Chestnut (Castanea crenata) Inner-Shell Extract Attenuates Barium-Chloride-Induced Injury and Denervation-Induced Atrophy in Skeletal Muscle of Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chestnut Inner-Shell Extract Preparation
2.2. Animals
2.3. Establishment of BaCl2-Induced Muscle Injury Model and Treatment
2.4. Establishment of Muscle Denervation Model and Treatment
2.5. Grip Strength Test
2.6. Histological Analysis
2.7. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Assay
2.8. Immunoblotting
2.9. Cell Culture
2.10. Cell Viability Assay
2.11. Statistical Analyses
3. Results
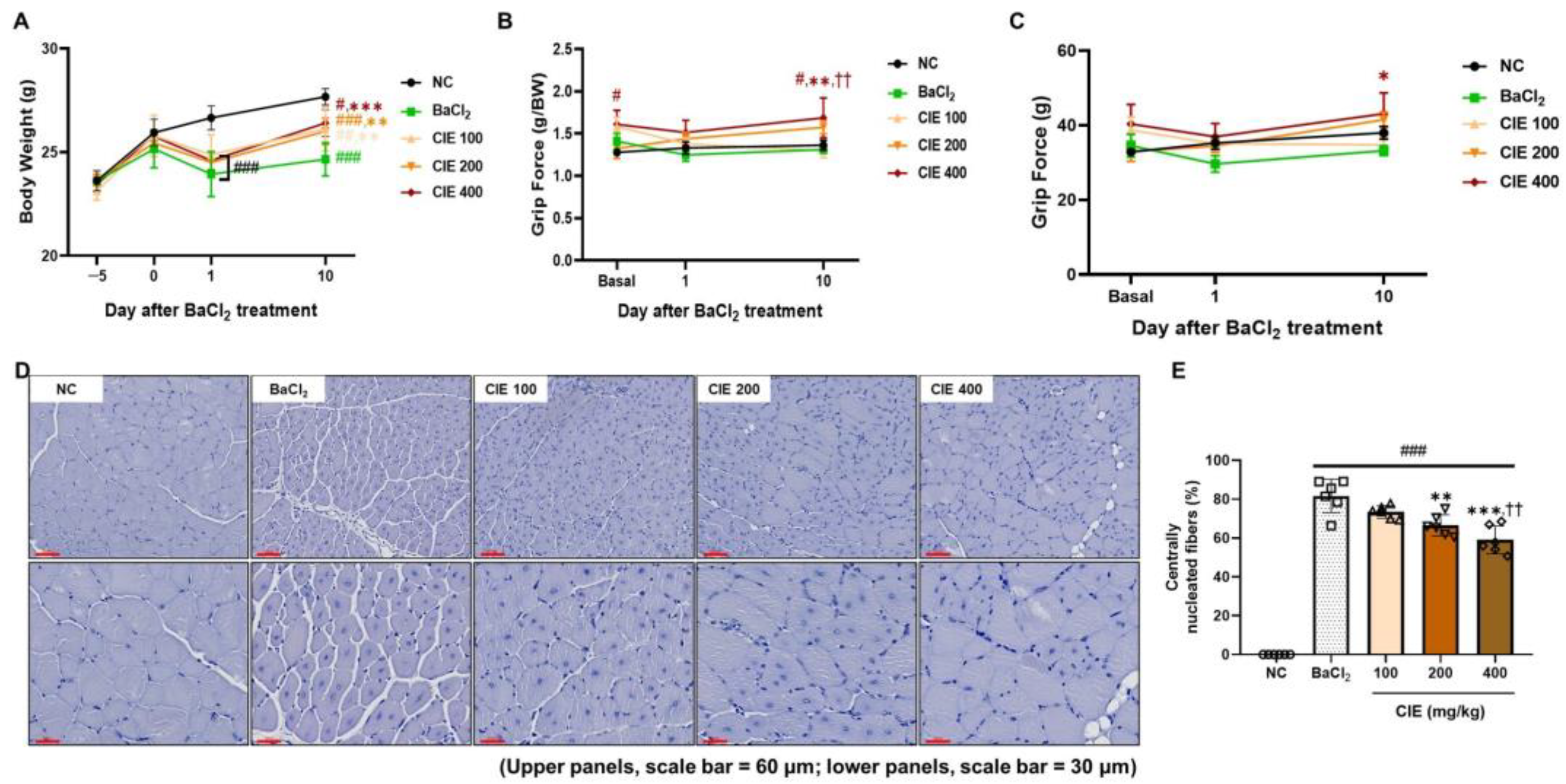
3.1. CIE Improves the TA Muscle Mass in Two Different Models
3.2. CIE Ameliorates Skeletal Muscle Function After the BaCl2-Induced Muscle Damage
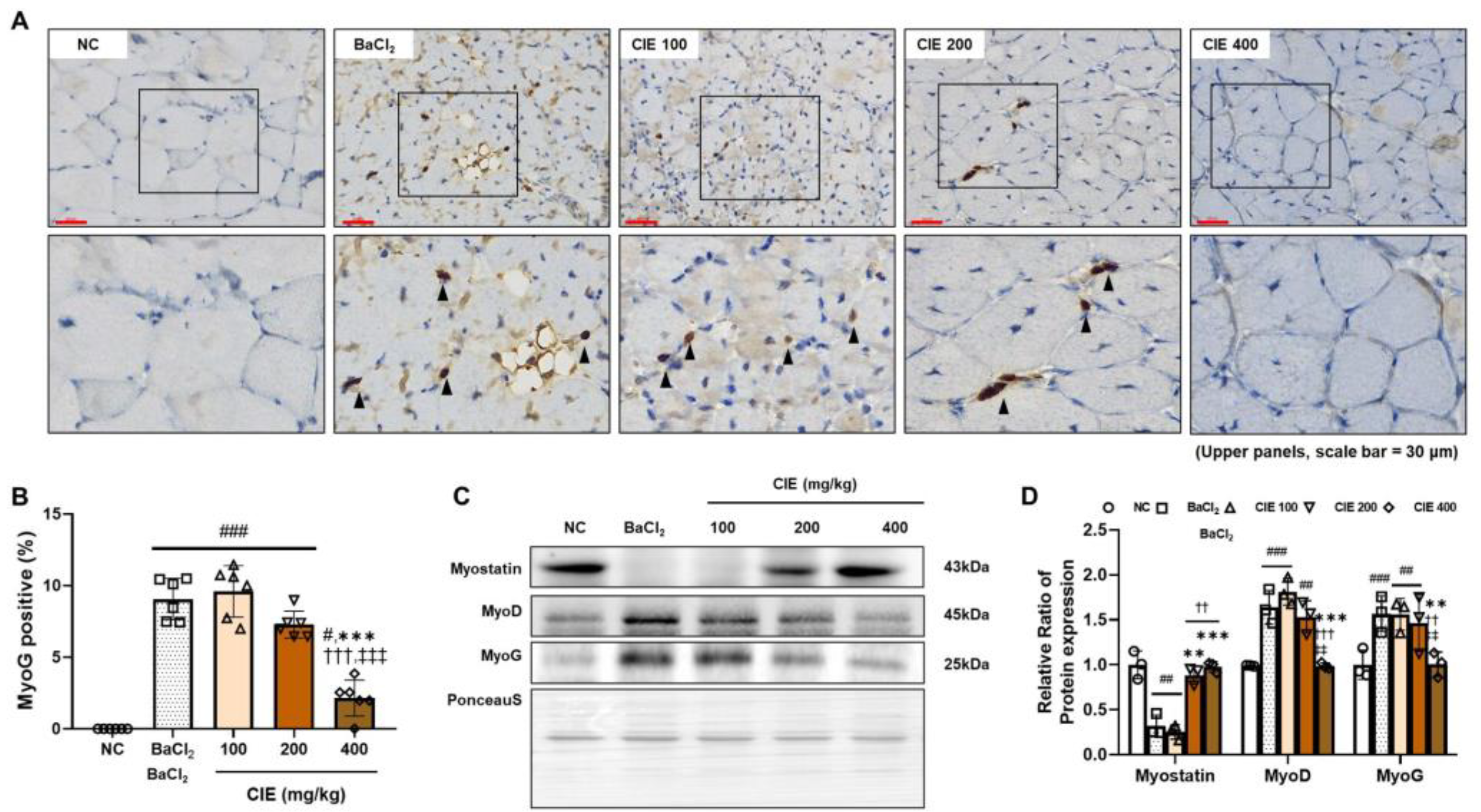
3.3. CIE Modulates MyoD, MyoG, and Myostatin Levels in TA Muscle After the BaCl2-Induced Muscle Damage
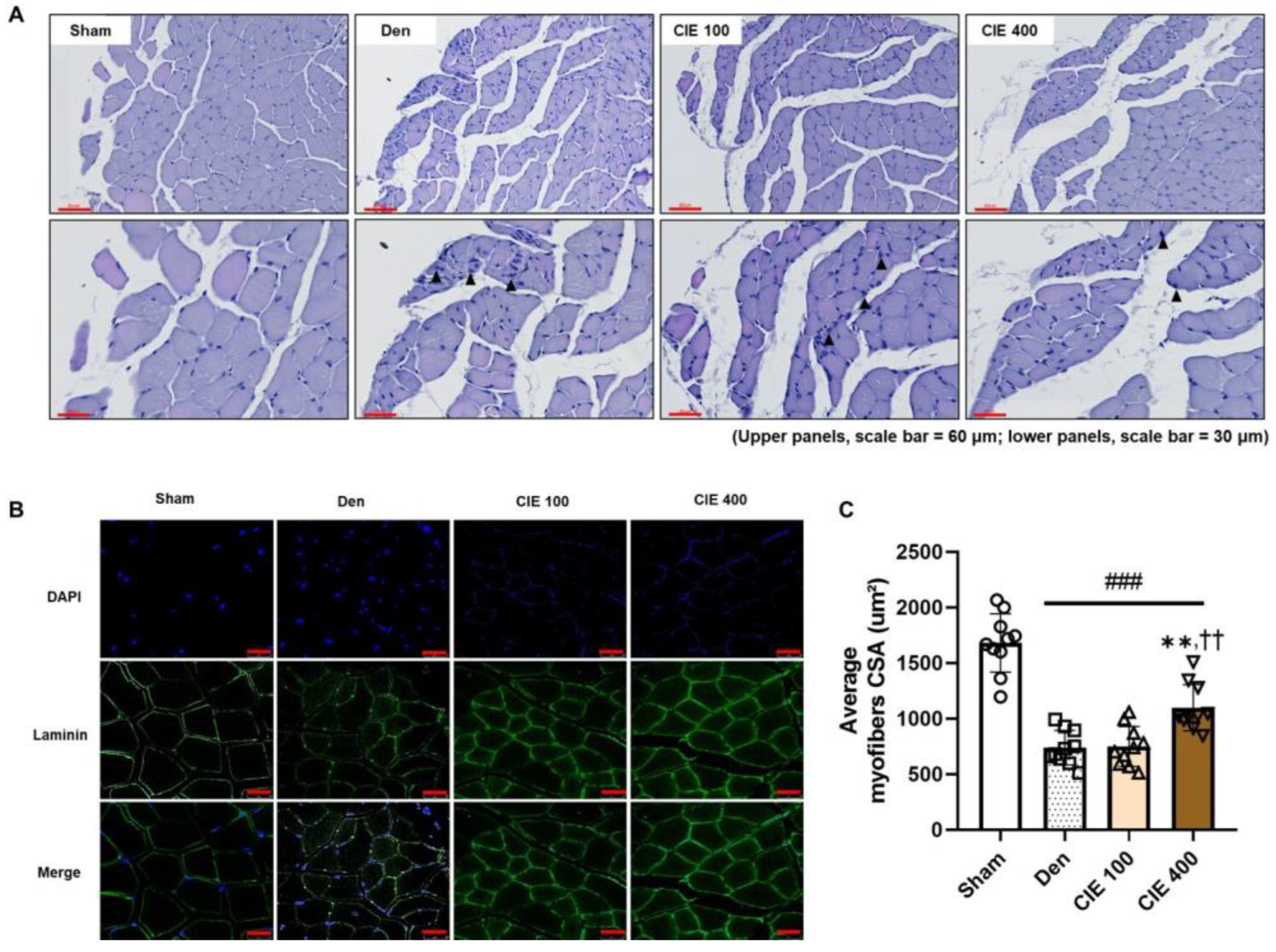
3.4. CIE Ameliorates Denervation-Induced Muscle Atrophy in TA Muscle
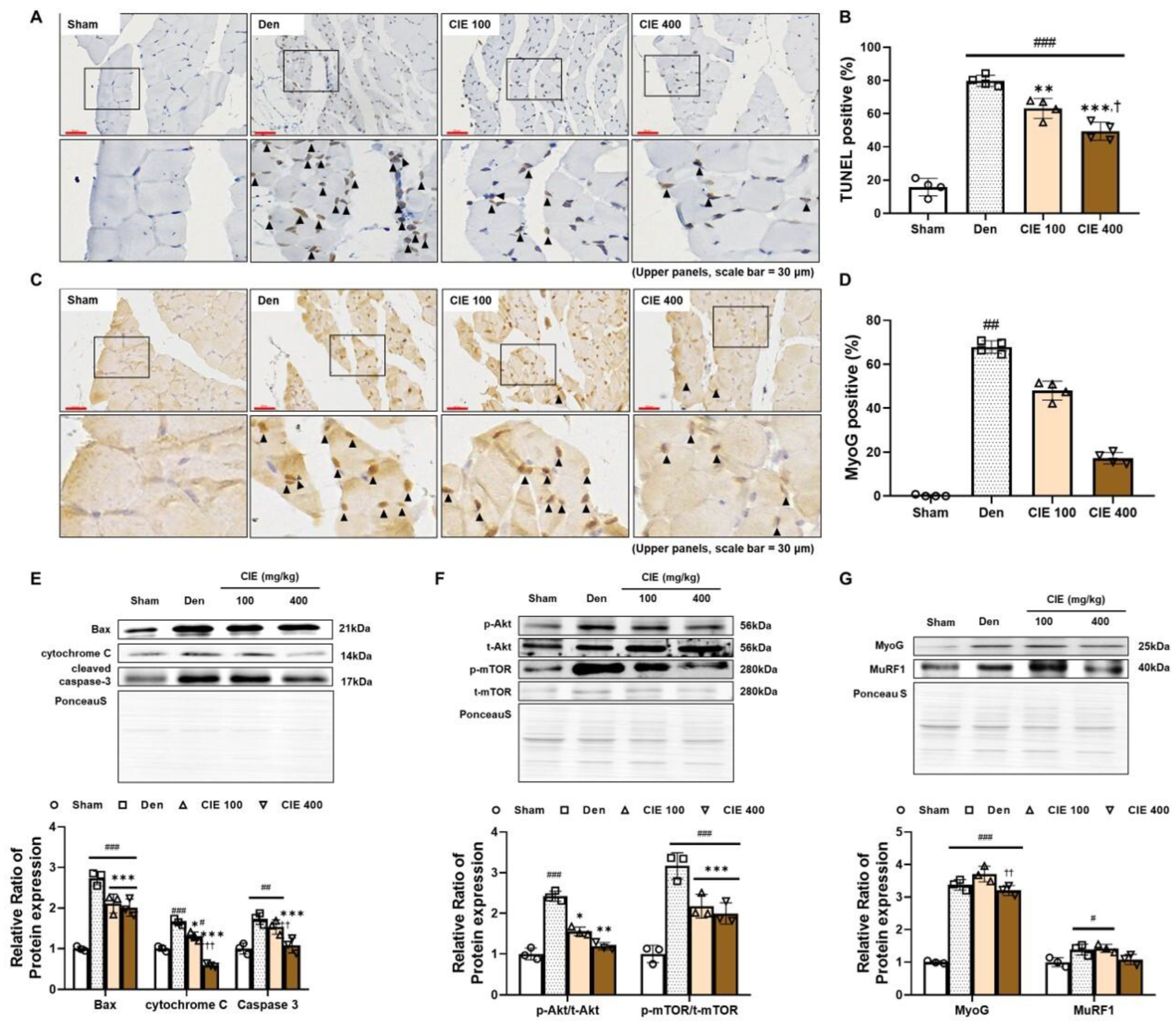
3.5. CIE Attenuates Denervation-Induced Apoptosis in TA Muscle
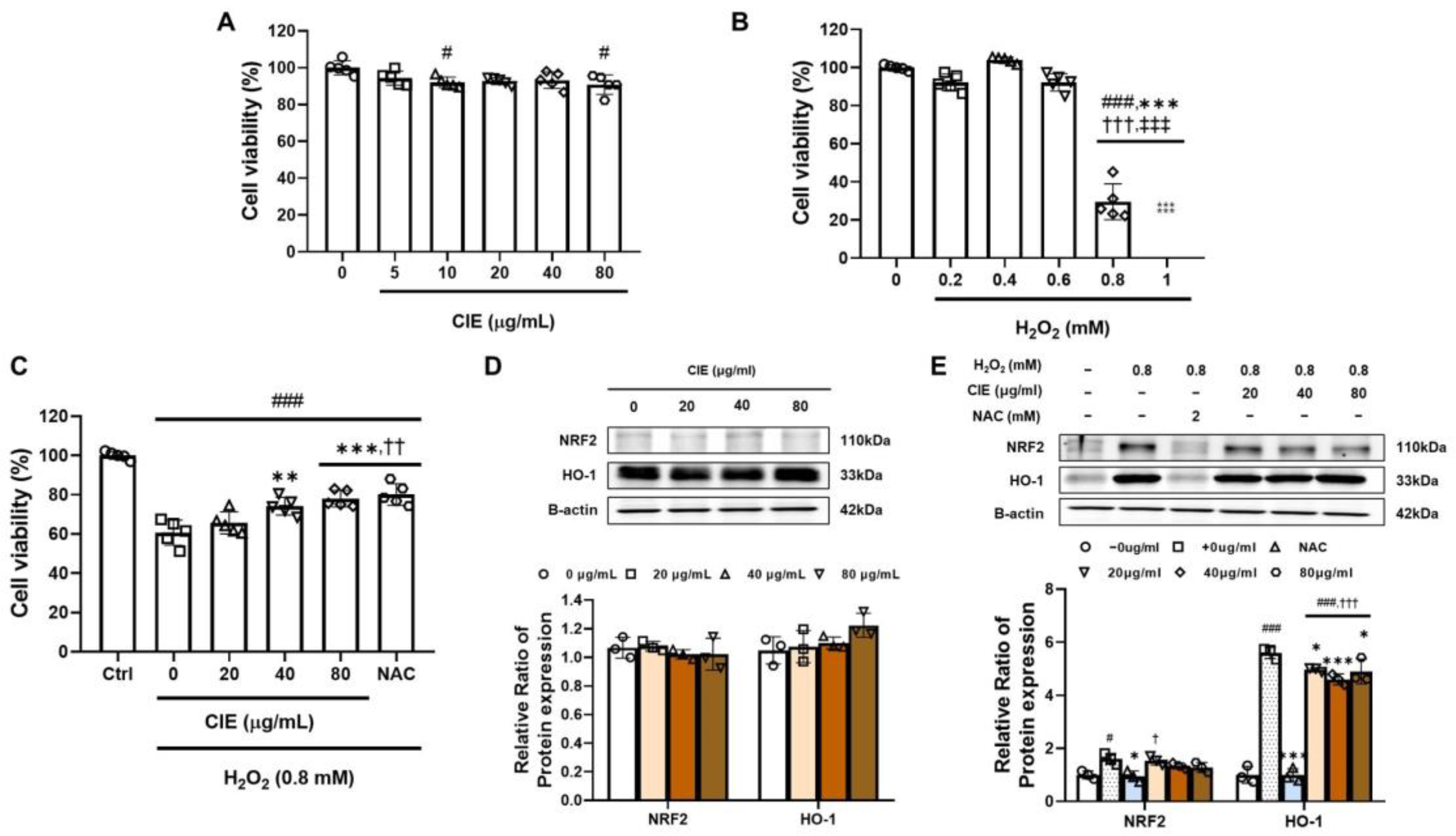
3.6. CIE Attenuates Oxidative Stress from H2O2 in C2C12 Myoblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| BaCl2 | Barium chloride |
| Bax | Bcl-2 associated X protein |
| CIE | Chestnut inner shell extract |
| CSA | Cross-sectional area |
| Den | Denervation operated group |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | Dimethyl sulfoxide |
| H&E | Hematoxylin and eosin |
| H2O2 | Hydrogen peroxide |
| HO-1 | Heme oxygenase 1 |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| mTOR | Mammalian target of rapamycin |
| MuRF1 | Muscle RING-finger protein-1 |
| MyoD | Myogenic differentiation 1 |
| MyoG | Myogenin |
| NAC | N-acetyl-L-cysteine |
| NC | Normal control |
| NRF2 | Nuclear factor erythroid 2 |
| PBS | Phosphate-buffered saline |
| PO | Per os |
| PVDF | Polyvinylidene difluoride |
| RIPA | Radio immunoprecipitation assay |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
| TA | Tibialis anterior |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
References
- Carlson, B. (Ed.) Muscle disorders. In Muscle Biology, 1st ed.; Academic Press: San Diego, CA, USA, 2021; pp. 143–161. [Google Scholar]
- Olivé, M.; Ferrer, I. Bcl-2 and bax immunohistochemistry in denervation-reinnervation and necrosis-regeneration of rat skeletal muscles. Muscle Nerve 2000, 23, 1862–1867. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.W.; Capel, A.J.; Rimington, R.P.; Wheeler, P.; Leonard, A.N.; Bishop, N.C.; Davies, O.G.; Lewis, M.P. Bioengineered human skeletal muscle capable of functional regeneration. BMC Biol. 2020, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.B.; Norton, C.E.; Jacobsen, N.L.; Fernando, C.A.; Cornelison, D.D.W.; Segal, S.S. Barium chloride injures myofibers through calcium-induced proteolysis with fragmentation of motor nerves and microvessels. Skelet. Muscle 2019, 9, 27. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial calcium: Effects of its imbalance in disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Xie, W.Q.; He, M.; Yu, D.J.; Wu, Y.X.; Wang, X.H.; Lv, S.; Xiao, W.F.; Li, Y.S. Mouse models of sarcopenia: Classification and evaluation. J. Cachexia Sarcopenia Muscle 2021, 12, 538–554. [Google Scholar] [CrossRef]
- Scalabrin, M.; Pollock, N.; Staunton, C.A.; Brooks, S.V.; McArdle, A.; Jackson, M.J.; Vasilaki, A. Redox responses in skeletal muscle following denervation. Redox. Biol. 2019, 26, 101294. [Google Scholar] [CrossRef]
- Hwangbo, H.; Park, C.; Bang, E.; Kim, H.S.; Bae, S.J.; Kim, E.; Jung, Y.; Leem, S.H.; Seo, Y.R.; Kim, G.Y.; et al. Morroniside protects C2C12 myoblasts from oxidative damage caused by ROS-mediated mitochondrial damage and induction of endoplasmic reticulum stress. Biomol. Ther. 2024, 32, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.B.; Park, D.H.; Kang, J.H. Experimental models of sarcopenia: Bridging molecular mechanism and therapeutic strategy. Cells 2020, 9, 1385. [Google Scholar] [CrossRef]
- Chun, S.E.; Lee, S.H.; Shin, Y.J.; Shin, S.H. Herbal medicine for sarcopenia: A systematic review of randomized controlled trials. J. Int. Korean Med. 2023, 44, 1118–1138. [Google Scholar] [CrossRef]
- Saeed, M.; Tasleem, M.; Haque, A.; Shoaib, A.; Rizvi, S.M.D. Muscular dystrophies and therapeutic potential of medicinal plants. JDR 2025, 4, e20240112. [Google Scholar] [CrossRef]
- Bagherniya, M.; Mahdavi, A.; Shokri-Mashhadi, N.; Banach, M.; Von Haehling, S.; Johnston, T.P.; Sahebkar, A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2772–2790. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, J.W.; Kim, J.H.; Kim, C.Y.; Ko, J.W.; Kim, T.W. Protective effects of chestnut (Castanea crenata) inner shell extract in macrophage-driven emphysematous lesion induced by cigarette smoke condensate. Nutrients 2023, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Frati, A.; Landi, D.; Marinelli, C.; Gianni, G.; Fontana, L.; Migliorini, M.; Pierucci, F.; Garcia-Gil, M.; Meacci, E. Nutraceutical properties of chestnut flours: Beneficial effects on skeletal muscle atrophy. Food Funct. 2014, 5, 2870–2882. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Quan, N.V.; Tu Anh, T.T.; Anh, L.H.; Minh, T.N. Phenolic Compositions and Antioxidant Properties in Bark, Flower, Inner Skin, Kernel and Leaf Extracts of Castanea crenata Sieb. et Zucc. Antioxidants 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, C.; Deng, B.; Liu, M. Ellagic acid attenuates muscle atrophy in STZ-induced diabetic mice. Physiol. Res. 2022, 71, 631–641. [Google Scholar] [CrossRef]
- Yu, L.; Tian, D.; Su, Z.; Zhang, L.; Guo, S.; Zhu, W.; Fang, Y.; Wang, P.; Zhang, N. Gallic acid alleviates exercise-induced muscle damage by inhibiting mitochondrial oxidative stress and ferroptosis. J. Transl. Med. 2025, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.S.; Kim, J.W.; Kim, J.H.; Kim, C.Y.; Ko, J.W.; Kim, T.W. Korean red ginseng suppresses mitochondrial apoptotic pathway in denervation-induced skeletal muscle atrophy. J. Ginseng Res. 2024, 48, 52–58. [Google Scholar] [CrossRef]
- Zheng, Y.; Lunn, A.; Gao, J.; Chen, H.; Yao, Y. Quantitative evaluation of hindlimb grip strength in mice as a measure of neuromuscular function. MethodsX 2024, 14, 103118. [Google Scholar] [CrossRef]
- El-Habta, R.; Andersson, G.; Kingham, P.J.; Backman, L.J. Anti-apoptotic effect of adipose tissue-derived stromal vascular fraction in denervated rat muscle. Stem. Cell Res. Ther. 2021, 12, 162. [Google Scholar] [CrossRef]
- Fortes, M.A.S.; Marzuca-Nassr, G.N.; Vitzel, K.F.; da Justa Pinheiro, C.H.; Newsholme, P.; Curi, R. Housekeeping proteins: How useful are they in skeletal muscle diabetes studies and muscle hypertrophy models? Anal. Biochem. 2016, 504, 38–40. [Google Scholar] [CrossRef]
- Wang, Q.; Han, W.; Ma, C.; Wang, T.; Zhong, J. Western blot normalization: Time to choose a proper loading control seriously. Electrophoresis 2023, 44, 854–863. [Google Scholar] [PubMed]
- Meyer, G.A. Evidence of induced muscle regeneration persists for years in the mouse. Muscle Nerve 2018, 58, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Kostyunina, D.S.; Ivanova, A.D.; Smirnova, O.V. Myostatin: Twenty years later. Hum. Physiol. 2018, 44, 88–101. [Google Scholar]
- Macpherson, P.C.D.; Wang, X.; Goldman, D. Myogenin regulates denervation-dependent muscle atrophy in mouse soleus muscle. J. Cell Biochem. 2011, 112, 2149–2159. [Google Scholar] [CrossRef]
- Dekeyser, G.J.; Clary, C.R.; Otis, J.S. Chronic alcohol ingestion delays skeletal muscle regeneration following injury. Regen Med. Res. 2013, 1, 2. [Google Scholar] [CrossRef]
- Musarò, A. The basis of muscle regeneration. Adv. Biol. 2014, 2014, 612471. [Google Scholar]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms Regulating Muscle Regeneration: Insights into the Interrelated and Time-Dependent Phases of Tissue Healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef]
- Jung, H.W.; Choi, J.H.; Jo, T.; Shin, H.; Suh, J.M. Systemic and local phenotypes of barium chloride induced skeletal muscle injury in mice. Ann. Geriatr. Med. Res. 2019, 23, 83–89. [Google Scholar] [CrossRef]
- Cisterna, B.A.; Cardozo, C.; Sáez, J.C. Neuronal involvement in muscular atrophy. Front. Cell Neurosci. 2014, 8, 405. [Google Scholar]
- Wang, B.B.; Guo, C.; Sun, S.Q.; Zhang, X.N.; Li, Z.; Li, W.J.; Li, D.Z.; Schumacher, M.; Liu, S. Comparison of the nerve regeneration capacity and characteristics between sciatic nerve crush and transection injury models in rats. Biomed. Env. Sci. 2023, 36, 160–173. [Google Scholar]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cell Mol. Life Sci. 2019, 76, 2559–2570. [Google Scholar] [CrossRef]
- Mendler, L.; Zádor, E.; Ver Heyen, M.; Dux, L.; Wuytack, F. Myostatin levels in regenerating rat muscles and in myogenic cell cultures. J. Muscle Res. Cell Motil. 2000, 21, 551–563. [Google Scholar] [CrossRef]
- Adhihetty, P.J.; O’Leary, M.F.N.; Chabi, B.; Wicks, K.L.; Hood, D.A. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J. Appl. Physiol. 2007, 102, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, E.M.; Andres-Mateos, E.; Mejias, R.; Simmers, J.L.; Mi, R.; Park, J.S.; Ying, S.; Hoke, A.; Lee, S.J.; Cohn, R.D. Denervation atrophy is independent from Akt and mTOR activation and is not rescued by myostatin inhibition. Dis. Model Mech. 2014, 7, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO, and regulation of apoptosis. Biochim. Biophys. Acta. Mol. Cell Res. 2011, 1813, 1978–1986. [Google Scholar] [CrossRef]
- Elwej, A.; Chaabane, M.; Ghorbel, I.; Chelly, S.; Boudawara, T.; Zeghal, N. Effects of barium graded doses on redox status, membrane bound ATPases and histomorphological aspect of the liver in adult rats. Toxicol. Mech. Methods 2017, 27, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K. Can antioxidants protect against disuse muscle atrophy? Sports Med. 2014, 44, 155–165. [Google Scholar]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and detrimental effects of reactive oxygen species on lifespan: A comprehensive review of comparative and experimental studies. Front. Cell. Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, G.; Wang, K.; Yang, J.; Shen, Y.; Yang, X.; Chen, X.; Yao, X.; Gu, X.; Qi, L.; et al. Oxidative stress: Roles in skeletal muscle atrophy. Biochem. Pharmacol. 2023, 214, 115664. [Google Scholar] [CrossRef]
- Ji, L.L.; Yeo, D. Mitochondrial dysregulation and muscle disuse atrophy. F1000Res 2019, 8, 1621. [Google Scholar] [CrossRef]
- Lee, D.; Kook, S.H.; Ji, H.; Lee, S.A.; Choi, K.C.; Lee, K.Y.; Lee, J.C. N-acetyl cysteine inhibits H2O2-mediated reduction in the mineralization of MC3T3-E1 cells by down-regulating Nrf2/HO-1 pathway. BMB Rep. 2015, 48, 636–641. [Google Scholar] [CrossRef] [PubMed]
| NC | BaCl2 | CIE 100 | CIE 200 | CIE 400 | |
|---|---|---|---|---|---|
| BW before CIE treatment (g) | 22.0 ± 0.4 | 21.8 ± 0.3 | 22.6 ± 0.6 | 21.8 ± 0.5 | 22.1 ± 0.6 |
| BW 15 days after CIE treatment (g) | 26.2 ± 0.8 | 25.0 ± 0.6 | 26.1 ± 1.1 | 24.7 ± 0.8 | 25.7 ± 1.9 |
| Injured TA weight (mg) | 55.6 ± 7.6 | 52.8 ± 5.9 | 52.0 ± 10.7 | 53.2 ± 5.7 | 54.6 ± 9.2 |
| Injured/non-injured TA weight | 1.2 ± 0.3 | 0.9 ± 0.1 # | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.3 |
| Sham | Den | CIE 100 | CIE 400 | |
|---|---|---|---|---|
| BW before surgery (g) | 20.7 ± 1.2 | 21.4 ± 1.0 | 21.1 ± 0.4 | 20.8 ± 0.9 |
| BW 1 week after surgery (g) | 21.4 ± 1.2 | 21.2 ± 0.9 | 20.7 ± 0.6 | 21.8 ± 0.7 |
| BW 2 week after surgery (g) | 21.8 ± 0.8 | 22.0 ± 1.3 | 21.5 ± 1.7 | 22.6 ± 1.1 |
| BW 3 week after surgery (g) | 24.1 ± 1.5 | 23.9 ± 1.2 | 22.9 ± 1.4 | 23.6 ± 1.3 |
| BW 4 week after surgery (g) | 23.8 ± 1.3 | 23.4 ± 1.2 | 23.2 ± 1.0 | 23.6 ± 1.3 |
| Denervated TA weight (mg) | 39.7 ± 3.5 | 28.4 ± 5.0 | 33.7 ± 2.8 | 33.0 ± 5.1 |
| Denervated/non-denervated TA weight | 1.0 ± 0.1 | 0.7 ± 0.1 ## | 0.6 ± 0.1 | 0.9 ± 0.1 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Chung, E.-H.; Kim, J.-W.; Jeong, J.-S.; Kim, C.-Y.; Lee, S.-H.; Ko, J.-W.; Lim, J.-O.; Kim, T.-W. Chestnut (Castanea crenata) Inner-Shell Extract Attenuates Barium-Chloride-Induced Injury and Denervation-Induced Atrophy in Skeletal Muscle of Mice. Nutrients 2025, 17, 2116. https://doi.org/10.3390/nu17132116
Kim J-H, Chung E-H, Kim J-W, Jeong J-S, Kim C-Y, Lee S-H, Ko J-W, Lim J-O, Kim T-W. Chestnut (Castanea crenata) Inner-Shell Extract Attenuates Barium-Chloride-Induced Injury and Denervation-Induced Atrophy in Skeletal Muscle of Mice. Nutrients. 2025; 17(13):2116. https://doi.org/10.3390/nu17132116
Chicago/Turabian StyleKim, Jin-Hwa, Eun-Hye Chung, Jeong-Won Kim, Ji-Soo Jeong, Chang-Yeop Kim, Su-Ha Lee, Je-Won Ko, Je-Oh Lim, and Tae-Won Kim. 2025. "Chestnut (Castanea crenata) Inner-Shell Extract Attenuates Barium-Chloride-Induced Injury and Denervation-Induced Atrophy in Skeletal Muscle of Mice" Nutrients 17, no. 13: 2116. https://doi.org/10.3390/nu17132116
APA StyleKim, J.-H., Chung, E.-H., Kim, J.-W., Jeong, J.-S., Kim, C.-Y., Lee, S.-H., Ko, J.-W., Lim, J.-O., & Kim, T.-W. (2025). Chestnut (Castanea crenata) Inner-Shell Extract Attenuates Barium-Chloride-Induced Injury and Denervation-Induced Atrophy in Skeletal Muscle of Mice. Nutrients, 17(13), 2116. https://doi.org/10.3390/nu17132116





