The Gut’s Secret Code: Bowel Microbiota as a Biomarker for Adaptation
Highlights
- Microbiota Changes After Resection Influence Outcomes in SBS: Extensive small bowel resection induces significant gut microbiota changes—particularly increasing Gram-negative Proteobacteria—that are linked to negative outcomes such as liver disease and poor growth. However, they can also support adaptation and energy salvage, highlighting the microbiota’s complex influence on SBS prognosis.
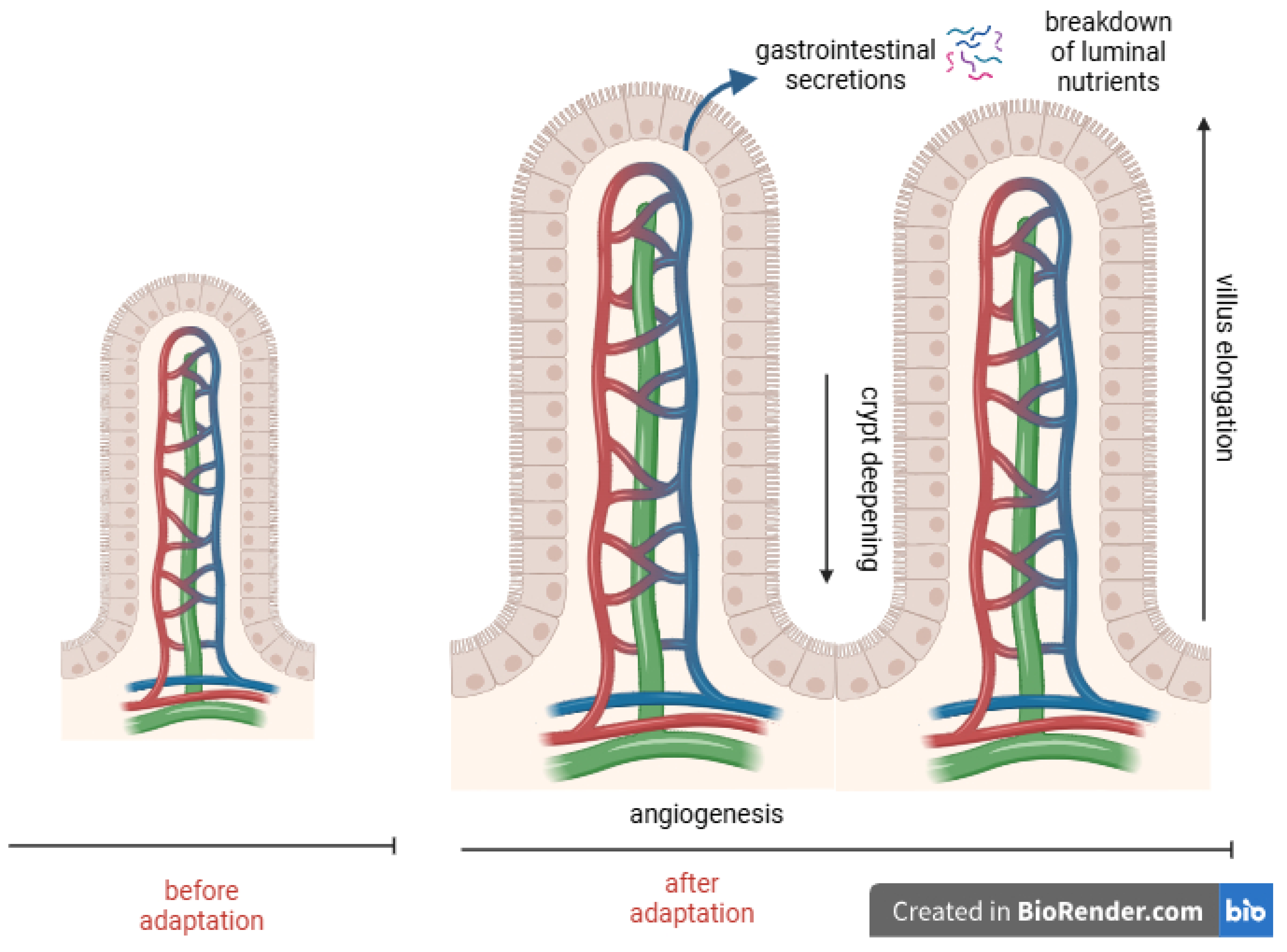
- Intestinal Adaptation—Phases, Mechanisms, and Key Factors: Intestinal adaptation occurs in three phases—acute, remodeling, and maintenance. The ileum demonstrates superior adaptive potential, and the preservation of the colon and ileocecal valve, in addition to early oral feeding, are crucial for adaption. Exclusive parenteral nutrition, however, can impair mucosal structure and adaptation.
- Modern Therapies—Hormonal, Nutritional, and Surgical Innovations: Therapeutic advances in SBS include utilzing GLP-2 analogues to promote adaptation and reduce parenteral nutrition; using growth hormone, EGF, IGF-1, and glutamine to support mucosal growth; and adopting surgical strategies such as intestinal lengthening and continuity restoration to maximize absorption. Transplantation is reserved as a last-resort treatment.
Abstract
1. Introduction
- Beneficial and Harmful Effects of Gut Microbiota
- Microbiota Dysbiosis and Its Role in Bowel Syndromes
- Objectives and Scope of the Current Review
2. Intestinal Failure
2.1. Microbiota Alterations After Intestinal Resection
2.2. Intestinal Adaptation and Rehabilitation: A Complex Process
2.2.1. Phases of Intestinal Adaptation
2.2.2. Role of Intestinal Anatomy
2.2.3. Importance of Enteral Nutrition
2.2.4. Individual Variability and Hormonal and Nutritional Mediator of Adaptation
2.2.5. Advances in Therapeutic Approaches and Surgical Rehabilitation
3. Discussion
3.1. Microbiota as Biomarkers of Bowel Adaptation
3.2. Microbial Imbalance, Fecal Lactate, and Probiotic Interventions
3.3. The Role of Gut Microbiota Preservation and Probiotic Therapy in Intestinal Adaptation Post Small Bowel Resection
3.4. Limitations of Microbiome Research in Intestinal Failure
3.4.1. Sampling Methods
3.4.2. Sequencing Approaches
3.4.3. Confounding Factors
3.4.4. Cohort Heterogeneity and Study Design
3.4.5. Recommendations for Future Research: Key Challenges and Future Directions
Need for Standardized Protocols in Sample Collection and Processing
- Sampling Site and Method:
- Storage and Handling:
- Future Outlook:
Leveraging Multi-Omics Approaches
- Future Outlook:
Rigorous Control of Confounding Variables
- Future Outlook:
Need for Larger, Multicenter, Longitudinal Studies
- Longitudinal Designs:
- Multicenter Collaboration:
- Standardized Data Collection:
- Future Outlook:
Validation of Microbial Biomarkers and Causal Mechanisms
- Future Outlook:
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CIF | Chronic intestinal failure |
| EGF | Epidermal growth factor |
| GH | Growth hormone |
| GLP-1 | Glucagon-like-peptide-1 |
| GLP-2 | Glucagon-like-peptide-2 |
| IF | Intestinal failure |
| IGF-1 | Insulin-like growth factor |
| MAPKs | Mitogen-activated protein kinase |
| PN | Parenteral nutrition |
| PYY | Peptide YY |
| SBS | Short bowel syndrome |
| TGF-α | Transforming growth factor-alpha |
References
- Kastl, A.J., Jr.; Terry, N.A.; Wu, G.D.; Albenberg, L.G. The structure and function of the human small intestinal microbiota: Current understanding and future directions. Cell Mol. Gastroenterol. Hepatol. 2019, 9, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Recent advances in understanding the structure and function of the human microbiome. Front. Microbiol. 2022, 13, 825338. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Wang, J.; Li, J. Fecal microbiota signatures of adult patients with different types of short bowel syndrome. J. Gastroenterol. Hepatol. 2017, 32, 1949–1957. [Google Scholar] [CrossRef]
- Solar, H.; Ortega, M.L.; Gondolesi, G. Current status of chronic intestinal failure management in adults. Nutrients 2024, 16, 2648. [Google Scholar] [CrossRef]
- Ossola, M.; Ferrocino, I.; Franciosa, I.; Aimasso, U.; Cravero, L.; Bonciolini, A.; Cardenia, V.; Merlo, F.D.; Anrò, M.; Chiarotto, A.; et al. Does microbiome matter in chronic intestinal failure due to type 1 short bowel syndrome in adults? Nutrients 2024, 16, 2282. [Google Scholar] [CrossRef]
- Gillard, L.; Mayeur, C.; Robert, V.; Pingenot, I.; Le Beyec, J.; Bado, A.; Lepage, P.; Thomas, M.; Joly, F. Microbiota is involved in post-resection adaptation in humans with short bowel syndrome. Front. Physiol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Matarese, L.; Harvin, G. Parenteral nutrition for short bowel syndrome: Practical aspects. In Short Bowel Syndrome: Practical Approach to Management; DiBaise, J.K., Parrish, C.R., Thompson, J.S., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 197–205. [Google Scholar]
- Bielawska, B.; Allard, J.P. Parenteral nutrition and intestinal failure. Nutrients 2017, 9, 466. [Google Scholar] [CrossRef]
- Neelis, E.; de Koning, B.; Rings, E.; Wijnen, R.; Nichols, B.; Hulst, J.; Gerasimidis, K. The gut microbiome in patients with intestinal failure: Current evidence and implications for clinical practice. JPEN J. Parenter. Enter. Nutr. 2019, 43, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Boutte, H.J., Jr.; Chen, J.; Wylie, T.N.; Wylie, K.M.; Xie, Y.; Geisman, M.; Prabu, A.; Gazit, V.; Tarr, P.I.; Levin, M.S.; et al. Fecal microbiome and bile acid metabolome in adult short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G154–G168. [Google Scholar] [CrossRef]
- Piper, H.G.; Fan, D.; Coughlin, L.A.; Ho, E.X.; McDaniel, M.M.; Channabasappa, N.; Kim, J.; Kim, M.; Zhan, X.; Xie, Y.; et al. Severe gut microbiota dysbiosis is associated with poor growth in patients with short bowel syndrome. JPEN J. Parenter. Enter. Nutr. 2017, 41, 1202–1212. [Google Scholar] [CrossRef]
- Davidovics, Z.H.; Carter, B.A.; Luna, R.A.; Hollister, E.B.; Shulman, R.J.; Versalovic, J. The fecal microbiome in pediatric patients with short bowel syndrome. JPEN J. Parenter. Enter. Nutr. 2016, 40, 1106–1113. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Lu, L.; Yan, W.; Tao, Y.; Zhou, K.; Jia, J.; Cai, W. Alterations in intestinal microbiota relate to intestinal failure-associated liver disease and central line infections. J. Pediatr. Surg. 2017, 52, 1318–1326. [Google Scholar] [CrossRef]
- Korpela, K.; Mutanen, A.; Salonen, A.; Savilahti, E.; de Vos, W.M.; Pakarinen, M.P. Intestinal microbiota signatures associated with histological liver steatosis in pediatric-onset intestinal failure. JPEN J. Parenter. Enter. Nutr. 2017, 41, 238–248. [Google Scholar] [CrossRef]
- Engstrand Lilja, H.; Wefer, H.; Nystrom, N.; Finkel, Y.; Engstrand, L. Intestinal dysbiosis in children with short bowel syndrome is associated with impaired outcome. Microbiome 2015, 3, 18. [Google Scholar] [CrossRef]
- Joly, F.; Mayeur, C.; Brunei, A.; Noordine, M.L.; Meylheuc, T.; Langella, P.; Messing, B.; Duée, P.H.; Cherbuy, C.; Thomas, M. Drastic changes in fecal and mucosa-associated microbiota in adult patients with short bowel syndrome. Biochimie 2010, 92, 753–761. [Google Scholar] [CrossRef]
- Marchix, J.; Goddard, G.; Helmrath, M.A. Host-gut microbiota crosstalk in intestinal adaptation. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 149–162. [Google Scholar] [CrossRef]
- Helmrath, M.A.; Fong, J.J.; Dekaney, C.M.; Henning, S.J. Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G215–G222. [Google Scholar] [CrossRef]
- Warner, B.W. The pathogenesis of resection-associated intestinal adaptation. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.C.; Swietlicki, E.A.; Wang, J.L. Regulation of PC4/TIS7 expression in adapting remnant intestine after resection. Am. J. Physiol. 1998, 275, G506–G513. [Google Scholar] [CrossRef] [PubMed]
- Sacks, A.I.; Warwick, G.J.; Barnard, J.A. Early proliferative events following intestinal resection in the rat. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 158–164. [Google Scholar] [PubMed]
- Cadena, M.E.; Vinck, E.E.; Santivañez, J.J.; Vergara Gómez, A. Biomarkers for intestinal failure in short bowel syndrome: A new era in GI rehabilitation. Rev. Colomb. Cir. 2019, 34, 234–242. [Google Scholar]
- Buchman, A.L. Intestinal failure and rehabilitation. Gastroenterol. Clin. N. Am. 2018, 47, 327–340. [Google Scholar] [CrossRef]
- Staun, M.; Pironi, L.; Bozzetti, F.; Baxter, J.; Forbes, A.; Joly, F.; Jeppesen, P.; Moreno, J.; Hébuterne, X.; Pertkiewicz, M.; et al. ESPEN guidelines on parenteral nutrition: Home parenteral nutrition (HPN) in adult patients. Clin. Nutr. 2009, 28, 467–479. [Google Scholar] [CrossRef]
- Cavicchi, M.; Beau, P.; Crenn, P.; Degott, C.; Messing, B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann. Intern. Med. 2000, 132, 525–532. [Google Scholar] [CrossRef]
- Seguy, D.; Vahedi, K.; Kapel, N.; Souberbielle, J.C.; Messing, B. Low-dose growth hormone in adult home parenteral nutrition-dependent short bowel syndrome patients: A positive study. Gastroenterology 2003, 124, 293–302. [Google Scholar] [CrossRef]
- Jonkers-Schuitema, C.F.; Wanten, G.; Szczygieł, B.; Forbes, A.; Kunecki, M.; Olewiński, M. Leczenie żywieniowe po rozległym wycięciu jelita (zespół krótkiego jelita). In Podstawy Żywienia Klinicznego; Sobotka, L., Ed.; Scientifica: Kraków, Poland, 2013; pp. 553–567. [Google Scholar]
- Gallant, K.D.; DiBaise, J.K.; Parrish, C.R. Clinical and nutritional assessment in the patient with short bowel syndrome. In Short Bowel Syndrome: Practical Approach to Management; DiBaise, J.K., Parrish, C.R., Thompson, J.S., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 115–127. [Google Scholar]
- Naberhuis, J.; Tappenden, K.A. Intestinal adaptation: The contemporary treatment goal for short bowel syndrome. In Short Bowel Syndrome: Practical Approach to Management; DiBaise, J.K., Parrish, C.R., Thompson, J.S., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 43–54. [Google Scholar]
- Höllwarth, M.E. Short bowel syndrome: Pathophysiological and clinical aspects. Pathophysiology 1999, 6, 1–19. [Google Scholar] [CrossRef]
- Mayeur, C.; Gratadoux, J.J.; Bridonneau, C.; Chegdani, F.; Larroque, B.; Kapel, N.; Corcos, O.; Thomas, M.; Joly, F. Faecal D/L lactate ratio is a metabolic signature of microbiota imbalance in patients with short bowel syndrome. PLoS ONE 2013, 8, e54335. [Google Scholar] [CrossRef]
- Budinska, E.; Gojda, J.; Heczkova, M.; Bratova, M.; Dankova, H.; Wohl, P.; Bastova, H.; Lanska, V.; Kostovcik, M.; Dastych, M. Microbiomeand metabolome profiles associated with different types of short bowel syndrome: Implications for treatment. JPEN J. Parenter. Enter. Nutr. 2020, 44, 105–118. [Google Scholar] [CrossRef] [PubMed]
- McMellen, M.E.; Wakeman, D.; Longshore, S.W.; McDuffie, L.A.; Warner, B.W. Growth factor: Possible roles for clinical management of the short bowel syndrome. Semin. Pediatr. Surg. 2010, 19, 35–43. [Google Scholar] [CrossRef]
- Sundaram, A.; Koutkia, P.; Apovian, C.M. Nutritional management of short bowel syndrome in adults. J. Clin. Gastroenterol. 2002, 34, 207–220. [Google Scholar] [CrossRef]
- Parrish, C.R. The clinician’s guide to short bowel syndrome. Pract. Gastroenterol. 2005, 8, 67–105. [Google Scholar]
- Seetharam, P.; Rodrigues, G. Short bowel syndrome: A review of management options. Saudi J. Gastroenterol. 2011, 17, 229–235. [Google Scholar]
- Kim, M.H.; Kim, H. The role of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef]
- Tappenden, K.A. Intestinal adaptation following resection. JPEN J. Parenter. Enter. Nutr. 2014, 38, 23–31. [Google Scholar] [CrossRef]
- O’Keefe, S. Nutritional issues in the short bowel syndrome—Total parenteral nutrition, enteral nutrition and the role of transplantation. Nestle Nutr. Inst. Workshop Ser. 2015, 82, 75–90. [Google Scholar]
- Rubin, D.C.; Levin, M.S. Mechanisms of intestinal adaptation. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 237–248. [Google Scholar] [CrossRef]
- Pironi, L. Intestinal adaptation and rehabilitation in adults with short bowel syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 457–461. [Google Scholar] [CrossRef]
- Drucker, D.J.; Erlich, P.; Asa, S.L.; Brubaker, P.L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc. Natl. Acad. Sci. USA 1996, 93, 7911–7916. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B. Teduglutide, a novel glucagon-like peptide 2 analog, in the treatment of patients with short bowel syndrome. Ther. Adv. Gastroenterol. 2012, 5, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.B.; Askov-Hansen, C.; Naimi, R.M.; Brandt, C.F.; Hartmann, B.; Holst, J.J.; Mortensen, P.B.; Jeppesen, P.B. Acute effects of continuous infusions of glucagon-like peptide (GLP)-1, GLP-2 and the combination (GLP-1+GLP-2) on intestinal absorption in short bowel syndrome (SBS) patients: A placebo-controlled study. Regul. Pept. 2013, 184, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.S. Surgical considerations in the short bowel syndrome. In Short Bowel Syndrome: Practical Approach to Management; DiBaise, J.K., Parrish, C.R., Thompson, J.S., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 263–276. [Google Scholar]
- Intestinal Transplant Registry Report. Intestinal Transplantation Association—Section of the Transplantation Society. Intestinal Transplantation Registry Report. Available online: https://intestinalregistry.org/images/registries/IITR%20Final%20Slides%207-3-2023.pdf (accessed on 2 March 2025).
- Xiao, Y.; Wang, W.; Lu, Y.; Zhang, L.; Chen, H.; Li, J. Multi-omics analysis reveals Lactobacillus and indolelactic acid as signatures of intestinal adaptation in short bowel syndrome. J. Clin. Med. 2022, 11, 1932. [Google Scholar]
- Wang, W.; Lu, Y.; Wu, B.; Peng, S.; Cai, W.; Xiao, Y. Lactobacillus plantarum supplementation alleviates liver and intestinal injury in parenteral nutrition-fed neonatal piglets. JPEN J. Parenter. Enteral Nutr. 2022, 46, 1932–1943. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, J.; Yao, D.; Guo, F.; Li, Y. Altered fecal microbiome and metabolome profiles in rat models of short bowel syndrome. Front. Microbiol. 2023, 14, 1185463. [Google Scholar] [CrossRef]
- Pereira-Fantini, P.M.; Byars, S.G.; Pitt, J.; Lapthorne, S.; Fouhy, F.; Cotter, P.D.; Bines, J.E. Unravelling the metabolic impact of SBS-associated microbial dysbiosis: Insights from the piglet short bowel syndrome model. Sci. Rep. 2017, 7, 43326. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Jiang, L.; Wang, Y.; Cai, W. Multi-omics unravels metabolic alterations in the ileal mucosa of neonatal piglets receiving total parenteral nutrition. Metabolites 2023, 13, 555. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Gao, B.; Yan, J.; Cai, W.; Jiang, J. Untargeted metabolomics reveal parenteral nutrition-associated alterations in pediatric patients with short bowel syndrome. Metabolites 2022, 12, 600. [Google Scholar] [CrossRef]
- Narula, R.K.; El Shafei, A.; Ramaiah, D.; Schmitz, P.G. D-lactic acidosis 23 years after jejuno-ileal bypass. Am. J. Kidney Dis. 2000, 36, e9. [Google Scholar] [CrossRef]
- Chowdhury, F.; Hill, L.; Shah, N.; Popov, J.; Cheveldayoff, P.; Pai, N. Intestinal microbiome in short bowel syndrome: Diagnostic and therapeutic opportunities. Curr. Opin. Gastroenterol. 2023, 39, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Joly, F. Intestinal microbiota in short bowel syndrome. Gastroenterol. Clin. Biol. 2010, 34 (Suppl. 1), S37–S43. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, Y.; Sugiyama, M.; Hashizume, K.; Yuki, N.; Morotomi, M.; Tanaka, R. Experience of long-term synbiotic therapy in seven short bowel patients with refractory enterocolitis. J. Pediatr. Surg. 2004, 39, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Patole, S.K.; Rao, S. Role of probiotics in short bowel syndrome in infants and children—A systematic review. Nutrients 2013, 5, 679–699. [Google Scholar] [CrossRef]
- Quince, C.; Walker, A.W.; Simpson, J.T.; Loman, N.J.; Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 2017, 35, 833–844. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Cleminson, J.S.; Young, G.R.; Campbell, D.I.; Campbell, F.; Gennery, A.R.; Berrington, J.E.; Stewart, C.J. Gut microbiome in paediatric short bowel syndrome: A systematic review and sequencing re-analysis. Pediatr. Res. 2025, in press. [CrossRef]
| Factor | Key Mediators/Hormones/Processes |
|---|---|
| Extent and Location of Resection (presence of colon and ileum) | Presence of colon and ileum significantly influences adaptation capacity. The colon can salvage calories via fermentation and fluid absorption. |
| Patient’s Age | Younger age is associated with better adaptive capacity. |
| Pharmacological Treatment | Use of growth factors and medications that stimulate adaptation (e.g., GLP-2 analogues, antisecretory drugs). |
| Nutritional Therapy | Enteral nutrition stimulates mucosal hyperplasia, gastrointestinal hormone secretion, and pancreaticobiliary secretions. |
| Hyperphagia | Increased oral intake promotes adaptation by providing luminal nutrients. |
| Mediator | Role in Adaptation |
|---|---|
| GLP-2 (Glucagon-like Peptide-2) | Stimulates mucosal growth, slows intestinal transit, enhances absorption. |
| IGF-1 (Insulin-like Growth Factor-1) | Promotes mucosal proliferation and nutrient absorption. |
| EGF (Epidermal Growth Factor) | Stimulates epithelial cell proliferation and repair. |
| TGF-α (Transforming Growth Factor-alpha) | Supports mucosal healing and growth. |
| PYY (Peptide YY) | Slows gastric emptying and intestinal transit, enhancing absorption. |
| GH (Growth Hormone) | Enhances intestinal growth and function. |
| GLP-1 (Glucagon-like Peptide-1) | Modulates motility and insulin secretion, contributes to adaptation. |
| Secretion of Pancreatic Juice and Bile | Facilitates digestion and nutrient absorption, trophic effects on mucosa. |
| Bacterial Strain | Impact on SBS/Intestinal Adaptation |
|---|---|
| Proteobacteria | Dominant in intestinal failure patients; associated with longer duration of parenteral nutrition (PN); often pathogenic. |
| Enterobacteriaceae | Increased abundance linked to longer PN duration. |
| Lactobacillus (e.g., L. plantarum) | Dominance associated with both shorter and longer PN duration (conflicting findings). High abundance linked to increased stomal output. May help reduce pathogenic overgrowth and support growth. |
| Clostridium cluster XIVa | Presence associated with earlier PN weaning and shorter PN duration. |
| Bacteroidetes | Higher relative abundance in SBS group III patients; potentially beneficial. |
| Prevotella | Increased in patients with high stomal output (≥1500 mL/day). |
| Erysipelatoclostridium | Inversely correlated with stomal output (lower abundance with high output). |
| Intestinibacter | Reduced abundance in patients with high stomal output. |
| Lactate-producing bacteria | Increased presence in patients with fecal lactate accumulation, associated with risk of D-lactic acidosis. |
| Lactate-consuming bacteria | Decreased presence in patients with fecal lactate accumulation, contributing to D-lactic acidosis risk. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braszczyńska-Sochacka, J.; Sochacki, J.; Mik, M. The Gut’s Secret Code: Bowel Microbiota as a Biomarker for Adaptation. Nutrients 2025, 17, 2117. https://doi.org/10.3390/nu17132117
Braszczyńska-Sochacka J, Sochacki J, Mik M. The Gut’s Secret Code: Bowel Microbiota as a Biomarker for Adaptation. Nutrients. 2025; 17(13):2117. https://doi.org/10.3390/nu17132117
Chicago/Turabian StyleBraszczyńska-Sochacka, Joanna, Jakub Sochacki, and Michał Mik. 2025. "The Gut’s Secret Code: Bowel Microbiota as a Biomarker for Adaptation" Nutrients 17, no. 13: 2117. https://doi.org/10.3390/nu17132117
APA StyleBraszczyńska-Sochacka, J., Sochacki, J., & Mik, M. (2025). The Gut’s Secret Code: Bowel Microbiota as a Biomarker for Adaptation. Nutrients, 17(13), 2117. https://doi.org/10.3390/nu17132117






