Fermented Fruits, Vegetables, and Legumes in Metabolic Syndrome: From Traditional Use to Functional Foods and Medical Applications
Abstract
1. Introduction
2. Selection of Relevant Publications
3. Fermentation
4. Fermented Fruits
4.1. Table Olives
- Greek olives are harvested in the purple stage of maturation, debittered by long soaking in brine (up to 1 year), and then fermented. To correct the color losses caused by the diffusion of anthocyanins, ferrous gluconate can be added. The final product is characterized by a sour, fermented, and salty flavor and a low pH (~4).
- Green Spanish olives are harvested in a green stage of maturation, debittered by lye treatment, and fermented in brine for up to 7 months. The ready-to-go product remains green and has a sensory profile and pH similar to natural Greek olives.
- California-style olives are obtained by an artificial method. First, the olives in a green stage of maturation undergo a debittering process by lye treatment. To obtain a black-colored product, the fruit is then oxidized by air. Lye treatment and oxidation are repeated several times, and then ferrous gluconate is added to fix the black color of the olives. The debittering process lasts only one week, and the fermentation process is omitted. The final product is characterized by an earthy, soapy, and buttery taste, a high pH (5.8–7.9), and may contain acrylamide.
4.1.1. Nutrients and Phytocompounds
4.1.2. Microbiota
4.1.3. The Relevance of Table Olives in the Context of MetS
4.2. Capers
4.2.1. Nutrients and Phytocompounds
4.2.2. Microbiota
4.2.3. The Relevance of Capers in the Context of MetS
5. Fermented Vegetables
5.1. Kimchi
5.1.1. Nutrients and Phytocompounds
5.1.2. Microbiota
5.1.3. The Relevance of Kimchi in the Context of MetS
5.2. Fermented Cabbage and Sauerkraut
5.2.1. Nutrients and Phytocompounds
5.2.2. Microbiota
5.2.3. The Relevance of Sauerkraut and Fermented Cabbage in the Context of MetS
6. Fermented Legumes
6.1. Fermented Soybean Products
6.1.1. Tempeh
Nutrients and Phytocompounds
Microbiota
The Relevance of Tempeh in the Context of MetS
6.1.2. Natto
Nutrients and Phytocompounds
Microbiota
The Relevance of Natto in the Context of MetS
6.1.3. Cheonggukjang
Nutrients and Phytocompounds
Microbiota
The Relevance of CGJ in the Context of MetS
6.1.4. Kochujang
Nutrients and Phytzocompounds
Microbiota
The Relevance of Kochujang in the Context of MetS
6.1.5. Doenjang
Nutrients and Phytocompounds
Microbiota
The Relevance of Doenjang in the Context of MetS
6.2. Other Examples of Fermented Legumes
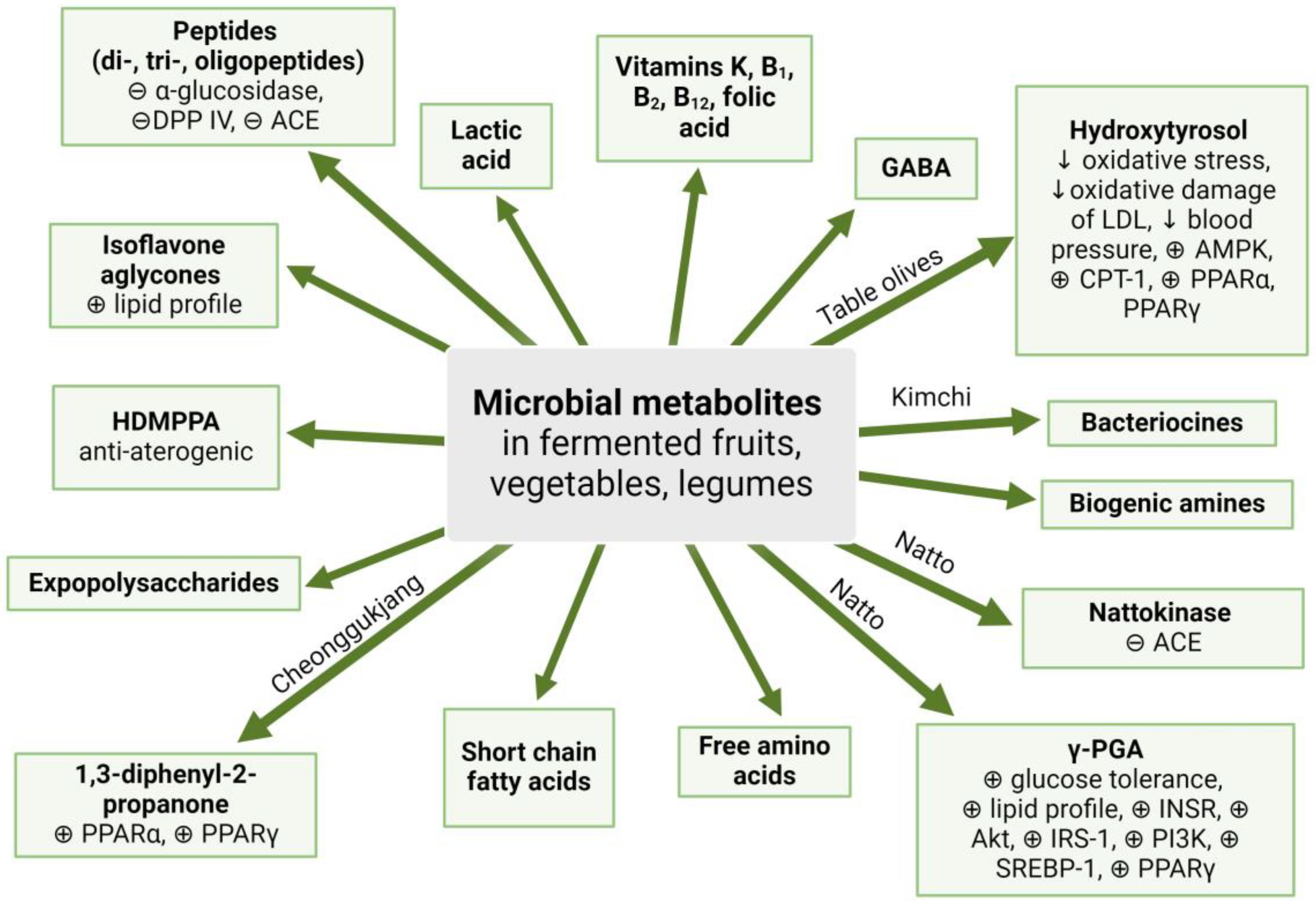
7. Microbial Metabolites
8. New Trends in Fermented Foods
9. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MetS | Metabolic syndrome |
| CVD | Cardiovascular disease |
| LAB | Lactic acid bacteria |
| GRAS | Generally Recognized as Safe |
| HT | Hydroxytyrosol |
| AMPK | AMP-activated protein kinase |
| ApoB | Apolipoprotein B |
| ACC | Acetyl-CoA carboxylase |
| DGAT | Diacylglycerol acyltransferase |
| HMGCR | 3-Hydroxy-3-methyl-glutaryl-CoA reductase |
| FFA | Free fatty acids |
| HDMPPA | 3-(40-Hydroxyl-30,50-dimethoxyphenyl) propionic acid |
| TG | Triglycerides |
| BP | Blood pressure |
| BMI | Body Mass Index |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| QUICKI | Quantitative Insulin Sensitivity Check Index |
| TLR 4 | Toll-like receptor 4 |
| NF-κB | Nuclear factor-κB |
| CGJ | Cheonggukjang |
| DJ | Doenjang |
| OGTT | Oral Glucose Tolerance Tests |
| DPP IV | Dipeptidyl peptidase 4 |
| ACE | Angiotensin-converting enzyme |
| AX | Arabinoxylan |
| γ-PGA | γ-polyglutamic acid |
| NK | Nattokinase |
| HFD | High-fat diet |
| CEBP/α | CCAAT element binding protein α |
| HSL | Hormone-sensitive lipase |
| KJ | Kochujang |
| TNF-α | Tumor necrosis factor α |
| MCP-1 | Monocyte chemoattractant protein-1 |
| AGT | Angiotensinogen |
| LXRα | Liver X receptor alpha gene |
| SREBP-1c | Sterol regulatory element-binding protein 1c gene |
| SREBP-2 | Sterol regulatory element-binding protein 2 gene |
| FAS | Fatty acid synthase gene |
| Ldlr | Low density lipoprotein receptor gene |
| Agpat5 | 1-Acyl-sn-glycerol-3-phosphate acyltransferase 5 gene |
| Acox2 | Acyl-CoA oxidase gene |
| Akt | Protein kinase B |
| GLP-1 | Glucagon-like peptide 1 gene |
| UCP1 | Uncoupling protein 1 gene |
| Me | Malic enzyme |
| G6pdx | Glucose-6-phosphate dehydrogenase X-linked |
| Aco | Acyl-CoA oxidase gene |
| PEPCK | Phosphoenolpyruvate carboxykinase 1 |
References
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Singh, R.; Zogg, H.; Wei, L.; Bartlett, A.; Ghoshal, U.C.; Rajender, S.; Ro, S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J. Neurogastroenterol. Motil. 2021, 27, 19–34. [Google Scholar] [CrossRef]
- Dobrowolski, P.; Prejbisz, A.; Kuryłowicz, A.; Baska, A.; Burchardt, P.; Chlebus, K.; Dzida, G.; Jankowski, P.; Jaroszewicz, J.; Jaworski, P.; et al. Metabolic Syndrome—A New Definition and Management Guidelines. Arter. Hypertens. 2022, 26, 99–121. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Song, E.; Ang, L.; Lee, H.W.; Kim, M.-S.; Kim, Y.J.; Jang, D.; Lee, M.S. Effects of Kimchi on Human Health: A Scoping Review of Randomized Controlled Trials. J. Ethn. Foods 2023, 10, 7. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Sayın, F.K.; Alkan, S.B. The Effect Of Pickling On Total Phenolic Contents And Antioxidant Activity Of 10 Vegetables. Food Health 2015, 1, 135–141. [Google Scholar] [CrossRef]
- Verni, M.; Verardo, V.; Rizzello, C.G. How Fermentation Affects the Antioxidant Properties of Cereals and Legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef]
- Gänzle, M.G. Food Fermentations for Improved Digestibility of Plant Foods—An Essential Ex Situ Digestion Step in Agricultural Societies? Curr. Opin. Food Sci. 2020, 32, 124–132. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Mickowska, B. Effect of Controlled Lactic Acid Fermentation on Selected Bioactive and Nutritional Parameters of Tempeh Obtained from Unhulled Common Bean (Phaseolus vulgaris) Seeds. J. Sci. Food Agric. 2014, 94, 359–366. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Ghnimi, S.; Guizani, N. Vegetable Fermentation and Pickling. In Handbook of Vegetables and Vegetable Processing, 2nd ed.; Sinha, N.K., Ed.; John Wiley & Sons: Ames, IA, USA, 2018; Volume 1, pp. 407–427. ISBN 9781119098935. [Google Scholar]
- Kim, H.-Y.; Park, K.-Y. Clinical Trials of Kimchi Intakes on the Regulation of Metabolic Parameters and Colon Health in Healthy Korean Young Adults. J. Funct. Foods 2018, 47, 325–333. [Google Scholar] [CrossRef]
- Song, H.S.; Whon, T.W.; Kim, J.; Lee, S.H.; Kim, J.Y.; Kim, Y.B.; Choi, H.-J.; Rhee, J.-K.; Roh, S.W. Microbial Niches in Raw Ingredients Determine Microbial Community Assembly during Kimchi Fermentation. Food Chem. 2020, 318, 126481. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Chen, L.; Dishisha, T.; Enshasy, H.E. Lactic Acid Bacteria: From Starter Cultures to Producers of Chemicals. FEMS Microbiol. Lett. 2018, 365, fny213. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Dahal, N.R.; Ndungutse, V. Bacillus Fermentation of Soybean: A Review. J. Food Sci. Technol. Nepal 2010, 6, 1–9. [Google Scholar] [CrossRef]
- Tamang, J.P. Biochemical And Modern Identification Techniques|Microfloras of Fermented Foods. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 249–252. ISBN 9780123847331. [Google Scholar]
- Anal, A.K. Quality Ingredients and Safety Concerns for Traditional Fermented Foods and Beverages from Asia: A Review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Perpetuini, G.; Prete, R.; Garcia-Gonzalez, N.; Khairul Alam, M.; Corsetti, A. Table Olives More than a Fermented Food. Foods 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Borges, N.; Pinho, O. Table Olives and Health: A Review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Mitchell, A.E. Reducing Phenolics Related to Bitterness in Table Olives. J. Food Qual. 2018, 2018, 3193185. [Google Scholar] [CrossRef]
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Portilha-Cunha, M.F.; Macedo, A.C.; Malcata, F.X. A Review on Adventitious Lactic Acid Bacteria from Table Olives. Foods 2020, 9, 948. [Google Scholar] [CrossRef]
- Argyri, K.; Doulgeraki, A.I.; Manthou, E.; Grounta, A.; Argyri, A.A.; Nychas, G.-J.E.; Tassou, C.C. Microbial Diversity of Fermented Greek Table Olives of Halkidiki and Konservolia Varieties from Different Regions as Revealed by Metagenomic Analysis. Microorganisms 2020, 8, 1241. [Google Scholar] [CrossRef]
- De Castro, A.; Sánchez, A.H.; López-López, A.; Cortés-Delgado, A.; Medina, E.; Montaño, A. Microbiota and Metabolite Profiling of Spoiled Spanish-Style Green Table Olives. Metabolites 2018, 8, 73. [Google Scholar] [CrossRef]
- Simões, L.; Fernandes, N.; Teixeira, J.; Abrunhosa, L.; Dias, D.R. Brazilian Table Olives: A Source of Lactic Acid Bacteria with Antimycotoxigenic and Antifungal Activity. Toxins 2023, 15, 71. [Google Scholar] [CrossRef]
- Aksay, O.; Selli, S.; Kelebek, H. LC-DAD-ESI-MS/MS-Based Assessment of the Bioactive Compounds in Fresh and Fermented Caper (Capparis spinosa) Buds and Berries. Food Chem. 2021, 337, 127959. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, T.; Wang, C. Capparis spinosa L. as a Potential Source of Nutrition and Its Health Benefits in Foods: A Comprehensive Review of Its Phytochemistry, Bioactivities, Safety, and Application. Food Chem. 2023, 409, 135258. [Google Scholar] [CrossRef]
- Francesca, N.; Barbera, M.; Martorana, A.; Saiano, F.; Gaglio, R.; Aponte, M.; Moschetti, G.; Settanni, L. Optimised Method for the Analysis of Phenolic Compounds from Caper (Capparis spinosa L.) Berries and Monitoring of Their Changes during Fermentation. Food Chem. 2016, 196, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-W.; Kim, K.-H.; Nam, Y.-D.; Roh, S.W.; Kim, M.-S.; Jeon, C.O.; Oh, H.-M.; Bae, J.-W. Analysis of Yeast and Archaeal Population Dynamics in Kimchi Using Denaturing Gradient Gel Electrophoresis. Int. J. Food Microbiol. 2008, 126, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Whon, T.W.; Roh, S.W.; Jeon, C.O. Unraveling Microbial Fermentation Features in Kimchi: From Classical to Meta-Omics Approaches. Appl. Microbiol. Biotechnol. 2020, 104, 7731–7744. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.H.; Noh, J.S.; Han, J.-S.; Kim, H.J.; Han, E.-S.; Song, Y.O. Kimchi, a Fermented Vegetable, Improves Serum Lipid Profiles in Healthy Young Adults: Randomized Clinical Trial. J. Med. Food 2013, 16, 223–229. [Google Scholar] [CrossRef]
- Gaudioso, G.; Weil, T.; Marzorati, G.; Solovyev, P.; Bontempo, L.; Franciosi, E.; Bertoldi, L.; Pedrolli, C.; Tuohy, K.M.; Fava, F. Microbial and Metabolic Characterization of Organic Artisanal Sauerkraut Fermentation and Study of Gut Health-Promoting Properties of Sauerkraut Brine. Front. Microbiol. 2022, 13, 929738. [Google Scholar] [CrossRef]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical Composition and Sensory Quality of Sauerkraut Produced from Different Cabbage Varieties. LWT 2021, 136, 110325. [Google Scholar] [CrossRef]
- Georgieva, A.; Petkova, M.; Todorova, E.; Gotcheva, V.; Angelov, A. Isolation and Selection of Sauerkraut Lactic Acid Bacteria Producing Exopolysaccharides. BIO Web Conf. 2023, 58, 02001. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Li, F.; Shi, H.; He, M.; Ge, J.; Ling, H.; Cheng, K. Analysis of Microbial Diversity and Metabolites in Sauerkraut Products with and without Microorganism Addition. Foods 2023, 12, 1164. [Google Scholar] [CrossRef]
- Ciska, E.; Honke, J.; Drabińska, N. Changes in Glucosinolates and Their Breakdown Products during the Fermentation of Cabbage and Prolonged Storage of Sauerkraut: Focus on Sauerkraut Juice. Food Chem. 2021, 365, 130498. [Google Scholar] [CrossRef]
- Satora, P.; Celej, D.; Skotniczny, M.; Trojan, N. Identifying Yeast Occurring in Commercial and Farm-Made Sauerkraut. Żywność Nauka. Technologia. Jakość 2017, 113, 27–36. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Zhao, A.; Wu, Y.; Zhang, Y.; Yang, X. Quantitative Analyses for Several Nutrients and Volatile Components during Fermentation of Soybean by Bacillus subtilis Natto. Food Chem. 2022, 374, 131725. [Google Scholar] [CrossRef]
- Liu, W.-T.; Huang, C.-L.; Liu, R.; Yang, T.-C.; Lee, C.-L.; Tsao, R.; Yang, W.-J. Changes in Isoflavone Profile, Antioxidant Activity, and Phenolic Contents in Taiwanese and Canadian Soybeans during Tempeh Processing. LWT 2023, 186, 115207. [Google Scholar] [CrossRef]
- Handoyo, T.; Morita, N. Structural and Functional Properties of Fermented Soybean (Tempeh) by Using Rhizopus oligosporus. Int. J. Food Prop. 2006, 9, 347–355. [Google Scholar] [CrossRef]
- Hartanti, A.T.; Rahayu, G.; Hidayat, I. Rhizopus Species from Fresh Tempeh Collected from Several Regions in Indonesia. HAYATI J. Biosci. 2015, 22, 136–142. [Google Scholar] [CrossRef][Green Version]
- Radita, R.; Suwanto, A.; Kurosawa, N.; Wahyudi, A.; Rusmana, I. Firmicutes Is the Predominant Bacteria in Tempeh. Int. Food Res. J. 2018, 25, 2313–2320. [Google Scholar]
- Lim, S.L.; Tay, S.T. Diversity and Killer Activity of Yeasts in Malaysian Fermented Food Samples. Trop. Biomed. 2011, 28, 438–443. [Google Scholar]
- Wikandari, R.; Millati, R.; Lennartsson, P.R.; Harmayani, E.; Taherzadeh, M.J. Isolation and Characterization of Zygomycetes Fungi from Tempe for Ethanol Production and Biomass Applications. Appl. Biochem. Biotechnol. 2012, 167, 1501–1512. [Google Scholar] [CrossRef]
- He, M.T.; Howell, K.S. Vitamin-B12 Enrichment in Tempeh by Co-Culture with Propionibacterium freudenreichii during Fermentation. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kim, I.-S.; Hwang, C.-W.; Yang, W.-S.; Kim, C.-H. Current Perspectives on the Physiological Activities of Fermented Soybean-Derived Cheonggukjang. Int. J. Mol. Sci. 2021, 22, 5746. [Google Scholar] [CrossRef]
- Piao, Y.-Z.; Eun, J.-B. Physicochemical Characteristics and Isoflavones Content during Manufacture of Short-Time Fermented Soybean Product (Cheonggukjang). J. Food Sci. Technol. 2020, 57, 2190–2197. [Google Scholar] [CrossRef]
- Park, M.K.; Cho, I.H.; Lee, S.; Choi, H.-K.; Kwon, D.-Y.; Kim, Y.-S. Metabolite Profiling of Cheonggukjang, a Fermented Soybean Paste, during Fermentation by Gas Chromatography-Mass Spectrometry and Principal Component Analysis. Food Chem. 2010, 122, 1313–1319. [Google Scholar] [CrossRef]
- Arulkumar, R.; Jung, H.-J.; Noh, S.-G.; Park, D.; Chung, H.-Y. Cheonggukjang-Specific Component 1,3-Diphenyl-2-Propanone as a Novel PPARα/γ Dual Agonist: An In Vitro and In Silico Study. Int. J. Mol. Sci. 2021, 22, 10884. [Google Scholar] [CrossRef]
- Mun, E.-G.; Park, J.E.; Cha, Y.-S. Effects of Doenjang, a Traditional Korean Soybean Paste, with High-Salt Diet on Blood Pressure in Sprague–Dawley Rats. Nutrients 2019, 11, 2745. [Google Scholar] [CrossRef]
- Lee, H.Y.; Haque, M.A.; Cho, D.Y.; Jeong, J.B.; Lee, J.H.; Lee, G.Y.; Jang, M.Y.; Lee, J.H.; Cho, K.M. Comparison of Microbial Diversity and Metabolites on Household and Commercial Doenjang. Food Chem. X 2024, 21, 101101. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Jung, E.-S.; Choi, E.-K.; Jeong, D.-Y.; Jo, S.-W.; Jin, J.-H.; Lee, J.-M.; Park, B.-H.; Chae, S.-W. Supplementation with Aspergillus oryzae-Fermented Kochujang Lowers Serum Cholesterol in Subjects with Hyperlipidemia. Clin. Nutr. 2015, 34, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Ha, G.; Yang, H.-J.; Ryu, M.-S.; Jeong, S.-J.; Jeong, D.-Y.; Park, S. Bacterial Community and Anti-Cerebrovascular Disease-Related Bacillus Species Isolated from Traditionally Made Kochujang from Different Provinces of Korea. Microorganisms 2021, 9, 2238. [Google Scholar] [CrossRef]
- Park, S.-J.; Chang, J.-H.; Cha, S.-K.; Moon, G.-S. Analysis of the Bacterial Composition During Kochujang, a Korean Traditional Fermented Hot Pepper-Soybean Paste, Fermentation. Food Sci. Biotechnol. 2009, 18, 1035–1037. [Google Scholar]
- Nam, Y.R.; Won, S.B.; Chung, Y.-S.; Kwak, C.S.; Kwon, Y.H. Inhibitory Effects of Doenjang, Korean Traditional Fermented Soybean Paste, on Oxidative Stress and Inflammation in Adipose Tissue of Mice Fed a High-Fat Diet. Nutr. Res. Pract. 2015, 9, 235–241. [Google Scholar] [CrossRef]
- Boskou, D.; Camposeo, S.; Clodoveo, M.L. Table Olives as Sources of Bioactive Compounds. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Urbana, IL, USA, 2015; pp. 217–259. ISBN 978-1630670412. [Google Scholar]
- Salis, C.; Papadakis, I.E.; Hagidimitriou, M. Identification and Quantification of Phenolic Compounds in Fresh and Processed Table Olives of Cv. ‘Kalamata’. Not. Bot. Horti. Agrobo. 2021, 49, 12394. [Google Scholar] [CrossRef]
- Marsilio, V.; Seghetti, L.; Iannucci, E.; Russi, F.; Lanza, B.; Felicioni, M. Use of a Lactic Acid Bacteria Starter Culture during Green Olive (Olea europaea L Cv Ascolana Tenera) Processing. J. Sci. Food Agric. 2005, 85, 1084–1090. [Google Scholar] [CrossRef]
- Cocolin, L.; Alessandria, V.; Botta, C.; Gorra, R.; Filippis, F.D.; Ercolini, D.; Rantsiou, K. NaOH-Debittering Induces Changes in Bacterial Ecology during Table Olives Fermentation. PLoS ONE 2013, 8, e69074. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.A.; Bozoudi, D.; Tsaltas, D. Enterococci Isolated from Cypriot Green Table Olives as a New Source of Technological and Probiotic Properties. Fermentation 2018, 4, 48. [Google Scholar] [CrossRef]
- Martorana, A.; Alfonzo, A.; Gaglio, R.; Settanni, L.; Corona, O.; La Croce, F.; Vagnoli, P.; Caruso, T.; Moschetti, G.; Francesca, N. Evaluation of Different Conditions to Enhance the Performances of Lactobacillus pentosus OM13 during Industrial Production of Spanish-Style Table Olives. Food Microbiol. 2017, 61, 150–158. [Google Scholar] [CrossRef]
- Pistarino, E.; Aliakbarian, B.; Casazza, A.A.; Paini, M.; Cosulich, M.E.; Perego, P. Combined Effect of Starter Culture and Temperature on Phenolic Compounds during Fermentation of Taggiasca Black Olives. Food Chem. 2013, 138, 2043–2049. [Google Scholar] [CrossRef]
- de Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol Protects from Aging Process via AMPK and Autophagy; a Review of Its Effects on Cancer, Metabolic Syndrome, Osteoporosis, Immune-Mediated and Neurodegenerative Diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper Respiratory Tract Health” (ID 3468), “Can Help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial Effects of Dietary Supplementation with Olive Oil, Oleic Acid, or Hydroxytyrosol in Metabolic Syndrome: Systematic Review and Meta-Analysis. Free Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L.; Panzella, L.; Napolitano, A.; d’Ischia, M. 5-S-Lipoylhydroxytyrosol, a Multidefense Antioxidant Featuring a Solvent-Tunable Peroxyl Radical-Scavenging 3-Thio-1,2-Dihydroxybenzene Motif. J. Org. Chem. 2013, 78, 9857–9864. [Google Scholar] [CrossRef] [PubMed]
- Diallinas, G.; Rafailidou, N.; Kalpaktsi, I.; Komianou, A.C.; Tsouvali, V.; Zantza, I.; Mikros, E.; Skaltsounis, A.L.; Kostakis, I.K. Hydroxytyrosol (HT) Analogs Act as Potent Antifungals by Direct Disruption of the Fungal Cell Membrane. Front. Microbiol. 2018, 9, 2624. [Google Scholar] [CrossRef]
- Officioso, A.; Panzella, L.; Tortora, F.; Alfieri, M.L.; Napolitano, A.; Manna, C. Comparative Analysis of the Effects of Olive Oil Hydroxytyrosol and Its 5-S-Lipoyl Conjugate in Protecting Human Erythrocytes from Mercury Toxicity. Oxid. Med. Cell. Longev. 2018, 2018, 9042192. [Google Scholar] [CrossRef]
- Priore, P.; Siculella, L.; Gnoni, G.V. Extra Virgin Olive Oil Phenols Down-Regulate Lipid Synthesis in Primary-Cultured Rat-Hepatocytes. J. Nutr. Biochem. 2014, 25, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z.; Wang, Y.; Weber, P.; Wertz, K.; Sharman, E.; et al. Hydroxytyrosol Promotes Mitochondrial Biogenesis and Mitochondrial Function in 3T3-L1 Adipocytes. J. Nutr. Biochem. 2010, 21, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-López, J.; Ruiz-Medina, A.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Phytochemical Profile and Antioxidant Activity of Caper Berries (Capparis spinosa L.): Evaluation of the Influence of the Fermentation Process. Food Chem. 2018, 250, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Khavasi, N.; hosein Somi, M.; Khadem, E.; Faramarzi, E.; Ayati, M.H.; Fazljou, S.M.B.; Torbati, M. Effect of Daily Caper Fruit Pickle Consumption on Disease Regression in Patients with Non-Alcoholic Fatty Liver Disease: A Double-Blinded Randomized Clinical Trial. Adv. Pharm. Bull. 2017, 7, 645–650. [Google Scholar] [CrossRef][Green Version]
- Perna, S.; Rafique, A.; Rondanelli, M.; Allehdan, S.; Riso, P.; Marino, M. Effect of Caper Fruit (Capparis spinosa L.) Consumption on Liver Enzymes, Lipid Profile, Fasting Plasma Glucose, and Weight Loss. A Systematic Review and a Preliminary Meta-Analysis of Randomized Controlled Trials. Biomed. Pharmacother. 2023, 168, 115638. [Google Scholar] [CrossRef]
- Özcan, M.M.; Uslu, N. The Effect of Fermentation with Different Additives on Bioactive Compounds, Antioxidant Activity, Phenolic Component, Fatty Acid Composition and Mineral Substance Contents of Capers Fruits. Food Meas. 2023, 17, 3896–3908. [Google Scholar] [CrossRef]
- Pérez Pulido, R.; Ben Omar, N.; Abriouel, H.; Lucas López, R.; Martínez Cañamero, M.; Gálvez, A. Microbiological Study of Lactic Acid Fermentation of Caper Berries by Molecular and Culture-Dependent Methods. Appl. Environ. Microbiol. 2005, 71, 7872–7879. [Google Scholar] [CrossRef]
- Özcan, M.M. Pickling and Storage of Caperberries (Capparis Spp.). Z. Lebensm. Unters. Forsch. 1999, 208, 379–382. [Google Scholar] [CrossRef]
- Khavasi, N.; Somi, M.; Khadem, E.; Ayati, M.H.; Torbati, M.; Fazljou, S. Daily Consumption of the Capparis Spinosa Reduces Some Atherogenic Indices in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Clinical Trial. Iran Red Crescent Med. J. 2018, 20, e63446. [Google Scholar] [CrossRef]
- Sardari, S.; Fallahi, F.; Emadi, F.; Davati, A.; Khavasi, N.; Gholamifesharaki, M.; Esmaeili, S.S. Daily Consumption of Caper Fruit Along With Atorvastatin Has Synergistic Effects in Hyperlipidemic Patients: Randomized Clinical Trial. Galen Med. J. 2019, 8, e1345. [Google Scholar] [CrossRef]
- Kim, E.K.; An, S.-Y.; Lee, M.-S.; Kim, T.H.; Lee, H.-K.; Hwang, W.S.; Choe, S.J.; Kim, T.-Y.; Han, S.J.; Kim, H.J.; et al. Fermented Kimchi Reduces Body Weight and Improves Metabolic Parameters in Overweight and Obese Patients. Nutr. Res. 2011, 31, 436–443. [Google Scholar] [CrossRef]
- Park, J.-A.; Tirupathi Pichiah, P.B.; Yu, J.-J.; Oh, S.-H.; Daily, J.W.; Cha, Y.-S. Anti-Obesity Effect of Kimchi Fermented with Weissella koreensis OK1-6 as Starter in High-Fat Diet-Induced Obese C57BL/6J Mice. J. Appl. Microbiol. 2012, 113, 1507–1516. [Google Scholar] [CrossRef]
- An, S.-Y.; Lee, M.S.; Jeon, J.Y.; Ha, E.S.; Kim, T.H.; Yoon, J.Y.; Ok, C.-O.; Lee, H.-K.; Hwang, W.-S.; Choe, S.J.; et al. Beneficial Effects of Fresh and Fermented Kimchi in Prediabetic Individuals. Ann. Nutr. Metab. 2013, 63, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Pichiah, P.B.T.; Kim, M.-J.; Cha, Y.-S. Cheonggukjang, a Soybean Paste Fermented with B. licheniformis-67 Prevents Weight Gain and Improves Glycemic Control in High Fat Diet Induced Obese Mice. J. Clin. Biochem. Nutr. 2016, 59, 31–38. [Google Scholar] [CrossRef]
- Kim, D.-J.; Jeong, Y.-J.; Kwon, J.-H.; Moon, K.-D.; Kim, H.-J.; Jeon, S.-M.; Lee, M.-K.; Park, Y.B.; Choi, M.-S. Beneficial Effect of Chungkukjang on Regulating Blood Glucose and Pancreatic β-Cell Functions in C75BL/KsJ- Db/Db Mice. J. Med. Food 2008, 11, 215–223. [Google Scholar] [CrossRef]
- Soh, J.-R.; Shin, D.-H.; Kwon, D.Y.; Cha, Y.-S. Effect of Cheonggukjang Supplementation upon Hepatic Acyl-CoA Synthase, Carnitine Palmitoyltransferase I, Acyl-CoA Oxidase and Uncoupling Protein 2 mRNA Levels in C57BL/6J Mice Fed with High Fat Diet. Genes Nutr. 2008, 2, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.N.; Choi, J.H.; Cha, Y.S.; Muthaiya, M.J.; Lee, C.H. Effect of Fermented Soybean Product (Cheonggukjang) Intake on Metabolic Parameters in Mice Fed a High-fat Diet. Mol. Nutr. Food. Res. 2013, 57, 1886–1891. [Google Scholar] [CrossRef]
- Park, E.-S.; Choi, S.-K.; Zhang, X.-H.; Choi, U.-K.; Seo, J.-S. Effect of Cheonggukjang Made with Germinated Soybean on Lipid Contents and Fecal Excretion of Neutral Steroids in Rats Fed a High Cholesterol Diet. Food Sci. Biotechnol. 2013, 22, 15–21. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, H.J.; Kim, M.J.; Kang, S.; Kim, D.S.; Daily, J.W.; Jeong, D.Y.; Kwon, D.Y.; Park, S. Standardized Chungkookjang, Short-Term Fermented Soybeans with Bacillus lichemiformis, Improves Glucose Homeostasis as Much as Traditionally Made Chungkookjang in Diabetic Rats. J. Clin. Biochem. Nutr. 2013, 52, 49–57. [Google Scholar] [CrossRef]
- Byun, M.-S.; Yu, O.-K.; Cha, Y.-S.; Park, T.-S. Korean Traditional Chungkookjang Improves Body Composition, Lipid Profiles and Atherogenic Indices in Overweight/Obese Subjects: A Double-Blind, Randomized, Crossover, Placebo-Controlled Clinical Trial. Eur. J. Clin. Nutr. 2016, 70, 1116–1122. [Google Scholar] [CrossRef]
- Kim, J. Anti-Obesity and Anti-Inflammation Effects of Cheonggukjang in C57Bl/6 Mice with High Fat Diet Induced Obesity. J. Life Sci. 2017, 27, 1357–1368. [Google Scholar] [CrossRef]
- Back, H.-I.; Kim, S.-R.; Yang, J.-A.; Kim, M.-G.; Chae, S.-W.; Cha, Y.-S. Effects of Chungkookjang Supplementation on Obesity and Atherosclerotic Indices in Overweight/Obese Subjects: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Med. Food 2011, 14, 532–537. [Google Scholar] [CrossRef]
- Shin, S.-K.; Kwon, J.-H.; Jeong, Y.-J.; Jeon, S.-M.; Choi, J.-Y.; Choi, M.-S. Supplementation of Cheonggukjang and Red Ginseng Cheonggukjang Can Improve Plasma Lipid Profile and Fasting Blood Glucose Concentration in Subjects with Impaired Fasting Glucose. J. Med. Food 2011, 14, 108–113. [Google Scholar] [CrossRef]
- Han, A.L.; Jeong, S.-J.; Ryu, M.-S.; Yang, H.-J.; Jeong, D.-Y.; Seo, Y.-B. Evaluation of Body Changes and the Anti-Obesity Effect after Consumption of Korean Fermented Food, Cheonggukjang: Randomized, Double-Blind Clinical Trial. Foods 2023, 12, 2190. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Wu, B.-H.; Chu, Y.-L.; Chang, W.-C.; Wu, M.-C. Effects of Tempeh Fermentation with Lactobacillus plantarum and Rhizopus oligosporus on Streptozotocin-Induced Type II Diabetes Mellitus in Rats. Nutrients 2018, 10, 1143. [Google Scholar] [CrossRef]
- Masdar, H.; Satriyasumatri, T.; Hakiki, M.R.; Rafisyahputra, M.; Juananda, D. Histological Apperarance of Diabetes-Rat Pancreas Administrated by Soybean Compared to Tempeh. AIP Conf. Proc. 2019, 2108, 020012. [Google Scholar] [CrossRef]
- Dewi, L.; Lestari, L.A.; Astiningrum, A.N.; Fadhila, V.; Amala, N.; Bakrie, M.A.; Hidayah, N. Tempeh and Red Ginger Flour for Hypercholesterolemic Rats. Nutr. Food Sci. 2021, 51, 41–49. [Google Scholar] [CrossRef]
- Kusuma, R.; Widada, J.; Huriyati, E.; Julia, M. Naturally Acquired Lactic Acid Bacteria from Fermented Cassava Improves Nutrient and Anti-Dysbiosis Activity of Soy Tempeh. Open Access Maced. J. Med. Sci. 2021, 9, 1148–1155. [Google Scholar] [CrossRef]
- Prasetyastuti, P.; Ghozali, D.S. The Effects of Soyferment-Tempeh on Lipid Profile, Retinol-Binding Protein 4 (RBP4), and Phosphoenolpyruvate Carboxykinase (PEPCK) Gene Expression in Type 2 Diabetic Mice. Indones. J. Pharm. 2021, 32, 193–200. [Google Scholar] [CrossRef]
- Wicaksono, H.; Prasetyastuti, P.; Hastuti, P.; Sadewa, A.H. The Effect of Fermented Tempeh Aerobic Anaerobic (FETAA) on Pancreatic Duodenal Homeobox 1 (Pdx1) Gene Expression and HOMA-Beta Index in Diabetic Mice. Acta Biochim. 2021, 4, 11. [Google Scholar] [CrossRef]
- Su, H.; Chen, W.; Lu, J.; Chao, H.; Liang, Y.; Haruka, S.; Hsu, W.; Wu, M.; Tsai, M. The Effects of Using Tempeh as a Supplement for Type 2 Diabetes. Food Sci. Nutr. 2023, 11, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Zahra, F.A.; Fulyani, F.; Maharani, N.; Anjani, G.; Noer, E.R. Effects of Fermented Tempeh Using Rhizopus oligosporus and Lactobacillus rhamnosus GG on Body Weight, Lee Index, High Sensitivity C Reactive Protein and Lipid Profile of Obese Rats. J. Biomed. Transl. Res. 2023, 9, 31–38. [Google Scholar] [CrossRef]
- Afifah, D.N.; Nabilah, N.; Supraba, G.T.; Pratiwi, S.N.; Nuryanto; Sulchan, M. The Effects of Tempeh Gembus, an Indonesian Fermented Food, on Lipid Profiles in Women with Hyperlipidemia. Curr. Nutr. Food Sci. 2020, 16, 56–64. [Google Scholar] [CrossRef]
- Isnawati, M.; Larasati, M.D.; Muninggar, D.L.P.; Afifah, D.N.; Suromo, L.B.; Sulchan, M. The Effect of Processed Tempeh Gembus Administration on Blood Glucose in Obese Women. IOP Conf. Ser. Earth Environ. Sci. 2020, 519, 012034. [Google Scholar] [CrossRef]
- Su, H.-K.; Tsai, M.-H.; Chao, H.-R.; Wu, M.-L.; Lu, J.-H. Data on Effect of Tempeh Fermentation on Patients with Type II Diabetes. Data Brief 2021, 38, 107310. [Google Scholar] [CrossRef]
- Zulaikha, H.N.; Dijaya Muliadi, R.; Kartawidjajaputra, F.; Antono, L. Cholesterol-Lowering Effect of Soy Nuts and Tempeh on Hypercholesterolemic Subjects. J. Funct. Food Nutraceutical 2023, 4, 95–102. [Google Scholar] [CrossRef]
- Iwai, K.; Nakaya, N.; Kawasaki, Y.; Matsue, H. Antioxidative Functions of Natto, A Kind of Fermented Soybeans: Effect on LDL Oxidation and Lipid Metabolism in Cholesterol-Fed Rats. J. Agric. Food Chem. 2002, 50, 3597–3601. [Google Scholar] [CrossRef]
- Park, K.-J.; Kang, J.-I.; Kim, T.-S.; Yeo, I.-H. The Antithrombotic and Fibrinolytic Effect of Natto in Hypercholesterolemia Rats. Prev. Nutr. Food Sci. 2012, 17, 78–82. [Google Scholar] [CrossRef]
- Kushida, M.; Okouchi, R.; Iwagaki, Y.; Asano, M.; Du, M.X.; Yamamoto, K.; Tsuduki, T. Fermented Soybean Suppresses Visceral Fat Accumulation in Mice. Mol. Nutr. Food Res. 2018, 62, 1701054. [Google Scholar] [CrossRef]
- Tamura, M.; Watanabe, J.; Noguchi, T.; Nishikawa, T. High Poly-γ-Glutamic Acid-Containing Natto Improves Lipid Metabolism and Alters Intestinal Microbiota in Mice Fed a High-Fat Diet. J. Clin. Biochem. Nutr. 2024, 74, 47–56. [Google Scholar] [CrossRef]
- Taniguchi-Fukatsu, A.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and Viscous Vegetables in a Japanese-Style Breakfast Improved Insulin Sensitivity, Lipid Metabolism and Oxidative Stress in Overweight Subjects with Impaired Glucose Tolerance. Br. J. Nutr. 2012, 107, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Seong, S.-H.; Dae, Y.K.; Hee, S.S.; Cha, Y.-S. Fermented Kochujang Supplement Shows Anti-Obesity Effects by Controlling Lipid Metabolism in C57BL/6J Mice Fed High Fat Diet. Food Sci. Biotechnol. 2008, 17, 336–342. [Google Scholar]
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, Y.S.; Shin, D.W.; Park, S. Kochujang, a Korean Fermented Red Pepper plus Soybean Paste, Improves Glucose Homeostasis in 90% Pancreatectomized Diabetic Rats. Nutrition 2009, 25, 790–799. [Google Scholar] [CrossRef]
- Lee, Y.; Cha, Y.-S.; Park, Y.; Lee, M. PPARγ2 C1431T Polymorphism Interacts with the Antiobesogenic Effects of Kochujang, a Korean Fermented, Soybean-Based Red Pepper Paste, in Overweight/Obese Subjects: A 12-Week, Double-Blind Randomized Clinical Trial. J. Med. Food 2017, 20, 610–617. [Google Scholar] [CrossRef]
- Han, A.L.; Jeong, S.-J.; Ryu, M.-S.; Yang, H.-J.; Jeong, D.-Y.; Park, D.-S.; Lee, H.K. Anti-Obesity Effects of Traditional and Commercial Kochujang in Overweight and Obese Adults: A Randomized Controlled Trial. Nutrients 2022, 14, 2783. [Google Scholar] [CrossRef] [PubMed]
- Park, N.Y.; Rico, C.W.; Lee, S.C.; Kang, M.Y. Comparative Effects of Doenjang Prepared from Soybean and Brown Rice on the Body Weight and Lipid Metabolism in High Fat-Fed Mice. J. Clin. Biochem. Nutr. 2012, 51, 235–240. [Google Scholar] [CrossRef][Green Version]
- Kim, M.-S.; Kim, B.; Park, H.; Ji, Y.; Holzapfel, W.; Kim, D.-Y.; Hyun, C.-K. Long-Term Fermented Soybean Paste Improves Metabolic Parameters Associated with Non-Alcoholic Fatty Liver Disease and Insulin Resistance in High-Fat Diet-Induced Obese Mice. Biochem. Biophys. Res. Commun. 2018, 495, 1744–1751. [Google Scholar] [CrossRef]
- Woo, H.; Han, A.; Park, J.E.; Cha, Y.-S. Korean Fermented Soybean Paste (Doenjang) Has Anti-Obesity and Anti-Hypertensive Effects via the Renin-Angiotensin System (RAS) in High-Fat Diet-Induced Obese Rats. PLoS ONE 2023, 18, e0291762. [Google Scholar] [CrossRef]
- Cha, Y.-S.; Yang, J.-A.; Back, H.-I.; Kim, S.-R.; Kim, M.-G.; Jung, S.-J.; Song, W.O.; Chae, S.-W. Visceral Fat and Body Weight Are Reduced in Overweight Adults by the Supplementation of Doenjang, a Fermented Soybean Paste. Nutr. Res. Pract. 2012, 6, 520–526. [Google Scholar] [CrossRef]
- Surya, R.; Lee, A.G.-Y. Exploring the Philosophical Values of Kimchi and Kimjang Culture. J. Ethn. Foods 2022, 9, 20. [Google Scholar] [CrossRef]
- Lee, S.-J.; Jeon, H.-S.; Yoo, J.-Y.; Kim, J.-H. Some Important Metabolites Produced by Lactic Acid Bacteria Originated from Kimchi. Foods 2021, 10, 2148. [Google Scholar] [CrossRef]
- Jung, S.J.; Kim, M.J.; Chae, S.W. Quality and Functional Characteristics of Kimchi Made with Organically Cultivated Young Chinese Cabbage (Olgari-Baechu). J. Ethn. Foods 2016, 3, 150–158. [Google Scholar] [CrossRef]
- Kim, H.J.; Kwon, M.S.; Hwang, H.; Choi, H.-S.; Lee, W.-J.; Choi, S.-P.; Jo, H.; Hong, S.W. A Review of the Health Benefits of Kimchi Functional Compounds and Metabolites. Microbiol. Biotechnol. Lett. 2023, 51, 353–373. [Google Scholar] [CrossRef]
- Kim, R.M.; Lee, K.-J.; Young Kim, H.; Hee Kim, J.; Kim, Y.-B.; Sok, D.-E. Effect of Various Kimchi Extracts on the Hepatic Glutathione S-Transferase Activity of Mice. Int. Food Res. 1998, 31, 389–394. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Yong, S.; Lee, M.J.; Park, S.J.; Yun, Y.-R.; Park, S.-H.; Lee, M.-A. Changes in Volatile and Non-Volatile Compounds of Model Kimchi through Fermentation by Lactic Acid Bacteria. LWT 2019, 105, 118–126. [Google Scholar] [CrossRef]
- Lee, M.-A.; Choi, Y.-J.; Lee, H.; Hwang, S.; Lee, H.J.; Park, S.J.; Chung, Y.B.; Yun, Y.-R.; Park, S.-H.; Min, S.; et al. Influence of Salinity on the Microbial Community Composition and Metabolite Profile in Kimchi. Fermentation 2021, 7, 308. [Google Scholar] [CrossRef]
- Park, J.E.; Oh, S.-H.; Cha, Y.-S. Lactobacillus brevis OPK-3 from Kimchi Prevents Obesity and Modulates the Expression of Adipogenic and Pro-Inflammatory Genes in Adipose Tissue of Diet-Induced Obese Mice. Nutrients 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Song, J.-L.; Park, E.-S.; Ju, J.; Kim, H.-Y.; Park, K.-Y. Anti-Obesity Effects of Starter Fermented Kimchi on 3T3-L1 Adipocytes. Prev. Nutr. Food Sci. 2015, 20, 298–302. [Google Scholar] [CrossRef]
- Lim, S.; Moon, J.H.; Shin, C.M.; Jeong, D.; Kim, B. Effect of Lactobacillus Sakei, a Probiotic Derived from Kimchi, on Body Fat in Koreans with Obesity: A Randomized Controlled Study. Endocrinol. Metab. 2020, 35, 425–434. [Google Scholar] [CrossRef]
- Oh, M.-R.; Jang, H.-Y.; Lee, S.-Y.; Jung, S.-J.; Chae, S.-W.; Lee, S.-O.; Park, B.-H. Lactobacillus plantarum HAC01 Supplementation Improves Glycemic Control in Prediabetic Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2337. [Google Scholar] [CrossRef]
- Seo, S.H.; Hong, J.; Son, I.H.; Han, Y.H.; Hyun, T. Association of Korean fermented cabbage kimchi consumption with an incidence of metabolic syndrome: 10-year follow-up results of the Korean Genome and Epidemiology Study. J. Nutr. Health 2019, 52, 569. [Google Scholar] [CrossRef]
- Peñas, E.; Martinez-Villaluenga, C.; Frias, J. Sauerkraut: Production, Composition, and Health Benefits. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2017; pp. 557–576. ISBN 9780128023099. [Google Scholar]
- Zhou, H.; Wang, S.; Liu, W.; Chang, L.; Zhu, X.; Mu, G.; Qian, F. Probiotic Properties of Lactobacillus Paraplantarum LS-5 and Its Effect on Antioxidant Activity of Fermented Sauerkraut. Food Biosci. 2023, 52, 102489. [Google Scholar] [CrossRef]
- Zhou, Q.; Zang, S.; Zhao, Z.; Li, X. Dynamic Changes of Bacterial Communities and Nitrite Character during Northeastern Chinese Sauerkraut Fermentation. Food Sci. Biotechnol. 2017, 27, 79–85. [Google Scholar] [CrossRef]
- Erdoğan, A.K.; Filiz, B.E. Menaquinone Content and Antioxidant Properties of Fermented Cabbage Products: Effect of Different Fermentation Techniques and Microbial Cultures. J. Funct. Foods 2023, 102, 105467. [Google Scholar] [CrossRef]
- Satora, P.; Strnad, S. Impact of Selected Yeast Strains on Quality Parameters of Obtained Sauerkraut. Appl. Sci. 2024, 14, 3462. [Google Scholar] [CrossRef]
- Shankar, T.; Palpperumal, S.; Kathiresan, D.; Sankaralingam, S.; Balachandran, C.; Baskar, K.; Hashem, A.; Alqarawi, A.A.; Abd_Allah, E.F. Biomedical and Therapeutic Potential of Exopolysaccharides by Lactobacillus paracasei Isolated from Sauerkraut: Screening and Characterization. Saudi J. Biol. Sci. 2021, 28, 2943–2950. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, X.; Li, S.; Li, C.; Li, D.; Yang, Z. Evaluation of Probiotic Properties of Lactobacillus Plantarum Strains Isolated from Chinese Sauerkraut. World J. Microbiol. Biotechnol. 2013, 29, 489–498. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, Y.; Wang, Y.; Wang, L.; Zhang, L.; Zhao, Z.; Li, S. Lactobacillus plantarum S9 Alleviates Lipid Profile, Insulin Resistance, and Inflammation in High-Fat Diet-Induced Metabolic Syndrome Rats. Sci. Rep. 2022, 12, 15490. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lin, F.; Zhu, X.; Zhang, C.; Jiang, M.; Lu, Z. An Exopolysaccharide from Lactobacillus plantarum H31 in Pickled Cabbage Inhibits Pancreas α-Amylase and Regulating Metabolic Markers in HepG2 Cells by AMPK/PI3K/Akt Pathway. Int. J. Biol. Macromol. 2020, 143, 775–784. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef]
- Medic, J.; Atkinson, C.; Hurburgh, C.R. Current Knowledge in Soybean Composition. J. Americ. Oil Chem. Soc. 2014, 91, 363–384. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hamaguchi, M.; Fukui, M. Fermented Soybean Foods and Diabetes. J. Diabetes Investig. 2023, 14, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Sajjadi, F.; Haghighatdoost, F. Effects of Soy Consumption on Metabolic Parameters in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis. EXCLI J. 2021, 20, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.M.; Passoth, V.; Eklund-Jonsson, C.; Alminger, M.L.; Schnürer, J. Rhizopus oligosporus and Yeast Co-Cultivation during Barley Tempeh Fermentation—Nutritional Impact and Real-Time PCR Quantification of Fungal Growth Dynamics. Food Microbiol. 2007, 24, 393–402. [Google Scholar] [CrossRef]
- Sitanggang, A.B.; Lesmana, M.; Budijanto, S. Membrane-Based Preparative Methods and Bioactivities Mapping of Tempe-Based Peptides. Food Chem. 2020, 329, 127193. [Google Scholar] [CrossRef]
- Ahnan-Winarno, A.D.; Cordeiro, L.; Winarno, F.G.; Gibbons, J.; Xiao, H. Tempeh: A Semicentennial Review on Its Health Benefits, Fermentation, Safety, Processing, Sustainability, and Affordability. Comp. Rev. Food Sci. Food Safe 2021, 20, 1717–1767. [Google Scholar] [CrossRef]
- Mani, V.; Ming, L.C. Tempeh and Other Fermented Soybean Products Rich in Isoflavones. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2017; pp. 453–474. ISBN 9780128023099. [Google Scholar]
- Setiawan, S.I.; Sudirman, A.R.; Aditia, D.A.; Yonathan, K.; Dwijayanti, A. Potency of Indonesian Soybean Tempeh in Enhancement of Gut Roseburia Spp. and Bifidobacterium Spp. to Prevent Type II Diabetes Mellitus and Hyperlipidemia: Fried or Steamed? In Proceedings of the 8th Conference of Indonesian Students Association in South Korea, Daejeon, Republic of Korea, 5–6 September 2015. [Google Scholar] [CrossRef]
- Liem, I.T.; Steinkraus, K.H.; Cronk, T.C. Production of Vitamin B-12 in Tempeh, a Fermented Soybean Food. Appl. Environ. Microbiol. 1977, 34, 773–776. [Google Scholar] [CrossRef]
- Syida, W.K.; Noriham, A.; Normah, I.; Yusuf, M. Changes in Chemical Composition and Amino Acid Content of Soy Protein Isolate (SPI) from Tempeh. Int. Food Res. J. 2018, 25, 1528–1533. [Google Scholar]
- van der Riet, W.B.; Wight, A.W.; Cilliers, J.J.L.; Datel, J.M. Food Chemical Analysis of Tempeh Prepared from South African-Grown Soybeans. Food Chem. 1987, 25, 197–206. [Google Scholar] [CrossRef]
- Ruiz-Terán, F.; Owens, J.D. Chemical and Enzymic Changes During the Fermentation of Bacteria-Free Soya Bean Tempe. J. Sci. Food Agric. 1996, 71, 523–530. [Google Scholar] [CrossRef]
- Rizal, S.; Kustyawati, M.E.; Murhadi; Hasanudin, U. The Growth of Yeast and Fungi, the Formation of β-Glucan, and the Antibacterial Activities during Soybean Fermentation in Producing Tempeh. Int. J. Food Sci. 2021, 2021, 6676042. [Google Scholar] [CrossRef] [PubMed]
- Tamam, B.; Syah, D.; Suhartono, M.T.; Kusuma, W.A.; Tachibana, S.; Lioe, H.N. Proteomic Study of Bioactive Peptides from Tempe. J. Biosci. Bioeng. 2019, 128, 241–248. [Google Scholar] [CrossRef]
- Rachmawati, P.A.; Afifah, D.N.; Rustanti, N.; Anjani, G.; Juniarto, A.Z. The Effect of Tempeh Gembus on Malondialdehyde and Superoxide Dismutase Enzyme Levels in Rats with Diet-Induced Metabolic Syndrome. J. Biomed. Transl. Res. 2022, 8, 91–98. [Google Scholar] [CrossRef]
- Watanabe, N.; Fujimoto, K.; Aoki, H. Antioxidant Activities of the Water-Soluble Fraction in Tempeh-like Fermented Soybean (GABA-Tempeh). Int. J. Food Sci. Nutr. 2007, 58, 577–587. [Google Scholar] [CrossRef]
- Astawan, M.; Rahmawati, I.S.; Cahyani, A.P.; Wresdiyati, T.; Putri, S.P.; Fukusaki, E. Comparison Between the Potential of Tempe Flour Made from Germinated and Nongerminated Soybeans in Preventing Diabetes Mellitus. HAYATI J. Biosci. 2020, 27, 16. [Google Scholar] [CrossRef]
- Blandino, G.; Inturri, R.; Lazzara, F.; Di Rosa, M.; Malaguarnera, L. Impact of Gut Microbiota on Diabetes Mellitus. Diabetes Metab. 2016, 42, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K.; Kezuka, M.; Oshiro, N.; Chan, H.T. Natto and Miso: An Overview on Their Preparation, Bioactive Components Andhealth-Promoting Effects. Food Res. 2021, 5, 446–452. [Google Scholar] [CrossRef]
- Kiuchi, K. Miso & Natto. Food Cult. 2001, 3, 7–10. [Google Scholar]
- Afzaal, M.; Saeed, F.; Islam, F.; Ateeq, H.; Asghar, A.; Shah, Y.A.; Ofoedu, C.E.; Chacha, J.S. Nutritional Health Perspective of Natto: A Critical Review. Biochem. Res. Int. 2022, 2022, 5863887. [Google Scholar] [CrossRef]
- Lan, G.; Li, C.; He, L.; Zeng, X.; Zhu, Q. Effects of Different Strains and Fermentation Method on Nattokinase Activity, Biogenic Amines, and Sensory Characteristics of Natto. J. Food Sci. Technol. 2020, 57, 4414–4423. [Google Scholar] [CrossRef]
- de Melo, F.C.B.C.; Zaia, C.T.B.V.; Celligoi, M.A.P.C. Levan from Bacillus subtilis Natto: Its Effects in Normal and in Streptozotocin-Diabetic Rats. Braz. J. Microbiol. 2012, 43, 1613–1619. [Google Scholar] [CrossRef]
- Kubo, Y.; Rooney, A.P.; Tsukakoshi, Y.; Nakagawa, R.; Hasegawa, H.; Kimura, K. Phylogenetic Analysis of Bacillus Subtilis Strains Applicable to Natto (Fermented soybean) Production. Appl. Environ. Microbiol. 2011, 77, 6463–6469. [Google Scholar] [CrossRef]
- Li, D.; Hou, L.; Hu, M.; Gao, Y.; Tian, Z.; Fan, B.; Li, S.; Wang, F. Recent Advances in Nattokinase-Enriched Fermented Soybean Foods: A Review. Foods 2022, 11, 1867. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Yu, B. Poly-γ-Glutamic Acid: Recent Achievements, Diverse Applications and Future Perspectives. Trends Food Sci. Technol. 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Tang, C.; Wang, C.; Liu, C.; Chen, Q.; Yang, K.; Gu, Y.; Lei, P.; Xu, H.; et al. Antidiabetic Effects and Mechanism of γ-Polyglutamic Acid on Type II Diabetes Mice. Int. J. Biol. Macromol. 2024, 261, 129809. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-H.; Son, W.-C.; Lee, S.-E.; Kim, B.-H. Anti-Obesity Effects of Poly-γ-Glutamic Acid with or without Isoflavones on High-Fat Diet Induced Obese Mice. Biosci. Biotechnol. Biochem. 2013, 77, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Hori, S.; Inose, A.; Kobori, M. Effects of γ-Polyglutamic Acid on Blood Glucose and Caecal Short Chain Fatty Acids in Adult Male Mice. Food Nutr. Sci. 2020, 11, 8–22. [Google Scholar] [CrossRef]
- Keziah, S.M.; Devi, C.S. Fibrinolytic and ACE Inhibitory Activity of Nattokinase Extracted from Bacillus subtilis VITMS 2: A Strain Isolated from Fermented Milk of Vigna Unguiculata. Protein J. 2021, 40, 876–890. [Google Scholar] [CrossRef]
- Murakami, K.; Yamanaka, N.; Ohnishi, K.; Fukayama, M.; Yoshino, M. Inhibition of Angiotensin I Converting Enzyme by Subtilisin NAT (Nattokinase) in Natto, a Japanese Traditional Fermented Food. Food Funct. 2012, 3, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Lenninger, M.; Ero, M.P.; Benson, K.F. Consumption of Nattokinase Is Associated with Reduced Blood Pressure and von Willebrand Factor, a Cardiovascular Risk Marker: Results from a Randomized, Double-Blind, Placebo-Controlled, Multicenter North American Clinical Trial. Integr. Blood Press. Control 2016, 9, 95–104. [Google Scholar] [CrossRef]
- Kim, J.Y.; Gum, S.N.; Paik, J.K.; Lim, H.H.; Kim, K.-C.; Ogasawara, K.; Inoue, K.; Park, S.; Jang, Y.; Lee, J.H. Effects of Nattokinase on Blood Pressure: A Randomized, Controlled Trial. Hypertens. Res. 2008, 31, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-J.; Lin, C.-S.; Lee, M.-Y. Lipid-Lowering Effect of Nattokinase in Patients with Primary Hypercholesterolemia. Acta Cardiol. Sin. 2009, 25, 26–30. [Google Scholar]
- Sun, R.; Niu, H.; Li, Y.; Sun, M.; Hua, M.; Miao, X.; Su, Y.; Wang, J.; Li, D.; Wang, Y. Fermented Natto Powder Alleviates Obesity by Regulating LXR Pathway and Gut Microbiota in Obesity Rats. J. Appl. Microbiol. 2024, 135, lxae003. [Google Scholar] [CrossRef]
- Wang, P.; Gao, X.; Li, Y.; Wang, S.; Yu, J.; Wei, Y. Bacillus Natto Regulates Gut Microbiota and Adipose Tissue Accumulation in a High-Fat Diet Mouse Model of Obesity. J. Funct. Foods 2020, 68, 103923. [Google Scholar] [CrossRef]
- Araki, R.; Fujie, K.; Yuine, N.; Watabe, Y.; Maruo, K.; Suzuki, H.; Hashimoto, K. The Possibility of Suppression of Increased Postprandial Blood Glucose Levels by Gamma-Polyglutamic Acid-Rich Natto in the Early Phase after Eating: A Randomized Crossover Pilot Study. Nutrients 2020, 12, 915. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Yamada, T.; Maruo, K.; Araki, A.; Miyakawa, R.; Suzuki, H.; Hashimoto, K. Gamma-Polyglutamic Acid-Rich Natto Suppresses Postprandial Blood Glucose Response in the Early Phase after Meals: A Randomized Crossover Study. Nutrients 2020, 12, 2374. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Takahashi, M.; Sekimizu, K. Polysaccharides of a Fermented Food, Natto, Suppress Sucrose-Induced Hyperglycemia in an in Vivo Evaluation System and Inhibit Glucose Uptake by Human Intestinal Cells. Drug Discov. Ther. 2020, 14, 8–13. [Google Scholar] [CrossRef]
- Sato, K.; Miyasaka, S.; Tsuji, A.; Tachi, H. Isolation and Characterization of Peptides with Dipeptidyl Peptidase IV (DPPIV) Inhibitory Activity from Natto Using DPPIV from Aspergillus oryzae. Food Chem. 2018, 261, 51–56. [Google Scholar] [CrossRef]
- Sun, R.; Li, D.; Sun, M.; Miao, X.; Jin, X.; Xu, X.; Su, Y.; Xu, H.; Wang, J.; Niu, H. Bacillus Natto Ameliorates Obesity by Regulating PI3K/AKT Pathways in Rats. Biochem. Biophys. Res. Commun. 2022, 603, 160–166. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Xu, P.; Guo, S.; Zhou, T.; Li, N. Soy Protein Degradation Drives Diversity of Amino-Containing Compounds via Bacillus Subtilis Natto Fermentation. Food Chem. 2022, 388, 133034. [Google Scholar] [CrossRef]
- Tamang, J.P.; Das, S.; Kharnaior, P.; Pariyar, P.; Thapa, N.; Jo, S.-W.; Yim, E.-J.; Shin, D.-H. Shotgun Metagenomics of Cheonggukjang, a Fermented Soybean Food of Korea: Community Structure, Predictive Functionalities and Amino Acids Profile. Food Res. Int. 2022, 151, 110904. [Google Scholar] [CrossRef]
- Kim, E.J.; Hong, J.Y.; Shin, S.R.; Moon, Y.-S.; Yoon, K. Analysis of the Taste Components and Antioxidant Properties of Cheonggukjang Containing Korean Red Ginseng. Food Sci. Biotechnol. 2009, 18, 53–59. [Google Scholar]
- Jeon, A.R.; Lee, J.H.; Mah, J.-H. Biogenic Amine Formation and Bacterial Contribution in Cheonggukjang, a Korean Traditional Fermented Soybean Food. LWT 2018, 92, 282–289. [Google Scholar] [CrossRef]
- Kim, H.-J.; Hwang, J.-T.; Kim, M.J.; Yang, H.-J.; Sung, M.J.; Kim, S.-H.; Park, S.; Gu, E.-J.; Park, Y.; Kwon, D.Y. The Inhibitory Effect of Saponin Derived from Cheonggukjang on Adipocyte Differentiation In Vitro. Food Sci. Biotechnol. 2014, 23, 1273–1278. [Google Scholar] [CrossRef]
- Cho, K.M.; Lim, H.-J.; Kim, M.-S.; Kim, D.S.; Hwang, C.E.; Nam, S.H.; Joo, O.S.; Lee, B.W.; Kim, J.K.; Shin, E.-C. Time Course Effects of Fermentation on Fatty Acid and Volatile Compound Profiles of Cheonggukjang Using New Soybean Cultivars. J. Food Drug Anal. 2017, 25, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim-Eun, H.; Kim, Y.-S. The Potentials of Bacillus licheniformis Strains for Inhibition of B. cereus Growth and Reduction of Biogenic Amines in Cheonggukjang (Korean Fermented Unsalted Soybean Paste). Food Control 2017, 79, 87–93. [Google Scholar] [CrossRef]
- Kim, D.; Jin, Y.H.; Mah, J.-H. Biogenic Amine Reduction by Food Additives in Cheonggukjang, a Korean Fermented Soybean Paste, Fermented with Tyramine-Producing Heterogeneous Bacterial Species. Heliyon 2024, 10, e26135. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, S.-Y.; Ko, J.-M.; Jeong, D.-Y.; Kim, Y.-S. Biological Activities of Cheonggukjang Prepared with Several Soybean Cultivars. Food Sci. Biotechnol. 2012, 21, 475–483. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Jang, J.S.; Hong, S.M.; Lee, J.E.; Sung, S.R.; Park, H.R.; Park, S. Long-Term Consumption of Fermented Soybean-Derived Chungkookjang Enhances Insulinotropic Action Unlike Soybeans in 90% Pancreatectomized Diabetic Rats. Eur. J. Nutr. 2007, 46, 44–52. [Google Scholar] [CrossRef]
- Hwang, J.W.; Do, H.J.; Kim, O.Y.; Chung, J.H.; Lee, J.-Y.; Park, Y.S.; Hwang, K.Y.; Seong, S.-I.; Shin, M.-J. Fermented Soy Bean Extract Suppresses Differentiation of 3T3-L1 Preadipocytes and Facilitates Its Glucose Utilization. J. Funct. Foods 2015, 15, 516–524. [Google Scholar] [CrossRef]
- Yang, H.J.; Kwon, D.Y.; Moon, N.R.; Kim, M.J.; Kang, H.J.; Jung, D.Y.; Park, S. Soybean Fermentation with Bacillus Licheniformis Increases Insulin Sensitizing and Insulinotropic Activity. Food Funct. 2013, 4, 1675. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Park, S.-L.; Hwang, J.-T.; Yi, S.-H.; Nam, Y.-D.; Lim, S.-I. Antidiabetic Effect of Morinda citrifolia (Noni) Fermented by Cheonggukjang in KK-Ay Diabetic Mice. Evid.-Based Complement. Altern. Med. 2012, 2012, 163280. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.-D.; Park, S.; Lim, S.-I. Microbial Composition of the Korean Traditional Food “Kochujang” Analyzed by a Massive Sequencing Technique. J. Food Sci. 2012, 77, M250–M256. [Google Scholar] [CrossRef]
- Das, D.; Wann, S.B.; Kalita, J.; Manna, P. Insight into the Efficacy Profile of Fermented Soy Foods against Diabetes. Food Biosci. 2023, 53, 102665. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Yang, H.-J.; Yang, H.G.; Ryu, M.S.; Ha, G.; Jeong, D.Y.; Park, S. Inverse Association of Daily Fermented Soybean Paste (“Jang”) Intake with Metabolic Syndrome Risk, Especially Body Fat and Hypertension, in Men of a Large Hospital-Based Cohort. Front. Nutr. 2023, 10, 1122945. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Lee, B.-H.; Kim, J.-H.; Kim, N.-S. Concentration of Phytoestrogens in Soybeans and Soybean Products in Korea. J. Sci. Food Agric. 2000, 80, 1709–1712. [Google Scholar] [CrossRef]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in Metabolic Syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef]
- Ahn, I.-S.; Do, M.-S.; Kim, S.-O.; Jung, H.-S.; Kim, Y.-I.; Kim, H.-J.; Park, K.-Y. Antiobesity Effect of Kochujang (Korean Fermented Red Pepper Paste) Extract in 3T3-L1 Adipocytes. J. Med. Food 2006, 9, 15–21. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, M.J.; Kim, K.S.; Lee, J.E.; Hong, S.P. In Vitro Antidiabetic and Antiobesity Activities of Traditional Kochujang and Doenjang and Their Components. Prev. Nutr. Food Sci. 2019, 24, 274–282. [Google Scholar] [CrossRef]
- Lee, H.Y.; Cho, D.Y.; Jung, J.G.; Kim, M.J.; Jeong, J.B.; Lee, J.H.; Lee, G.Y.; Jang, M.Y.; Lee, J.H.; Haque, M.A.; et al. Comparisons of Physicochemical Properties, Bacterial Diversities, Isoflavone Profiles and Antioxidant Activities on Household and Commercial Doenjang. Molecules 2023, 28, 3516. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Ryu, M.-S.; Yang, H.-J.; Wu, X.-H.; Jeong, D.-Y.; Park, S.-M. Bacterial Distribution, Biogenic Amine Contents, and Functionalities of Traditionally Made Doenjang, a Long-Term Fermented Soybean Food, from Different Areas of Korea. Microorganisms 2021, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.-H.; Hong, S.-P. Characteristics of Bacterial Strains with Desirable Flavor Compounds from Korean Traditional Fermented Soybean Paste (Doenjang). Molecules 2021, 26, 5067. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Lee, H.; Song, Y.; Cha, J. Taxonomic Variations of Bacterial and Fungal Communities Depending on Fermentation Temperature in Traditional Korean Fermented Soybean Food, Doenjang. J. Microbiol. Biotechnol. 2024, 34, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U.; Baraniak, B.; Bochnak, J. Peptides Obtained from Fermented Faba Bean Seeds (Vicia faba) as Potential Inhibitors of an Enzyme Involved in the Pathogenesis of Metabolic Syndrome. LWT 2019, 105, 306–313. [Google Scholar] [CrossRef]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and Antihypertensive Properties of Liquid and Solid State Fermented Lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Yeap, S.K.; Mohd Ali, N.; Mohd Yusof, H.; Alitheen, N.B.; Beh, B.K.; Ho, W.Y.; Koh, S.P.; Long, K. Antihyperglycemic Effects of Fermented and Nonfermented Mung Bean Extracts on Alloxan-Induced-Diabetic Mice. J. Biomed. Biotechnol. 2012, 2012, 285430. [Google Scholar] [CrossRef]
- Yeap, S.K.; Beh, B.K.; Ho, W.Y.; Mohd Yusof, H.; Mohamad, N.E.; Ali, N.M.; Jaganath, I.B.; Alitheen, N.B.; Koh, S.P.; Long, K. In Vivo Antioxidant and Hypolipidemic Effects of Fermented Mung Bean on Hypercholesterolemic Mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 508029. [Google Scholar] [CrossRef]
- Lin, N.-N.; Lee, Y.-F.; Chi, Y.-J.; Wang, M.-F.; Chan, Y.-C.; Chan, K.-C.; Chen, Y.-J.; Chiu, Y.-T. Bacillus subtilis -Fermented Red Bean (Red Bean Natto) Reduces Hyperlipidemia Levels in Hamsters Fed an Atherogenic Diet: Red Bean Natto Reduces Hyperlipidemia in Hamsters. J. Food Biochem. 2017, 41, e12264. [Google Scholar] [CrossRef]
- Pino, A.; Vaccalluzzo, A.; Solieri, L.; Romeo, F.V.; Todaro, A.; Caggia, C.; Arroyo-López, F.N.; Bautista-Gallego, J.; Randazzo, C.L. Effect of Sequential Inoculum of Beta-Glucosidase Positive and Probiotic Strains on Brine Fermentation to Obtain Low Salt Sicilian Table Olives. Front. Microbiol. 2019, 10, 174. [Google Scholar] [CrossRef]
- Vaccalluzzo, A.; Pino, A.; De Angelis, M.; Bautista-Gallego, J.; Romeo, F.V.; Foti, P.; Caggia, C.; Randazzo, C.L. Effects of Different Stress Parameters on Growth and on Oleuropein-Degrading Abilities of Lactiplantibacillus plantarum Strains Selected as Tailored Starter Cultures for Naturally Table Olives. Microorganisms 2020, 8, 1607. [Google Scholar] [CrossRef]
- Lalas, S.; Athanasiadis, V.; Gortzi, O.; Bounitsi, M.; Giovanoudis, I.; Tsaknis, J.; Bogiatzis, F. Enrichment of Table Olives with Polyphenols Extracted from Olive Leaves. Food Chem. 2011, 127, 1521–1525. [Google Scholar] [CrossRef]
- Bahuguna, A.; Shukla, S.; Lee, J.S.; Bajpai, V.K.; Kim, S.-Y.; Huh, Y.S.; Han, Y.-K.; Kim, M. Garlic Augments the Functional and Nutritional Behavior of Doenjang, a Traditional Korean Fermented Soybean Paste. Sci. Rep. 2019, 9, 5436. [Google Scholar] [CrossRef] [PubMed]

| Product | Raw Ingredient | Characteristic Microorganisms | Main Bioactive Compounds | Country/Region of Origin | References |
|---|---|---|---|---|---|
| Table olives | Olive fruits | Bacteria: L. acidipiscis, L. brevis, L. casei, L. coryniformis, L. fermentum, L. helveticus, L. paracasei, L. parafarraginis, L. paraplantarum, L. pentosus, L. plantarum, L. rhamnosus, E. faecalis, E. faecium, Lc. formosensis, Lc. lactis, Leu. mesenteroides, Ped. acidilactici, Ped. damnosus, S. inulinus, S. terrae, St. thermophilus, W. paramesenteroides, W. hellenica Yeasts: Wickerhamomyces anomalus, Wickerhamomyces sydowiorum, Saccharomyces cerevisiae, Pichia kluyveri, Pichia membranifaciens, Brettanomyces custersianus, Candida orthopsilosis, Candida tropicalis, Debaryomyces hansenii | Phenolic compounds: hydroxytyrosol, tyrosol, rutin, luteolin, cyanidin, and delphinidin glucosides (only in black olives) Iridoids: oleuropein, verbascoside Organic acids: lactic acid SCFAs: acetic acid, butyric acid Triterpenic acids: maslinic acid, oleanolic acid MUFA: Oleic acid Sterols: β-sitosterol, Δ5-avenasterol | Mediterranean countries (Spain, Portugal, Greece, and Italy) | [23,27,28,29,30] |
| Caper | Caper berries | Bacteria: L. plantarum, L. Paraplantarum, L. Pentosus, L. Fermentum, L. Brevis, Ped. Pentosaceus, Ped. Acidilactici, E. faecium Yeasts: Aureobasidium pullulans | Phenolic compounds: quercetin, quercetin glycosides (mainly rutin), kaempferol, kaempferol glycosides, isorhamnetin, isorhamnetin glycosides, myricetin, ferulic acid, vanillic acid, epicatechin | Mediterranean countries | [31,32,33] |
| Kimchi | Chinese cabbage with other vegetables and condiments | Bacteria: Leu. mesenteroides, Leu. gasicomitatum, Leu. gasicomitatum, Leu. kimchii, Leu. miyukkimchii, L. brevis, L. plantarum, L. kimchii, L. kimchiensis, L. koreensis, W. koreensis, Lc. kimchii, Tetragenococcus spp., St. faecalis, Ped. cerevisiae, Bacillus spp. Yeasts: Lodderomyces spp., Candida spp., Trichosporon spp., Saccharomyces spp., Pichia spp. | Sulfur compounds: S-methlycysteinsulfoxide, S-allylcysteinsulfoxide Sterols: α-sitosterol Amino acids: ornithine Others: capsaicin, HDMPPA, gingerol | Korea | [34,35,36] |
| Sauerkraut/ Fermented cabbage | Cabbage | Bacteria: L. plantarum, Leu. mesenteroides, Ped. pentosaceus, Levilactobacillus spp., Paucilactobacillus spp., Secundilactobacillus spp. Yeasts: Cryptococcus macerans, Debaryomyces hansenii, Pichia fermentans, Wickerhamomyces anomalus, Rhodotorula mucilaginosa | Organic acids: lactic acid, malic acid SCFAs: acetic acid, propionic acid, butyric acid Biogenic amines: tyramine, putrescine Amino acids: alanine, leucine Exopolysaccharides Esters: ethyl acetate, ethyl lactate Pyrazines: 2,5-dimethylpyrazine Sulfur compounds: allyl isothiocyanate, dimethyl sulfide Terpenes: geranyl acetone Others: dimethyl sulfoxide, uracil, ascorbinogen | Europe, USA, China | [37,38,39,40,41,42] |
| Natto | Soybean | Bacillus subtilis var. natto | Peptides and amino acids Exopolysaccharides: levan Isoflavones: daidzein, genistein, glycitein, and corresponding β-glucosides Ketones: acetoin Pyrazines: di, tri, tetramethyl pyrazines Biogenic amines: spermine, followed by spermidine, tyramine SCFAs: acetic acid, isobutyric acid, isovaleric acid Others: γ-PGA, phenol, 2-metoxyphenol | China, Japan | [43] |
| Tempeh | Soybean | Bacteria: E. cecorum, L. agilis, L. fermentum, L. mucosae, L. delbrucki, Acetobacter indonesiensis, Weisella spp., Enterococcus spp., Leuconostoc spp., Paenibacillus spp., Bacillus spp. Yeasts: Pichia guilliermondii, Candida tropicalis, Pichia norvegensis, Sporopachydermia lactativora, Trichosporon asahii Molds: R. microsporus, R. delemar, R. oligosporus, R. oryzae, R. stolonifer, Mucor spp., Rhizomucor spp. | Isoflavones: daidzein, genistein, glycitein, and corresponding β-glucosides Peptides and amino acids SCFAs Others: GABA | Indonesia (also introduced in Japan, India, Europe, and Africa) | [44,45,46,47,48,49,50] |
| Cheonggukjang | Soybean | Bacteria: B. piscis, B. coagulans, B. carboniphilus, B. hisashii, Ab. aneurinilyticus, S. equorum, B. subtilis, B. licheniformis, B. amylolquefaciens, B. haynessi, B. velazensis | Biogenic amines: tyramine, β-phenylethylamine, putrescine Saponins: soyasapogenol A and B Ketones: 1,3-diphenyl-2-propanone Organic acids: lactic acid, 2-hydroxyglutaric acid Pyrazines Aromatic compounds: 4 -(nonafluoro-tert-butyl) nitrobenzene Isoflavones: daidzein, genistein, glycitein, and corresponding β-glucosides Others: GABA, γ-PGA | Korea | [51,52,53,54] |
| Doenjang | Soybean | Bacteria: B. paralicheniformis, B. subtilis, B. acidicola, B. dabaoshanensis, B. idriensis, B. carboniphilus, B. aerius, B. crescens, E. hirae, E. phoeniculicola, Ped. acidilacti, E. faecalis, Ped. claussenii, B. licheniformis, B. athrophaeus, Enterobacter soli, Lentibacillus sp., Enterobacter sp. Yeasts: Zygosaccharomyces pseudorouxii, Zygosaccharomyces mellis, Candida versatilis, Ogatea polymorpha, Saccharomyces cerevisiae | Isoflavones: daidzein, genistein, glycitein, and corresponding β-glucosides Amino acids: glutamic acid Fatty acids: isovaleric acid, Volatile compounds: 3-methyl butanal, benzeneacetaldehyde, α-curcumene, β-sesquiphellandrene, diallyl disulfide Pyrazines Others: GABA | Korea | [55,56] |
| Kochujang | Soybean | Bacteria: B. amyloquefaciens, B. carboniphilus, B. circulans, B. coagulans, B. lentus, B. licheniformis, B. megaterium, B. pumilus, B. stearothermophilus, B. sonorensis, B. subtilis, B. thuringiensis, Aneurinibacillus thermoaerophilus, Brevibacillus borstelensis, E. faecalis, E. faecium, L. delbrucki, L. fermentum, L. fructivorans, L. gassei, L. halophilus, L. plantarum, L. salivarius, L. sakei, L. paracasei, W. confuse Yeasts: Saccharomyces cerevisiae, Zygosaccharomyces rouxii | Isoflavones Others: capsaicin | Korea | [57,58,59,60] |
| Conclusions: The microbial communities of table olives, capers, kimchi, and sauerkraut consist mostly of LAB, including L. plantarum and Pediococcus spp., as well as yeasts. In fermented soybean products, mostly Bacillus species have been identified. In tempeh, molds such as Rhizopus spp. and Mucor spp. have also been identified. The most frequently identified groups of bioactive compounds in fermented foods include organic acids, SCFAs, GABA, free amino acids, and biogenic amines. Olives and capers are dietary sources of phenolic compounds, primarily flavonoids. A characteristic group of compounds found exclusively in table olives are iridoids. Kimchi and sauerkraut are produced from fermented cabbage and, as a result, are rich in sulfur compounds. Fermented soybean products typically contain isoflavone aglycones (daidzein, genistein, glycitein), peptides, and amino acids. A unique and health-promoting compound, γ-PGA, has been reported in natto and cheonggukjang. | |||||
| Product | n | Animals/ Patients | Length of Study | Intervention | Control | Health-Promoting Effect 1 | References |
|---|---|---|---|---|---|---|---|
| Caper fruit pickle | 44 | Subjects with a BMI of 25–35 kg/m2 and NAFLD | 12 weeks | Caper fruit pickle (40–50 g/day) and consultation with a nutritionist | Consultation with a nutritionist | Anti-obesity effect: ↓ BW, ↓ BMI (compared to baseline) No hypolipidemic effect: Ø TC, Ø TG, Ø LDL, Ø HDL | [77] |
| Caper fruit pickle | 44 | Subjects with a BMI of 25–35 kg/m2 and NAFLD | 12 weeks | Caper fruit pickle (40–50 g/day) and consultation with a nutritionist | Consultation with a nutritionist | No anti-inflammatory effect: Ø hs-CRP Anti-obesity effect: ↓ waist circumference Hypolipidemic effect: ↓ LDL/HDL, ↓TG/HDL, ↓ TC/HDL (compared to baseline) No anti-diabetic effect: Ø insulin, Ø HOMA-IR | [82] |
| Caper fruit pickle | 60 | Subjects newly diagnosed with hyperlipidemia and prescribed low-dose atorvastatin | 8 weeks | Caper fruit pickle (40–50 g/day) and 10 mg atorvastatin | 10 mg atorvastatin | Hypolipidemic effect: ↓ TC, ↓ LDL, ↓ TG, ↑ HDL | [83] |
| Kimchi | 22 | Obese and overweight subjects (crossover study) | 4 weeks | Kimchi (300 g/day) | Baseline | Anti-inflammatory effect: Ø CRP, Ø IL-6, Ø TNF-α, ↓ MCP-1 Anti-obesity effect: ↓ BW, ↓ BMI, ↓ WHR, ↓ body fat, Ø adiponectin, ↓ leptin Anti-diabetic effect: ↓ FBG, ↓ FBI, No hypotensive effect: Ø systolic BP, Ø diastolic BP Hypolipidemic effect: ↓ TC, Ø LDL, Ø HDL, Ø TG | [84] |
| Kimchi fermented with Weissella koreensis OK1-6 | 7 | Obese mice | 12 weeks | HFD with kimchi powder (3%) | HFD | Anti-obesity effect: ↓ BW, ↓ epididymal adipose tissue, ↓ leptin Anti-diabetic effect: ↓ insulin Hypolipidemic effect: ↓ TC, Ø TG | [85] |
| Kimchi | 21 | Prediabetic subjects (crossover study) | 8 weeks | Kimchi (300 g/day) | Baseline | No anti-inflammatory effect: Ø IL-1β, Ø IL-6, Ø IL-10, Ø TNF-α, Ø MCP-1, Ø CRP, Ø FGF-21 Anti-obesity effect: ↓ BW, ↓ BMI, ↓ WC, ↓ body fat (%, kg), Ø adiponectin Anti-diabetic effect: ↓ HbA1c, Ø FBG, ↓ FBI, ↓ HOMA-IR, ↑ Matsuda Index, ↑ QUICKI, ↑ DI, Ø IGI Hypotensive effect: ↓ systolic BP, ↓ diastolic BP | [86] |
| Kimchi | 100 | Healthy subjects | 1 week | Kimchi (210 g/day) | Kimchi (15 g/day) | Antioxidative effect: ↑ TAC (statistically significant in both groups, but not between groups) Anti-diabetic effect: ↓ FBG (statistically significant in both groups and between groups) Hypolipidemic effect: ↓ TC, ↓ LDL, ↓ TG (statistically significant in both groups, but not between groups) | [36] |
| Kimchi | 28 | Healthy subjects | 4 weeks | Standardized or functional kimchi with additional condiments and probiotics (210 g/day) | Baseline | Anti-inflammatory effect: ↓ IL-6, Ø hs- CRP, Ø MCP-1, Ø TNF-α (both standardized and functional kimchi) Anti-obesity effect: ↓ body fat (%), ↑ skeletal muscle mass, ↑ adiponectin, Ø BW, Ø WHR, Ø BMI, Ø body fat (kg), Ø leptin (statistically significant only in functional kimchi group) Hypolipidemic effect: ↓ LDL, ↑ HDL (both groups), ↓ TG, ↓ TC (only in functional kimchi group) | [16] |
| Cheonggukjang (CGJ) | 100 | 90% of pancreatectomized diabetic rats | 8 weeks | Soybean or CGJ (amount not given) | Diabetic rats, diabetic rats with rosiglitazone, non-diabetic rats | No anti-obesity effect: Ø BW, Ø food intake Anti-diabetic effect: ↓ glucose, ↓ insulin, improved OGTT, Ø β cell area (CGJ and soybean groups), ↓ β cell size and mass (only CGJ group), Ø β cells apoptosis | [87] |
| Cheonggukjang | 30 | C57BL/KsJ-db/db mice | 6 weeks | Diet containing CGJ (5 g/100 g) | Diabetic mice, diabetic mice with rosiglitazone | Anti-obesity effect: ↓ BW, ↓ weight gain, ↓ food efficiency ratio, Ø food intake Anti-diabetic effect: ↓ glucose, ↓ HbA1c, Ø leptin, Ø insulin, ↓ glucagon, ↑ insulin (pancreatic tissue), Ø glucagon (pancreatic tissue) | [88] |
| Cheonggukjang | 30 | Mice | 12 weeks | HFD with CGJ (40%) | Normal diet, HFD | Anti-obesity effect: ↑ food intake, ↓ weight gain, ↓ epididymal fat, ↓ back fat Hypolipidemic effect: ↓ TC, ↓ TG | [89] |
| Cheonggukjang | 38 | Obese mice | 13 weeks | HFD with 30% cooked soybean powder or 30% CGJ powder | Normal diet, HFD | Hypolipidemic effect: Ø TG, ↓ TC (significant only for HFD compared to HFD + 30% CGJ powder) | [90] |
| Cheonggukjang | 32 | Rats | 4 weeks | HFD with CGJ (20%) or CGJ made from soybean germinated under light or dark conditions (20%) | HFD | No anti-obesity effect: Ø BW, Ø food intake, Ø food efficiency ratio Hypolipidemic effect: Ø TC, ↓ LDL, ↓ TG, ↑ HDL (only for CGJ from soybean germinated under light conditions) | [91] |
| Cheonggukjang | 80 | Diabetic rats (induced by partial pancreatectomy) | 8 weeks | HFD with cooked soybeans (10%) or traditional CGJ or CGJ fermented with a starter (B. lichemiformis) (10%) | Nondiabetic rats, diabetic rats | Anti-obesity effect: ↓ BW (only soybean), ↓ epididymal fat, ↓ caloric intake (all groups), ↓ leptin (only soybean) Anti-diabetic effect: ↓ FBG (all treatments), ↑ insulin, ↑ β cell area (both CGJ groups), ↓ β cell size (all groups), ↓ β cell apoptosis (all groups) | [92] |
| Cheonggukjang | 87 | Overweight/obese subjects (BMI ≥ 23 kg/m2 or WC ≥ 80 cm for women or ≥90 cm for men) (crossover study) | 12 weeks | CGJ fermented with B. licheniformis (70 g/day) | Baseline, placebo | Anti-inflammatory effect: ↓ hsCRP (women, compared to baseline) Anti-obesity effect: Ø BW, Ø BMI, Ø body fat (kg), Ø lean body mass, Ø WC, Ø HC, ↓ WHR (significant only for women compared between groups), ↓ body fat (%) (significant only for women, compared to baseline and placebo) Hypolipidemic effect: ↑ TC (men, compared to baseline), Ø TC (women, compared to baseline), Ø LDL, ↑ TG (compared to baseline), Ø HDL, Ø FFA, ↑ ApoA1 (compared to baseline), ↓ ApoB (women, compared to baseline), ↓ ApoB/ApoA1 (compared to baseline) | [93] |
| Cheonggukjang | 40 | Obese mice | 13 weeks | HFD with unfermented soybean (30%) or CGJ fermented with γ-PGA producing starter strain (B. licheniformis-67) (30%) | Normal diet and HFD | Anti-obesity effect: ↓ BW, ↓ weight gain, ↓ food efficiency ratio, ↓ leptin (significant compared to soybean and CGJ group), ↓ epidydymal fat (only CGJ group) Anti-diabetic effect: ↓ FBG, improved OGTT (only in CGJ group), Ø insulin Hypolipidemic effect: ↓TC (significant only for CGJ group), ↓ HDL, Ø TG (both soybean and CGJ groups) | [87] |
| Cheonggukjang | 30 | Obese mice | 13 weeks | HFD with CGJ (10%) | Normal diet and HFD | Anti-inflammatory effect: ↓ MCP-1, ↓ TNF-α mRNA expression Anti-obesity effect: ↓ BW, ↓ weight gain, Ø food intake, ↓ food efficiency ratio, Ø epididymal fat, ↓ perirenal fat, ↓ leptin, ↑ adiponectin (all parameters significant compared to HFD, but leptin level also significant compared to baseline), ↓ adipocyte size Anti-diabetic effect: ↓ glucose, ↓ insulin Hypolipidemic effect: ↓ TC, ↓ TG | [94] |
| Cheonggukjang | 55 | Overweight/obese subjects with a BMI ≥ 25 kg/m2 | 12 weeks | Freeze-dried CGJ (26 g/day) | Baseline and placebo | No anti-obesity effect: Ø total fat, Ø visceral fat, Ø subcutaneous fat, Ø visceral subcutaneous ratio Hypolipidemic effect: Ø TC, Ø TG, Ø LDL, Ø HDL, Ø FFA, Ø ApoA1, ↓ ApoB (significant compared to baseline and placebo), Ø ApoB/ApoA1 | [95] |
| Cheonggukjang | 45 | Subject with impaired fasting blood glucose | 8 weeks | CGJ (20 g/day) or CGJ with red ginseng (20 g/day) | Placebo (starch 2 g/day) | Antioxidative effect: ↓ TBARS Anti-diabetic effect: ↓ FBG Hypolipidemic: ↓ TC, ↓ LDL, Ø HDL, Ø lipoprotein (a), ↓ Apo B/ApoA1 (only CGJ group) | [96] |
| Cheonggukjang | 59 | Obese subjects with a BMI ≥ 25 kg/m2 | 8 weeks | Freeze-dried traditional CGJ with a high microorganism content, with a low microorganism content, and commercial CGJ (3 g/day) | Baseline | No anti-inflammatory effect: Ø IL-6, Ø haptoglobin, Ø hs-CRP No anti-obesity effect: Ø HC, Ø WHR, Ø VF, Ø SF, Ø V/S No hypolipidemic effect: Ø TC, Ø LDL, Ø HDL No anti-diabetic effect: Ø glucose, Ø insulin, Ø HOMA-IR, Ø QUICKI (the same effect on each type of supplementation) | [97] |
| Tempeh | 48 | STZ-induced diabetic rats | 14 weeks | STZ + HFD with cooked soybean or tempeh or tempeh fermented with probiotics (L. plantarum and R. oligosporus) (40 mg/kg BW/day) | Control diet, STZ + HFD or STZ + HFD + pioglitazone | Anti-diabetic effect: improved OGTT, ↓ AC glucose, ↓ HbA1C, ↓ insulin, ↓ HOMA-IR Hypolipidemic effect: ↓ TC, ↓TG, ↓ LDL, ↓ FFA, ↑ HDL | [98] |
| Tempeh | 20 | STZ-induced rats | 30 days | Tempeh powder (200 mg/kg BW/day) | Nondiabetic rats, diabetic rats | Anti-diabetic effect: ↓ insulitis, no effect on the diameter of pancreatic Langerhans islets | [99] |
| Tempeh | 18 | Rats | 3 weeks | High cholesterol diet and tempeh flour (0.95 g/100 g BW/day) | Standard diet, high cholesterol diet | Antioxidative effect: ↓ MDA Anti-obesity effect: ↓ BW, Ø food intake Hypolipidemic effect: ↓ TC, ↓ TG, ↑ HDL | [100] |
| Tempeh | 30 | STZ-induced rats | 4 weeks | Tempeh or tempeh fermented with fermented cassava tuber extract in amounts that provide 15 or 30% of protein | Nondiabetic rats, diabetic rats | Anti-diabetic effect: ↓ FBG (statistically significant compared to diabetic control, effect was slightly stronger according to tempeh fermented with cassava extract) | [101] |
| Tempeh | 30 | STZ-induced diabetic mice | 3 weeks | Tempeh (10, 20, or 40 mg/100 g BW/day) | Nondiabetic mice, diabetic mice, diabetic mice with metformin | Anti-diabetic effect: ↓ blood glucose Hypolipidemic effect: ↓ TC, ↓ LDL, ↓ TG, ↑ HDL | [102] |
| Tempeh fermented in both aerobic and anaerobic conditions | 20 | STZ-induced diabetic mice | 3 weeks | Tempeh (10, 20, or 40 mg/100 g BW/day) | Nondiabetic mice, diabetic mice | Anti-diabetic effect: ↓ blood glucose, ↑ insulin, ↓ insulin secretion (HOMA-β) (statistically significant compared to diabetic control, glucose level was statistically significant also compared to baseline) | [103] |
| Tempeh | 18 | Db/db obese diabetic mice | 12 weeks | Tempeh (300 mg/kg or 600 mg/kg) | Db/db obese diabetic mice | Anti-obesity effect: ↓ BW (for 600 mg/kg, compared to baseline and 300 mg/kg) Anti-diabetic effect: ↓ blood glucose Hypolipidemic effect: ↓ lipid accumulation in adipocytes (for 600 mg) | [104] |
| Tempeh | 30 | Obese rats | 4 weeks | High-fat sucrose diet and freeze-dried tempeh fermented with R. oligosporus (60 mg/kg BW) or R. oligosporus and L. rhamnosus GG co-fermented tempeh in dose 60 mg/kg BW or 120 mg/kg BW | Standard diet, high-fat sucrose diet, and high-fat fructose diet with orlistat (120 mg/kg BW) | Anti-inflammatory effect: ↓ hsCRP (all interventions compared to negative control) Anti-obesity effect: ↓ BW (all interventions compared to negative control) Hypolipidemic effect: ↓ TC, ↓ LDL, ↓ TG, ↑ HDL (all interventions compared to negative control) | [105] |
| Tempeh gembus | 41 | Women with hyperlipidemia | 2 weeks | Tempeh gembus (103 or 206 g/day) and nutrition education | Nutrition education, baseline | Hypolipidemic effect: ↓ TC, ↓ LDL (significant only compared to baseline, no between groups), Ø TG, Ø HDL | [106] |
| Tempeh | 40 | Obese women with BMI ≥ 25 kg/m2 | 4 weeks | Processed tempeh (150 g/day) | Control | Anti-obesity effect: ↓ WC, Ø BW, Ø BMI No anti-diabetic effect: Ø FBG | [107] |
| Tempeh | 35 | Patients with type 2 diabetes | 12 weeks | Freeze-dried tempeh (2 g/day) | Baseline | Hypolipidemic effect: ↓ TG, Ø TC, Ø LDL, Ø HDL Anti-diabetic effect: ↓ HbA1c, Ø AC sugar | [108] |
| Tempeh | 24 | Subjects with TC ≥ 4.92 mmol/L | 6 weeks | Tempeh (66 g/day) | Control group and baseline (n of subjects in control group = 3) | Anti-obesity effect: ↓ BW Hypolipidemic effect: ↓WC (statistically significant compared to baseline, no between groups); Ø fat (%), Ø visceral fat (%), Ø TC | [109] |
| Natto fractions | 18 | Rats | 3 weeks | Diet containing 1% cholesterol and with 9% low-molecular-weight viscous substance from natto (LMWVS) or 9% soybean water extract (SWE) from soybean from natto | Diet with 1% cholesterol | Ani-oxidative effect: Ø Cu/Zn-SOD, Ø CAT, Ø GSH, ↓ TBARS, ↑ Mn-SOD (only LMWVS), ↑ GSH-Px (only SWE) No anti-obesity effect: Ø BW Hypolipidemic effect: Ø TC, Ø LDL, Ø HDL, Ø free cholesterol, Ø FFA, ↓ TG (both fractions) | [110] |
| Natto | 50 | Rats | 4 weeks | Diet with 1% cholesterol and natto powder (750 mg/day or 1500 mg/day) | Diet, diet with 1% cholesterol, diet with 1% cholesterol, and aspirin (100 mg/day) | No anti-obesity effect: Ø BW Hypolipidemic effect: Ø TG, ↓ TC (only group with supplementation in dose 1500 mg/day) | [111] |
| Natto | 30 | Mice | 4 weeks | HFD with natto (2.5% or 5%) or soybean (2.5% or 5%) | Control diet, HFD | Antioxidative effect: ↓ TBARS Anti-obesity effect: ↓ BW (natto 2.5%, natto 5%, soybean 5%), Ø food intake, ↓ total fat tissue Anti-diabetic effect: ↓ glucose (natto 5%, soybean 2.5%, soybean 5%), ↓ insulin (natto 5%), ↓ HOMA-IR (natto 2.5%) Hypolipidemic effect: Ø TG, ↓ TC (natto 2.5%, natto 5%, soybean 2.5%) | [112] |
| Natto | 21 | Mice | 6 weeks | HFD with cooked soybean (15%) or tempeh (15%) | HFD | No anti-obesity effect: Ø BW, Ø visceral fat, Ø food intake Anti-diabetic effect: ↓ glucose (only the tempeh group compared to HFD) Hypolipidemic effect: Ø TC, Ø FFA, Ø HDL, ↓ TG (only tempeh group compared to HFD) | [113] |
| Natto | 11 | Overweight subjects with impaired glucose tolerance (crossover study) | 2 weeks | Breakfast consisting of white rice, natto, okra, and Japanese yam | Breakfast consisted of white rice, boiled soybeans, potatoes, broccoli; baseline | Antioxidative effect: ↓ MDA-LDL (significant between groups) No anti-obesity effect: Ø BW, Ø adiponectin, Ø leptin Anti-diabetic effect: Ø HOMA-IR, Ø OGTT (glucose), Ø HbA1c, Ø 1,5-anhydroglucitol, Ø FBG, Ø FBI, ↓ hyperinsulinemia, ↑ CISI (statistically significant only compared to baseline, no between groups) Hypolipidemic effect: ↓ TC, ↓ LDL, Ø TG, Ø HDL | [114] |
| Kochujang (KJ) | 30 | Mice | 12 weeks | HFD with KJ (22%) | Normal diet, HFD | Anti-obesity effect: ↓ BW, ↓ epididymal fat, ↓ back fat, ↑ food intake Anti-diabetic effect: ↓ glucose Hypolipidemic effect: ↓ TC, ↓ LDL, Ø HDL | [115] |
| Kochujang | 60 | 90% of pancreatectomized diabetic rats | 8 weeks | HFD with KJ (5%) fermented in a traditional method or a modern method (with A. sojae, B. subtilis) | Diabetic rats, non-diabetic rats | Anti-obesity effect: ↓ epididymal fat, ↓ leptin, ↑ caloric intake (both groups) Anti-diabetic effect: ↓ glucose, ↓ insulin, improved OGTT (both groups) | [116] |
| Kochujang | 26 | Hyperlipidemic subjects | 12 weeks | Dried KJ fermented with A. oryzae (34.5 g/day) | Placebo, baseline | Hypolipidemic effect: ↓ TC (both compared to baseline and placebo), ↓ LDL (only compared to baseline), Ø HDL, Ø TG | [57] |
| Kochujang | 53 | Overweight or obese adults with BMI ≥ 23 kg/m2 or WHR ≥ 0.90 for males or ≥0.85 for females | 12 weeks | Dried KJ (32 g/day) | Placebo, baseline | Antioxidant effect: Ø ORAC, Ø GSH, ↓ TRAP (compared to baseline), Ø SOD, ↓ CAT No anti-obesity effect: Ø BMI, Ø WHR, Ø WC, Ø visceral fat, Ø subcutaneous fat No anti-diabetic effect: Ø glucose, Ø insulin, Ø HOMA-IR, Ø HbA1c Hypolipidemic effect: Ø TC, Ø LDL, Ø HDL, Ø FFA, ↓ TG (compared to placebo), ↓ ApoA1, ↓ApoB (compared to baseline) No hypotensive effect: Ø SBP, Ø DBP | [117] |
| Kochujang | 48 | Overweight adults with BMI ≥ 23 kg/m2 | 6 weeks | Freeze-dried KJ (19 g/day): traditional with a high content of beneficial microorganisms (HTK), or traditional containing a low content of beneficial microorganisms (LTK), or a commercial KJ (CK) | Baseline | No anti-inflammatory effect: Ø hs-CRP Anti-obesity effect: ↓ hip circumference, ↓WHR (only CK group), ↓ visceral fat (only HTK group), ↓ WC (HTK and CK groups) No anti-diabetic effect: Ø adiponectin, Ø leptin, Ø subcutaneous fat, Ø BMI, Ø BW, Ø body fat (kg), body fat (%), Ø glucose Hypolipidemic effect: ↓ TC, ↓ LDL, ↓ TG, ↓ HDL (significant only for HTK) | [118] |
| Doenjang (DJ) | 24 | Mice | 8 weeks | HFD with DJ (amount not given) | Normal diet, HFD | Anti-inflammatory effect: ↓ TNF-α Anti-obesity effect: ↓ BW, ↓ food efficiency ratio, Ø food intake, Ø epididymal fat, Ø leptin, Ø adiponectin Hypolipidemic effect: ↓ TG, ↓ FFA, Ø TC, Ø HDL | [119] |
| Doenjang | 47 | Mice | 11 weeks | HFD with steamed soybeans (11.7%) or DJ (14.4%) | Low-fat diet, HFD | Anti-obesity effect: ↓ BW, ↓ epididymal fat, ↓ leptin (compared to HFD, only for DJ group, not in soybean group), Ø adipocyte size | [60] |
| Doenjang | 28 | Mice | 14 weeks | HFD with short-term fermented soybean paste with starter (A. oryzae) (SFSP) or long-term fermented soybean paste (LFSP) | Normal diet, HFD | Anti-obesity effect: ↓ BW, ↓ subcutaneous fat (only LFSP group) Anti-diabetic effect: ↑ insulin sensitivity in liver and skeletal muscle (only LFSP group), Ø insulin Hypolipidemic effect: ↓ TG (only LFSP group), Ø TC | [120] |
| Doenjang | 18 | Rats | 8 weeks | High-salt diet (8% NaCl) with freeze-dried DJ (water solution) | Normal diet, high-salt diet (8% NaCl) | No anti-obesity effect: Ø BW Hypotensive effect: ↓ SBP, ↓ serum renin | [55] |
| Doenjang | 24 | Rats | 13 weeks | HFD with DJ (containing 8% of salt) | Normal diet and HFD, and HFD with 8% salt | Anti-obesity effect: ↓ BW, ↓ fat weight/BW, ↓ adipocyte size Anti-diabetic effect: ↓ glucose (lower than both the normal diet and HFD group) Hypotensive effect: ↓ SBP, ↓ serum aldosterone, Ø serum renin, Ø serum angiotensin II | [121] |
| Doenjang | 51 | Adults with BMI ≥ 23 kg/m2 or WHR ≥ 0.90 for males or ≥0.85 for females | 12 weeks | Dried DJ (9.9 g/day) | Placebo and baseline | Anti-obesity effect: ↓ BW, ↓ body fat (kg), ↓ body fat (%), visceral fat (cm2) (compared both to baseline and placebo), ↓ WHR, ↓ total fat (cm2), ↓ subcutaneous fat (compared only to baseline) Hypolipidemic effect: Ø TG, Ø HDL, Ø FFA ↓ TC, ↓ LDL, ↓ ApoA1, ↓ ApoB (compared only to baseline) | [122] |
| Conclusions: Fermented caper fruits, kimchi, and DJ are fermented products that may be beneficial in reducing body weight. CGJ and natto appear to be the most well-studied products and are considered potential superfoods for patients with diabetes. Consumption of CGJ has been shown to reduce blood glucose levels, improve OGTT results, and positively affect β-cell function. Tempeh consumption has been associated with reductions in blood glucose levels and HbA1c in both human and animal models. The lipid profile may be improved following the consumption of fermented soybean products, including tempeh, natto, and KJ. To the best of our knowledge, there are no studies evaluating the effects of table olives and sauerkraut consumption on metabolic parameters in either animal or human models. | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernacka, K.; Sozański, T.; Kucharska, A.Z. Fermented Fruits, Vegetables, and Legumes in Metabolic Syndrome: From Traditional Use to Functional Foods and Medical Applications. Nutrients 2025, 17, 1989. https://doi.org/10.3390/nu17121989
Bernacka K, Sozański T, Kucharska AZ. Fermented Fruits, Vegetables, and Legumes in Metabolic Syndrome: From Traditional Use to Functional Foods and Medical Applications. Nutrients. 2025; 17(12):1989. https://doi.org/10.3390/nu17121989
Chicago/Turabian StyleBernacka, Karolina, Tomasz Sozański, and Alicja Z. Kucharska. 2025. "Fermented Fruits, Vegetables, and Legumes in Metabolic Syndrome: From Traditional Use to Functional Foods and Medical Applications" Nutrients 17, no. 12: 1989. https://doi.org/10.3390/nu17121989
APA StyleBernacka, K., Sozański, T., & Kucharska, A. Z. (2025). Fermented Fruits, Vegetables, and Legumes in Metabolic Syndrome: From Traditional Use to Functional Foods and Medical Applications. Nutrients, 17(12), 1989. https://doi.org/10.3390/nu17121989








