Microencapsulated Propionate and Butyrate Improved Energy Balance and Gut Microbiota Composition in Diet-Induced Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Food Intake, Energy Expenditure, Body Weight and Composition Measurements
2.3. Fecal and Cecal Microbiota Extraction, 16S Sequencing and Bioinformatics
2.4. Metabolomics Assay
2.5. Statistical Analysis
3. Results
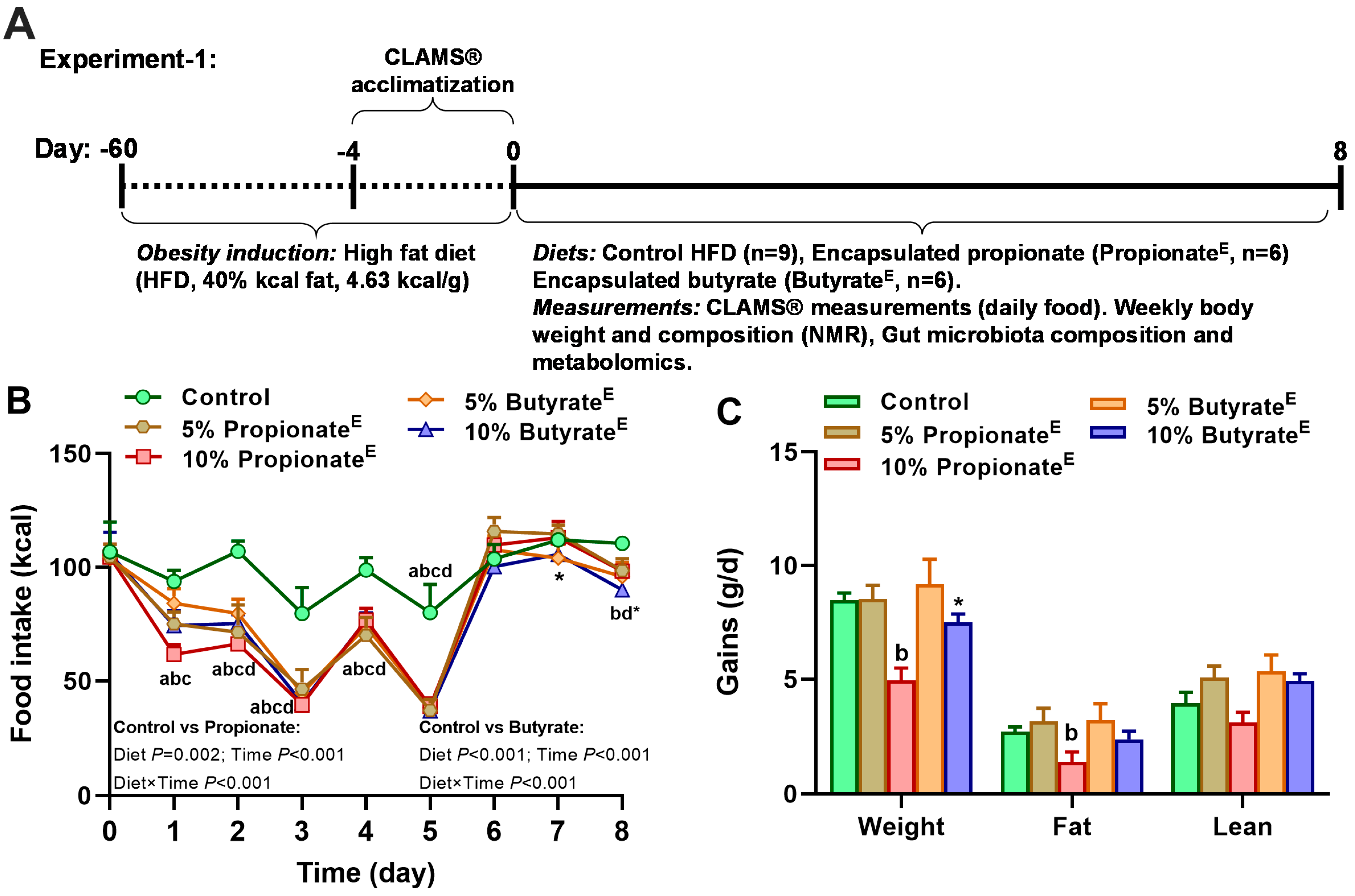
3.1. Microencapsulated Propionate and Butyrate Dose-Dependently Reduced Food Intake and Energy Expenditure, Shifted Substrate Utilization Toward Lipids and Decreased Body Weight Gains
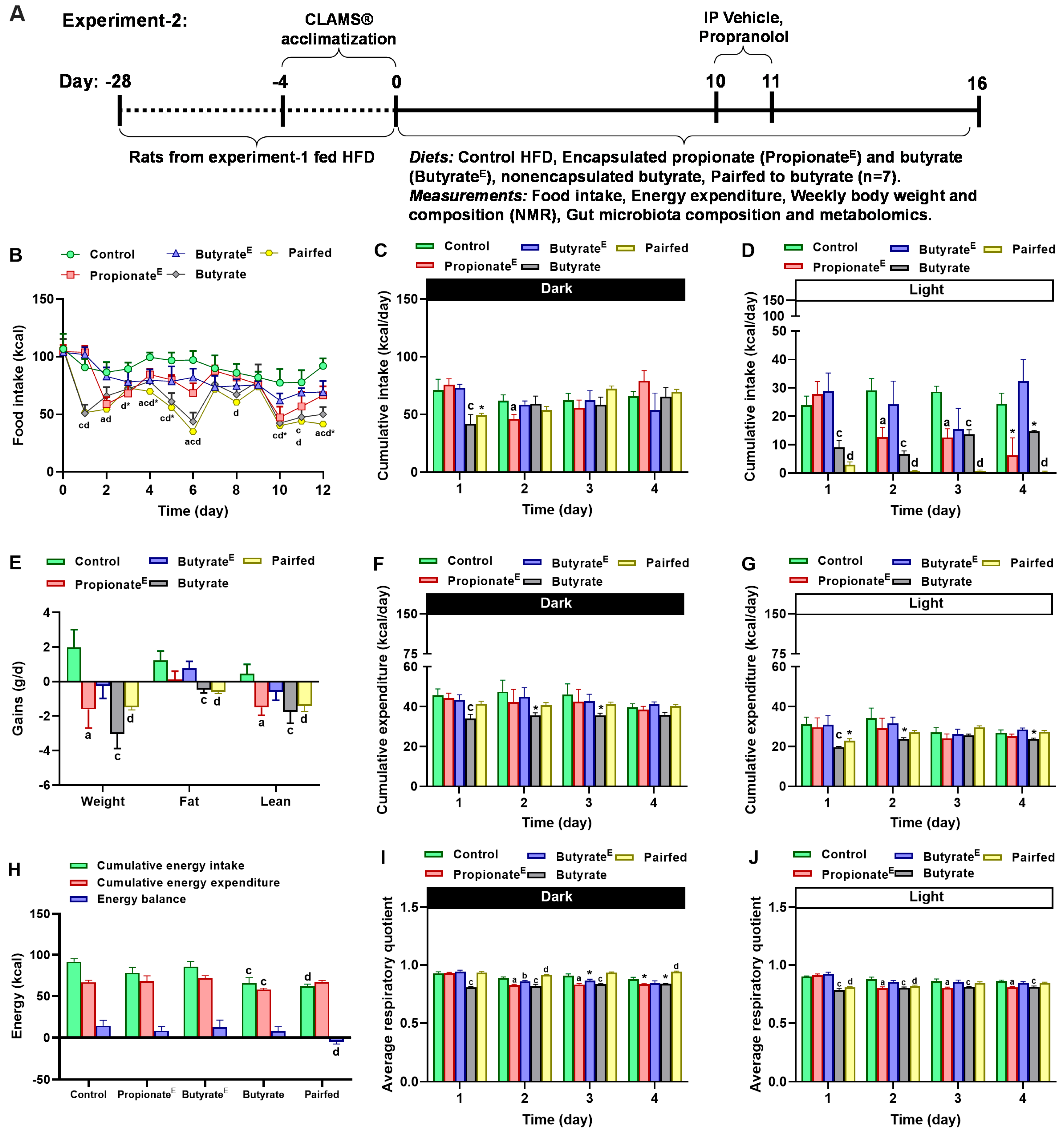
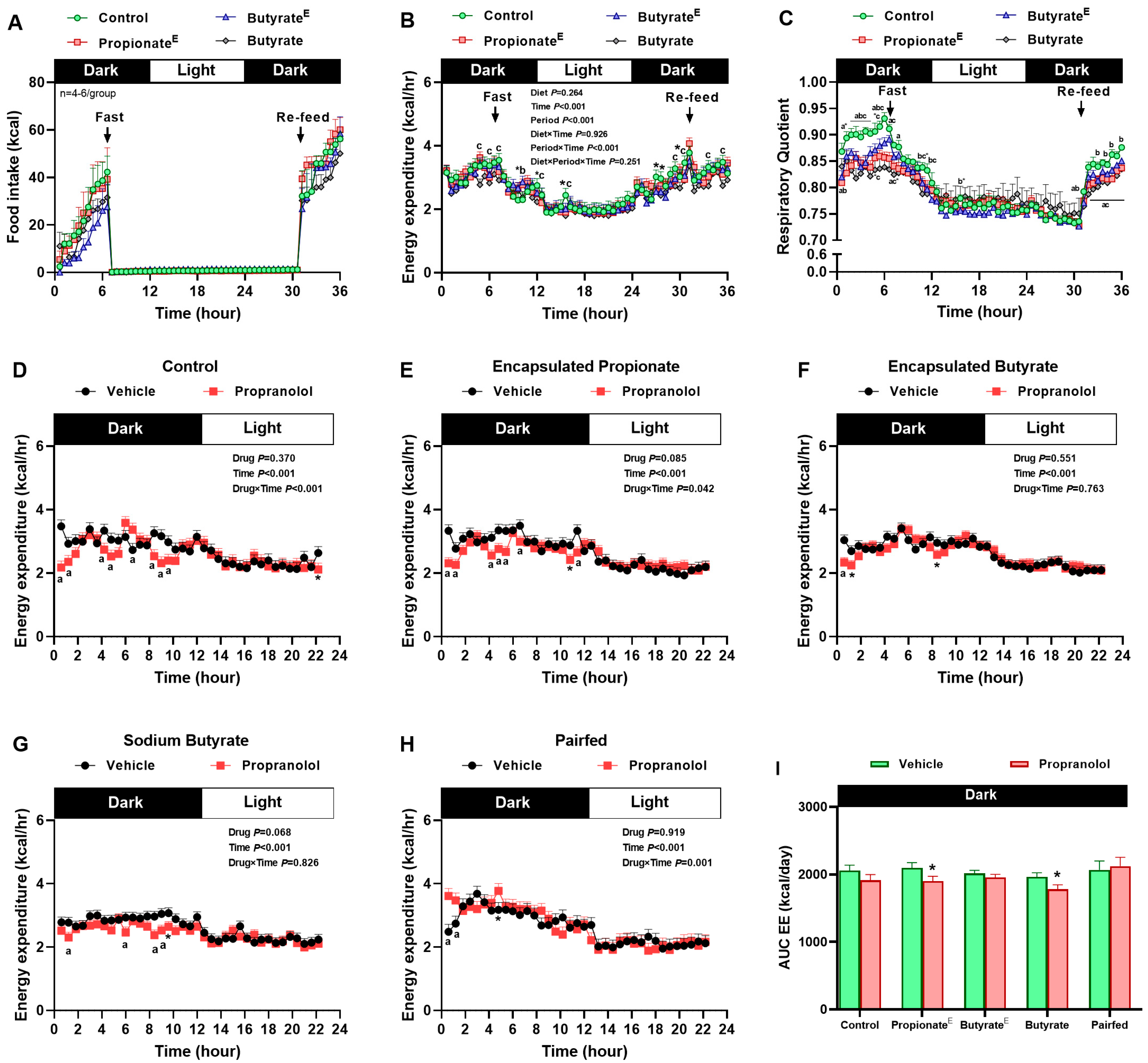
3.2. Microencapsulated Propionate and Non-Encapsulated Butyrate Increased Lipid Utilization, Decreased Fasting Energy Expenditure, Yet, Sustained Sympathetically Mediated Fed Energy Expenditure
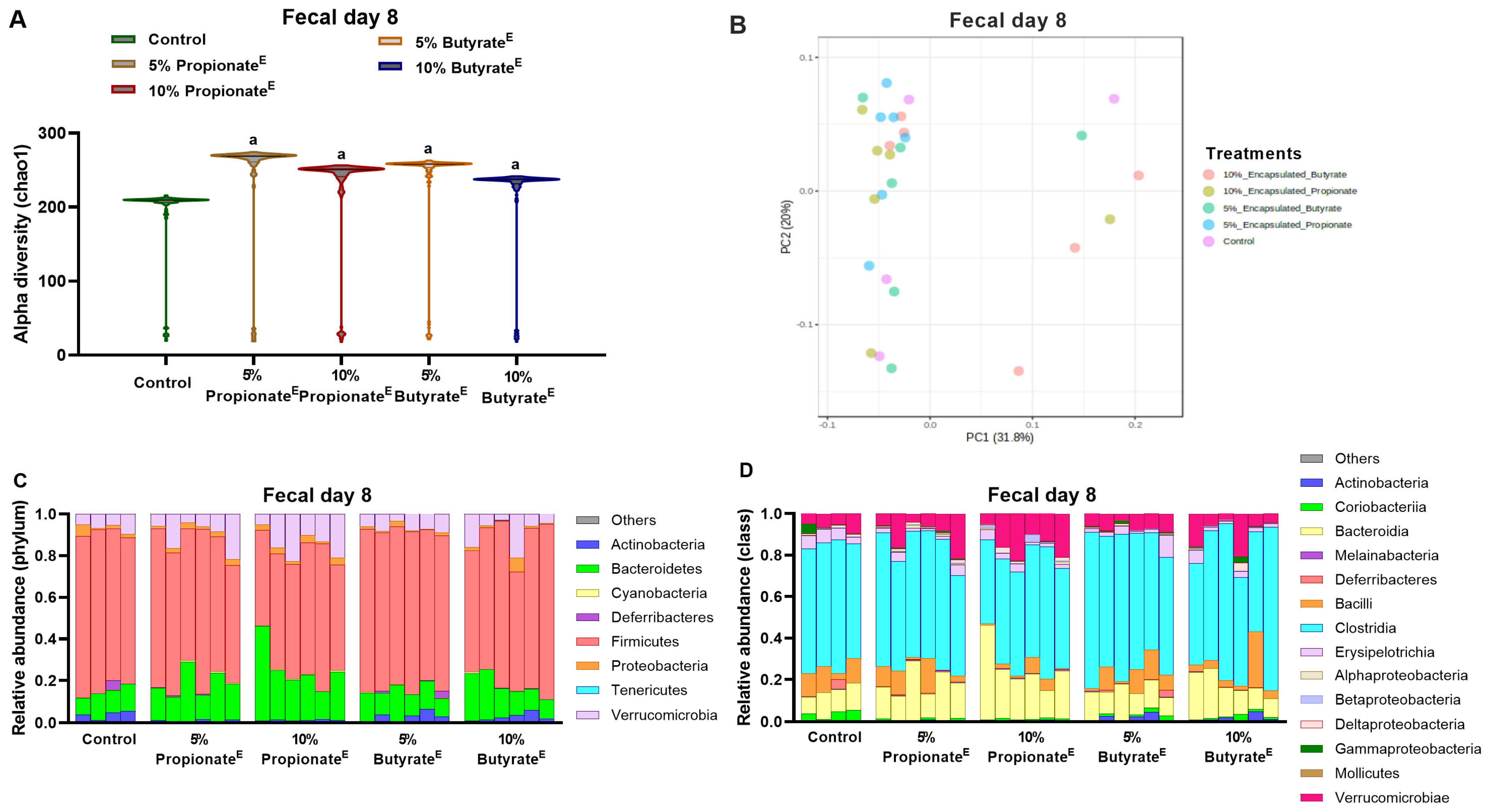
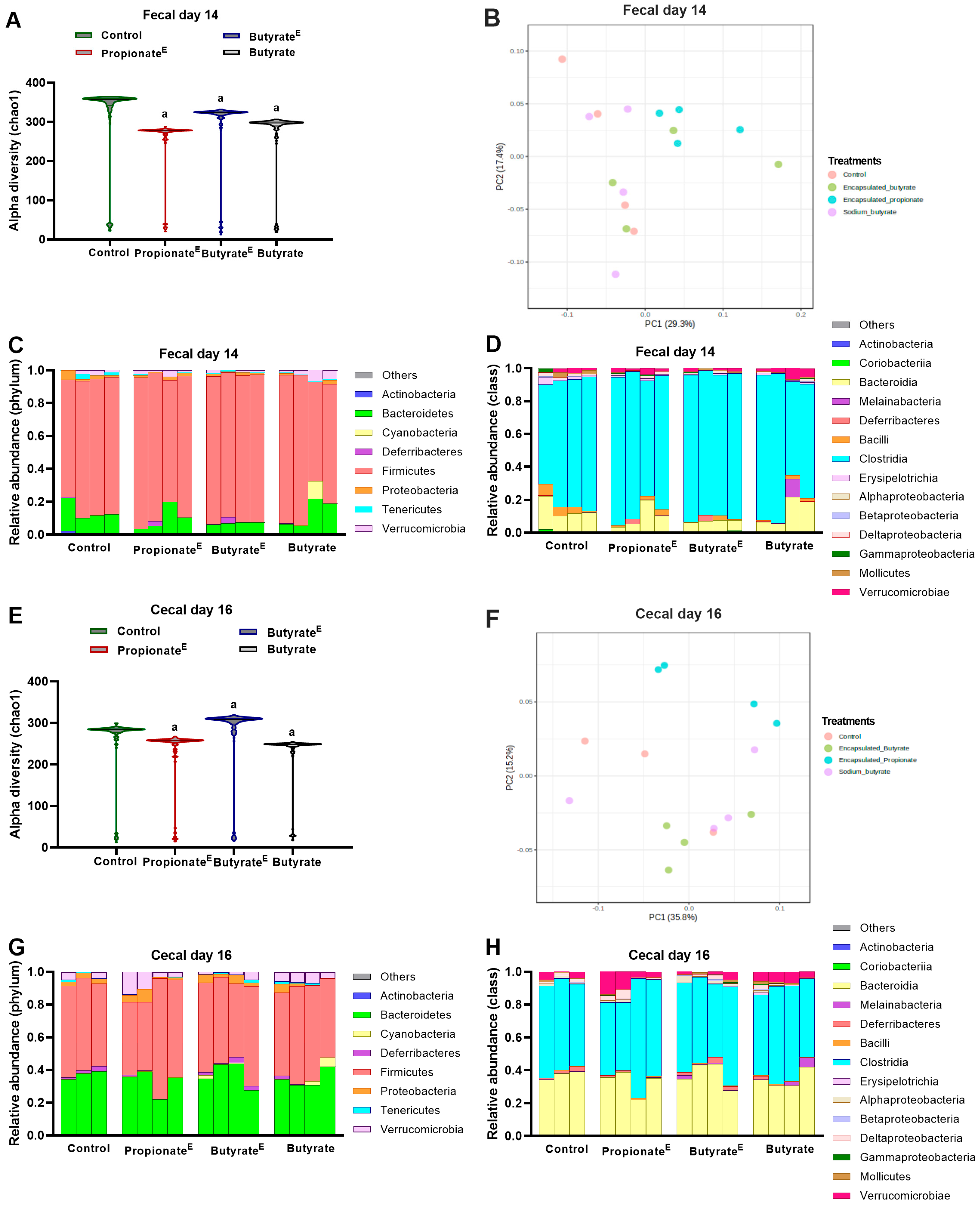
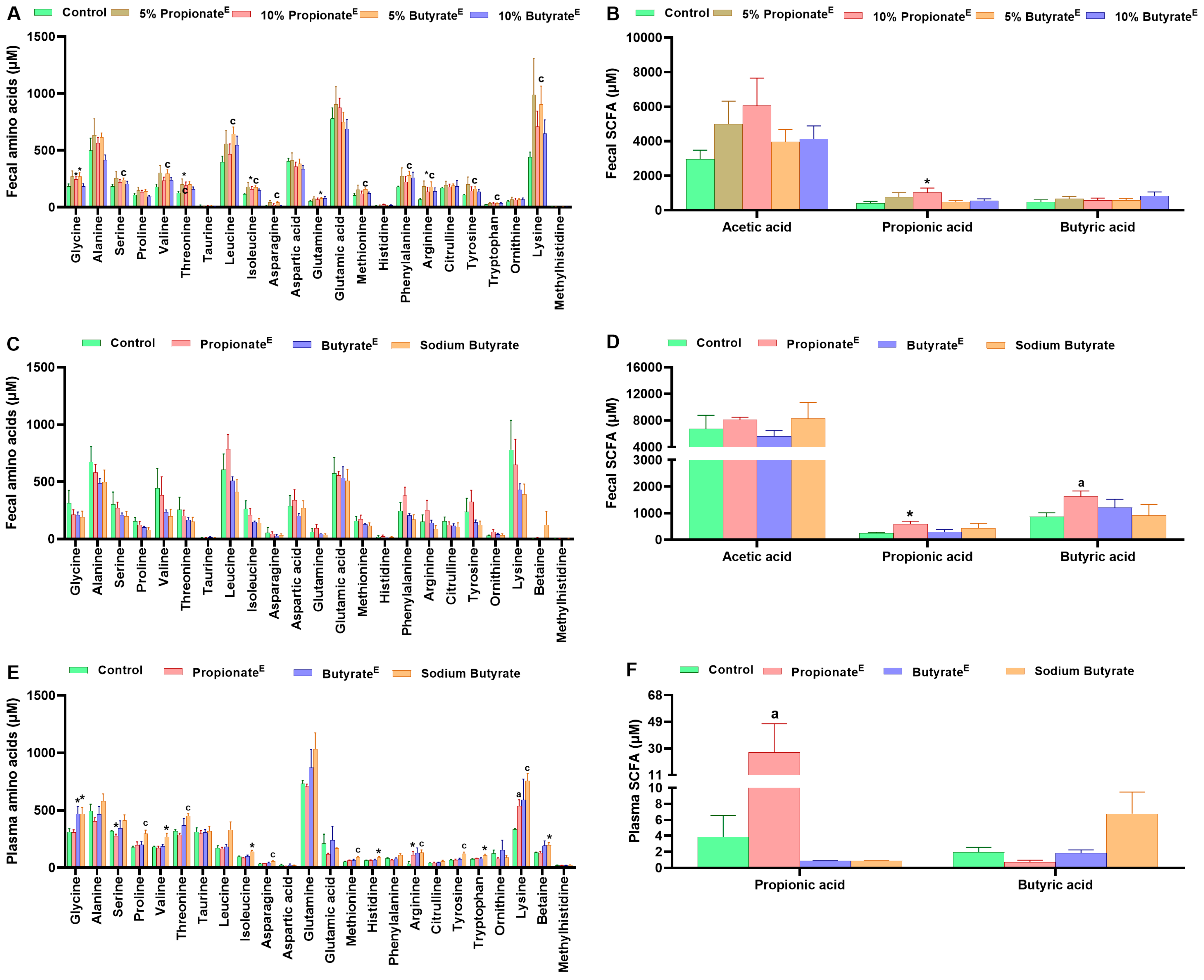
3.3. Microencapsulated Propionate and Butyrate Altered Gut Microbiota Composition and Metabolite Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Sharma, R.; Frost, G. Propionate. Anti-obesity and satiety enhancing factor? Appetite 2011, 56, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Güler, M.S.; Arslan, S.; Ağagündüz, D.; Cerqua, I.; Pagano, E.; Canani, R.B.; Capasso, R. Butyrate: A potential mediator of obesity and microbiome via different mechanisms of actions. Food Res. Int. 2024, 199, 115420. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Chambers, E.; Morrison, D.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Matheus, V.; Monteiro, L.; Oliveira, R.; Maschio, D.; Collares-Buzato, C. Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice. Exp. Biol. Med. 2017, 242, 1214–1226. [Google Scholar] [CrossRef]
- Fang, W.; Xue, H.; Chen, X.; Chen, K.; Ling, W. Supplementation with sodium butyrate modulates the composition of the gut microbiota and ameliorates high-fat diet-induced obesity in mice. J. Nutr. 2019, 149, 747–754. [Google Scholar] [CrossRef]
- Amiri, P.; Hosseini, S.A.; Saghafi-Asl, M.; Roshanravan, N.; Tootoonchian, M. Expression of PGC-1alpha, PPAR-alpha and UCP1 genes, metabolic and anthropometric factors in response to sodium butyrate supplementation in patients with obesity: A triple-blind, randomized placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2025, 79, 249–257. [Google Scholar] [CrossRef]
- Liljeberg, H.; Björck, I. Delayed gastric emptying rate as a potential mechanism for lowered glycemia after eating sourdough bread: Studies in humans and rats using test products with added organic acids or an organic salt. Am. J. Clin. Nutr. 1996, 64, 886–893. [Google Scholar] [CrossRef]
- Liljeberg, H.G.; Lönner, C.H.; Björck, I.M. Sourdough fermentation or addition of organic acids or corresponding salts to bread improves nutritional properties of starch in healthy humans. J. Nutr. 1995, 125, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Preston, T.; Tedford, C.; Brignardello, J.; Garcia-Perez, I.; Holmes, E.; Wallis, G.A.; Morrison, D.J.; Frost, G.S. Effects of inulin propionate ester incorporated into palatable food products on appetite and resting energy expenditure: A randomised crossover study. Nutrients 2019, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Darzi, J.; Frost, G.S.; Robertson, M.D. Effects of a novel propionate-rich sourdough bread on appetite and food intake. Eur. J. Clin. Nutr. 2012, 66, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, G.; Östman, E.M.; Liljeberg, H.G.; Kallinen, N.; Björgell, O.; Björck, I.M.; Almér, L.-O. Measurements of the gastric emptying rate by use of ultrasonography: Studies in humans using bread with added sodium propionate. Am. J. Clin. Nutr. 2001, 74, 254–258. [Google Scholar] [CrossRef]
- Todesco, T.; Rao, A.V.; Bosello, O.; Jenkins, D. Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am. J. Clin. Nutr. 1991, 54, 860–865. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Aspey, K.; Chen, Y.; Khan, S.; Morrison, D.J.; Frost, G. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes Obes. Metab. 2018, 20, 1034–1039. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.-Z.; Zhang, R.-N.; He, C.-X.; Chen, G.-Y.; Liu, C.; Chen, Y.-W.; Fan, J.-G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60. [Google Scholar] [CrossRef]
- Zhai, S.; Qin, S.; Li, L.; Zhu, L.; Zou, Z.; Wang, L. Dietary butyrate suppresses inflammation through modulating gut microbiota in high-fat diet-fed mice. FEMS Microbiol. Lett. 2019, 366, fnz153. [Google Scholar] [CrossRef]
- Song, B.; Zhong, Y.; Zheng, C.; Li, F.; Duan, Y.; Deng, J. Propionate alleviates high-fat diet-induced lipid dysmetabolism by modulating gut microbiota in mice. J. Appl. Microbiol. 2019, 127, 1546–1555. [Google Scholar] [CrossRef]
- Brütting, C.; Lara Bisch, M.; Brandsch, C.; Hirche, F.; Stangl, G.I. Impact of dietary propionate on fructose-induced changes in lipid metabolism, gut microbiota and short-chain fatty acids in mice. Int. J. Food Sci. Nutr. 2021, 72, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.G., Jr.; Soergel, K.H.; Wood, C.M. Absorption of short chain fatty acids from the human jejunum. Gastroenterology 1976, 70, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Ruppin, H.; Bar-Meir, S.; Soergel, K.H.; Wood, C.M.; Schmitt, M.G., Jr. Absorption of short-chain fatty acids by the colon. Gastroenterology 1980, 78, 1500–1507. [Google Scholar] [CrossRef]
- Van der Schoot, V.; Haer-Wigman, L.; Feenstra, I.; Tammer, F.; Oerlemans, A.J.; van Koolwijk, M.P.; van Agt, F.; Arens, Y.H.; Brunner, H.G.; Vissers, L.E. Lessons learned from unsolicited findings in clinical exome sequencing of 16,482 individuals. Eur. J. Hum. Genet. 2022, 30, 170–177. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef]
- Hijova, E.; Chmelarova, A. Short chain fatty acids and colonic health. Bratisl. Lekárske Listy 2007, 108, 354. [Google Scholar]
- Blaak, E.; Canfora, E.; Theis, S.; Frost, G.; Groen, A.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the intestinal epithelium: Modulation of proliferation and inflammation in homeostasis and disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Williams, L.M.; Cao, S. Harnessing and delivering microbial metabolites as therapeutics via advanced pharmaceutical approaches. Pharmacol. Ther. 2024, 256, 108605. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Polyviou, T.; MacDougall, K.; Chambers, E.; Viardot, A.; Psichas, A.; Jawaid, S.; Harris, H.; Edwards, C.; Simpson, L.; Murphy, K. Randomised clinical study: Inulin short-chain fatty acid esters for targeted delivery of short-chain fatty acids to the human colon. Aliment. Pharmacol. Ther. 2016, 44, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [PubMed]
- Banasiewicz, T.; Krokowicz, L.; Stojcev, Z.; Kaczmarek, B.F.; Kaczmarek, E.; Maik, J.; Marciniak, R.; Krokowicz, P.; Walkowiak, J.; Drews, M. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Color. Dis. 2013, 15, 204–209. [Google Scholar] [CrossRef]
- Krokowicz, L.; Stojcev, Z.; Kaczmarek, B.F.; Kociemba, W.; Kaczmarek, E.; Walkowiak, J.; Krokowicz, P.; Drews, M.; Banasiewicz, T. Microencapsulated sodium butyrate administered to patients with diverticulosis decreases incidence of diverticulitis--a prospective randomized study. Int. J. Color. Dis. 2014, 29, 387–393. [Google Scholar] [CrossRef]
- Lewandowski, K.; Kaniewska, M.; Karlowicz, K.; Rosolowski, M.; Rydzewska, G. The effectiveness of microencapsulated sodium butyrate at reducing symptoms in patients with irritable bowel syndrome. Przegląd Gastroenterol. 2022, 17, 28–34. [Google Scholar] [CrossRef]
- Vernero, M.; De Blasio, F.; Ribaldone, D.G.; Bugianesi, E.; Pellicano, R.; Saracco, G.M.; Astegiano, M.; Caviglia, G.P. The Usefulness of Microencapsulated Sodium Butyrate Add-On Therapy in Maintaining Remission in Patients with Ulcerative Colitis: A Prospective Observational Study. J. Clin. Med. 2020, 9, 3941. [Google Scholar] [CrossRef]
- Coppola, S.; Nocerino, R.; Paparo, L.; Bedogni, G.; Calignano, A.; Di Scala, C.; de Giovanni di Santa Severina, A.F.; De Filippis, F.; Ercolini, D.; Berni Canani, R. Therapeutic Effects of Butyrate on Pediatric Obesity: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2244912. [Google Scholar] [CrossRef]
- Khosravi, Z.; Hadi, A.; Tutunchi, H.; Asghari-Jafarabadi, M.; Naeinie, F.; Roshanravan, N.; Ostadrahimi, A.; Fadel, A. The effects of butyrate supplementation on glycemic control, lipid profile, blood pressure, nitric oxide level and glutathione peroxidase activity in type 2 diabetic patients: A randomized triple -blind, placebo-controlled trial. Clin. Nutr. ESPEN 2022, 49, 79–85. [Google Scholar] [CrossRef]
- Li, H.; Zhao, L.; Liu, S.; Zhang, Z.; Wang, X.; Lin, H. Propionate inhibits fat deposition via affecting feed intake and modulating gut microbiota in broilers. Poult. Sci. 2020, 100, 235. [Google Scholar] [CrossRef]
- Yang, T.; Sun, Y.; Dai, Z.; Liu, J.; Xiao, S.; Liu, Y.; Wang, X.; Yang, S.; Zhang, R.; Yang, C. Microencapsulated sodium butyrate alleviates immune injury and intestinal problems caused by clostridium perfringens through gut microbiota. Animals 2023, 13, 3784. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, T.; Li, P.; Cheng, S.; Casper, D.P. Effects of Essential Oil and/or Encapsulated Butyrate on Fecal Microflora in Neonatal Holstein Calves. Animals 2023, 13, 3523. [Google Scholar] [CrossRef] [PubMed]
- Makowski, Z.; Lipiński, K.; Mazur-Kuśnirek, M. The Effects of sodium butyrate, coated sodium butyrate, and butyric acid glycerides on nutrient digestibility, gastrointestinal function, and fecal microbiota in turkeys. Animals 2022, 12, 1836. [Google Scholar] [CrossRef] [PubMed]
- Bernad-Roche, M.; Bellés, A.; Grasa, L.; Casanova-Higes, A.; Mainar-Jaime, R.C. Effects of dietary supplementation with protected sodium butyrate on gut microbiota in growing-finishing pigs. Animals 2021, 11, 2137. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Avirineni, B.S.; Singh, A.; Zapata, R.C.; Stevens, R.D.; Phillips, C.D.; Chelikani, P.K. Diets Containing Egg or Whey Protein and Inulin Fiber Improve Energy Balance and Modulate Gut Microbiota in Exercising Obese Rats. Mol. Nutr. Food Res. 2022, 66, e2100653. [Google Scholar] [CrossRef]
- Singh, A.; Zapata, R.C.; Pezeshki, A.; Reidelberger, R.D.; Chelikani, P.K. Inulin fiber dose-dependently modulates energy balance, glucose tolerance, gut microbiota, hormones and diet preference in high-fat-fed male rats. J. Nutr. Biochem. 2018, 59, 142–152. [Google Scholar] [CrossRef]
- Singh, A.; Zapata, R.C.; Pezeshki, A.; Workentine, M.L.; Chelikani, P.K. Host genetics and diet composition interact to modulate gut microbiota and predisposition to metabolic syndrome in spontaneously hypertensive stroke-prone rats. FASEB J. 2019, 33, 6748–6766. [Google Scholar] [CrossRef]
- Singh, A.; Pezeshki, A.; Zapata, R.C.; Yee, N.J.; Knight, C.G.; Tuor, U.I.; Chelikani, P.K. Diets enriched in whey or casein improve energy balance and prevent morbidity and renal damage in salt-loaded and high-fat-fed spontaneously hypertensive stroke-prone rats. J. Nutr. Biochem. 2016, 37, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Avirineni, B.S.; Singh, A.; Zapata, R.C.; Phillips, C.D.; Chelikani, P.K. Dietary whey and egg proteins interact with inulin fiber to modulate energy balance and gut microbiota in obese rats. J. Nutr. Biochem. 2022, 99, 108860. [Google Scholar] [CrossRef] [PubMed]
- Zapata, R.C.; Singh, A.; Pezeshki, A.; Avirineni, B.S.; Patra, S.; Chelikani, P.K. Low-Protein Diets with Fixed Carbohydrate Content Promote Hyperphagia and Sympathetically Mediated Increase in Energy Expenditure. Mol. Nutr. Food Res. 2019, 63, e1900088. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Foroutan, A.; Fitzsimmons, C.; Mandal, R.; Piri-Moghadam, H.; Zheng, J.; Guo, A.; Li, C.; Guan, L.L.; Wishart, D.S. The Bovine Metabolome. Metabolites 2020, 10, 233. [Google Scholar] [CrossRef]
- Even, P.C.; Nadkarni, N.A. Indirect calorimetry in laboratory mice and rats: Principles, practical considerations, interpretation and perspectives. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2012, 303, R459–R476. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Ma, Y.; Peng, S.; Tang, N.; Cheng, Y. Encapsulation of butyrate using low-alkali konjac gel induced by ethanol for colonic delivery. Food Hydrocoll. Health 2021, 1, 100046. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, A.H.; Lett, A.M.; Frost, G.; Chambers, E.S. Regulation of energy expenditure and substrate oxidation by short-chain fatty acids. J. Endocrinol. 2019, 242, R1–R8. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef]
- Tschöp, M.H.; Speakman, J.R.; Arch, J.R.; Auwerx, J.; Brüning, J.C.; Chan, L.; Eckel, R.H.; Farese, R.V., Jr.; Galgani, J.E.; Hambly, C. A guide to analysis of mouse energy metabolism. Nat. Methods 2012, 9, 57–63. [Google Scholar] [CrossRef]
- Canfora, E.E.; van der Beek, C.M.; Jocken, J.W.; Goossens, G.H.; Holst, J.J.; Olde Damink, S.W.; Lenaerts, K.; Dejong, C.H.; Blaak, E.E. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: A randomized crossover trial. Sci. Rep. 2017, 7, 2360. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Hamaker, B.R. Tuning expectations to reality: Don’t expect increased gut microbiota diversity with dietary fiber. J. Nutr. 2023, 153, 3156–3163. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the role of Erysipelotrichaceae in the human host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.; Brummer, R.J. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Procaccianti, G.; De Bastiani, R.; Turroni, S.; D’Amico, F.; Allegretta, L.; Antonino, N.; Baldi, E.; Casamassima, C.; Casella, G.; et al. Micro-encapsulated and colonic-release sodium butyrate modulates gut microbiota and improves abdominal pain in patients with symptomatic uncomplicated diverticular disease. Front. Med. 2025, 12, 1487892. [Google Scholar] [CrossRef]
- Albouery, M.; Bretin, A.; Buteau, B.; Gregoire, S.; Martine, L.; Gambert, S.; Bron, A.M.; Acar, N.; Chassaing, B.; Bringer, M.A. Soluble Fiber Inulin Consumption Limits Alterations of the Gut Microbiota and Hepatic Fatty Acid Metabolism Caused by High-Fat Diet. Nutrients 2021, 13, 1037. [Google Scholar] [CrossRef]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef]
| Control | PropionateE (5%) | PropionateE (10%) | ButyrateE (5%) | ButyrateE (10%) | Sodium Butyrate | |
|---|---|---|---|---|---|---|
| Ingredients (g kg−1) | ||||||
| Corn starch a | 220 | 168 | 116 | 189 | 158 | 158 |
| Fructose b | 150 | 150 | 150 | 150 | 150 | 150 |
| Sucrose c | 150 | 150 | 150 | 150 | 150 | 150 |
| Casein High Nitrogen (80-mesh) b | 176 | 176 | 176 | 176 | 176 | 176 |
| Corn oil d | 60 | 60 | 60 | 60 | 60 | 60 |
| Lard e | 145 | 147 | 149 | 126 | 107 | 107 |
| α-Cellulose b | 50 | 50 | 50 | 50 | 50 | 50 |
| Biotin b | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
| L-Cystine b | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Choline bitartrate b | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| AIN-93-MX b | 35 | 35 | 35 | 35 | 35 | 35 |
| AIN-93-VX b | 10 | 10 | 10 | 10 | 10 | 10 |
| Sodium Propionate f | 0 | 50 | 100 | 0 | 0 | 0 |
| Sodium Butyrate fg | 0 | 0 | 0 | 50 | 100 | 100 |
| tert-Butylhydroquinone (tBHQ) in oil b | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 |
| Vanilla Extract h | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Total amount (g) | 1000.32 | 1000.32 | 1000.32 | 1000.32 | 1000.32 | 1000.32 |
| Composition | ||||||
| Carbohydrate (% kcal) * | 45% | 40% | 36% | 42% | 40% | 40% |
| Protein (% kcal) * | 15.21% | 15.21% | 15.21% | 15.21% | 15.21% | 15.21% |
| Fat (% kcal) * | 40% | 44% | 49% | 43% | 45% | 45% |
| Energy density (kcal/g) * | 4.629 | 4.629 | 4.629 | 4.629 | 4.629 | 4.629 |
| Total Fiber (% wt) | 5% | 5% | 5% | 5% | 5% | 5% |
| Fat (% g/kg) | 20% | 26% | 31% | 24% | 27% | 27% |
| Mineral:Calories ratio | 0.0076 | 0.0076 | 0.0076 | 0.0076 | 0.0076 | 0.0076 |
| Vitamin:Calories ratio | 0.0022 | 0.0022 | 0.0022 | 0.0022 | 0.0022 | 0.0022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patra, S.; Chelikani, P.K. Microencapsulated Propionate and Butyrate Improved Energy Balance and Gut Microbiota Composition in Diet-Induced Obese Rats. Nutrients 2025, 17, 2180. https://doi.org/10.3390/nu17132180
Patra S, Chelikani PK. Microencapsulated Propionate and Butyrate Improved Energy Balance and Gut Microbiota Composition in Diet-Induced Obese Rats. Nutrients. 2025; 17(13):2180. https://doi.org/10.3390/nu17132180
Chicago/Turabian StylePatra, Souvik, and Prasanth K. Chelikani. 2025. "Microencapsulated Propionate and Butyrate Improved Energy Balance and Gut Microbiota Composition in Diet-Induced Obese Rats" Nutrients 17, no. 13: 2180. https://doi.org/10.3390/nu17132180
APA StylePatra, S., & Chelikani, P. K. (2025). Microencapsulated Propionate and Butyrate Improved Energy Balance and Gut Microbiota Composition in Diet-Induced Obese Rats. Nutrients, 17(13), 2180. https://doi.org/10.3390/nu17132180





