The Role of the Gut Microbiota in Mental Health and Cognitive Function in Patients with Coronary Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology Background
2.2. Review Procedure and Search Strategy
2.3. Source Selection
3. Gut Microbiota
3.1. The Gut Microbiota in the Context of Cardiovascular Disease
3.1.1. Hypertension and the Gut Microbiota
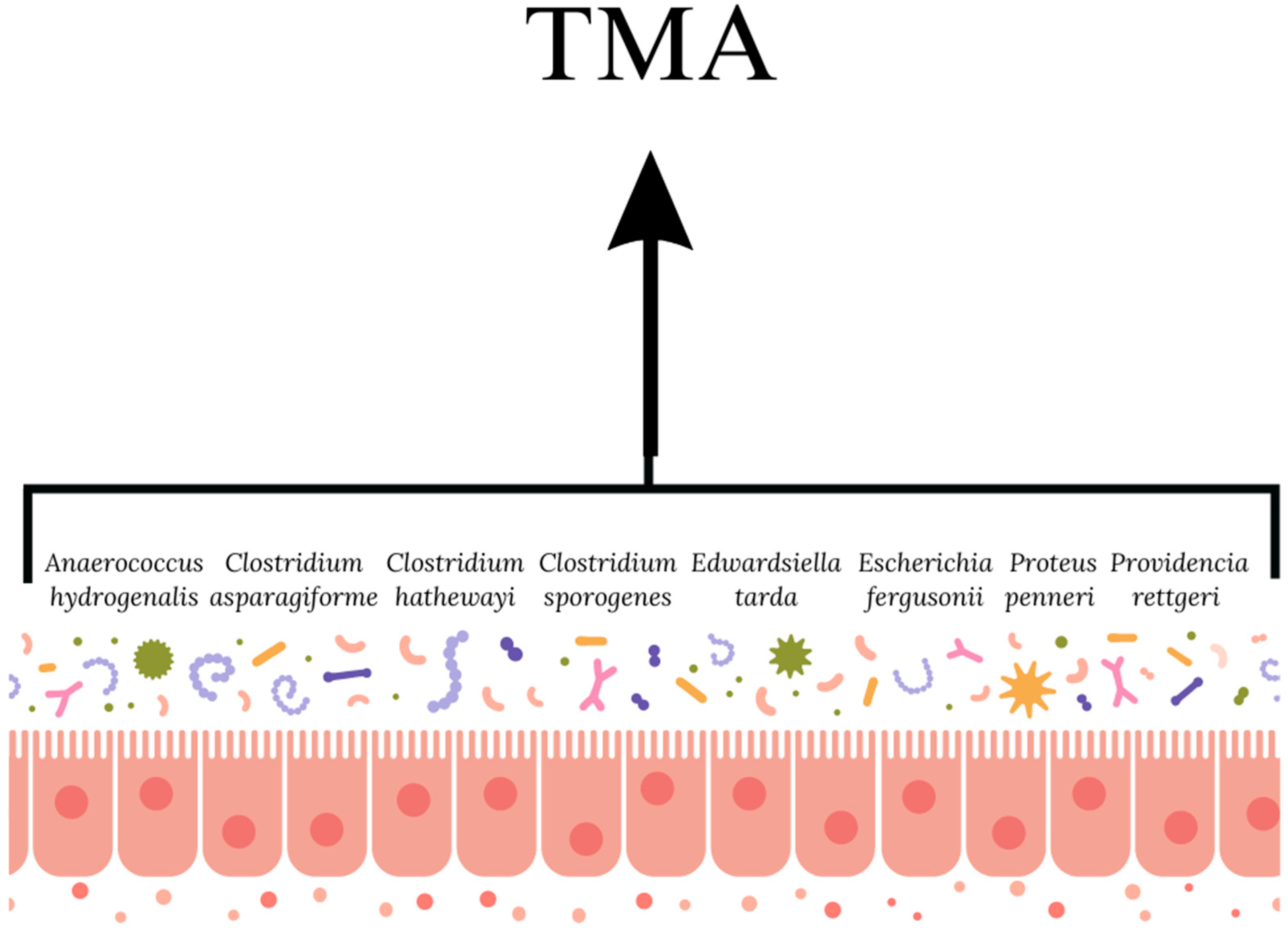
3.1.2. Coronary Artery Atherosclerosis and the Gut Microbiota
3.2. Gut Microbiota in Cognitive Impairment
4. Gut Microbiota of Patients with Coronary Artery Atherosclerosis and Cognitive Impairment
5. Modulation of the Gut Microbiota in Relation to CAD
5.1. Dietary Fiber
5.2. Probiotics
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| AD | Alzheimer’s disease |
| BBB | Blood–brain barrier |
| CAD | Coronary artery atherosclerosis |
| CVD | Cardiovascular diseases |
| DASH | Dietary Approaches to Stop Hypertension |
| HPA | Hypothalamic–pituitary–adrenal axis |
| LPS | Lipopolysaccharide |
| SCFAs | Short-chain fatty acids |
| TMAO | Trimethylamine N-oxide |
References
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Díaz, J.; Aragón-Vela, J.; Muñoz-Quezada, S.; Tercedor-Sánchez, L.; Abadia-Molina, F. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef]
- Liu, H.; Zhuang, J.; Tang, P.; Li, J.; Xiong, X.; Deng, H. The Role of the Gut Microbiota in Coronary Heart Disease. Curr. Atheroscler. Rep. 2020, 22, 77. [Google Scholar] [CrossRef]
- Krupa-Kotara, K.; Helisz, P.; Gwioździk, W.; Grajek, M. The Importance of the Microbiota in Shaping Women’s Health—The Current State of Knowledge. Appl. Microbiol. 2023, 3, 11–34. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Aadil, R.M. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Moazzami, K.; Sullivan, S.; Lima, B.B.; Kim, J.H.; Hammadah, M.; Almuwaqqat, Z.; Shah, A.J.; Hajjar, I.; Goldstein, F.C.; Levey, A.I.; et al. Mental stress-induced myocardial ischemia and cognitive impairment in coronary atherosclerosis. J. Psychosom. Res. 2021, 141, 110342. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediators Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef]
- Malinowska, M.; Tokarz-Deptuła, B.; Deptuła, W. Mikrobiom człowieka. Post. Mikrobiol. 2017, 56, 33–42. [Google Scholar]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Hur, H.J.; Wu, X.; Yang, H.J.; Kim, M.J.; Lee, K.H.; Hong, M.; Kim, M.S. Beneficial effects of a low-glycemic diet on serum metabolites and gut microbiota in obese women with Prevotella and Bacteriodes enterotypes: A randomized clinical trial. Front. Nutr. 2022, 9, 861880. [Google Scholar] [CrossRef]
- Di Pierro, F.A. Possible Perspective about the Compositional Models, Evolution, and Clinical Meaning of Human Enterotypes. Microorganisms 2021, 9, 2341. [Google Scholar] [CrossRef]
- La Sala, L.; Pontiroli, A.E. Prevention of Diabetes and Cardiovascular Disease in Obesity. Int. J. Mol. Sci. 2020, 21, 8178. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EbioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Piccioni, A.; de Cunzo, T.; Valletta, F.; Covino, M.; Rinninella, E.; Raoul, P.; Zanza, C.; Mele, M.C.; Franceschi, F. Gut Microbiota and Environment in Coronary Artery Disease. Int. J. Environ. Res. Public Health 2021, 18, 4242. [Google Scholar] [CrossRef]
- Qian, B.; Zhang, K.; Li, Y.; Sun, K. Update on gut microbiota in cardiovascular diseases. Front. Cell Infect. Microbiol. 2022, 12, 1059349. [Google Scholar] [CrossRef]
- Paeslack, N.; Mimmler, M.; Becker, S.; Gao, Z.; Khuu, M.P.; Mann, A.; Malinarich, F.; Regen, T.; Reinhardt, C. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids 2022, 54, 1339–1356. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Jiang, H. Gut microbiota in atherosclerosis: Focus on trimethylamine N-oxide. Apmis 2020, 128, 353–366. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M. Trimethylamine N-oxide generated by the gut microbiota is associated with vascular inflammation: New insights into atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Hu, X.; Niu, H.; Tian, R.; Wang, H.; Pang, H.; Jiang, L.; Qiu, B.; Chen, X.; et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome 2019, 7, 68. [Google Scholar] [CrossRef]
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Matey-Hernandez, M.L.; Keehn, L.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Kuo, C.F.; et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Heart J. 2018, 39, 2390–2397. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Choroszy, M.; Litwinowicz, K.; Bednarz, R.; Roleder, T.; Lerman, A.; Toya, T.; Kamiński, K.; Sawicka-Śmiarowska, E.; Niemira, M.; Sobieszczańska, B. Human Gut Microbiota in Coronary Artery Disease: A Systematic Review and Meta-Analysis. Metabolites 2022, 12, 1165. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Kuijer, E.J.; Steenbergen, L. The microbiota-gut-brain axis in hippocampus-dependent learning and memory: Current state and future challenges. Neurosci. Biobehav. Rev. 2023, 152, 105296. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The microbiota-gut-brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell Mol. Life Sci. 2022, 79, 80. [Google Scholar] [CrossRef]
- Gołyszny, M.; Obuchowicz, E. Are neuropeptides relevant for the mechanism of action of SSRIs? Neuropeptides 2019, 75, 1–17. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Ma, S.R.; Zhao, Z.X.; Pan, L.B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood-brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lappalainen, L.; Rajamaki, B.; Tolppanen, A.M.; Hartikainen, S. Coronary artery revascularizations and cognitive decline—A systematic review. Curr. Probl. Cardiol. 2022, 47, 100960. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Wang, Q.; Liu, F.; Xian, Y.; Xu, F.; Liu, Y. Gut microbiota in combination with blood metabolites reveals characteristics of the disease cluster of coronary artery disease and cognitive impairment: A Mendelian randomization study. Front. Immunol. 2024, 14, 1308002. [Google Scholar] [CrossRef]
- Koszewicz, M.; Jaroch, J.; Brzecka, A.; Ejma, M.; Budrewicz, S.; Mikhaleva, L.M.; Aliev, G. Dysbiosis is one of the risk factor for stroke and cognitive impairment and potential target for treatment. Pharmacol. Res. 2021, 164, 105277. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Liu, H.; Xu, H.; Liu, F.; Song, H.; Zhao, X.; Li, H. Dysregulation of Ruminococcaceae and Megamonas could be predictive markers for rapid progression of mild cognitive impairment. Microb. Pathog. 2023, 183, 106272. [Google Scholar] [CrossRef]
- Toya, T.; Corban, M.T.; Marrietta, E.; Horwath, I.E.; Lerman, L.O.; Murray, J.A.; Lerman, A. Coronary artery disease is associated with an altered gut microbiome composition. PLoS ONE 2020, 15, e0227147. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Wang, Q.; Liu, F.; Xu, F.; Liu, Y. Mendelian randomization study reveals a causal relationship between coronary artery disease and cognitive impairment. Front. Cardiovasc. Med. 2023, 10, 1150432. [Google Scholar] [CrossRef]
- Constantino-Jonapa, L.A.; Espinoza-Palacios, Y.; Escalona-Montaño, A.R.; Hernández-Ruiz, P.; Amezcua-Guerra, L.M.; Amedei, A.; Aguirre-García, M.M. Contribution of Trimethylamine N-Oxide (TMAO) to Chronic Inflammatory and Degenerative Diseases. Biomedicines 2023, 11, 431. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Gong, L.; Wang, H.; Zhu, X.; Dong, Q.; Yu, Q.; Mao, B.; Meng, L.; Zhao, Y.; Liu, X. Nomogram to Predict Cognitive Dysfunction After a Minor Ischemic Stroke in Hospitalized-Population. Front. Aging Neurosci. 2021, 13, 637363. [Google Scholar] [CrossRef]
- Connell, E.; Le Gall, G.; Pontifex, M.G.; Sami, S.; Cryan, J.F.; Clarke, G.; Müller, M.; Vauzour, D. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 2022, 17, 43. [Google Scholar] [CrossRef]
- Bremner, J.D.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapaport, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef]
- Wang, Q.J.; Shen, Y.E.; Wang, X.; Fu, S.; Zhang, X.; Zhang, Y.N.; Wang, R.T. Concomitant memantine and Lactobacillus plantarum treatment attenuates cognitive impairments in APP/PS1 mice. Aging 2020, 12, 628–649. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.; Xia, R.; Li, C. Gut microbiota in coronary artery disease: A friend or foe? Biosci. Rep. 2020, 40, BSR20200454. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Li, Y.; Hu, Y.; Franke, A.A.; Liang, L.; Hu, F.B.; Chan, A.T.; Mukamal, K.J.; Rimm, E.B.; et al. Gut microbiota-derived metabolites and risk of coronary artery disease: A prospective study among US men and women. Am. J. Clin. Nutr. 2021, 114, 238–247. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Sampson, L.; Wang, M.; Manson, J.E.; Rimm, E.; Sun, Q. Lignan Intake and Risk of Coronary Heart Disease. J. Am. Coll. Cardiol. 2021, 78, 666–678. [Google Scholar] [CrossRef]
- Coutinho-Wolino, K.S.; de FCardozo, L.F.; de Oliveira Leal, V.; Mafra, D.; Stockler-Pinto, M.B. Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur. J. Nutr. 2021, 60, 3567–3584. [Google Scholar] [CrossRef]
- Li, Q.; Chen, H.; Zhang, M.; Wu, T.; Liu, R. Altered short chain fatty acid profiles induced by dietary fiber intervention regulate AMPK levels and intestinal homeostasis. Food Funct. 2019, 10, 7174–7187. [Google Scholar] [CrossRef]
- Simó, C.; García-Cañas, V. Dietary bioactive ingredients to modulate the gut microbiota-derived metabolite TMAO. New opportunities for functional food development. Food Funct. 2020, 11, 6745–6776. [Google Scholar] [CrossRef]
- Wiese, G.N.; Biruete, A.; Moorthi, R.N.; Moe, S.M.; Lindemann, S.R.; Hill Gallant, K.M. Plant-Based Diets, the Gut Microbiota, and Trimethylamine N-Oxide Production in Chronic Kidney Disease: Therapeutic Potential and Methodological Considerations. J. Ren. Nutr. 2021, 31, 121–131. [Google Scholar] [CrossRef]
- Cantero, M.A.; Guedes, M.R.A.; Fernandes, R.; Lollo, P.C.B. Trimethylamine N-oxide reduction is related to probiotic strain specificity: A systematic review. Nutr. Res. 2022, 104, 29–35. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, M.; Huang, N.; Yuan, Z. Meta-analysis of the effect of probiotics or synbiotics on the risk factors in patients with coronary artery disease. Front. Cardiovasc. Med. 2023, 10, 1154888. [Google Scholar] [CrossRef]
| Enterotype I | Enterotype II | Enterotype III | |
|---|---|---|---|
| Predominant bacterial type | Bacteroidetes | Prevotella | Ruminococcus |
| Type of bacteria | Parabacteroides, Alistipes, Bilophila | Desulfovibrio, Succinivibrio. | Ruminoccocus Akkermansia *, Methanobrevibacter * |
| Type of bacteria potentially negatively associated (e.g., with inflammatory processes or dysbiosis) | Methanobrevibacter | Akkermansia | Prevotella |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helisz, P.; Krupa-Kotara, K.; Gwioździk, W.; Głogowska-Ligus, J. The Role of the Gut Microbiota in Mental Health and Cognitive Function in Patients with Coronary Atherosclerosis. Nutrients 2025, 17, 2311. https://doi.org/10.3390/nu17142311
Helisz P, Krupa-Kotara K, Gwioździk W, Głogowska-Ligus J. The Role of the Gut Microbiota in Mental Health and Cognitive Function in Patients with Coronary Atherosclerosis. Nutrients. 2025; 17(14):2311. https://doi.org/10.3390/nu17142311
Chicago/Turabian StyleHelisz, Paulina, Karolina Krupa-Kotara, Weronika Gwioździk, and Joanna Głogowska-Ligus. 2025. "The Role of the Gut Microbiota in Mental Health and Cognitive Function in Patients with Coronary Atherosclerosis" Nutrients 17, no. 14: 2311. https://doi.org/10.3390/nu17142311
APA StyleHelisz, P., Krupa-Kotara, K., Gwioździk, W., & Głogowska-Ligus, J. (2025). The Role of the Gut Microbiota in Mental Health and Cognitive Function in Patients with Coronary Atherosclerosis. Nutrients, 17(14), 2311. https://doi.org/10.3390/nu17142311