Phytother. Res. 2021, 35, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Leng, B. Cynaropicrin Averts the Oxidative Stress and Neuroinflammation in Ischemic/Reperfusion Injury Through the Modulation of NF-kB. Appl. Biochem. Biotechnol. 2023, 195, 5424–5438. [Google Scholar] [CrossRef] [PubMed]
- Nucera, S.; Scarano, F.; Macrì, R.; Mollace, R.; Gliozzi, M.; Carresi, C.; Ruga, S.; Serra, M.; Tavernese, A.; Caminiti, R.; et al. The Effect of an Innovative Combination of Bergamot Polyphenolic Fraction and Cynara cardunculus L. Extract on Weight Gain Reduction and Fat Browning in Obese Mice. Int. J. Mol. Sci. 2024, 25, 191. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Corradino, R.; Celano, M.; D’Agostino, M.; Maiuolo, J.; Oliverio, M.; Procopio, A.; Iannone, M.; Rotiroti, D.; Russo, D. Antiproliferative and antioxidant effects on breast cancer cells of oleuropein and its semisynthetic peracetylated derivatives. Food Chem. 2011, 127, 0308–8146. [Google Scholar] [CrossRef]
- Liman, R.; Çoban, F.K.; Ciğerci, I.H.; İbrahim, B.; Bozkurt, S. Antiangiogenic and Apoptotic Effects of Oleuropein on Breast Cancer Cells. J. Pharm. Res. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Yan, C.M.; Chai, E.Q.; Cai, H.Y.; Miao, G.Y.; Ma, W. Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol. Med. Rep. 2015, 11, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, S.; Vecchio, E.; Battaglia, A.M.; Oliverio, M.; Nardi, M.; Procopio, A.; Costanzo, F.; Biamonte, F.; Faniello, M.C. The Double-Edged Sword of Oleuropein in Ovarian Cancer Cells: From Antioxidant Functions to Cytotoxic Effects. Int. J. Mol. Sci. 2023, 24, 842. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; et al. The Effects of Bergamot Polyphenolic Fraction, Cynara cardunculus and Olea europea L. Extract on Doxorubicin-Induced Cardiotoxicity. Nutrients 2021, 13, 2158. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Bernardini, C.; Oppedisano, F.; La Mantia, D.; Trombetti, F.; Palma, E.; Forni, M.; Mollace, V.; Romeo, G.; Troisio, I.; et al. The Impairment of Cell Metabolism by Cardiovascular Toxicity of Doxorubicin Is Reversed by Bergamot Polyphenolic Fraction Treatment in Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 8977. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Nucera, S.; Scicchitano, M.; Bosco, F.; et al. Effects of Bergamot Polyphenols on Mitochondrial Dysfunction and Sarcoplasmic Reticulum Stress in Diabetic Cardiomyopathy. Nutrients 2021, 13, 2476. [Google Scholar] [CrossRef] [PubMed]
- Tuerxun, H.; Zhao, Y.; Li, Y.; Liu, X.; Wen, S.; Zhao, Y. Resveratrol alleviates testicular toxicity induced by anti-PD-1 through regulating the NRF2-SLC7A11-GPX4 pathway. Front. Immunol. 2025, 16, 1529991. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Dey, D.; Biswas, P.K.; Rahaman, T.I.; Saha, S.; Parvez, A.; Khan, D.A.; Lily, N.J.; Saha, K.; Sohel, M.; et al. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int. J. Mol. Sci. 2022, 23, 11746. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Bhattacharya, H.; Bhattacharyya, C.; Chakraborty, P.; Fleishman, J.; Alexiou, A.; Papadakis, M.; Jha, S.K. Nrf2/Keap1/ARE regulation by plant secondary metabolites: A new horizon in brain tumor management. Cell Commun. Signal. 2024, 15, 497. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Chen, Y.; Dong, Y. Unraveling the AMPK-SIRT1-FOXO Pathway: The In-Depth Analysis and Breakthrough Prospects of Oxidative Stress-Induced Diseases. Antioxidants 2025, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.U.; Sangeetha, D. Therapeutic potentials and targeting strategies of quercetin on cancer cells: Challenges and future prospects. Phytomedicine 2024, 133, 155902. [Google Scholar] [CrossRef] [PubMed]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; et al. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Merecz-Sadowska, A.; Sikora, J.; Dudzic, M.; Wiertek-Płoszaj, N.; Picot, L.; Śliwiński, T.; Kowalczyk, T. Flavonoids and their derivatives as DNA topoisomerase inhibitors with anti-cancer activity in various cell models: Exploring a novel mode of action. Pharmacol. Res. 2024, 209, 107457. [Google Scholar] [CrossRef] [PubMed]
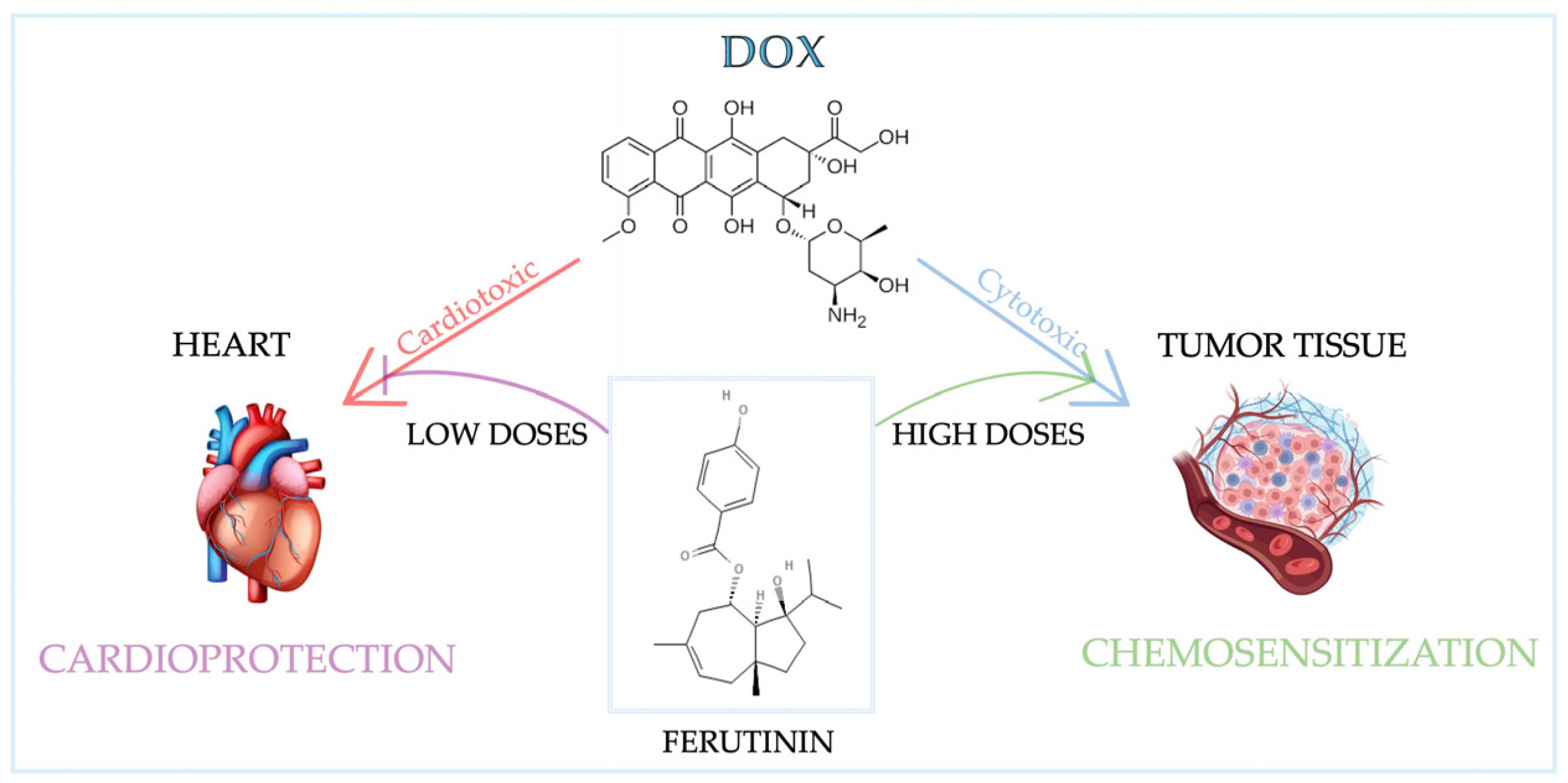
- Macrì, R.; Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Nucera, S.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic Effects. Molecules 2020, 25, 5768. [Google Scholar] [CrossRef] [PubMed]
- Macrì, R.; Bava, I.; Scarano, F.; Mollace, R.; Musolino, V.; Gliozzi, M.; Greco, M.; Foti, D.; Tucci, L.; Maiuolo, J.; et al. In Vitro Evaluation of Ferutinin Rich-Ferula communis L., ssp. glauca, Root Extract on Doxorubicin-Induced Cardiotoxicity: Antioxidant Properties and Cell Cycle Modulation. Int. J. Mol. Sci. 2023, 24, 12735. [Google Scholar] [CrossRef]
- Maiuolo, J.; Musolino, V.; Guarnieri, L.; Macrì, R.; Coppoletta, A.R.; Cardamone, A.; Serra, M.; Gliozzi, M.; Bava, I.; Lupia, C.; et al. Ferula communis L. (Apiaceae) Root Acetone-Water Extract: Phytochemical Analysis, Cytotoxicity and In Vitro Evaluation of Estrogenic Properties. Plants 2022, 11, 1905. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Miceli, N.; Davì, F.; Bava, I.; Tucci, L.; Ragusa, S.; Taviano, M.F.; Musolino, V.; Gliozzi, M.; Carresi, C.; et al. Ferula communis Root Extract: In Vitro Evaluation of the Potential Additive Effect with Chemotherapy Tamoxifen in Breast Cancer (MCF-7) Cells Part II. Plants 2023, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Safi, R.; Hamade, A.; Bteich, N.; El Saghir, J.; Assaf, M.D.; El-Sabban, M.; Najjar, F. A ferutinin analogue with enhanced potency and selectivity against ER-positive breast cancer cells in vitro. Biomed. Pharmacother. 2018, 105, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Macri, R.; Oppedisano, F.; Scarano, F.; Caterina Zito, M.; et al. The Effect of Ferula communis Extract in Escherichia coli Lipopolysaccharide-Induced Neuroinflammation in Cultured Neurons and Oligodendrocytes. Int. J. Mol. Sci. 2021, 22, 7910. [Google Scholar] [CrossRef] [PubMed]
- Adorisio, S.; Muscari, I.; Fierabracci, A.; Thi Thuy, T.; Marchetti, M.C.; Ayroldi, E.; Delfino, D.V. Biological effects of bergamot and its potential therapeutic use as an anti-inflammatory, antioxidant, and anticancer agent. Pharm. Biol. 2023, 61, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Sharma, Y.; Kumar, D. Unveiling the link between chronic inflammation and cancer. Metabol. Open. 2025, 25, 100347. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, F.; Wu, Y.; Zhu, Y.; Jiang, Y.; Wu, Q.; Dong, Z.; Liu, K. Inflammation in cancer: Therapeutic opportunities from new insights. Mol. Cancer 2025, 24, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, L.; Chang, W.; Zhang, Y. The crosstalk between the gut microbiota and tumor immunity: Implications for cancer progression and treatment outcomes. Front. Immunol. 2023, 13, 1096551. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.S.; Mustafa, T.; Connell, J.P.; Grande-Allen, K.J. Tumor necrosis factor alpha and interleukin 1 beta suppress myofibroblast activation via nuclear factor kappa B signaling in 3D-cultured mitral valve interstitial cells. Acta Biomater. 2021, 127, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ahmed, Y.; Lafdil, F. Novel insights into the impact of liver inflammatory responses on primary liver cancer development. Liver Res. 2023, 7, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef] [PubMed]
- Minarovits, J. Human tumor viruses: Induction of three-dimensional alterations in the host genome structure. Front. Microbiol. 2023, 14, 1280210. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhu, S.; Wang, Y.; Zhao, Y.; Yan, K.; Li, X.; Wang, X.; He, C.; Ding, C.; Chen, Y.; et al. Effect of inflammation on association between cancer and coronary artery disease. BMC Cardiovasc. Disord. 2024, 24, 72. [Google Scholar] [CrossRef] [PubMed]
- Mora Barthelmess, R.; Stijlemans, B.; Van Ginderachter, J.A. Hallmarks of Cancer Affected by the MIF Cytokine Family. Cancers 2023, 15, 395. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.P.; Mark, K.G.; Leslie, K.; Pao, W.; Motoi, N.; Gerald, W.L.; Travis, W.D.; Bornmann, W.; Veach, D.; Clarkson, B.; et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J. Clin. Investig. 2007, 117, 3846–3856. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Malerba, E.; Susca, N.; Favoino, E.; Perosa, F.; Brunori, G.; Prete, M.; Racanelli, V. Endothelial cells in tumor microenvironment: Insights and perspectives. Front. Immunol. 2024, 15, 1367875. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y. Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front. Immunol. 2018, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, L.; Pancione, M.; Votino, C.; Colangelo, T.; Lupo, A.; Novellino, E.; Lavecchia, A.; Colantuoni, V. Emerging role of the β-catenin-PPARγ axis in the pathogenesis of colorectal cancer. World J. Gastroenterol. 2014, 20, 7137–7151. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.; Wang, M.; Wang, X.; Yang, K.; Xie, F.; Liao, Z.; Wei, P. PPAR-γ Modulators as Current and Potential Cancer Treatments. Front. Oncol. 2021, 11, 737776. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, A.; Ziemichód, W.; Herbet, M.; Piątkowska-Chmiel, I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients 2023, 15, 1436. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Lombardo, G.E.; Bruschetta, G.; Rapisarda, A.; Maugeri, A.; Navarra, M. Bergamot Byproducts: A Sustainable Source to Counteract Inflammation. Nutrients 2024, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Mollace, R.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Ruga, S.; Zito, M.C.; et al. Oxidative Stress Triggers Defective Autophagy in Endothelial Cells: Role in Atherothrombosis Development. Antioxidants 2021, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Parafati, M.; Lascala, A.; La Russa, D.; Mignogna, C.; Trimboli, F.; Morittu, V.M.; Riillo, C.; Macirella, R.; Mollace, V.; Brunelli, E.; et al. Bergamot Polyphenols Boost Therapeutic Effects of the Diet on Non-Alcoholic Steatohepatitis (NASH) Induced by “Junk Food”: Evidence for Anti-Inflammatory Activity. Nutrients 2018, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Gallyas, F., Jr.; Sumegi, B. Mitochondrial Protection by PARP Inhibition. Int. J. Mol. Sci. 2020, 21, 2767. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yu, L.; Qu, X.; Huang, T. The role of peroxisome proliferator-activated receptors in the tumor microenvironment, tumor cell metabolism, and anticancer therapy. Front. Pharmacol. 2023, 14, 1184794. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Z.; Wu, H.; Wang, B.; Ouyang, Y.; Liu, J.; Zheng, X.; Zhang, H.; Li, X.; Feng, X.; et al. Adipocyte-rich microenvironment promotes chemoresistance via upregulation of peroxisome proliferator-activated receptor gamma/ABCG2 in epithelial ovarian cancer. Int. J. Mol. Med. 2024, 53, 37. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Pitna, D.B.; Kimura, K.; Yoshimoto, Y.; Uchiyama, T.; Mori, T.; Kondo, R.; Hara, S.; Egoshi, Y.; Yamaguchi, S.; et al. Total synthesis of cynaropicrin. Org. Biomol. Chem. 2021, 19, 6038–6044. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X. Cynaropicrin attenuates inflammatory cytokines in LPS-induced RAW264.7 cells and ovalbumin-induced asthmatic mice. J. Biochem. Mol. Toxicol. 2024, 38, e23836. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, A.; Giordani, C.; Bonacci, S.; Giuliani, A.; Ramini, D.; Matacchione, G.; Sabbatinelli, J.; Di Valerio, S.; Pacetti, D.; Procopio, A.D.; et al. Anti-Inflammatory Effects of Olive Leaf Extract and Its Bioactive Compounds Oleacin and Oleuropein-Aglycone on Senescent Endothelial and Small Airway Epithelial Cells. Antioxidants 2023, 12, 1509. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, B.; Di Liberto, D.; Pratelli, G.; Rizzo, C.; Barbera, L.; Lauricella, M.; Carlisi, D.; Maggio, A.; Palumbo Piccionello, A.; D’Anneo, A.; et al. Extra Virgin Olive Oil Polyphenol-Enriched Extracts Exert Antioxidant and Anti-Inflammatory Effects on Peripheral Blood Mononuclear Cells from Rheumatoid Arthritis Patients. Antioxidants 2025, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Mirsanei, Z.; Heidari, N.; Hazrati, A.; Asemani, Y.; Niknam, B.; Yousefi, Z.; Jafari, R. Oleuropein reduces LPS-induced inflammation via stimulating M2 macrophage polarization. Biomed. Pharmacother. 2023, 163, 114857. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Chioccioli, S.; Monaco, N.; Peppicelli, S.; Andreucci, E.; Urciuoli, S.; Romani, A.; Luceri, C.; Tortora, K.; Calorini, L.; et al. Oleuropein-Rich Leaf Extract as a Broad Inhibitor of Tumour and Macrophage iNOS in an Apc Mutant Rat Model. Antioxidants 2021, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.X.; Zhang, Y.H.; Guo, R.N.; Zhao, S.N. Inhibition of MEK/ERK/STAT3 signaling in oleuropein treatment inhibits myocardial ischemia/reperfusion. Int. J. Mol. Med. 2018, 42, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Karakoç, M.D.; Sekkin, S. Effects of Oleuropein on Epirubicin and Cyclophosphamide Combination Treatment in Rats. Turk. J. Pharm. Sci. 2021, 18, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sánchez, M.; Toral, M.; Martín-García, B.; Gómez-Caravaca, A.M.; Arráez-Román, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Bukhari, S.A.; Chauhdary, Z.; Akhter, N.; Noreen, R. Effect of resveratrol and quercetin on SFRP4 as a biomarker of diabesity: In silico and in vivo studies. Biochem. Biophys. Res. Commun. 2025, 761, 151748. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cen, F.; Tian, F.; Li, M.J.; Zhang, Q.; Shen, H.Y.; Shen, X.C.; Zhou, M.M.; Du, J. Combination treatment with quercetin and resveratrol attenuates high fat diet-induced obesity and associated inflammation in rats via the AMPKα1/SIRT1 signaling pathway. Exp. Ther. Med. 2017, 14, 5942–5948. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, W.; Yu, B.; Liang, H.; Mao, S.; Hu, X.; Feng, Y.; Xu, J.; Chu, L. Quercetin improves cerebral ischemia/reperfusion injury by promoting microglia/macrophages M2 polarization via regulating PI3K/Akt/NF-κB signaling pathway. Biomed. Pharmacother. 2023, 168, 115653. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Wu, C.; Chen, J.; Wo, D.; Ren, D.N.; Yan, H.; Peng, L.; Zhu, W. Resveratrol prevents Ang II-induced cardiac hypertrophy by inhibition of NF-κB signaling. Biomed. Pharmacother. 2023, 165, 115275. [Google Scholar] [CrossRef] [PubMed]
- Geroushi, A.; Auzi, A.A.; Elhwuegi, A.S.; Elzawam, F.; Elsherif, A.; Nahar, L.; Sarker, S.D. Antiinflammatory sesquiterpenes from the root oil of Ferula hermonis. Phytother. Res. 2011, 25, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, L.; Hei, R.; Li, X.; Cai, H.; Wu, X.; Zheng, Q.; Cai, C. CDK inhibitors in cancer therapy, an overview of recent development. Am. J. Cancer Res. 2021, 11, 1913–1935. [Google Scholar] [PubMed]
- Ozaki, T.; Nakagawara, A. Role of p53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xiong, R.; Zhou, Z.; Zhang, C.; Han, Y.; Shi, T.; Qiu, J.; Zhang, R. ZFP57 promotes ovarian cancer progression by transcriptionally regulating BRCA1 and managing G1 checkpoint. J. Cancer 2023, 14, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, W.; Chang, Y.; He, Z.; Wu, M.; Zheng, H.; Ke, X.; Lv, M.; Liu, Q.; Liu, Q.; et al. CEP192 is a novel prognostic marker and correlates with the immune microenvironment in hepatocellular carcinoma. Front. Immunol. 2022, 13, 950884. [Google Scholar] [CrossRef] [PubMed]
- Gheghiani, L.; Wang, L.; Zhang, Y.; Moore, X.T.R.; Zhang, J.; Smith, S.C.; Tian, Y.; Wang, L.; Turner, K.; Jackson-Cook, C.K.; et al. PLK1 Induces Chromosomal Instability and Overrides Cell-Cycle Checkpoints to Drive Tumorigenesis. Cancer Res. 2021, 81, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Corno, A.; Cordeiro, M.H.; Allan, L.A.; Lim, Q.W.; Harrington, E.; Smith, R.J.; Saurin, A.T. A bifunctional kinase-phosphatase module balances mitotic checkpoint strength and kinetochore-microtubule attachment stability. EMBO J. 2023, 42, 112630. [Google Scholar] [CrossRef] [PubMed]
- Łukasik, P.; Załuski, M.; Gutowska, I. Cyclin-Dependent Kinases (CDK) and Their Role in Diseases Development-Review. Int. J. Mol. Sci. 2021, 22, 2935. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Marano, L. Aging, Cancer, and Inflammation: The Telomerase Connection. Int. J. Mol. Sci. 2024, 25, 8542. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.; Trub, A.; Ahn, A.; Taylor, M.; Ambani, K.; Chan, K.T.; Lu, K.H.; Mahendra, C.A.; Blyth, C.; Coulson, R.; et al. INX-315, a Selective CDK2 Inhibitor, Induces Cell Cycle Arrest and Senescence in Solid Tumors. Cancer Discov. 2024, 14, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Bhaskar, R.; Ghosh, S.; Yarlagadda, B.; Singh, K.K.; Verma, P.; Sengupta, S.; Mladenov, M.; Hadzi-Petrushev, N.; Stojchevski, R.; et al. Exploring the Genetic Orchestra of Cancer: The Interplay Between Oncogenes and Tumor-Suppressor Genes. Cancers 2025, 17, 1082. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Russo, C.; Musumeci, L.; Lombardo, G.E.; De Sarro, G.; Barreca, D.; Cirmi, S.; Navarra, M. The Anticancer Effect of a Flavonoid-Rich Extract of Bergamot Juice in THP-1 Cells Engages the SIRT2/AKT/p53 Pathway. Pharmaceutics 2022, 14, 2168. [Google Scholar] [CrossRef]
- Delle Monache, S.; Sanità, P.; Trapasso, E.; Ursino, M.R.; Dugo, P.; Russo, M.; Ferlazzo, N.; Calapai, G.; Angelucci, A.; Navarra, M. Mechanisms underlying the anti-tumoral effects of Citrus Bergamia juice. PLoS ONE 2013, 8, 61484. [Google Scholar] [CrossRef] [PubMed]
- Navarra, M.; Femia, A.P.; Romagnoli, A.; Tortora, K.; Luceri, C.; Cirmi, S.; Ferlazzo, N.; Caderni, G. A flavonoid-rich extract from bergamot juice prevents carcinogenesis in a genetic model of colorectal cancer, the Pirc rat (F344/NTac-Apcam1137). Eur. J. Nutr. 2020, 59, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Lepore, S.M.; Maggisano, V.; Lombardo, G.E.; Maiuolo, J.; Mollace, V.; Bulotta, S.; Russo, D.; Celano, M. Antiproliferative Effects of Cynaropicrin on Anaplastic Thyroid Cancer Cells. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Xi, J.; Zhong, M.; Chen, F.; Zhao, H.; Zhang, B.; Fang, J. Cynaropicrin Induces Cell Cycle Arrest and Apoptosis by Inhibiting PKM2 to Cause DNA Damage and Mitochondrial Fission in A549 Cells. J. Agric. Food Chem. 2021, 69, 13557–13567. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, H.R.; Zago, L.; Difonzo, G.; Pasqualone, A.; Caponio, F.; Ferraz da Costa, D.C. Olive Leaves as a Source of Anticancer Compounds: In Vitro Evidence and Mechanisms. Molecules 2024, 29, 4249. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hou, H.; Zhang, Y.; Fang, Z.; Li, Y.; Zhang, L.; Wang, Y.; Zhang, S.; Zhang, H.; Jin, Q.; et al. Oleuropein and its hydrolysate from extra virgin olive oil inhibit breast cancer cells proliferation interfering with the PI3K-AKT signal pathway. J. Funct. Foods 2023, 110, 1756–4646. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Gene Expression Alterations Associated with Oleuropein-Induced Antiproliferative Effects and S-Phase Cell Cycle Arrest in Triple-Negative Breast Cancer Cells. Nutrients 2020, 12, 3755. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.A.; Pozo-Guisado, E.; Alvarez-Barrientos, A.; Fernandez-Salguero, P.M.; Castellón, E.A. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J. Androl. 2007, 28, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Adhami, V.M.; Afaq, F.; Feyes, D.K.; Mukhtar, H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin. Cancer Res. 2001, 7, 1466–1473. [Google Scholar] [PubMed]
- Bailon-Moscoso, N.; Cevallos-Solorzano, G.; Romero-Benavides, J.C.; Orellana, M.I. Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr. Genom. 2017, 18, 106–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cai, G.; Zhang, C.; Li, H.; Nie, Y.; Yu, S.; Zhang, B.; Wu, M.; Luo, W.; Liu, J.; et al. Resveratrol suppresses growth and VCAN expression in a Cancer-associated fibroblast-breast Cancer hybrid organoid. Int. Immunopharmacol. 2025, 153, 114451. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, D.; Ragazzi, E.; Vianello, C.; Caparrotta, L.; Montopoli, M. Effect of Quercetin on Cell Cycle and Cyclin Expression in Ovarian Carcinoma and Osteosarcoma Cell Lines. Nat. Prod. Commun. 2015, 10, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.K.; Lee, E.J.; Kim, H.C.; Kim, J.H. Induction of G(1)/S phase arrest and apoptosis by quercetin in human osteosarcoma cells. Arch. Pharm. Res. 2010, 33, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Son, H.K.; Kim, D. Quercetin Induces Cell Cycle Arrest and Apoptosis in YD10B and YD38 Oral Squamous Cell Carcinoma Cells. Asian Pac. J. Cancer Prev. 2023, 24, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.A.; Ababneh, N.A.; Tarawneh, N.; Ismail, M.A.; Awidi, A.; Abdalla, S. Antitumor Effects of Quercetin and Luteolin in A375 Cutaneous Melanoma Cell Line Are Mediated by Upregulation of P-ERK, c-Myc, and the Upstream GPER. Life 2025, 15, 417. [Google Scholar] [CrossRef] [PubMed]
- Keshmirshekan, F.; Mohamadi-Zarch, S.M.; Bagheri, S.M. A Review of Anticancer Potential of Conferone, Diversin and Ferutinin; Which One is Stronger for Cancer Therapy? Anti-Cancer Agents Med. Chem. 2025, 25, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut Microbiome-Brain Alliance: A Landscape View into Mental and Gastrointestinal Health and Disorders. ACS Chem. Neurosci. 2023, 14, 1717–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Ola, M.; Rolling, T.; Tosini, N.L.; Joshowitz, S.; Littmann, E.R.; Amoretti, L.A.; Fontana, E.; Wright, R.J.; Miranda, E.; et al. High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat. Med. 2020, 26, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, A.B.; Klug, J.; Mesko, M.; Gao, I.H.; Lipkin, S.M.; Shen, X.; Iliev, I.D. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 2022, 185, 3807–3822.e12. [Google Scholar] [CrossRef] [PubMed]
- Galeano Niño, J.L.; Wu, H.; LaCourse, K.D.; Kempchinsky, A.G.; Baryiames, A.; Barber, B.; Futran, N.; Houlton, J.; Sather, C.; Sicinska, E.; et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 2022, 611, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xia, H.; Tan, X.; Shi, C.; Ma, Y.; Meng, D.; Zhou, M.; Lv, Z.; Wang, S.; Jin, Y. Intratumoural microbiota: A new frontier in cancer development and therapy. Signal Transduct. Target. Ther. 2024, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, K.; Ji, L.; Li, Y. Intratumoral microbiota, fatty acid metabolism, and tumor microenvironment constitute an unresolved trinity in colon adenocarcinoma. Sci. Rep. 2025, 15, 2568. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Redondo, E.; Rodríguez-Cabezas, M.E.; Gálvez, J.; Rodríguez-Nogales, A. Systematic review: The gut microbiota as a link between colorectal cancer and obesity. Obes. Rev. 2025, 26, 13872. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cheng, M.; Liu, J.; Cui, M.; Yin, B.; Liang, J. Research progress on the impact of intratumoral microbiota on the immune microenvironment of malignant tumors and its role in immunotherapy. Front. Immunol. 2024, 15, 1389446. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.J.; Zhu, H.R.; Jin, Y.J.; Liu, P.; Yu, X.W.; Zhang, Y.R. Correlation between gut microbiota and tumor immune microenvironment: A bibliometric and visualized study. World J. Clin. Oncol. 2025, 16, 101611. [Google Scholar] [CrossRef] [PubMed]
- Jovel, J.; Patterson, J.; Wang, W.; Hotte, N.; O’Keefe, S.; Mitchel, T.; Perry, T.; Kao, D.; Mason, A.L.; Madsen, K.L.; et al. Characterization of the Gut Microbiome Using 16S or Shotgun Metagenomics. Front. Microbiol. 2016, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Investig. 2022, 132, 154944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Riehl, L.; Fürst, J.; Kress, M.; Rykalo, N. The importance of the gut microbiome and its signals for a healthy nervous system and the multifaceted mechanisms of neuropsychiatric disorders. Front. Neurosci. 2024, 17, 1302957. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, F.; Wu, G. Potential effects of gut microbiota on host cancers: Focus on immunity, DNA damage, cellular pathways, and anticancer therapy. ISME J. 2023, 17, 1535–1551. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Qi, P.; Shu, L.; Ding, Y.; Zeng, P.; Wen, G.; Xiong, Y.; Deng, H. Dysbiosis and extraintestinal cancers. J. Exp. Clin. Cancer Res. 2025, 44, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cai, Q.; Tian, Z.; Chen, W.; Tang, H. Crosstalk between Gut Microbiota and Cancer Immunotherapy: Present Investigations and Future Perspective. Research 2025, 8, 0600. [Google Scholar] [CrossRef] [PubMed]
- McGill, C.R.; Fulgoni, V.L., 3rd; Devareddy, L. Ten-year trends in fiber and whole grain intakes and food sources for the United States population: National Health and Nutrition Examination Survey 2001–2010. Nutrients 2015, 7, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Qi, Q.; Wang, Z.; Usyk, M.; Sotres-Alvarez, D.; Mattei, J.; Tamez, M.; Gellman, M.D.; Daviglus, M.; Hu, F.B.; et al. The Gut Microbiome Modifies the Association Between a Mediterranean Diet and Diabetes in USA Hispanic/Latino Population. J. Clin. Endocrinol. Metab. 2022, 107, e924–e934. [Google Scholar] [CrossRef] [PubMed]
- Zeb, F.; Naqeeb, H.; Osaili, T.; Faris, M.E.; Ismail, L.C.; Obaid, R.S.; Naja, F.; Radwan, H.; Hasan, H.; Hashim, M.; et al. Molecular crosstalk between polyphenols and gut microbiota in cancer prevention. Nutr. Res. 2024, 124, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Y.; Zhang, J.; Zhang, Y.; He, W.; Ju, J.; Wu, Y.; Wang, Y. The effect of resveratrol, curcumin and quercetin combination on immuno-suppression of tumor microenvironment for breast tumor-bearing mice. Sci. Rep. 2023, 13, 13278. [Google Scholar] [CrossRef] [PubMed]
- Mandle, H.B.; Jenab, M.; Gunter, M.J.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; Zhang, J.; Sugier, P.E.; Rothwell, J.; Severi, G.; et al. Inflammation and gut barrier function-related genes and colorectal cancer risk in western European populations. Mutagenesis 2025, 40, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Zhang, C.J.; Wang, L.Z.; Xie, F.S.; Wu, H.Y.; Li, T.; Bian, C.W.; Wu, R.L. Lipopolysaccharide promotes cancer cell migration and invasion through METTL3/PI3K/AKT signaling in human cholangiocarcinoma. Heliyon 2024, 10, 29683. [Google Scholar] [CrossRef] [PubMed]
- Mollace, A.; Coluccio, M.L.; Donato, G.; Mollace, V.; Malara, N. Cross-talks in colon cancer between RAGE/AGEs axis and inflammation/immunotherapy. Oncotarget 2021, 12, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy Rajalakshmi, R.; Melians, M.A.; Pon, F.F.; Cosio, D.S.; Buvarahamurthy, V.; Jayakumar, A.R.; Paidas, M.J. Advantages and Disadvantages of Nutraceuticals; Springer Nature: Singapore, 2023; pp. 245–286. [Google Scholar] [CrossRef]
- Shaman, J.A. The Future of Pharmacogenomics: Integrating Epigenetics, Nutrigenomics, and Beyond. J. Pers. Med. 2024, 14, 1121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Xing, X.; Wang, S. Health benefits of dietary polyphenols: Insight into interindividual variability in absorption and metabolism. Curr. Opin. Food Sci. 2022, 48, 2214–7993. [Google Scholar] [CrossRef]
- Favari, C.; Rinaldi de Alvarenga, J.F.; Sánchez-Martínez, L.; Tosi, N.; Mignogna, C.; Cremonini, E.; Manach, C.; Bresciani, L.; Del Rio, D.; Mena, P. Factors driving the inter-individual variability in the metabolism and bioavailability of (poly)phenolic metabolites: A systematic review of human studies. Redox Biol. 2024, 71, 103095. [Google Scholar] [CrossRef] [PubMed]
- Sahraeian, S.; Rashidinejad, A.; Golmakani, M.-T. Recent advances in the conjugation approaches for enhancing the bioavailability of polyphenols. Food Hydrocoll. 2024, 146, 109221. [Google Scholar] [CrossRef]
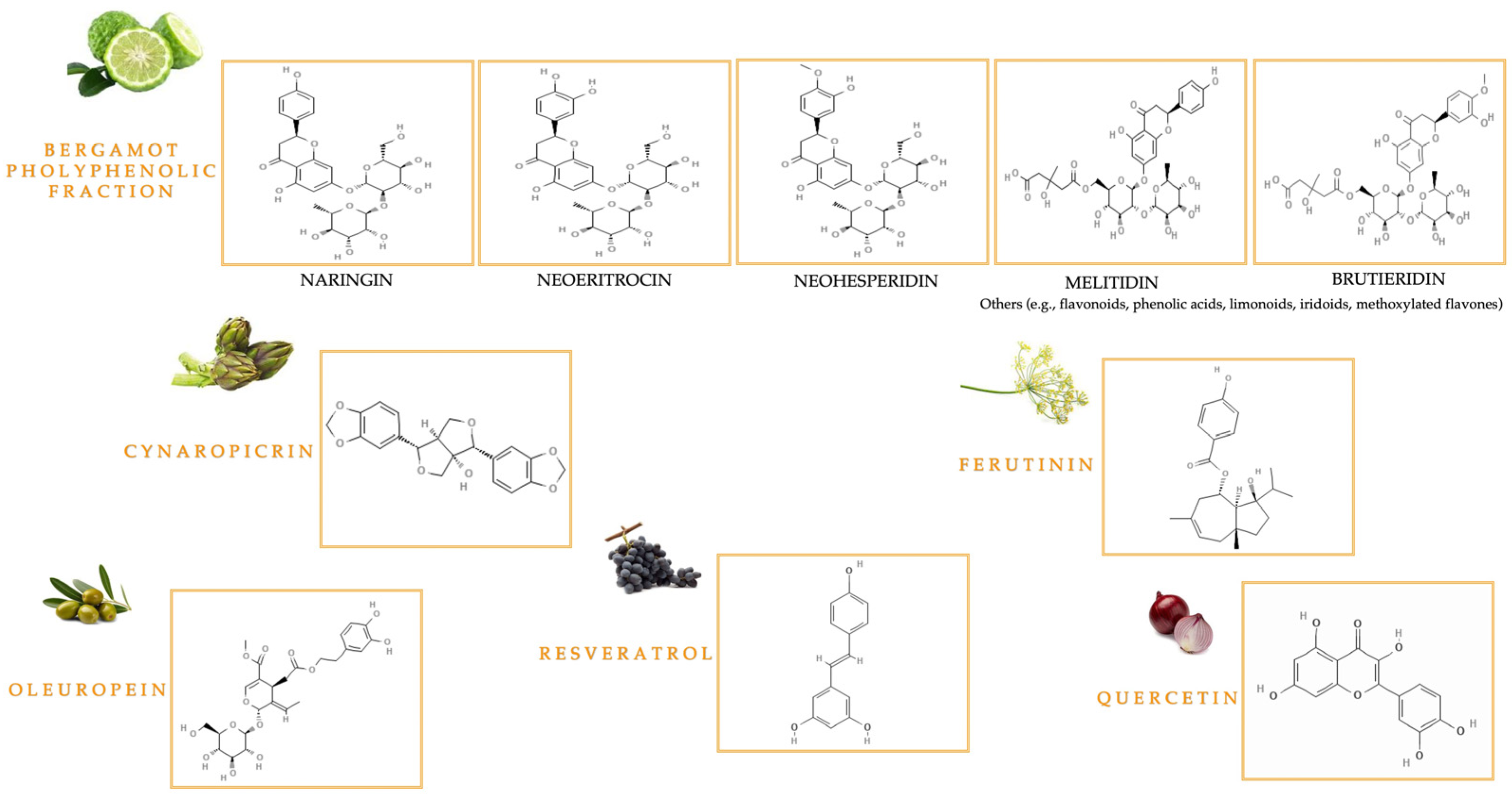
| Nutraceuticals | Bioactive Compounds | Main Sources | Biological Effects | References |
|---|---|---|---|---|
| Citrus bergamia Risso & Poiteau extract | Naringin, neohesperidin, neoeriocitrin, C-glucoside, flavanone O-glycosides, rhoifolin, 40-O-glucoside, neodiosmin, rhoifolin, poncirin limonene, linalool, linalyl acetate | Bergamot juice (BJ), bergamot essential oil (BEO) | Hypolipemic, hypoglycaemic anti-inflammatory, antioxidant, anticancer | [44,45,46,47] |
| Cynara cardunculus L. extract | Cynaropicrin (cyn), chlorogenic acid, dicaffeoylquinic acids, luteolin, inulin | Leaves, flowers, roots, by-products | Hepatoprotective, antioxidant, antimicrobial, antiobesity, chemopreventive | [48,49] |
| Olea Europaea L. extract | Hydroxytyrosol, oleuropein, tyrosol, oleic acid, omega-3, omega-6 | Olive oil (OO), olive leaves, Olive mill wastewater (OMWW) | Antioxidant, cardioprotective, antitumoral, anti-inflammatory, anti-aging, antibacterial, prevention of metabolic disorders and chronic diseases | [50,51,52,53] |
| Quercetin | Flavonol (free and glycosylated forms) | Onions, apples, berries, broccoli, tea, cherries, tomatoes, asparagus, peas, grapes, coriander seeds | Antioxidant, anti-inflammatory, antiproliferative, antiviral, cardioprotective, antiaging, prevention of metabolic disorders, chronic diseases, platelet aggregation, lipid peroxidation, and capillary permeability, modulating the composition of the gut microbiota | [54,55] |
| Resveratrol | Trans-RV | Grapes skin, red wines, blueberry, cranberry, peanuts, bilberry | Antioxidant, anti-inflammatory, pro-apoptotic, antitumor, telomerase inhibition, improves gut barrier | [30,56,57,58] |
| Ferula Communis L. extract | Ferutinin, sesquiterpenes, ferulenol, ferulone A and B, flavonoids | Roots, rhizomes, latex | Selective estrogen receptor modulator (SERM-like), antiproliferative, dose-dependent estrogenic effect; antioxidant effects, antidiabetic, antimicrobial, cytotoxic actions | [59,60] |
| Nutraceutical | Low Dose Effect (Protective/Antioxidant) | High Dose Effect (Pro-Oxidant/Anticancer) | Mechanism | References |
|---|---|---|---|---|
| Bergamot polyphenolic fraction | ↓ MDA/ROS; ↑ SOD/GPX; ↓ 3-NT/LOX-1; ↑ protective effect. | Synergistic support in redox balance, not directly cytotoxic. | ↓ peroxynitrite; ↑ antioxidant enzymes (cardiomyocytes, liver). | [108,109,110,111,113,128,143] |
| Cynaropicrin | ↑ Nrf2/SOD/CAT/GSH-PX; antioxidant in A375, brain, liver tissues. | Inhibits TxR → ↑ ROS → apoptosis in cancer cells. | Activates antioxidant genes (GCL, HMOX-1), pro-apoptotic in melanoma, neural, liver cells. | [117,118,119] |
| Oleuropein | ↑ SOD/GPX/GRX/CAT; ↓ ROS; protects membrane and ER integrity. | ↑ ROS, mitochondrial dysfunction, cyt C release; apoptotic in MCF-7, HepG2, HEY cancer cells. | Dose- and cell-type specific redox modulation. | [124,125] |
| Quercetin | Activates Nrf2; ↑ GSH/SOD/GPX; inhibits Topo II; protection from Dox cardiotoxicity. | ↑ ROS; DNA damage; cyt C release; apoptosis via intrinsic/extrinsic pathways. | Caspase cascade, KEAP1 oxidation, ARE activation, Topo II inhibition. | [132] |
| Resveratrol | ↑ SIRT1/AMPK/FOXO3a, antioxidant genes; prevents Dox toxicity. | ↑ ROS; mitochondrial permeability transition pore (mPTP) opening; apoptosis through caspase activation. | Mitochondrial depolarization; dual effect depending on dose and exposure time. | [133,134,135] |
| Ferutinin | Antioxidant, phytoestrogenic; ↓ ROS/MDA; protects H9c2 and neural cells. | ↑ ROS, ↑ Bax, cyt C; apoptosis in MCF-7, MDA-MB-231 BC cells. | SERM-like; mitochondrial apoptosis; cell-selective effects. | [138,139,140,141,142] |
| Natural Compound/Plant | Cell Cycle Phase | Molecular Mechanism | Cell Type | References |
|---|---|---|---|---|
| Citrus bergamia | G1/S | Silent mating type information regulation 2 homolog 2 (SIRT2)/Akt/p53 ↑ apoptosis | THP-1, SH-SY5Y | [196,197,198] |
| Cynaropicrin | G2/M | ↓ Pyruvate kinase M2 (PKM2)/PARP | CAL-62, 8505C, SW1736, A549 | [199,200] |
| Oleuropein | G1/S | ↑ p21, p53, CKIs | MCF-7, MDA-MB-231, MDA-MB-468 | [201,202,203] |
| Resveratrol | S | ↑ p53/p21/p27/Bax/p53 upregulated modulator of apoptosis (PUMA) ↓ cyclins D/E ↓ cyclin-dependent kinase 2, 4, 6 (CDK2/4/6) | C-3, LNCaP, A431, T47D | [204,205,206,207,208] |
| Quercetin | G1/G2/M | ↑ p21/p27 ↓ CDK2/6, cyclins A/E/D ↓ Rb phosphorylation | HOS, OSCC, T47D, A375, p39, YD10B, YD38, OSCC, KON | [209,210,211,212] |
| Ferutinin | ↓ G1 and restoring cell cycle | ↑ apoptosis ↑ protective effect | H9c2 | [138,140,213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altomare, C.; Macrì, R.; Serra, M.; Ussia, S.; Ritorto, G.; Maiuolo, J.; Muscoli, C.; Perri, E.; Mollace, V. The Potential of Nutraceutical Supplementation in Counteracting Cancer Development and Progression: A Pathophysiological Perspective. Nutrients 2025, 17, 2354. https://doi.org/10.3390/nu17142354
Altomare C, Macrì R, Serra M, Ussia S, Ritorto G, Maiuolo J, Muscoli C, Perri E, Mollace V. The Potential of Nutraceutical Supplementation in Counteracting Cancer Development and Progression: A Pathophysiological Perspective. Nutrients. 2025; 17(14):2354. https://doi.org/10.3390/nu17142354
Chicago/Turabian StyleAltomare, Carmen, Roberta Macrì, Maria Serra, Sara Ussia, Giovanna Ritorto, Jessica Maiuolo, Carolina Muscoli, Enzo Perri, and Vincenzo Mollace. 2025. "The Potential of Nutraceutical Supplementation in Counteracting Cancer Development and Progression: A Pathophysiological Perspective" Nutrients 17, no. 14: 2354. https://doi.org/10.3390/nu17142354
APA StyleAltomare, C., Macrì, R., Serra, M., Ussia, S., Ritorto, G., Maiuolo, J., Muscoli, C., Perri, E., & Mollace, V. (2025). The Potential of Nutraceutical Supplementation in Counteracting Cancer Development and Progression: A Pathophysiological Perspective. Nutrients, 17(14), 2354. https://doi.org/10.3390/nu17142354








