The Role of Vitamin D in Inflammatory Bowel Diseases: From Deficiency to Targeted Therapeutics and Precise Nutrition Strategies
Abstract
1. Introduction
2. Materials and Methods
3. Vitamin D: Metabolism and Role in Inflammatory Bowel Diseases
3.1. Vitamin D: Metabolism, Biological Functions, and Its Potential Role in IBD Pathogenesis
3.2. The Role of Vitamin D in Intestinal Epithelial Barrier Integrity in IBD
3.3. The Role of Vitamin D on Microbial Dysbiosis in IBD
3.4. The Role of Vitamin D on the Immune System in IBD
4. Vitamin D Deficiency in Patients with IBD: Prevalence and Predictors
5. Clinical Impact of Vitamin D Deficiency in IBD Patients
5.1. The Role of Vitamin D in IBD Onset
5.2. The Role of Vitamin D on IBD Outcomes
5.3. The Role of Vitamin D in Patients with IBD Treated with Advanced Therapy
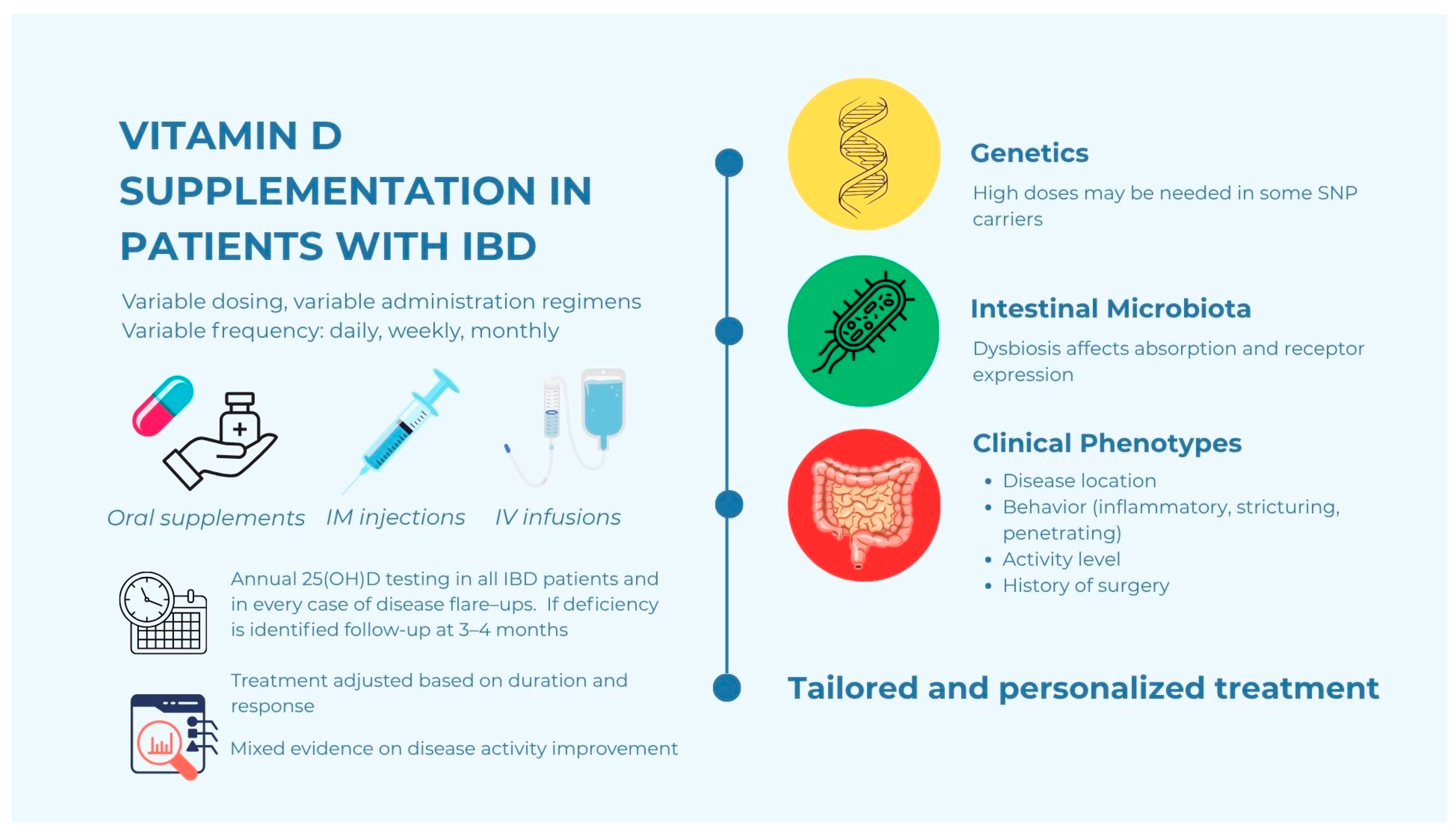
6. Strategies for Vitamin D Supplementation in IBD Patients
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| IBD | Inflammatory Bowel Disease |
| CD | Crohn’s Disease |
| UC | Ulcerative Colitis |
| 25(OH)D | 25-hydroxyvitamin D |
| VDR | Vitamin D Receptor |
| ZO | Zonula Occludens |
| BMI | Body Mass Index |
| IPAA | Ileal pouch-anal anastomosis |
| RCTs | Randomized Controlled Trials |
| HBI | Harvey-Bradshaw Index |
| CRP | C-reactive protein |
| hs-CRP | high-sensitivity CRP |
| FC | Fecal Calprotectin |
| IFX | Infliximab |
| SNPs | Single nucleotide polymorphisms |
| ADA | Adalimumab |
| VDZ | Vedolizumab |
| UST | Ustekinumab |
References
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and Pathogenesis of Inflammatory Bowel Disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Jin, Y.; Shao, X.; Xu, Y.; Ma, G.; Jiang, Y.; Xu, Y.; Jiang, Y.; Hu, D. Global, Regional, and National Burden of Inflammatory Bowel Disease, 1990–2021: Insights from the Global Burden of Disease 2021. Int. J. Color. Dis. 2024, 39, 139. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Munsick, C.; Bemiss, C.; Mahon, B.D. Biochemical and Molecular Action of Nutrients 1,25-Dihydroxycholecalciferol Prevents and Ameliorates Symptoms of Experimental Murine Inflammatory Bowel Disease 1. J. Nutr. 2000, 130, 2648–2652. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, G.; De Gregorio, C.; Palumbo, M.; Nunnari, G.; Malaguarnera, L. Immuno-Modulatory Effects of Vitamin D3 in Human Monocyte and Macrophages. Cell. Immunol. 2012, 280, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Meeker, S.; Seamons, A.; Maggio-Price, L.; Paik, J. Protective Links between Vitamin D, Inflammatory Bowel Disease and Colon Cancer. World J. Gastroenterol. 2016, 22, 933–948. [Google Scholar] [CrossRef]
- Capurso, G.; Tacelli, M.; Vanella, G.; Ponz de Leon Pisani, R.; Dell’Anna, G.; Abati, M.; Mele, R.; Lauri, G.; Panaitescu, A.; Nunziata, R.; et al. Managing Complications of Chronic Pancreatitis: A Guide for the Gastroenterologist. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 1267–1283. [Google Scholar] [CrossRef]
- Holmes, E.A.; Rodney Harris, R.M.; Lucas, R.M. Low Sun Exposure and Vitamin D Deficiency as Risk Factors for Inflammatory Bowel Disease, With a Focus on Childhood Onset. Photochem. Photobiol. 2019, 95, 105–118. [Google Scholar] [CrossRef]
- Kim, K.B.; Kim, H.W.; Lee, J.S.; Yoon, S.M. Inflammatory Bowel Disease and Vitamin D. Korean J. Gastroenterol. 2020, 76, 275–281. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin d Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Infantino, C.; Francavilla, R.; Vella, A.; Cenni, S.; Principi, N.; Strisciuglio, C.; Esposito, S. Role of Vitamin D in Celiac Disease and Inflammatory Bowel Diseases. Nutrients 2022, 14, 5154. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin d Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 362. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory Bowel Disease: Clinical Aspects and Established and Evolving Therapies. The Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Alhassan Mohammed, H.; Mirshafiey, A.; Vahedi, H.; Hemmasi, G.; Moussavi Nasl Khameneh, A.; Parastouei, K.; Saboor-Yaraghi, A.A. Immunoregulation of Inflammatory and Inhibitory Cytokines by Vitamin D3 in Patients with Inflammatory Bowel Diseases. Scand. J. Immunol. 2017, 85, 386–394. [Google Scholar] [CrossRef]
- Toruner, M.; Unal, N.G. Epigenetics of Inflammatory Bowel Diseases. Turk. J. Gastroenterol. 2023, 34, 437–448. [Google Scholar] [CrossRef]
- Tangestani, H.; Boroujeni, H.K.; Djafarian, K.; Emamat, H.; Shab-Bidar, S. Vitamin D and The Gut Microbiota: A Narrative Literature Review. Clin. Nutr. Res. 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Lu, R.; Wu, S.; Chatterjee, I.; Zhou, D.; Xia, Y.; Sun, J. Vitamin D Receptor Protects Against Dysbiosis and Tumorigenesis via the JAK/STAT Pathway in Intestine. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 729–746. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, T.; Shi, Y.; Tian, F.; Hu, H.; Deb, D.K.; Chen, Y.; Bissonnette, M.; Li, Y.C. Gut Epithelial Vitamin D Receptor Regulates Microbiota-Dependent Mucosal Inflammation by Suppressing Intestinal Epithelial Cell Apoptosis. Endocrinology 2018, 159, 967–979. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.Y.; Liu, H.H.; Yin, X.D.; Cao, L.T.; Xu, J.H.; Li, X.M.; Ye, D.Q.; Wang, J. Associations of Vitamin D Receptor Single Nucleotide Polymorphisms with Susceptibility to Systemic Sclerosis. Arch. Med. Res. 2019, 50, 368–376. [Google Scholar] [CrossRef]
- Gisbert-Ferrándiz, L.; Salvador, P.; Ortiz-Masiá, D.; Macías-Ceja, D.C.; Orden, S.; Esplugues, J.V.; Calatayud, S.; Hinojosa, J.; Barrachina, M.D.; Hernández, C. A Single Nucleotide Polymorphism in the Vitamin D Receptor Gene Is Associated with Decreased Levels of the Protein and a Penetrating Pattern in Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Tizaoui, K.; Hamzaoui, K. Association between VDR Polymorphisms and Rheumatoid Arthritis Disease: Systematic Review and Updated Meta-Analysis of Case-Control Studies. Immunobiology 2015, 220, 807–816. [Google Scholar] [CrossRef]
- Tomaszewska, W.M.; Siudziński, P.; Łyko, M.; Skoczylas, A.; Kurasz, J.; Maj, W.; Podlasiewicz, W.; Pala, K.; Dudziak, P.; Nowak, A.; et al. The Role of Vitamin D in the Pathomechanism of Inflammatory Bowel Disease (IBD) and Its Therapeutic Implications–A Literature Review. J. Educ. Health Sport 2025, 77, 56782. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.A.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Prevention and Management of Osteoporosis; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; ISBN 9241209216. [Google Scholar]
- Melamed, M.L.; Michos, E.D.; Post, W.; Astor, B. 25-Hydroxyvitamin D Levels and the Risk of Mortality in the General Population. Arch. Intern. Med. 2008, 168, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Durup, D.; Jørgensen, H.L.; Christensen, J.; Schwarz, P.; Heegaard, A.M.; Lind, B. A Reverse J-Shaped Association of All-Cause Mortality with Serum 25-Hydroxyvitamin D in General Practice: The CopD Study. J. Clin. Endocrinol. Metab. 2012, 97, 2644–2652. [Google Scholar] [CrossRef]
- Sadeghian, M.; Saneei, P.; Siassi, F.; Esmaillzadeh, A. Vitamin D Status in Relation to Crohn’s Disease: Meta-Analysis of Observational Studies. Nutrition 2016, 32, 505–514. [Google Scholar] [CrossRef]
- Fatahi, S.; Alyahyawi, N.; Albadawi, N.; Mardali, F.; Dara, N.; Sohouli, M.H.; Prabahar, K.; Rohani, P.; Koushki, N.; Sayyari, A.; et al. The Association between Vitamin D Status and Inflammatory Bowel Disease among Children and Adolescents: A Systematic Review and Meta-Analysis. Front. Nutr. 2023, 9, 1007725. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef]
- Chatu, S.; Chhaya, V.; Holmes, R.; Neild, P.; Kang, J.-Y.; Pollok, R.C.; Poullis, A. Factors Associated with Vitamin D Deficiency in a Multicultural Inflammatory Bowel Disease Cohort. Frontline Gastroenterol. 2013, 4, 51–56. [Google Scholar] [CrossRef]
- Pallav, K.; Riche, D.; May, W.L.; Sanchez, P.; Gupta, N.K. Predictors of Vitamin D Deficiency in Inflammatory Bowel Disease and Health: A Mississippi Perspective Retrospective Study. World J. Gastroenterol. 2017, 23, 638–645. [Google Scholar] [CrossRef]
- Domislović, V.; Vranešić Bender, D.; Barišić, A.; Brinar, M.; Ljubas Kelečić, D.; Rotim, C.; Novosel, M.; Matašin, M.; Krznarić, Ž. High prevalence of untreated and undertreated vitamin d deficiency and insufficiency in patients with inflammatory bowel disease. Acta Clin. Croat. 2020, 59, 109–118. [Google Scholar] [CrossRef]
- Tajika, M.; Matsuura, A.; Nakamura, T.; Suzuki, T.; Sawaki, A.; Kato, T.; Hara, K.; Ookubo, K.; Yamo, K.; Kato, M.; et al. Risk Factors for Vitamin D Deficiency in Patients with Crohn’s Disease. J. Gastroenterol. 2004, 39, 527–533. [Google Scholar] [CrossRef]
- Harries, A.D.; Brown, R.; Heatley, R.V.; Williams, L.A.; Woodhead, S.; Rhodes, J. Vitamin D Status in Crohn’s Disease: Association with Nutrition and Disease Activity. Gut 1985, 26, 1197–1203. [Google Scholar] [CrossRef]
- Gilman, J.; Shanahan, F.; Cashman, K.D. Determinants of Vitamin D Status in Adult Crohn’s Disease Patients, with Particular Emphasis on Supplemental Vitamin D Use. Eur. J. Clin. Nutr. 2006, 60, 889–896. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Bonovas, S. Environmental, Nutritional, and Socioeconomic Determinants of IBD Incidence: A Global Ecological Study. J. Crohns Colitis 2020, 14, 323–331. [Google Scholar] [CrossRef]
- Lim, W.C.; Hanauer, S.B.; Li, Y.C. Mechanisms of Disease: Vitamin D and Inflammatory Bowel Disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 308–315. [Google Scholar] [CrossRef]
- Rizvi, A.; Trivedi, P.; Bar-Mashiah, A.; Plietz, M.; Khaitov, S.; Sylla, P.; Greenstein, A.; Dubinsky, M.C.; Kayal, M. Vitamin D Deficiency Is Common in Patients with Ulcerative Colitis After Total Proctocolectomy with Ileal Pouch Anal Anastomosis. Inflamm. Bowel Dis. 2022, 28, 1924–1926. [Google Scholar] [CrossRef]
- Khanna, R.; Wu, X.; Shen, B. Low Levels of Vitamin D Are Common in Patients with Ileal Pouches Irrespective of Pouch Inflammation. J. Crohns Colitis 2013, 7, 525–533. [Google Scholar] [CrossRef]
- Navaneethan, U.; Venkatesh, P.G.K.; Shen, B. Risks and Benefits of Ileal Pouch-Anal Anastomosis for Ulcerative Colitis. Therapy 2011, 8, 83–99. [Google Scholar] [CrossRef]
- Abitbol, V.; Roux, C.; Guillemant, S.; Valleur, P.; Hautefeuille, P.; Dougados, M.; Couturier, D.; Chaussade, S. Bone Assessment in Patients with Ileal Pouch–Anal Anastomosis for Inflammatory Bowel Disease. Br. J. Surg. 1997, 84, 1551–1554. [Google Scholar] [CrossRef]
- Salemans, J.; Nagengast, F.; Tangerman, A.; Van Schaik, A.; De Haan, A.; Jansen, J. Postprandial Conjugated and Unconjugated Serum Bile Acid Levels after Proctocolectomy with Ileal Pouch-Anal Anastomosis. Scand. J. Gastroenterol. 1993, 28, 786–790. [Google Scholar] [CrossRef]
- Nasmyth, D.; Godwin, P.; Dixon, M.; Williams, N.; Johnston, D. Ileal Ecology after Pouch-Anal Anastomosis or Ileostomy. A Study of Mucosal Morphology, Fecal Bacteriology, Fecal Volatile Fatty Acids, and Their Interrelationship. Gastroenterology 1989, 96, 817–824. [Google Scholar] [CrossRef]
- Mallon, K.; Mcbride, C.; Doherty, P.G.; Burns, D.R. Dietary Risk Factors Associated with Onset of IBD: A Systematic Literature Review and Meta-Analysis. J. Crohns Colitis 2023, 17, i985–i986. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Higuchi, L.M.; Bao, Y.; Korzenik, J.R.; Giovannucci, E.L.; Richter, J.M.; Fuchs, C.S.; Chan, A.T. Higher Predicted Vitamin D Status Is Associated with Reduced Risk of Crohn’s Disease. Gastroenterology 2012, 142, 482–489. [Google Scholar] [CrossRef]
- Gubatan, J.M.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. P787 Vitamin D Status and Clinical Outcomes in Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. J. Crohns Colitis 2019, 13, S515. [Google Scholar] [CrossRef]
- Silva, M.C.; Furlanetto, T.W. Does Serum 25-Hydroxyvitamin D Decrease during Acute-Phase Response? A Systematic Review. Nutr. Res. 2015, 35, 91–96. [Google Scholar] [CrossRef]
- López-Muñoz, P.; Beltrán, B.; Sáez-González, E.; Alba, A.; Nos, P.; Iborra, M. Influence of Vitamin D Deficiency on Inflammatory Markers and Clinical Disease Activity in IBD Patients. Nutrients 2019, 11, 1059. [Google Scholar] [CrossRef]
- Caviezel, D.; Maissen, S.; Niess, J.H.; Kiss, C.; Hruz, P. High Prevalence of Vitamin D Deficiency among Patients with Inflammatory Bowel Disease. Inflamm. Intest. Dis. 2018, 2, 200–210. [Google Scholar] [CrossRef]
- Garg, M.; Rosella, O.; Lubel, J.S.; Gibson, P.R. Association of Circulating Vitamin D Concentrations with Intestinal but Not Systemic Inflammation in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 2634–2643. [Google Scholar] [CrossRef]
- Guzman, Y.; Samson, O.; Segal, J.P.; Limdi, J.K.; Hayee, B. P700 Effect of Vitamin D in Disease Activity Score and Clinical Relapse in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Crohns Colitis 2020, 14, S567–S568. [Google Scholar] [CrossRef]
- Gubatan, J.; Mitsuhashi, S.; Zenlea, T.; Rosenberg, L.; Robson, S.; Moss, A.C. Low Serum Vitamin D During Remission Increases Risk of Clinical Relapse in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2017, 15, 240–246.e1. [Google Scholar] [CrossRef]
- Valvano, M.; Magistroni, M.; Cesaro, N.; Carlino, G.; Monaco, S.; Fabiani, S.; Vinci, A.; Vernia, F.; Viscido, A.; Latella, G. Effectiveness of Vitamin D Supplementation on Disease Course in Inflammatory Bowel Disease Patients: Systematic Review With Meta-Analysis. Inflamm. Bowel Dis. 2024, 30, 281–291. [Google Scholar] [CrossRef]
- Pappa, H.M.; Langereis, E.J.; Grand, R.J.; Gordon, C.M. Prevalence and Risk Factors for Hypovitaminosis D in Young Patients with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 361–364. [Google Scholar] [CrossRef]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin d’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef]
- Ardesia, M.; Ferlazzo, G.; Fries, W.; Šebeková, K. Vitamin D and Inflammatory Bowel Disease. BioMed Res. Int. 2015, 2015, 470805. [Google Scholar] [CrossRef]
- Walker, C.; Stacey, W.; Ramkalawan, K.M.; Kafienah, Y.; Connaughton, R.; Breslin, N.; McNamara, D.; O’Donnell, S.; Ryan, B.; O’Connor, A. P165 Vitamin D Deficiency Is Associated with Poor Sleep Quality in Patients with Crohn’s Disease. J. Crohns Colitis 2023, 17, i324. [Google Scholar] [CrossRef]
- Sina, M.; Djegsi, A.; Pemaj, X.; Telaku, S.; Babameto, A.; Prifti, S. P683 Risk Factors for Fatigue in IBD Patients. J. Crohns Colitis 2023, 17, i814–i815. [Google Scholar] [CrossRef]
- Frigstad, S.O.; Høivik, M.L.; Jahnsen, J.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, Ø.; Bernklev, T.; Moum, B.; et al. Fatigue Is Not Associated with Vitamin D Deficiency in Inflammatory Bowel Disease Patients. World J. Gastroenterol. 2018, 24, 3293–3301. [Google Scholar] [CrossRef]
- Di Molfetta, I.V.; Bordoni, L.; Gabbianelli, R.; Sagratini, G.; Alessandroni, L. Vitamin D and Its Role on the Fatigue Mitigation: A Narrative Review. Nutrients 2024, 16, 221. [Google Scholar] [CrossRef]
- Winter, R.W.; Collins, E.; Cao, B.; Carrellas, M.; Crowell, A.M.; Korzenik, J.R. Higher 25-Hydroxyvitamin D Levels Are Associated with Greater Odds of Remission with Anti-Tumour Necrosis Factor-α Medications among Patients with Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2017, 45, 653–659. [Google Scholar] [CrossRef]
- Zator, Z.A.; Cantu, S.M.; Konijeti, G.G.; Nguyen, D.D.; Sauk, J.; Yajnik, V.; Ananthakrishnan, A.N. Pretreatment 25-Hydroxyvitamin D Levels and Durability of Anti-Tumor Necrosis Factor-α Therapy in Inflammatory Bowel Diseases. J. Parenter. Enter. Nutr. 2014, 38, 385–391. [Google Scholar] [CrossRef]
- Xia, S.L.; Min, Q.J.; Shao, X.X.; Lin, D.P.; Ma, G.L.; Wu, H.; Cao, S.G.; Jiang, Y. Influence of Vitamin D3 Supplementation on Infliximab Effectiveness in Chinese Patients with Crohn’s Disease: A Retrospective Cohort Study. Front. Nutr. 2021, 8, 739285. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chanchlani, N.; Smith, R.; Roberts, C.; Nice, R.; Mcdonald, T.J.; Hamilton, B.; Bewshea, C.; Kennedy, N.A.; Goodhand, J.R.; et al. P662 Pre-Treatment Vitamin D Concentrations Do Not Predict Therapeutic Outcome to Anti-TNF Therapies in Biologic-Naïve Patients with Active Luminal Crohn’s Disease. J. Crohns Colitis 2023, 17, i791–i793. [Google Scholar] [CrossRef]
- Abraham, B.P.; Fan, C.; Thurston, T.; Moskow, J.; Malaty, H.M. The Role of Vitamin D in Patients with Inflammatory Bowel Disease Treated with Vedolizumab. Nutrients 2023, 15, 4847. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Rubin, S.J.S.; Bai, L.; Haileselassie, Y.; Levitte, S.; Balabanis, T.; Patel, A.; Sharma, A.; Sinha, S.R.; Habtezion, A. Vitamin D Is Associated with A4β7+ Immunophenotypes and Predicts Vedolizumab Therapy Failure in Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 1980–1990. [Google Scholar] [CrossRef]
- De Vita, F.; Ribaldone, G.R.; D’Avolio, A.; Infusino, V.; Antonucci, M.; Caviglia, G.P.; Armandi, A.; Ceccarelli, L.; Costa, F.; Bottari, A.; et al. P1143 of Vitamin D Metabolism Gene Polymorphisms on Therapeutic Efficacy of Vedolizumab and ustekinumab in Inflammatory Bowel Disease. Heliyon 2024, 10, e27700. [Google Scholar] [CrossRef]
- Hlavaty, T.; Krajcovicova, A.; Payer, J. Vitamin D Therapy in Inflammatory Bowel Diseases: Who, in What Form, and How Much? J. Crohns Colitis 2015, 9, 198–209. [Google Scholar] [CrossRef]
- Wylon, K.; Drozdenko, G.; Krannich, A.; Heine, G.; Dölle, S.; Worm, M. Pharmacokinetic Evaluation of a Single Intramuscular High Dose versus an Oral Long-Term Supplementation of Cholecalciferol. PLoS ONE 2017, 12, e0169620. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; John Orav, E.; Staehelin, H.B.; Meyer, O.W.; Theiler, R.; Dick, W.; Willett, W.C.; Egli, A. Monthly High-Dose Vitamin D Treatment for the Prevention of Functional Decline a Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Guirguis-Blake, J.M.; Michael, Y.L.; Perdue, L.A.; Coppola, E.L.; Beil, T.L. Interventions to Prevent Falls in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA-J. Am. Med. Assoc. 2018, 319, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Stuart, A.L.; Elizabeth Williamson, B.J.; Simpson, J.A.; Kotowicz, M.A.; Doris Young, F.; Geoffrey Nicholson, F.C. Annual High-Dose Oral Vitamin D and Falls and Fractures in Older Women A Randomized Controlled Trial. Jama 2010, 303, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Vitamin D and the Parenteral Nutrition Patient. Gastroenterology 2009, 137, S79–S91. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R. Risk Assessment for Vitamin. Am. J. Clin. Nutr. 2007, 85, 6–18. [Google Scholar] [CrossRef]
- Billington, E.O.; Burt, L.A.; Rose, M.S.; Davison, E.M.; Gaudet, S.; Kan, M.; Boyd, S.K.; Hanley, D.A. Safety of High-Dose Vitamin D Supplementation: Secondary Analysis of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2020, 105, 1261–1273. [Google Scholar] [CrossRef]
- Webb, A.; DeCosta, B.; Holick, M. Sunlight Regulates the Cutaneous Production of Vitamin D3 by Causing Its Photodegradation. J. Clin. Endocrinol. Metab. 1989, 68, 882–887. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Raftery, T.; Martineau, A.R.; Greiller, C.L.; Ghosh, S.; McNamara, D.; Bennett, K.; Meddings, J.; O’Sullivan, M. Effects of Vitamin D Supplementation on Intestinal Permeability, Cathelicidin and Disease Markers in Crohn’s Disease: Results from a Randomised Double-Blind Placebo-Controlled Study. United Eur. Gastroenterol. J. 2015, 3, 294–302. [Google Scholar] [CrossRef]
- Bayoumi, M.; Berger, D.; Golden, R.; Fogg, L.; Keshavarzian, A.; Swanson, G. Initial Vitamin D Concentration Correlates with Disease Markers in Inflammatory Bowel Disease: Presidential Poster: 1863. Off. J. Am. Coll. Gastroenterol. 2015, 110, S791–S792. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Rejnmark, L.; Moss, A.C. Role of Vitamin D in the Natural History of Inflammatory Bowel Disease. J. Crohns Colitis 2018, 12, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Sohouli, M.H.; Farahmand, F.; Alimadadi, H.; Rahmani, P.; Motamed, F.; da Silva Magalhães, E.I.; Rohani, P. Vitamin D Therapy in Pediatric Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. World J. Pediatr. 2023, 19, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, R.; Ghorbaninejad, P.; Eslahi, M.; Sheikhi, L.; Abbasi, F.; Hasanzadeh, M.; Mohammadpour, S.; Milajerdi, A. Effects of Vitamin D Supplementation on Serum 25-Hydroxy Cholecalciferol in Inflammatory Bowel Diseases: A Meta-Analysis of Randomized Clinical Trials. Int. J. Prev. Med. 2024, 15, 65. [Google Scholar] [CrossRef]
- Yang, L.; Weaver, V.; Smith, J.P.; Bingaman, S.; Hartman, T.J.; Cantorna, M.T. Therapeutic Effect of Vitamin d Supplementation in a Pilot Study of Crohn’s Patients. Clin. Transl. Gastroenterol. 2013, 4, e33. [Google Scholar] [CrossRef]
- Sharifi, A.; Hosseinzadeh-Attar, M.J.; Vahedi, H.; Nedjat, S. A Randomized Controlled Trial on the Effect of Vitamin D3 on Inflammation and Cathelicidin Gene Expression in Ulcerative Colitis Patients. Saudi J. Gastroenterol. 2016, 22, 316–323. [Google Scholar] [CrossRef]
- Guzman-Prado, Y.; Samson, O.; Segal, J.P.; Limdi, J.K.; Hayee, B. Vitamin D Therapy in Adults with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, 1819–1830. [Google Scholar] [CrossRef]
- Garg, M.; Hendy, P.; Ding, J.N.; Shaw, S.; Hold, G.; Hart, A. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J. Crohns Colitis 2018, 12, 963–972. [Google Scholar] [CrossRef]
- Shirwaikar Thomas, A.; Criss, Z.K.; Shroyer, N.F.; Abraham, B.P. Vitamin D Receptor Gene Single Nucleotide Polymorphisms and Association with Vitamin D Levels and Endoscopic Disease Activity in Inflammatory Bowel Disease Patients: A Pilot Study. Inflamm. Bowel Dis. 2021, 27, 1263–1269. [Google Scholar] [CrossRef]
- Wang, J.; Mei, L.; Hao, Y.; Xu, Y.; Yang, Q.; Dai, Z.; Yang, Y.; Wu, Z.; Ji, Y. Contemporary Perspectives on the Role of Vitamin D in Enhancing Gut Health and Its Implications for Preventing and Managing Intestinal Diseases. Nutrients 2024, 16, 2352. [Google Scholar] [CrossRef]

| Pathogenic AXIS | Vitamin D Role | Consequence of Deficiency/ VDR Impairment |
|---|---|---|
| Epithelial Barrier Integrity | ↑ Tight junction proteins (claudins, occludins, ZO) via VDR signaling | ↓ Tight junction expression → ↑ gut permeability (“leaky gut”) → immune activation |
| Zonulin Regulation | ↓ Zonulin release → maintenance of tight junctions | ↑ Zonulin → tight junction disassembly → barrier dysfunction |
| Mucus Production | ↑ Goblet cell activity → ↑ mucosal layer protection | ↓ Mucus layer → increased exposure to luminal microbes |
| Microbial Composition | Promotes growth of SCFA-producing commensals (e.g., Faecalibacterium) | Dysbiosis: ↓ beneficial species, ↑ pathobionts (Enterobacteriaceae, Fusobacterium) |
| Antimicrobial Peptides | ↑ Cathelicidin, β-defensins | ↓ Antimicrobial defense → ↑ microbial translocation |
| Immune Modulation | Shifts from Th1/Th17 → Tregs; ↑ IL-10, TGF-β; ↓ TNF-α, IL-6, IL-17, IFN-γ | ↑ Pro-inflammatory cytokines and Th1/Th17 dominance → chronic intestinal inflammation |
| Risk Factor | Notes |
|---|---|
| High BMI (>30 kg/m2) | Associated with lower bioavailability of vitamin D |
| Non-Caucasian ethnicity | Likely due to reduced cutaneous synthesis from increased melanin |
| Longer disease duration | Chronic inflammation may impact nutrient absorption |
| Increased disease activity | Higher inflammatory burden linked to lower vitamin D levels |
| Smoking | Potential negative effect on nutrient absorption |
| Small bowel involvement (CD) | Reduces vitamin D absorption |
| Nutritional status & dietary restrictions | Reduced intake of vitamin D-rich foods |
| Seasonal variation & reduced sun exposure | Especially during disease flares or in sedentary individuals |
| Latitude | Higher incidence of IBD in high-latitude countries where sun exposure is reduced |
| Pharmacologic factors | Impairs absorption of fat-soluble vitamins (e.g., cholestyramine) |
| IBD-related surgery (e.g., ileum resection, IPAA) | Malabsorption risk persists even in absence of active disease or pouchitis |
| Author | Study Type | Therapy | Population | Key Findings |
|---|---|---|---|---|
| Winter et al. (2017) [64] | Retrospective | Anti-TNF (IFX, ADA) | 173 IBD patients | Low pre-treatment vitamin D levels associated with reduced likelihood of remission at 3 months after starting anti-TNF therapy. |
| Zator et al. (2014) [65] | Retrospective | Anti-TNF (IFX, ADA) | 101 IBD patients | Patients with insufficient vitamin D were more likely to discontinue anti-TNF therapy prematurely. |
| Xia et al. (2021) [66] | Retrospective cohort | IFX | Biologic-naïve Chinese CD patients | Vitamin D3 supplementation (125 IU/day) improved clinical remission at 54 weeks, especially in vitamin D-deficient patients. |
| Lin et al. (2023) [67] | Large prospective cohort | IFX, ADA | >1100 patients with luminal CD | Baseline vitamin D status not predictive of primary non-response to anti-TNF at week 14 or non-remission at week 54. |
| Abraham et al. (2023) [68] | Retrospective | VDZ | 88 IBD patients (44 UC, 44 CD) | Vitamin D ≥ 30 ng/mL predicted significant endoscopic improvement in UC patients and lower CRP levels in CD patients. |
| Gubatan et al. (2021) [69] | Translational + transcriptomic | VDZ | 48 VDZ-naïve IBD patients | Low serum 25[OH]D associated with α4β7+ immunophenotypes; vitamin D < 25 ng/mL linked to higher risk of primary non-response and treatment failure at 1 year. |
| De Vita et al. (2024) [70] | Prospective + genotyping | UST, VDZ | 103 IBD patients (67 CD, 36 UC) | SNPs in vitamin D-related genes (e.g., GC, CYP24A1) predicted differential responses to UST and VDZ; specific genotypes linked to poorer outcomes with VDZ. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Anna, G.; Fanizzi, F.; Zilli, A.; Furfaro, F.; Solitano, V.; Parigi, T.L.; Ciliberto, A.; Fanizza, J.; Mandarino, F.V.; Fuccio, L.; et al. The Role of Vitamin D in Inflammatory Bowel Diseases: From Deficiency to Targeted Therapeutics and Precise Nutrition Strategies. Nutrients 2025, 17, 2167. https://doi.org/10.3390/nu17132167
Dell’Anna G, Fanizzi F, Zilli A, Furfaro F, Solitano V, Parigi TL, Ciliberto A, Fanizza J, Mandarino FV, Fuccio L, et al. The Role of Vitamin D in Inflammatory Bowel Diseases: From Deficiency to Targeted Therapeutics and Precise Nutrition Strategies. Nutrients. 2025; 17(13):2167. https://doi.org/10.3390/nu17132167
Chicago/Turabian StyleDell’Anna, Giuseppe, Fabrizio Fanizzi, Alessandra Zilli, Federica Furfaro, Virginia Solitano, Tommaso Lorenzo Parigi, Ambra Ciliberto, Jacopo Fanizza, Francesco Vito Mandarino, Lorenzo Fuccio, and et al. 2025. "The Role of Vitamin D in Inflammatory Bowel Diseases: From Deficiency to Targeted Therapeutics and Precise Nutrition Strategies" Nutrients 17, no. 13: 2167. https://doi.org/10.3390/nu17132167
APA StyleDell’Anna, G., Fanizzi, F., Zilli, A., Furfaro, F., Solitano, V., Parigi, T. L., Ciliberto, A., Fanizza, J., Mandarino, F. V., Fuccio, L., Facciorusso, A., Donatelli, G., Allocca, M., Massironi, S., Annese, V., Peyrin-Biroulet, L., Danese, S., & D’Amico, F. (2025). The Role of Vitamin D in Inflammatory Bowel Diseases: From Deficiency to Targeted Therapeutics and Precise Nutrition Strategies. Nutrients, 17(13), 2167. https://doi.org/10.3390/nu17132167









