Histamine Metabolism in IBD: Towards Precision Nutrition
Abstract
1. Introduction
2. Methods
3. The Complex Interplay Between Histamine and IBD
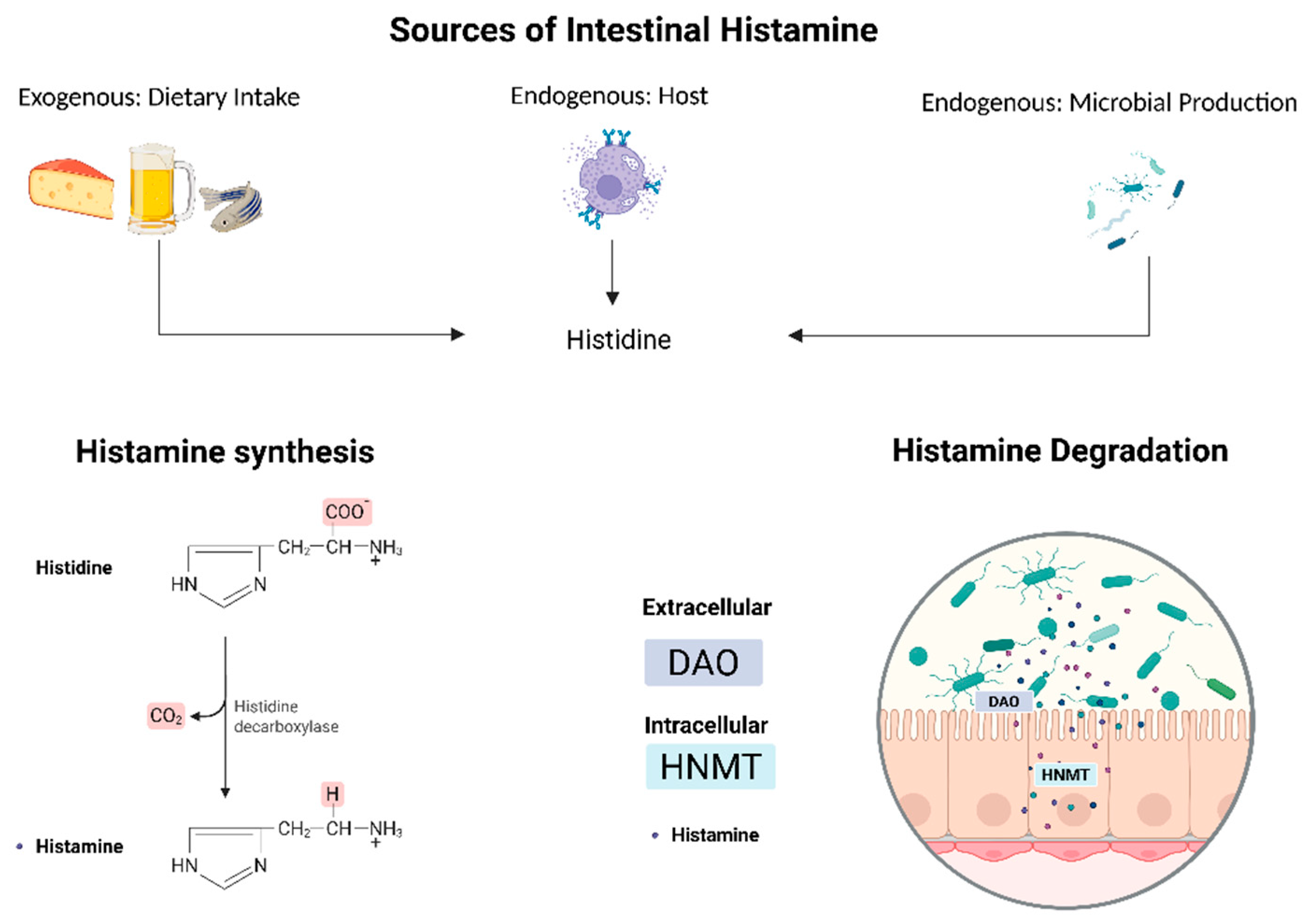
3.1. Endogenous Histamine Dynamics: Production, Degradation, and Enzyme Dysregulation in IBD
3.2. Histamine Receptors and Immune Modulation
4. Endogenous Microbial Factors: Microbial Dysbiosis and Histamine-Producing Bacteria
4.1. Microbial Dysbiosis in IBD
4.2. Histamine-Producing Bacteria in IBD
5. Towards Precision Nutrition in IBD: Low-Histamine Diet and Beyond
5.1. Modulating Exogenous Histamine Levels: Low-Histamine Diet
5.2. Modulation of Endogenous Histamine-Producing Bacteria
5.3. Role of Fiber and Histamine
5.4. Role of Yeast and Salt in IBD: Links to Histamine Metabolism
6. Discussion
7. Limitations
8. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCA | Anti-Saccharomyces cerevisiae Antibodies |
| BCFAs | Branched-Chain Fatty Acids |
| CDAI | Crohn’s Disease Activity Index |
| CDED | Crohn’s Disease Exclusion Diet |
| CD | Crohn’s Disease |
| CTT | Colonic Transit Time |
| DAO | Diamine Oxidase |
| DSS | Dextran Sodium Sulfate |
| EEN | Exclusive Enteral Nutrition |
| GIT | Gastrointestinal Tract |
| H2S | Hydrogen Sulfide |
| HDC | Histidine Decarboxylase |
| HIT | Histamine Intolerance |
| HNMT | Histamine-N-Methyltransferase |
| HRs | Histamine Receptors |
| IBS | Irritable Bowel Syndrome |
| iHMP | Integrative Human Microbiome Project |
| LHD | Low-Histamine Diet |
| MHA | Methylhistamine |
| NH3 | Ammonia |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PLC | Phospholipase C |
| PLP | Pyridoxal-5′-phosphate |
| SCFAs | Short-Chain Fatty Acids |
| SNPs | Single Nucleotide Polymorphisms |
| TLRs | Toll-Like Receptors |
| UC | Ulcerative Colitis |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| VH | Visceral Hyperactivity |
| SIBO | Small Intestinal Bacterial Overgrowth |
References
- Perler, B.K.; Ungaro, R.; Baird, G.; Mallette, M.; Bright, R.; Shah, S.; Shapiro, J.; Sands, B.E. Presenting Symptoms in Inflammatory Bowel Disease: Descriptive Analysis of a Community-Based Inception Cohort. BMC Gastroenterol. 2019, 19, 47. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s Disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Jess, T. Implications of the Changing Epidemiology of Inflammatory Bowel Disease in a Changing World. United Eur. Gastroenterol. J. 2022, 10, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Heller, C.; Moss, A.C.; Rubin, D.T. Overview to Challenges in IBD 2024–2029. Inflamm. Bowel. Dis. 2024, 30, S1–S4. [Google Scholar] [CrossRef]
- Duricova, D.; Burisch, J.; Jess, T.; Gower-Rousseau, C.; Lakatos, P.L. ECCO-EpiCom Age-Related Differences in Presentation and Course of Inflammatory Bowel Disease: An Update on the Population-Based Literature. J. Crohns Colitis 2014, 8, 1351–1361. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Vindigni, S.M.; Zisman, T.L.; Suskind, D.L.; Damman, C.J. The Intestinal Microbiome, Barrier Function, and Immune System in Inflammatory Bowel Disease: A Tripartite Pathophysiological Circuit with Implications for New Therapeutic Directions. Ther. Adv. Gastroenterol. 2016, 9, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Antoni, L.; Nuding, S.; Wehkamp, J.; Stange, E.F. Intestinal Barrier in Inflammatory Bowel Disease. World J. Gastroenterol. 2014, 20, 1165–1179. [Google Scholar] [CrossRef]
- Wang, M.; Shi, J.; Yu, C.; Zhang, X.; Xu, G.; Xu, Z.; Ma, Y. Emerging Strategy towards Mucosal Healing in Inflammatory Bowel Disease: What the Future Holds? Front. Immunol. 2023, 14, 1298186. [Google Scholar] [CrossRef]
- Crohn’s Disease-The Lancet. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)60026-9/fulltext (accessed on 19 June 2025).
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The Gut Microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative Colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kaur, S.; Ali, A.; Siahbalaei, Y.; Ahmad, U.; Nargis, F.; Pandey, A.K.; Singh, B. Association of Diamine Oxidase (DAO) Variants with the Risk for Migraine from North Indian Population. Meta. Gene 2020, 24, 100619. [Google Scholar] [CrossRef]
- Hong, S.M.; Baek, D.H. Diagnostic Procedures for Inflammatory Bowel Disease: Laboratory, Endoscopy, Pathology, Imaging, and Beyond. Diagnostics 2024, 14, 1384. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN Guideline on Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.C.; Opheim, R.; Kristensen, V.A.; Høivik, M.L.; Lund, C.; Aabrekk, T.B.; Johansen, I.; Holten, K.; Strande, V.; Bengtson, M.-B.; et al. Health-Related Quality of Life in Patients with Newly Diagnosed Inflammatory Bowel Disease: An Observational Prospective Cohort Study (IBSEN III). Qual. Life Res. 2023, 32, 2951–2964. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Spinelli, A.; Carvello, M.; Adamina, M.; Panis, Y.; Warusavitarne, J.; Tulchinsky, H.; Bemelman, W.A.; Kotze, P.G.; D’Hoore, A.; Lastikova, L.; et al. Patients’ Perceptions of Surgery for Inflammatory Bowel Disease. Color. Dis. 2021, 23, 2690–2698. [Google Scholar] [CrossRef]
- de Castro, M.M.; Corona, L.P.; Pascoal, L.B.; Miyamoto, J.É.; Ignacio-Souza, L.M.; de Lourdes Setsuko Ayrizono, M.; Torsoni, M.A.; Torsoni, A.S.; Leal, R.F.; Milanski, M. Dietary Patterns Associated to Clinical Aspects in Crohn’s Disease Patients. Sci. Rep. 2020, 10, 7033. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Russell, R.K.; Giachero, F.; Gkikas, K.; Tel, B.; Assa, A.; Bronsky, J.; de Ridder, L.; Hojsak, I.; Jenke, A.; et al. Precision Nutrition in Pediatric IBD: A Position Paper from the ESPGHAN Special Interest Group for Basic Science and Translational Research, the IBD Porto Group, and Allied Health Professionals. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 428–445. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Patel, R.H.; Mohiuddin, S.S. Biochemistry, Histamine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Branco, A.C.C.C.; Yoshikawa, F.S.Y.; Pietrobon, A.J.; Sato, M.N. Role of Histamine in Modulating the Immune Response and Inflammation. Mediat. Inflamm. 2018, 2018, 9524075. [Google Scholar] [CrossRef]
- Dvornikova, K.A.; Platonova, O.N.; Bystrova, E.Y. Inflammatory Bowel Disease: Crosstalk between Histamine, Immunity, and Disease. Int. J. Mol. Sci. 2023, 24, 9937. [Google Scholar] [CrossRef]
- Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD-Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445. [Google Scholar] [CrossRef]
- Parker, A.; Vaux, L.; Patterson, A.M.; Modasia, A.; Muraro, D.; Fletcher, A.G.; Byrne, H.M.; Maini, P.K.; Watson, A.J.M.; Pin, C. Elevated Apoptosis Impairs Epithelial Cell Turnover and Shortens Villi in TNF-Driven Intestinal Inflammation. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Enko, D. Histamine Intolerance Originates in the Gut. Nutrients 2021, 13, 1262. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Liu, Y.; Jia, H. Alterations of Gut Microbiota and Cytokines in Elevated Serum Diamine Oxidase Disorder. Medicine 2022, 101, e31966. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, S.; Imark, C.; Kneubühl, M. Biogenic Amines in Foods: Histamine and Food Processing. Inflamm. Res. 1999, 48, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.d.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- Tuck, C.J.; Biesiekierski, J.R.; Schmid-Grendelmeier, P.; Pohl, D. Food Intolerances. Nutrients 2019, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Schnedl, W.J.; Lackner, S.; Enko, D.; Schenk, M.; Holasek, S.J.; Mangge, H. Evaluation of Symptoms and Symptom Combinations in Histamine Intolerance. Intest. Res. 2019, 17, 427–433. [Google Scholar] [CrossRef]
- Okutan, G.; Ruiz Casares, E.; Perucho Alcalde, T.; Sánchez Niño, G.M.; Penadés, B.F.; Terrén Lora, A.; Torrente Estríngana, L.; López Oliva, S.; San Mauro Martín, I. Prevalence of Genetic Diamine Oxidase (DAO) Deficiency in Female Patients with Fibromyalgia in Spain. Biomedicines 2023, 11, 660. [Google Scholar] [CrossRef]
- Blasco-Fontecilla, H. Personalized Medicine: Unraveling the Potential of Diamine Oxidase Deficiency. J. Clin. Med. 2024, 13, 6797. [Google Scholar] [CrossRef]
- Duelo, A.; Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Ruiz-Casares, E.; Vidal-Carou, M.C.; Latorre-Moratalla, M.L. Pilot Study on the Prevalence of Diamine Oxidase Gene Variants in Patients with Symptoms of Histamine Intolerance. Nutrients 2024, 16, 1142. [Google Scholar] [CrossRef]
- Maintz, L.; Yu, C.-F.; Rodríguez, E.; Baurecht, H.; Bieber, T.; Illig, T.; Weidinger, S.; Novak, N. Association of Single Nucleotide Polymorphisms in the Diamine Oxidase Gene with Diamine Oxidase Serum Activities. Allergy 2011, 66, 893–902. [Google Scholar] [CrossRef]
- Smolinska, S.; Winiarska, E.; Globinska, A.; Jutel, M. Histamine: A Mediator of Intestinal Disorders—A Review. Metabolites 2022, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, M.; Elek, M.; Stark, H. Chemical Probes for Histamine Receptor Subtypes. In The Functional Roles of Histamine Receptors; Yanai, K., Passani, M.B., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 29–76. ISBN 978-3-031-16997-7. [Google Scholar]
- Selective Expression of Histamine Receptors H1R, H2R, and H4R, but Not H3R, in the Human Intestinal Tract|Gut. Available online: https://gut.bmj.com/content/55/4/498.short (accessed on 14 May 2025).
- Smolinska, S.; Jutel, M.; Crameri, R.; O’Mahony, L. Histamine and Gut Mucosal Immune Regulation. Allergy 2014, 69, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Deiteren, A.; Man, J.G.D.; Ruyssers, N.E.; Moreels, T.G.; Pelckmans, P.A.; Winter, B.Y.D. Histamine H4 and H1 Receptors Contribute to Postinflammatory Visceral Hypersensitivity. Gut 2014, 63, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Groeger, D.; Perez, N.R.; Schiavi, E.; Ferstl, R.; Frei, R.; Konieczna, P.; Akdis, C.A.; Jutel, M.; O’Mahony, L. Histamine Receptor 2 Is Required to Suppress Innate Immune Responses to Bacterial Ligands in Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2016, 22, 1575–1586. [Google Scholar] [CrossRef]
- Schirmer, B.; Neumann, D. The Function of the Histamine H4 Receptor in Inflammatory and Inflammation-Associated Diseases of the Gut. Int. J. Mol. Sci. 2021, 22, 6116. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Frei, S.M.; Stevens, R.L. The Multifaceted Mast Cell in Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2014, 20, 2364–2378. [Google Scholar] [CrossRef] [PubMed]
- Development of Dextran Sulphate Sodium-Induced Experimental Colitis Is Suppressed in Genetically Mast Cell-Deficient Ws/Ws Rats|Clinical and Experimental Immunology|Oxford Academic. Available online: https://academic.oup.com/cei/article-abstract/119/2/264/6461750 (accessed on 14 May 2025).
- Zhao, P.; Dong, L.; Luo, J.; Guan, H.; Ma, H.; Wang, X. Possible Role of Mast Cells and Neuropeptides in the Recovery Process of Dextran Sulfate Sodium-Induced Colitis in Rats. Chin. Med. Sci. J. 2013, 28, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Mirzahosseini, A.; Dalmadi, B.; Csutora, P. Histamine Receptor H4 Regulates Mast Cell Degranulation and IgE Induced FcεRI Upregulation in Murine Bone Marrow-Derived Mast Cells. Cell Immunol. 2013, 283, 38–44. [Google Scholar] [CrossRef]
- Zhang, Z.; Kurashima, Y. Two Sides of the Coin: Mast Cells as a Key Regulator of Allergy and Acute/Chronic Inflammation. Cells 2021, 10, 1615. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef]
- Mazzoni, A.; Young, H.A.; Spitzer, J.H.; Visintin, A.; Segal, D.M. Histamine Regulates Cytokine Production in Maturing Dendritic Cells, Resulting in Altered T Cell Polarization. J. Clin. Investig. 2001, 108, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Bene, L.; Sápi, Z.; Bajtai, A.; Buzás, E.; Szentmihályi, A.; Arató, A.; Tulassay, Z.; Falus, A. Partial Protection against Dextran Sodium Sulphate Induced Colitis in Histamine-Deficient, Histidine Decarboxylase Knockout Mice. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 171–176. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.-Q.; Yu, T.-Y.; Yin, Y.-Y.; Liu, Y.; Wang, X.-D.; He, Z.-G.; Yin, L.; Chen, C.-Q.; Li, J.-Y. Mast Cell Tryptase Promotes Inflammatory Bowel Disease–Induced Intestinal Fibrosis. Inflamm. Bowel. Dis. 2021, 27, 242–255. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A Decrease of the Butyrate-Producing Species Roseburia Hominis and Faecalibacterium Prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut Microbiota in the Pathogenesis of Inflammatory Bowel Disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut Microbiota, Inflammatory Bowel Disease and Colorectal Cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Iyer, N.; Corr, S.C. Gut Microbial Metabolite-Mediated Regulation of the Intestinal Barrier in the Pathogenesis of Inflammatory Bowel Disease. Nutrients 2021, 13, 4259. [Google Scholar] [CrossRef] [PubMed]
- Fenneman, A.C.; Weidner, M.; Chen, L.A.; Nieuwdorp, M.; Blaser, M.J. Antibiotics in the Pathogenesis of Diabetes and Inflammatory Diseases of the Gastrointestinal Tract. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 81–100. [Google Scholar] [CrossRef]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Larauche, M.; Duboc, H.; Earle, K.A.; Sonnenburg, E.D.; Ferreyra, J.A.; Higginbottom, S.K.; Million, M.; et al. Complex Interactions Among Diet, Gastrointestinal Transit, and Gut Microbiota in Humanized Mice. Gastroenterology 2013, 144, 967–977. [Google Scholar] [CrossRef]
- Brinck, J.E.; Sinha, A.K.; Laursen, M.F.; Dragsted, L.O.; Raes, J.; Uribe, R.V.; Walter, J.; Roager, H.M.; Licht, T.R. Intestinal pH: A Major Driver of Human Gut Microbiota Composition and Metabolism. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 1–18. [Google Scholar] [CrossRef]
- Procházková, N.; Falony, G.; Dragsted, L.O.; Licht, T.R.; Raes, J.; Roager, H.M. Advancing Human Gut Microbiota Research by Considering Gut Transit Time. Gut 2023, 72, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Silva, S.; Sabino, J.; Valles-Colomer, M.; Falony, G.; Kathagen, G.; Caenepeel, C.; Cleynen, I.; van der Merwe, S.; Vermeire, S.; Raes, J. Quantitative Microbiome Profiling Disentangles Inflammation- and Bile Duct Obstruction-Associated Microbiota Alterations across PSC/IBD Diagnoses. Nat. Microbiol. 2019, 4, 1826–1831. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. The Control and Consequences of Bacterial Fermentation in the Human Colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Yang, Y.; Hall, A.B.; Jiang, X. The Taxonomic Distribution of Histamine-Secreting Bacteria in the Human Gut Microbiome. BMC Genom. 2021, 22, 695. [Google Scholar] [CrossRef]
- Landete, J.M.; De Las Rivas, B.; Marcobal, A.; Muñoz, R. Updated Molecular Knowledge about Histamine Biosynthesis by Bacteria. Crit. Rev. Food Sci. Nutr. 2008, 48, 697–714. [Google Scholar] [CrossRef]
- Hemarajata, P.; Gao, C.; Pflughoeft, K.J.; Thomas, C.M.; Saulnier, D.M.; Spinler, J.K.; Versalovic, J. Lactobacillus Reuteri-Specific Immunoregulatory Gene rsiR Modulates Histamine Production and Immunomodulation by Lactobacillus Reuteri. J. Bacteriol. 2013, 195, 5567–5576. [Google Scholar] [CrossRef]
- Diaz, M.; del Rio, B.; Ladero, V.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Histamine Production in Lactobacillus Vaginalis Improves Cell Survival at Low pH by Counteracting the Acidification of the Cytosol. Int. J. Food Microbiol. 2020, 321, 108548. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Banicod, R.J.S.; Ntege, W.; Njiru, M.N.; Abubakar, W.H.; Kanthenga, H.T.; Javaid, A.; Khan, F. Production and Transformation of Biogenic Amines in Different Food Products by the Metabolic Activity of the Lactic Acid Bacteria. Int. J. Food Microbiol. 2025, 428, 110996. [Google Scholar] [CrossRef]
- Schelp, E.; Worley, S.; Monzingo, A.F.; Ernst, S.; Robertus, J.D. pH-Induced Structural Changes Regulate Histidine Decarboxylase Activity in Lactobacillus 30a1. J. Mol. Biol. 2001, 306, 727–732. [Google Scholar] [CrossRef]
- Lin, J.; Smith, M.P.; Chapin, K.C.; Baik, H.S.; Bennett, G.N.; Foster, J.W. Mechanisms of Acid Resistance in Enterohemorrhagic Escherichia Coli. Appl. Environ. Microbiol. 1996, 62, 3094–3100. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Duelo, A.; Veciana-Nogués, M.T.; Berlanga, M.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Intestinal Dysbiosis in Patients with Histamine Intolerance. Nutrients 2022, 14, 1774. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naïve Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Hrubisko, M.; Danis, R.; Huorka, M.; Wawruch, M. Histamine Intolerance—The More We Know the Less We Know. Nutrients 2021, 13, 2228. [Google Scholar] [CrossRef]
- Song, W.-B.; Lv, Y.-H.; Zhang, Z.-S.; Li, Y.-N.; Xiao, L.-P.; Yu, X.-P.; Wang, Y.-Y.; Ji, H.-L.; Ma, L. Soluble Intercellular Adhesion Molecule-1, D-Lactate and Diamine Oxidase in Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2009, 15, 3916–3919. [Google Scholar] [CrossRef] [PubMed]
- Schnedl, W.J.; Michaelis, S.; Enko, D.; Mangge, H. Fecal Calprotectin Elevations Associated with Food Intolerance/Malabsorption Are Significantly Reduced with Targeted Diets. Nutrients 2023, 15, 1179. [Google Scholar] [CrossRef]
- Honzawa, Y.; Nakase, H.; Matsuura, M.; Chiba, T. Clinical Significance of Serum Diamine Oxidase Activity in Inflammatory Bowel Disease: Importance of Evaluation of Small Intestinal Permeability. Inflamm. Bowel Dis. 2011, 17, E23–E25. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Zhao, M.; Giannetti, M.P.; Weller, E.; Hufdhi, R.; Novak, P.; Mendoza-Alvarez, L.B.; Hornick, J.; Lyons, J.J.; Glover, S.C.; et al. Distinct Small Intestine Mast Cell Histologic Changes in Patients With Hereditary Alpha-Tryptasemia and Mast Cell Activation Syndrome. Am. J. Surg. Pathol. 2021, 45, 997–1004. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Low-Histamine Diets: Is the Exclusion of Foods Justified by Their Histamine Content? Nutrients 2021, 13, 1395. [Google Scholar] [CrossRef]
- Chung, B.Y.; Park, S.Y.; Byun, Y.S.; Son, J.H.; Choi, Y.W.; Cho, Y.S.; Kim, H.O.; Park, C.W. Effect of Different Cooking Methods on Histamine Levels in Selected Foods. Ann. Dermatol. 2017, 29, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Jin, H.; Chen, L.; Ji, J.; Zhang, Z. Histamine Intolerance—A Kind of Pseudoallergic Reaction. Biomolecules 2022, 12, 454. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Rabell-González, J.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Biogenic Amines in Plant-Origin Foods: Are They Frequently Underestimated in Low-Histamine Diets? Foods 2018, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Maintz, L.; Novak, N. Histamine and Histamine Intolerance2. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Wagner, N.; Dirk, D.; Peveling-Oberhag, A.; Reese, I.; Rady-Pizarro, U.; Mitzel, H.; Staubach, P. A Popular Myth – Low-Histamine Diet Improves Chronic Spontaneous Urticaria–Fact or Fiction? J. Eur. Acad. Dermatol. Venereol. 2017, 31, 650–655. [Google Scholar] [CrossRef]
- Chen, H.; Nwe, P.-K.; Yang, Y.; Rosen, C.E.; Bielecka, A.A.; Kuchroo, M.; Cline, G.W.; Kruse, A.C.; Ring, A.M.; Crawford, J.M.; et al. A Forward Chemical Genetic Screen Reveals Gut Microbiota Metabolites That Modulate Host Physiology. Cell 2019, 177, 1217–1231.e18. [Google Scholar] [CrossRef]
- Kim, S.H.; Ben-Gigirey, B.; Barros-Velázquez, J.; Price, R.J.; An, H. Histamine and Biogenic Amine Production by Morganella Morganii Isolated from Temperature-Abused Albacore. J. Food Prot. 2000, 63, 244–251. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus Reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Duelo, A.; Veciana-Nogués, M.T.; Berlanga, M.; Vidal-Carou, M.C.; Latorre-Moratalla, M.L. The Dietary Treatment of Histamine Intolerance Reduces the Abundance of Some Histamine-Secreting Bacteria of the Gut Microbiota in Histamine Intolerant Women. A Pilot Study. Front. Nutr. 2022, 9, 1018463. [Google Scholar] [CrossRef]
- Lyte, M.; Daniels, K. A Microbial Endocrinology-Designed Discovery Platform to Identify Histamine-Degrading Probiotics: Proof of Concept in Poultry. Microorganisms 2025, 13, 751. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Bercik, P. Long-Term Personalized Low FODMAP Diet in IBS. Neurogastroenterol. Motil. 2022, 34, e14356. [Google Scholar] [CrossRef] [PubMed]
- Radi, G.; Campanti, A.; Diotallevi, F.; Martina, E.; Marani, A.; Offidani, A. A Systematic Review of Atopic Dermatitis: The Intriguing Journey Starting from Physiopathology to Treatment, from Laboratory Bench to Bedside. Biomedicines 2022, 10, 2700. [Google Scholar] [CrossRef]
- Schirmer, B.; Rezniczek, T.; Seifert, R.; Neumann, D. Proinflammatory Role of the Histamine H4 Receptor in Dextrane Sodium Sulfate-Induced Acute Colitis. Biochem. Pharmacol. 2015, 98, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Takeda, K. Manipulation of Epithelial Integrity and Mucosal Immunity by Host and Microbiota-Derived Metabolites. Eur. J. Immunol. 2020, 50, 921–931. [Google Scholar] [CrossRef]
- Shulpekova, Y.O.; Nechaev, V.M.; Popova, I.R.; Deeva, T.A.; Kopylov, A.T.; Malsagova, K.A.; Kaysheva, A.L.; Ivashkin, V.T. Food Intolerance: The Role of Histamine. Nutrients 2021, 13, 3207. [Google Scholar] [CrossRef]
- Tian, Z.; Zhuang, X.; Zhuo, S.; Zhu, Y.; Hu, S.; Zhao, M.; Tang, C.; Zhang, Z.; Li, X.; Ma, R.; et al. Dietary Inflammatory Potential Mediated Gut Microbiota and Metabolite Alterations in Crohn’s Disease: A Fire-New Perspective. Clin. Nutr. 2022, 41, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, M.; Del Vecchio, L.E.; Dargenio, P.; Kaitsas, F.; Rozera, T.; Porcari, S.; Gasbarrini, A.; Cammarota, G.; Ianiro, G. Histamine-Producing Bacteria and Their Role in Gastrointestinal Disorders. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 709–718. [Google Scholar] [CrossRef]
- Zonna, X.; Banta, C.; Hossein-Javaheri, N. The Association Between Crohn’s Disease and Patient Response to Yeast: A Review of the Literature. Gastroenterol. Insights 2024, 15, 1064–1074. [Google Scholar] [CrossRef]
- Kuang, R.; O’Keefe, S.J.D.; Ramos del Aguila de Rivers, C.; Koutroumpakis, F.; Binion, D.G. Is Salt at Fault? Dietary Salt Consumption and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2023, 29, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Mankaï, A.; Sakly, W.; Thabet, Y.; Achour, A.; Manoubi, W.; Ghedira, I. Anti-Saccharomyces Cerevisiae Antibodies in Patients with Systemic Lupus Erythematosus. Rheumatol. Int. 2013, 33, 665–669. [Google Scholar] [CrossRef]
- Dai, H.; Gao, X.-M. Elevated Levels of Serum Antibodies against Alpha-1, 6-Glucan in Patients with Systemic Lupus Erythematosus or Rheumatoid Arthritis. Protein Cell 2011, 2, 739–744. [Google Scholar] [CrossRef]
- Melayah, S.; Ghozzi, M.; Jemni, M.; Sakly, N.; Ghedira, I.; Mankaï, A. Anti-Saccharomyces Cerevisiae Antibodies in Rheumatoid Arthritis. Lab. Med. 2022, 53, 585–589. [Google Scholar] [CrossRef]
- Yazıcı, D.; Aydın, S.Z.; Yavuz, D.; Tarçın, Ö.; Deyneli, O.; Direskeneli, H.; Akalın, S. Anti-Saccaromyces Cerevisiae Antibodies (ASCA) Are Elevated in Autoimmune Thyroid Disease ASCA in Autoimmune Thyroid Disease. Endocrine 2010, 38, 194–198. [Google Scholar] [CrossRef]
- Main, J.; McKenzie, H.; Yeaman, G.R.; Kerr, M.A.; Robson, D.; Pennington, C.R.; Parratt, D. Antibody to Saccharomyces Cerevisiae (Bakers’ Yeast) in Crohn’s Disease. BMJ 1988, 297, 1105–1106. [Google Scholar] [CrossRef]
- Linares, D.M.; Martín, M.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic Amines in Dairy Products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef]
- Martini, G.R.; Tikhonova, E.; Rosati, E.; DeCelie, M.B.; Sievers, L.K.; Tran, F.; Lessing, M.; Bergfeld, A.; Hinz, S.; Nikolaus, S.; et al. Selection of Cross-Reactive T Cells by Commensal and Food-Derived Yeasts Drives Cytotoxic TH1 Cell Responses in Crohn’s Disease. Nat. Med. 2023, 29, 2602–2614. [Google Scholar] [CrossRef] [PubMed]
- Camus, M.; Esses, S.; Pariente, B.; Bourhis, L.L.; Douay, C.; Chardiny, V.; Mocan, I.; Benlagha, K.; Clave, E.; Toubert, A.; et al. Oligoclonal Expansions of Mucosal T Cells in Crohn’s Disease Predominate in NKG2D-Expressing CD4 T Cells. Mucosal Immunol. 2014, 7, 325–334. [Google Scholar] [CrossRef]
- Barclay, G.R.; McKenzie, H.; Pennington, J.; Parratt, D.; Pennington, C.R. The Effect of Dietary Yeast on the Activity of Stable Chronic Crohn’s Disease. Scand. J. Gastroenterol. 1992, 27, 196–200. [Google Scholar] [CrossRef]
- Urlep, D.; Orel, R.; Kunstek, P.; Benedik, E. Treatment of Active Crohn’s Disease in Children Using Partial Enteral Nutrition Combined with a Modified Crohn’s Disease Exclusion Diet: A Pilot Prospective Cohort Trial on Clinical and Endoscopic Outcomes. Nutrients 2023, 15, 4676. [Google Scholar] [CrossRef]
- Monteleone, I.; Marafini, I.; Dinallo, V.; Di Fusco, D.; Troncone, E.; Zorzi, F.; Laudisi, F.; Monteleone, G. Sodium Chloride–Enriched Diet Enhanced Inflammatory Cytokine Production and Exacerbated Experimental Colitis in Mice. J. Crohn’s Colitis 2017, 11, 237–245. [Google Scholar] [CrossRef]
- Wang, X.; Lang, F.; Liu, D. High-Salt Diet and Intestinal Microbiota: Influence on Cardiovascular Disease and Inflammatory Bowel Disease. Biology 2024, 13, 674. [Google Scholar] [CrossRef]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-Responsive Gut Commensal Modulates TH17 Axis and Disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High Salt Diet Exacerbates Colitis in Mice by Decreasing Lactobacillus Levels and Butyrate Production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Aguiar, S.L.F.; Miranda, M.C.G.; Guimarães, M.A.F.; Santiago, H.C.; Queiroz, C.P.; Cunha, P.d.S.; Cara, D.C.; Foureaux, G.; Ferreira, A.J.; Cardoso, V.N.; et al. High-Salt Diet Induces IL-17-Dependent Gut Inflammation and Exacerbates Colitis in Mice. Front. Immunol. 2018, 8, 1969. [Google Scholar] [CrossRef] [PubMed]
- Carriquiry, A.; Moshfegh, A.J.; Steinfeldt, L.C.; Cogswell, M.E.; Loustalot, F.; Zhang, Z.; Yang, Q.; Tian, N. Trends in the Prevalence of Excess Dietary Sodium Intake—United States, 2003–2010. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 1021–1025. [Google Scholar]
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults with Crohn’s Disease. Gastroenterology 2021, 161, 837–852e9. [Google Scholar] [CrossRef]
- Peng, Z.; Yi, J.; Liu, X. A Low-FODMAP Diet Provides Benefits for Functional Gastrointestinal Symptoms but Not for Improving Stool Consistency and Mucosal Inflammation in IBD: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2072. [Google Scholar] [CrossRef]
- Więcek, M.; Panufnik, P.; Kaniewska, M.; Lewandowski, K.; Rydzewska, G. Low-FODMAP Diet for the Management of Irritable Bowel Syndrome in Remission of IBD. Nutrients 2022, 14, 4562. [Google Scholar] [CrossRef]
- Bardacke, J.A.; Yarrow, L.; Rosenkranz, S.K. The Long-Term Effects of a Low–Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet for Irritable Bowel Syndrome Management. Curr. Dev. Nutr. 2023, 7, 101997. [Google Scholar] [CrossRef] [PubMed]
- Weisshof, R.; Chermesh, I. Micronutrient Deficiencies in Inflammatory Bowel Disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Siva, S.; Rubin, D.T.; Gulotta, G.; Wroblewski, K.; Pekow, J. Zinc Deficiency Is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Therkelsen, S.P.; Nentwich, I.; Nissen-Meyer, L.S.H.; Hetland, G. IgE-Sensitization to Food and Inhalant Allergens in IBD Patients Compared with Normal Blood Donors at Oslo University Hospital, Norway. Scand. J. Gastroenterol. 2019, 54, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Levo, Y.; Shalit, M.; Wollner, S.; Fich, A. Serum IgE Levels in Patients with Inflammatory Bowel Disease. Ann. Allergy 1986, 56, 85–87. [Google Scholar]
- Sánchez-Pérez, S.; Celorio-Sardà, R.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Vidal-Carou, M.C. 1-Methylhistamine as a Potential Biomarker of Food Histamine Intolerance. A Pilot Study. Front. Nutr. 2022, 9, 973682. [Google Scholar] [CrossRef]
- Muli, S.; Blumenthal, A.; Conzen, C.-A.; Benz, M.E.; Alexy, U.; Schmid, M.; Keski-Rahkonen, P.; Floegel, A.; Nöthlings, U. Association of Ultraprocessed Foods Intake with Untargeted Metabolomics Profiles in Adolescents and Young Adults in the DONALD Cohort Study. J. Nutr. 2024, 154, 3255–3265. [Google Scholar] [CrossRef]
- Comas-Basté, O.; Latorre-Moratalla, M.L.; Bernacchia, R.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. New Approach for the Diagnosis of Histamine Intolerance Based on the Determination of Histamine and Methylhistamine in Urine. J. Pharm. Biomed. Anal. 2017, 145, 379–385. [Google Scholar] [CrossRef]
- Beltrán-Ortiz, C.; Peralta, T.; Ramos, V.; Durán, M.; Behrens, C.; Maureira, D.; Guzmán, M.A.; Bastias, C.; Ferrer, P. Standardization of a Colorimetric Technique for Determination of Enzymatic Activity of Diamine Oxidase (DAO) and Its Application in Patients with Clinical Diagnosis of Histamine Intolerance. World Allergy Organ. J. 2020, 13, 100457. [Google Scholar] [CrossRef]
- Ahmadifar, S.; Le, T.C.; Marcocci, L.; Pietrangeli, P.; Mateescu, M.A. Zymographic Approach to Determine the Intrinsic Enzyme Specific Activity of Diamine Oxidase in Presence of Interfering Enzymes. Anal. Chim. Acta 2017, 975, 78–85. [Google Scholar] [CrossRef]
- van Odijk, J.; Weisheit, A.; Arvidsson, M.; Miron, N.; Nwaru, B.; Ekerljung, L. The Use of DAO as a Marker for Histamine Intolerance: Measurements and Determinants in a Large Random Population-Based Survey. Nutrients 2023, 15, 2887. [Google Scholar] [CrossRef]
- Rentzos, G.; Weisheit, A.; Ekerljung, L.; van Odijk, J. Measurement of Diamine Oxidase (DAO) during Low-Histamine or Ordinary Diet in Patients with Histamine Intolerance. Eur. J. Clin. Nutr. 2024, 78, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, D.S.; Shum, M.; Hsieh, J.; Blonski, W.; Greenwald, D.A. Non-Pulmonary Allergic Diseases and Inflammatory Bowel Disease: A Qualitative Review. World J. Gastroenterol. 2014, 20, 11023–11032. [Google Scholar] [CrossRef]
- Palacios, N.L.; Agúndez, J.A.G.; Mendoza, J.L.; García-Martín, E.; Martínez, C.; Fuentes Ferrer, M.E.; Díaz-Rubio, M. Analysis of a Non-Synonymous Single Nucleotide Polymorphism of the Human Diamine Oxidase Gene (Ref. SNP ID: Rs1049793) in Patients with Crohn’s Disease. Scand. J. Gastroenterol. 2009, 44, 1207–1212. [Google Scholar] [CrossRef]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A Novel Ruminococcus Gnavus Clade Enriched in Inflammatory Bowel Disease Patients. Genome. Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.B.; Almeida, M.; Juncker, A.S.; Rasmussen, S.; Li, J.; Sunagawa, S.; Plichta, D.R.; Gautier, L.; Pedersen, A.G.; Le Chatelier, E.; et al. Identification and Assembly of Genomes and Genetic Elements in Complex Metagenomic Samples without Using Reference Genomes. Nat. Biotechnol. 2014, 32, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Ezzatpour, S.; Mondragon Portocarrero, A.d.C.; Cardelle-Cobas, A.; Lamas, A.; López-Santamarina, A.; Miranda, J.M.; Aguilar, H.C. The Human Gut Virome and Its Relationship with Nontransmissible Chronic Diseases. Nutrients 2023, 15, 977. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.M.; Peng, Y.; Massimino, L.; Sin, Z.Y.; Parigi, T.L.; Facoetti, A.; Rahman, S.; Danese, S.; Ungaro, F. Gut Virome in Inflammatory Bowel Disease and Beyond. Gut 2024, 73, 350–360. [Google Scholar] [CrossRef] [PubMed]

| Receptor | G Protein Coupling | Expression | Molecular Mass (kDa) | Potential Role in IBD |
|---|---|---|---|---|
| H1R | Gαq | Enterocytes | 56 | Development of allergic reactions |
| H2R | Gαs | Enterocytes Gastric pancreatic cells | 40 | External secretion of hydrochloric acid Anti-inflammatory effects Innate immune response to microorganisms when histamine binds |
| H3R | Gi/o | Nervous system: hippocampus, cerebral cortex, and neurons of the basal ganglia | 48 | Pro-inflammatory activity |
| H4R | Gi/o | Small and large intestinal epithelium, bile, pancreatic duct | 44 | Mainly present in immune cells May contribute to the development of inflammatory reactions and hypersensitivity |
| Gene | Function | Role |
|---|---|---|
| hdcA | Histidine decarboxylase | Catalyzes the decarboxylation of histidine to histamine (PLP-dependent) |
| hdcP | Histidine/histamine antiporter | Imports histidine Exports histamine from the bacterial cytoplasm |
| hdcB | Maturation Protein | |
| hdcR | Transcriptional regulator | Part of the LysR-type transcriptional regulator (LTTR) regulating amino acid metabolism pathways |
| Low-Histamine | High-Histamine | Histamine-Releasing |
|---|---|---|
| Fresh dairy (ricotta, mozzarella, cottage cheese, milk) | Aged/fermented cheeses (parmesan, cheddar, blue cheese) | Citrus and tropical fruits (orange, lemon, pineapple, banana, avocado) |
| Fresh meat and fish (chicken, beef, trout, cod, etc.) | Fermented foods and drinks (kimchi, yogurt, kombucha, wine, beer) | Certain vegetables (tomato, eggplant, spinach, squash) |
| Soy and meat alternatives (e.g., coconut aminos) | Processed meats (bacon, salami, sausages) | Berries and chocolate (strawberries, raspberries, cocoa) |
| Unflavored distilled alcohol (vodka, gin) | Preserved fish (canned tuna, sardines, smoked mackerel) | Legumes, nuts, and wheat (chickpeas, peanuts, cashews, bread) |
| Fresh vegetables and grains (carrots, squash, rice, quinoa) | Fermented soy (miso, tofu, tempeh, soy sauce) | Spices (cinnamon, chili, paprika, curry) |
| Mild herbs and spices (oregano, basil, ginger, mustard) | Vinegar and vinegar-based condiments | Citric-acid-containing juices |
| Egg yolk or cooked egg white | Raw eggs | Seafood and raw egg white |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanta, D.; Katsamakas, E.; Gudiksen, A.M.B.; Jalili, M. Histamine Metabolism in IBD: Towards Precision Nutrition. Nutrients 2025, 17, 2473. https://doi.org/10.3390/nu17152473
Kanta D, Katsamakas E, Gudiksen AMB, Jalili M. Histamine Metabolism in IBD: Towards Precision Nutrition. Nutrients. 2025; 17(15):2473. https://doi.org/10.3390/nu17152473
Chicago/Turabian StyleKanta, Dimitra, Eleftherios Katsamakas, Anna Maia Berg Gudiksen, and Mahsa Jalili. 2025. "Histamine Metabolism in IBD: Towards Precision Nutrition" Nutrients 17, no. 15: 2473. https://doi.org/10.3390/nu17152473
APA StyleKanta, D., Katsamakas, E., Gudiksen, A. M. B., & Jalili, M. (2025). Histamine Metabolism in IBD: Towards Precision Nutrition. Nutrients, 17(15), 2473. https://doi.org/10.3390/nu17152473









