Modulating Gut Microbiota with Dietary Components: A Novel Strategy for Cancer–Depression Comorbidity Management
Abstract
1. Introduction
2. The Interactions Between Depression and Gut Microbiota
2.1. Gut Microbiota in Depressed Patients
2.2. The Effect of Gut Microbiota on Depression
3. Depression and Cancer
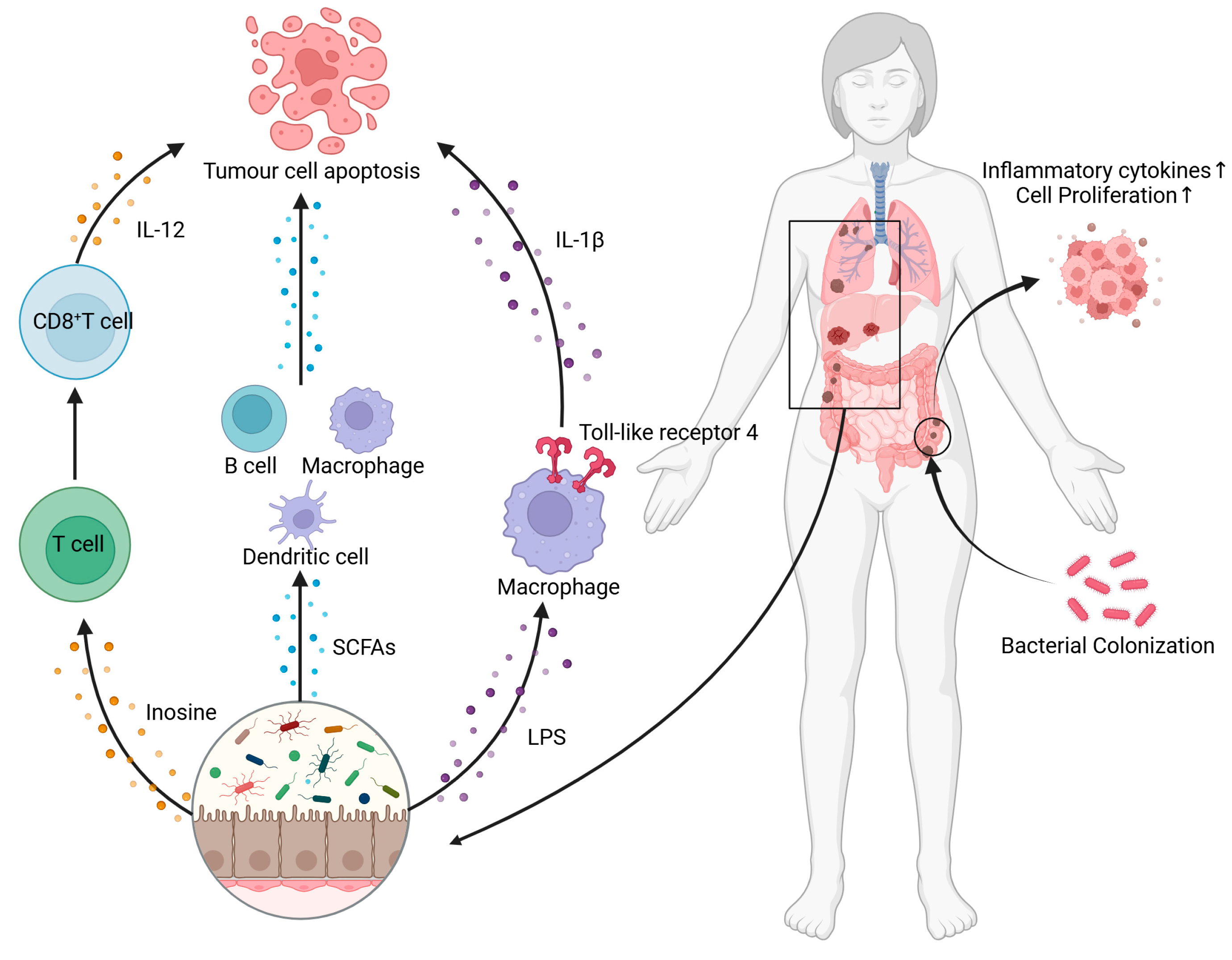
4. Interaction Between Cancer and Gut Microbiota
4.1. The Variation of Gut Microbiota in Cancer Patients
4.2. The Influence of Gut Microbiota on Cancer Development
5. Regulating Dietary Components Is a Promising Therapeutic Direction
5.1. Dietary Patterns
5.2. Probiotics and Prebiotics
5.3. Diet-Derived Phytochemicals
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCFAs | short-chain fatty acids |
| PNS | psychoneurological symptoms |
| MGB | microbiota–gut–brain |
| HPA | hypothalamic–pituitary–adrenal |
| 5-HT | 5-hydroxytryptamine |
| GABA | γ-aminobutyric acid |
| CRC | colorectal cancer |
| ROS | reactive oxygen species |
| DC | dendritic cells |
| PD-L1 | programmed cell death ligand 1 |
| BDNF | brain-derived neurotrophic factor |
| LBPs | Lycium barbarum polysaccharides |
| ITCs | isothiocyanates |
References
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut Microbiota in Obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Belkaid, Y.; Naik, S. Compartmentalized and Systemic Control of Tissue Immunity by Commensals. Nat. Immunol. 2013, 14, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fan, Z.; Yan, Q.; Pan, T.; Luo, J.; Wei, Y.; Li, B.; Fang, Z.; Lu, W. Gut Microbiota Determines the Fate of Dietary Fiber-Targeted Interventions in Host Health. Gut Microbes 2024, 16, 2416915. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut Microbiota-Derived Metabolites in the Regulation of Host Immune Responses and Immune-Related Inflammatory Diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Vliex, L.M.M.; Penders, J.; Nauta, A.; Zoetendal, E.G.; Blaak, E.E. The Individual Response to Antibiotics and Diet—Insights into Gut Microbial Resilience and Host Metabolism. Nat. Rev. Endocrinol. 2024, 20, 387–398. [Google Scholar] [CrossRef]
- Becker, S. A Historic and Scientific Review of Breast Cancer: The next Global Healthcare Challenge. Int. J. Gynecol. Obstet. 2015, 131, S36–S39. [Google Scholar] [CrossRef]
- Antoni, M.H.; Lutgendorf, S.K.; Cole, S.W.; Dhabhar, F.S.; Sephton, S.E.; McDonald, P.G.; Stefanek, M.; Sood, A.K. The Influence of Bio-Behavioural Factors on Tumour Biology: Pathways and Mechanisms. Nat. Rev. Cancer 2006, 6, 240–248. [Google Scholar] [CrossRef]
- Zong, J.; Wang, X.; Zhou, X.; Wang, C.; Chen, L.; Yin, L.; He, B.; Deng, Z. Gut-Derived Serotonin Induced by Depression Promotes Breast Cancer Bone Metastasis through the RUNX2/PTHrP/RANKL Pathway in Mice. Oncol. Rep. 2016, 35, 739–748. [Google Scholar] [CrossRef]
- O’Neill, S.; Posada Villa, J.; Medina Mora, M.E.; Al Hamzawi, A.O.; Piazza, M.; Tachimori, H.; Hu, C.; Lim, C.; Bruffaerts, R.; Lépine, J.P.; et al. Associations between DSM-IV Mental Disorders and Subsequent Self-Reported Diagnosis of Cancer. J. Psychosom. Res. 2014, 76, 207–212. [Google Scholar] [CrossRef]
- Gross, A.L.; Gallo, J.J.; Eaton, W.W. Depression and Cancer Risk: 24 Years of Follow-up of the Baltimore Epidemiologic Catchment Area Sample. Cancer Cause Control 2010, 21, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Lin, C.; Liao, K.; Chen, W. No Association between Depression and Risk of Hepatocellular Carcinoma in Older People in Taiwan. ISRN Psychiatry 2013, 2013, 901987. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Youn, S.; Vi, K.; Lee, S.; Lee, J.; Chung, S. The Prevalence of Depression among Patients with the Top Ten Most Common Cancers in South Korea. Psychiatry Investig. 2017, 14, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Mulick, A.; Magill, N.; Symeonides, S.; Gourley, C.; Burke, K.; Belot, A.; Quartagno, M.; van Niekerk, M.; Toynbee, M.; et al. Major Depression and Survival in People With Cancer. Psychosom. Med. 2021, 83, 410–416. [Google Scholar] [CrossRef]
- Shi, C.; Lamba, N.; Zheng, L.J.; Cote, D.; Regestein, Q.R.; Liu, C.M.; Tran, Q.; Routh, S.; Smith, T.R.; Mekary, R.A.; et al. Depression and Survival of Glioma Patients: A Systematic Review and Meta-Analysis. Clin. Neurol. Neurosurg. 2018, 172, 8–19. [Google Scholar] [CrossRef]
- Walker, J.; Magill, N.; Mulick, A.; Symeonides, S.; Gourley, C.; Toynbee, M.; van Niekerk, M.; Burke, K.; Quartagno, M.; Frost, C.; et al. Different Independent Associations of Depression and Anxiety with Survival in Patients with Cancer. J. Psychosom. Res. 2020, 138, 110218. [Google Scholar] [CrossRef]
- El-Sherif, R.A.M.; Shaban, A.H.; Abbas, F.A.; Alsirafy, S.A. Burden, Depression and Quality of Life in Carers of Newly Diagnosed Advanced Cancer Patients in Egypt. J. Pain Symptom Manag. 2024, 67, e403–e408. [Google Scholar] [CrossRef]
- Polityńska, B.; Pokorska, O.; Wojtukiewicz, A.M.; Sawicka, M.; Myśliwiec, M.; Honn, K.V.; Tucker, S.C.; Wojtukiewicz, M.Z. Is Depression the Missing Link between Inflammatory Mediators and Cancer? Pharmacol. Ther. 2022, 240, 108293. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the Immune System. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, W. Interaction of Microbiome and Immunity in Tumorigenesis and Clinical Treatment. Biomed. Pharmacother. 2022, 156, 113894. [Google Scholar] [CrossRef]
- Garrett, W.S. Cancer and the Microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.L.; Lyon, D.E.; Yoon, S.L.; Horgas, A.L. The Microbiome and Cancer Implications for Oncology Nursing Science. Cancer Nurs. 2016, 39, E56–E62. [Google Scholar] [CrossRef] [PubMed]
- Venkataramu, V.N.; Ghotra, H.K.; Chaturvedi, S.K. Management of Psychiatric Disorders in Patients with Cancer. Indian J. Psychiatry 2022, 64, S458–S472. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, S.; Liu, J.; Chen, F.; Li, X.; Wu, G. Gut Microbiota as a New Target for Anticancer Therapy: From Mechanism to Means of Regulation. npj Biofilms Microbiomes 2025, 11, 43. [Google Scholar] [CrossRef]
- Song, B.C.; Bai, J. Microbiome-Gut-Brain Axis in Cancer Treatment-Related Psychoneurological Toxicities and Symptoms: A Systematic Review. Support Care Cancer 2021, 29, 605–617. [Google Scholar] [CrossRef]
- Casey, D.A. Depression in Older Adults a Treatable Medical Condition. Prim. Care 2017, 44, 499–510. [Google Scholar] [CrossRef]
- Zheng, S.; Zhu, Y.; Wu, W.; Zhang, Q.; Wang, Y.; Wang, Z.; Yang, F. A Correlation Study of Intestinal Microflora and First-Episode Depression in Chinese Patients and Healthy Volunteers. Brain Behav. 2021, 11, e02036. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas Valero, M.; Noda, Y.; et al. Gut Microbiota and Major Depressive Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Chung, Y.E.; Chen, H.; Chou, H.L.; Chen, I.; Lee, M.S.; Chuang, L.; Liu, Y.; Liu, M.; Chen, C.; Wu, C.; et al. Exploration of Microbiota Targets for Major Depressive Disorder and Mood Related Traits. J. Psychiatr. Res. 2019, 111, 74–82. [Google Scholar] [CrossRef]
- Rong, H.; Xie, X.; Zhao, J.; Lai, W.; Wang, M.; Xu, D.; Liu, Y.; Guo, Y.; Xu, S.; Deng, W.; et al. Similarly in Depression, Nuances of Gut Microbiota: Evidences from a Shotgun Metagenomics Sequencing Study on Major Depressive Disorder versus Bipolar Disorder with Current Major Depressive Episode Patients. J. Psychiat. Res. 2019, 113, 90–99. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Gui, S.; Zhou, C.; Chen, J.; Yang, C.; Hu, Z.; Wang, H.; Zhong, X.; Zeng, L.; et al. Comparative Metaproteomics Analysis Shows Altered Fecal Microbiota Signatures in Patients with Major Depressive Disorder. Neuroreport 2018, 29, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, P.; Liu, Y.; Zhong, X.; Wang, H.; Guo, Y.; Xie, P. Sex Differences in Gut Microbiota in Patients with Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Cathomas, F.; Murrough, J.W.; Nestler, E.J.; Han, M.H.; Russo, S.J. Neurobiology of Resilience: Interface between Mind and Body. Biol. Psychiatry 2019, 86, 410–420. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef]
- Pearson Leary, J.; Zhao, C.; Bittinger, K.; Eacret, D.; Luz, S.; Vigderman, A.S.; Dayanim, G.; Bhatnagar, S. The Gut Microbiome Regulates the Increases in Depressive-Type Behaviors and in Inflammatory Processes in the Ventral Hippocampus of Stress Vulnerable Rats. Mol. Psychiatry 2020, 25, 1068–1079. [Google Scholar] [CrossRef]
- Bhatt, S.; Kanoujia, J.; Lakshmi, S.M.; Patil, C.R.; Gupta, G.; Chellappan, D.K.; Dua, K. Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol. Disord.-Drug Targets 2023, 22, 276–288. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.; Xing, C.; Liu, T. Pain Regulation by Gut Microbiota: Molecular Mechanisms and Therapeutic Potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Erritzoe, D.; Godlewska, B.R.; Rizzo, G.; Searle, G.E.; Agnorelli, C.; Lewis, Y.; Ashok, A.H.; Colasanti, A.; Boura, I.; Farrell, C.; et al. Brain Serotonin Release Is Reduced in Patients with Depression: A [11c]Cimbi-36 Positron Emission Tomography Study with a d-Amphetamine Challenge. Biol. Psychiatry 2023, 93, 1089–1098. [Google Scholar] [CrossRef]
- Juruena, M.F.; Bocharova, M.; Agustini, B.; Young, A.H. Atypical Depression and Non-Atypical Depression: Is HPA Axis Function a Biomarker? A Systematic Review. J. Affect. Disord. 2018, 233, 45–67. [Google Scholar] [CrossRef]
- Misiak, B.; Loniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA Axis Dysregulation in Severe Mental Illness: Can We Shift the Blame to Gut Microbiota? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.C.; Rochmawati, E.; Wiechula, R. Experiences and Perspectives of Suffering in Cancer: A Qualitative Systematic Review. Eur. J. Oncol. Nurs. 2021, 54, 102041. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Hou, L.; Cao, L.; Liu, G.; Yang, K. Effectiveness of Dignity Therapy for Patients with Advanced Cancer: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials. Depress. Anxiety 2020, 37, 234–246. [Google Scholar] [CrossRef]
- Nakamura, Z.M.; Deal, A.M.; Nyrop, K.A.; Chen, Y.T.; Quillen, L.J.; Brenizer, T.; Muss, H.B. Serial Assessment of Depression and Anxiety by Patients and Providers in Women Receiving Chemotherapy for Early Breast Cancer. Oncologist 2021, 26, 147–156. [Google Scholar] [CrossRef]
- Huehnchen, P.; van Kampen, A.; Boehmerle, W.; Endres, M. Cognitive Impairment after Cytotoxic Chemotherapy. Neuro-Oncol. Pract. 2020, 7, 11–21. [Google Scholar] [CrossRef]
- Ibrahim, E.Y.; Domenicano, I.; Nyhan, K.; Elfil, M.; Mougalian, S.S.; Cartmel, B.; Ehrlich, B.E. Cognitive Effects and Depression Associated with Taxane-Based Chemotherapy in Breast Cancer Survivors: A Meta-Analysis. Front. Oncol. 2021, 11, 642382. [Google Scholar] [CrossRef]
- Pinquart, M.; Duberstein, P.R. Depression and Cancer Mortality: A Meta-Analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef]
- Fervaha, G.; Izard, J.P.; Tripp, D.A.; Rajan, S.; Leong, D.P.; Siemens, D.R. Depression and Prostate Cancer: A Focused Review for the Clinician. Urol. Oncol. 2019, 37, 282–288. [Google Scholar] [CrossRef]
- Yu, S.; Li, W.; Tang, L.; Fan, X.; Yao, S.; Zhang, X.; Bi, Z.; Cheng, H. Depression in Breast Cancer Patients: Immunopathogenesis and Immunotherapy. Cancer Lett. 2022, 536, 215648. [Google Scholar] [CrossRef]
- Christodoulidis, G.; Konstantinos-Eleftherios, K.; Marina-Nektaria, K. Double Role of Depression in Gastric Cancer: As a Causative Factor and as Consequence. World J. Gastroenterol. 2024, 30, 1266–1269. [Google Scholar] [CrossRef]
- Michoglou, K.; Ravinthiranathan, A.; San Ti, S.; Dolly, S.; Thillai, K. Pancreatic Cancer and Depression. World J. Clin. Cases 2023, 11, 2631–2636. [Google Scholar] [CrossRef] [PubMed]
- Tosic Golubovic, S.; Binic, I.; Krtinic, D.; Djordjevic, V.; Conic, I.; Gugleta, U.; Andjelkovic Apostolovic, M.; Stanojevic, M.; Kostic, J. Risk Factors and Predictive Value of Depression and Anxiety in Cervical Cancer Patients. Medicina 2022, 58, 507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Shi, J.; Que, J.; Liu, J.; Lappin, J.M.; Leung, J.; Ravindran, A.V.; Chen, W.; Qiao, Y.; et al. Depression and Anxiety in Relation to Cancer Incidence and Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Mol. Psychiatry 2020, 25, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xu, C.; Yang, K.; Tang, S.; Tang, L.L.; Chen, L.; Li, W.; Mao, Y.; Ma, J. Causal Relationship between Genetically Predicted Depression and Cancer Risk: A Two-Sample Bi-Directional Mendelian Randomization. BMC Cancer 2022, 22, 353. [Google Scholar] [CrossRef]
- van Tuijl, L.A.; Basten, M.; Pan, K.Y.; Vermeulen, R.; Portengen, L.; de Graeff, A.; Dekker, J.; Geerlings, M.I.; Hoogendoorn, A.; Lamers, F.; et al. Depression, Anxiety, and the Risk of Cancer: An Individual Participant Data Meta-Analysis. Cancer 2023, 129, 3287–3299. [Google Scholar] [CrossRef]
- Nakhlband, A.; Farahzadi, R.; Saeedi, N.; Barzegar, H.; Montazersaheb, S.; Soofiyani, S.R. Bidirectional Relations between Anxiety, Depression, and Cancer: A Review. Curr. Drug Targets 2023, 24, 118–130. [Google Scholar] [CrossRef]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do Stress-Related Psychosocial Factors Contribute to Cancer Incidence and Survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Musselman, D.; Nemeroff, C. The Biology of Depression in Cancer and the Relationship between Depression and Cancer Progression. Int. Rev. Psychiatry 2014, 26, 16–30. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Eseadi, C.; Ngwu, M.O. Significance of Music Therapy in Treating Depression and Anxiety Disorders among People with Cancer. World J. Clin. Oncol. 2023, 14, 69–80. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, Y.; Wang, J.; Huang, A.; Liu, Y.; Han, Y.; Hu, D. The Association of Adverse Reactions and Depression in Cervical Cancer Patients Treated with Radiotherapy and/or Chemotherapy: Moderated Mediation Models. Front. Psychol. 2023, 14, 1207265. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Clark, J.; Dore, J. Fecal Microbiota Analysis: An Overview of Sample Collection Methods and Sequencing Strategies. Future Microbiol. 2015, 10, 1485–1504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Xia, Q. Role of Gut Microbiome in Cancer Immunotherapy: From Predictive Biomarker to Therapeutic Target. Exp. Hematol. Oncol. 2023, 12, 84. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pereira Marques, J.; Pinto Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric Microbial Community Profiling Reveals a Dysbiotic Cancer-Associated Microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bai, C.; Zhao, L.; Ge, Y.; Li, X. Characterization of the Fecal Microbiota in Gastrointestinal Cancer Patients and Healthy People. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2022, 24, 1134–1147. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, M.; Yao, Z.; Liang, W.; Li, Q.; Liu, J.; Yang, H.; Ji, Y.; Wei, W.; Tan, A.; et al. Breast Cancer in Postmenopausal Women Is Associated with an Altered Gut Metagenome. Microbiome 2018, 6, 136. [Google Scholar] [CrossRef]
- Liss, M.A.; White, J.R.; Goros, M.; Gelfond, J.; Leach, R.; Johnson Pais, T.; Lai, Z.; Rourke, E.; Basler, J.; Ankerst, D.; et al. Metabolic Biosynthesis Pathways Identified from Fecal Microbiome Associated with Prostate Cancer. Eur. Urol. 2018, 74, 575–582. [Google Scholar] [CrossRef]
- Matsushita, M.; Fujita, K.; Motooka, D.; Hatano, K.; Fukae, S.; Kawamura, N.; Tomiyama, E.; Hayashi, Y.; Banno, E.; Takao, T.; et al. The Gut Microbiota Associated with High-Gleason Prostate Cancer. Cancer Sci. 2021, 112, 3125–3135. [Google Scholar] [CrossRef]
- Zheng, C.; Lu, F.; Chen, B.; Yang, J.; Yu, H.; Wang, D.; Xie, H.; Chen, K.; Xie, Y.; Li, J.; et al. Gut Microbiome as a Biomarker for Predicting Early Recurrence of HBV-Related Hepatocellular Carcinoma. Cancer Sci. 2023, 114, 4717–4731. [Google Scholar] [CrossRef]
- Dai, M.; Lui, R.N.; Lau, L.H.S. The Role of Gut Microbiome and Fecal Microbiota Transplantation in Liver Cancer and Related Complications: Mechanisms and Therapeutic Potentials. Hepatoma Res. 2023, 9, 39. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, S.; Luo, J.; Dong, X.; Hao, Y.T.; Li, H.; Shan, L.; Zhou, Y.; Shi, H.; Zhang, Z.; et al. Alterations of Fecal Bacterial Communities in Patients with Lung Cancer. Am. J. Transl. Res. 2018, 10, 3171–3185. [Google Scholar] [PubMed]
- Kong, C.; Liang, L.; Liu, G.; Du, L.; Yang, Y.; Liu, J.; Shi, D.; Li, X.; Ma, Y. Integrated Metagenomic and Metabolomic Analysis Reveals Distinct Gut-Microbiome-Derived Phenotypes in Early-Onset Colorectal Cancer. Gut 2023, 72, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Burcharth, J.; Pommergaard, H.C. Linking Gut Microbiota to Colorectal Cancer. J. Cancer 2017, 8, 3378–3395. [Google Scholar] [CrossRef]
- Dai, Z.; Coker, O.O.; Nakatsu, G.; Wu, W.K.K.; Zhao, L.; Chen, Z.; Chan, F.K.L.; Kristiansen, K.; Sung, J.J.Y.; Wong, S.H.; et al. Multi-Cohort Analysis of Colorectal Cancer Metagenome Identified Altered Bacteria across Populations and Universal Bacterial Markers. Microbiome 2018, 6, 70. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, A.; Wu, X.; Deng, X.; Yang, J.; Xue, J. Heterogeneous Changes in Gut and Tumor Microbiota in Patients with Pancreatic Cancer: Insights from Clinical Evidence. BMC Cancer 2024, 24, 478. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, D.; Hou, P.; Shen, W.; Li, H.; Wang, T.; Liu, R. Dysbiosis of Gut Microbiota in Patients with Esophageal Cancer. Microb. Pathog. 2021, 150, 104709. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Z. Influence of Microbiota on Immunity and Immunotherapy for Gastric and Esophageal Cancers. Gastroenterol. Rep. 2020, 8, 206–214. [Google Scholar] [CrossRef]
- Liu, Y.; Baba, Y.; Ishimoto, T.; Gu, X.; Zhang, J.; Nomoto, D.; Okadome, K.; Baba, H.; Qiu, P. Gut Microbiome in Gastrointestinal Cancer: A Friend or Foe? Int. J. Biol. Sci. 2022, 18, 4101–4117. [Google Scholar] [CrossRef]
- Faitová, T.; Svanberg, R.; Da Cunha Bang, C.; Ilett, E.E.; Jørgensen, M.; Noguera Julian, M.; Paredes, R.; MacPherson, C.R.; Niemann, C.U. The Gut Microbiome in Patients with Chronic Lymphocytic Leukemia. Haematologica 2022, 107, 2238–2243. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Romanidou, G.; Voidarou, C.; Bezirtzoglou, E. Focus on the Gut-Kidney Axis in Health and Disease. Front. Med. 2021, 7, 620102. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hao, H.; Li, X.; Wang, Z. The Effect of Intestinal Flora on Immune Checkpoint Inhibitors in Tumor Treatment: A Narrative Review. Ann. Transl. Med. 2020, 8, 1097. [Google Scholar] [CrossRef]
- Ai, L.; Tian, H.; Chen, Z.; Chen, H.; Xu, J.; Fang, J. Systematic Evaluation of Supervised Classifiers for Fecal Microbiota-Based Prediction of Colorectal Cancer. Oncotarget 2017, 8, 9546–9556. [Google Scholar] [CrossRef]
- Scott, N.; Whittle, E.; Jeraldo, P.; Chia, N. A Systemic Review of the Role of Enterotoxic Bacteroides Fragilis in Colorectal Cancer. Neoplasia 2022, 29, 100797. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The Gut Microbiota Shapes Intestinal Immune Responses during Health and Disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Sui, H.; Zhang, L.; Gu, K.; Chai, N.; Ji, Q.; Zhou, L.; Wang, Y.; Ren, J.; Yang, L.; Zhang, B.; et al. YYFZBJS Ameliorates Colorectal Cancer Progression in ApcMin/+ Mice by Remodeling Gut Microbiota and Inhibiting Regulatory T-Cell Generation. Cell Commun. Signal 2020, 18, 113. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Wang, S.; Wei, J.; Qu, L.; Pan, L.; Xu, K. The Role of the Gut Microbiota and Probiotics Associated with Microbial Metabolisms in Cancer Prevention and Therapy. Front. Pharmacol. 2022, 13, 1025860. [Google Scholar] [CrossRef]
- Yan, X.; Bai, L.; Qi, P.; Lv, J.; Song, X.; Zhang, L. Potential Effects of Regulating Intestinal Flora on Immunotherapy for Liver Cancer. Int. J. Mol. Sci. 2023, 24, 11387. [Google Scholar] [CrossRef]
- Scheppach, W. To Keep Cancer in Check Influence of Microflora on the Development of Malignant Tumours. Aktuel. Ernahrungsmed. 2006, 31, S137–S139. [Google Scholar] [CrossRef]
- Rosadi, F.; Fiorentini, C.; Fabbri, A. Bacterial Protein Toxins in Human Cancers. Pathog. Dis. 2016, 74, ftv105. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.; Yousefi, B.; Kokhaei, P.; Arabkari, V.; Ghasemian, A. Current Information on the Association of Helicobacter Pylori with Autophagy and Gastric Cancer. J. Cell. Physiol. 2019, 234, 14800–14811. [Google Scholar] [CrossRef]
- Siegl, C.; Rudel, T. Modulation of P53 during Bacterial Infections. Nat. Rev. Microbiol. 2015, 13, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Miskeen, A.Y.; Hazari, Y.M.; Asrafuzzaman, S.; Fazili, K.M. Fusobacterium Nucleatum, Inflammation, and Immunity: The Fire within Human Gut. Tumor Biol. 2016, 37, 2805–2810. [Google Scholar] [CrossRef]
- Chaturvedi, R.; de Sablet, T.; Coburn, L.A.; Gobert, A.P.; Wilson, K.T. Arginine and Polyamines in Helicobacter Pylori-Induced Immune Dysregulation and Gastric Carcinogenesis. Amino Acids 2012, 42, 627–640. [Google Scholar] [CrossRef]
- Dieterich, L.C.; Bikfalvi, A. The Tumor Organismal Environment: Role in Tumor Development and Cancer Immunotherapy. Semin. Cancer Biol. 2020, 65, 197–206. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, X.; Yang, Z.; Yang, X.; Peng, Q. Bacteria and Tumor: Understanding the Roles of Bacteria in Tumor Genesis and Immunology. Microbiol. Res. 2022, 261, 127082. [Google Scholar] [CrossRef]
- Abdelaziz, D.H.A.; Khalil, H.; Cormet Boyaka, E.; Amer, A.O. The Cooperation between the Autophagy Machinery and the Inflammasome to Implement an Appropriate Innate Immune Response: Do They Regulate Each Other? Immunol. Rev. 2015, 265, 194–204. [Google Scholar] [CrossRef]
- Wang, S.; Xu, B.; Zhang, Y.; Chen, G.; Zhao, P.; Gao, Q.; Yuan, L. The Role of Intestinal Flora on Tumorigenesis, Progression, and the Efficacy of PD-1/PD-L1 Antibodies in Colorectal Cancer. Cancer Biol. Med. 2024, 21, 65–82. [Google Scholar] [CrossRef]
- Bruggemann, H.; Al Zeer, M.A. Bacterial Signatures and Their Inflammatory Potentials Associated with Prostate Cancer. Apmis 2020, 128, 80–91. [Google Scholar] [CrossRef]
- Hou, M.; Yu, Q.; Yang, L.; Zhao, H.; Jiang, P.; Qin, L.; Zhang, Q. The Role of Short-Chain Fatty Acid Metabolism in the Pathogenesis, Diagnosis and Treatment of Cancer. Front. Oncol. 2024, 14, 1451045. [Google Scholar] [CrossRef] [PubMed]
- Encarnacao, J.C.; Abrantes, A.M.; Pires, A.S.; Botelho, M.F. Revisit Dietary Fiber on Colorectal Cancer: Butyrate and Its Role on Prevention and Treatment. Cancer Metastasis Rev. 2015, 34, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Donovan, H.S.; Hagan, T.L.; Campbell, G.B.; Boisen, M.M.; Rosenblum, L.M.; Edwards, R.P.; Bovbjerg, D.H.; Horn, C.C. Nausea as a Sentinel Symptom for Cytotoxic Chemotherapy Effects on the Gut-Brain Axis among Women Receiving Treatment for Recurrent Ovarian Cancer: An Exploratory Analysis. Support Care Cancer 2016, 24, 2635–2642. [Google Scholar] [CrossRef]
- Paulsen, J.A.; Ptacek, T.S.; Carter, S.J.; Liu, N.; Kumar, R.; Hyndman, L.; Lefkowitz, E.J.; Morrow, C.D.; Rogers, L.Q. Gut Microbiota Composition Associated with Alterations in Cardiorespiratory Fitness and Psychosocial Outcomes among Breast Cancer Survivors. Support Care Cancer 2017, 25, 1563–1570. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut Microbiome-Wide Association Study of Depressive Symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Jordan, K.R.; Loman, B.R.; Bailey, M.T.; Pyter, L.M. Gut Microbiota-immune-brain Interactions in Chemotherapy-associated Behavioral Comorbidities. Cancer 2018, 124, 3990–3999. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Eng, T.; Shelton, J.; Khanna, N.; Scott, I.; Meador, R.; Bruner, D.W. Associations of the Gut Microbiome with Psychoneurological Symptom Cluster in Women with Gynecologic Cancers: A Longitudinal Study. Support Care Cancer 2023, 31, 626. [Google Scholar] [CrossRef]
- Ganci, M.; Suleyman, E.; Butt, H.; Ball, M. The Role of the Brain-Gut-Microbiota Axis in Psychology: The Importance of Considering Gut Microbiota in the Development, Perpetuation, and Treatment of Psychological Disorders. Brain Behav. 2019, 9, e01408. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-Term Dietary Patterns Are Associated with pro-Inflammatory and Anti-Inflammatory Features of the Gut Microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Kwon, C.; Ediriweera, M.K.; Kim Cho, S. Interplay between Phytochemicals and the Colonic Microbiota. Nutrients 2023, 15, 1989. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Illiano, P.; Brambilla, R.; Parolini, C. The Mutual Interplay of Gut Microbiota, Diet and Human Disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of Dietary Intervention on Human Diseases: Molecular Mechanisms and Therapeutic Potential. Signal Transduct. Target Ther. 2024, 9, 59. [Google Scholar] [CrossRef]
- Klement, R.J.; Pazienza, V. Impact of Different Types of Diet on Gut Microbiota Profiles and Cancer Prevention and Treatment. Medicina 2019, 55, 84. [Google Scholar] [CrossRef]
- Li, Y.; Lv, M.R.; Wei, Y.J.; Sun, L.; Zhang, J.X.; Zhang, H.G.; Li, B. Dietary Patterns and Depression Risk: A Meta-Analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef]
- Taylor, S.R.; Falcone, J.N.; Cantley, L.C.; Goncalves, M.D. Developing Dietary Interventions as Therapy for Cancer. Nat. Rev. Cancer 2022, 22, 452–466. [Google Scholar] [CrossRef]
- Mao, Y.Q.; Huang, J.T.; Zhang, S.L.; Kong, C.; Li, Z.-M.; Jing, H.; Chen, H.L.; Kong, C.Y.; Huang, S.H.; Cai, P.R.; et al. The Antitumour Effects of Caloric Restriction Are Mediated by the Gut Microbiome. Nat. Metab. 2023, 5, 96–110. [Google Scholar] [CrossRef]
- Ajona, D.; Ortiz Espinosa, S.; Lozano, T.; Exposito, F.; Calvo, A.; Valencia, K.; Redrado, M.; Remírez, A.; Lecanda, F.; Alignani, D.; et al. Short-Term Starvation Reduces IGF-1 Levels to Sensitize Lung Tumors to PD-1 Immune Checkpoint Blockade. Nat. Cancer 2020, 1, 75–85. [Google Scholar] [CrossRef]
- Nakamura, K.; Tonouchi, H.; Sasayama, A.; Ashida, K. A Ketogenic Formula Prevents Tumor Progression and Cancer Cachexia by Attenuating Systemic Inflammation in Colon 26 Tumor-Bearing Mice. Nutrients 2018, 10, 206. [Google Scholar] [CrossRef]
- Orillion, A.; Damayanti, N.P.; Shen, L.; Adelaiye Ogala, R.; Affronti, H.; Elbanna, M.; Chintala, S.; Ciesielski, M.; Fontana, L.; Kao, C.; et al. Dietary Protein Restriction Reprograms Tumor-Associated Macrophages and Enhances Immunotherapy. Clin. Cancer Res. 2018, 24, 6383–6395. [Google Scholar] [CrossRef]
- Li, T.; Tan, Y.T.; Chen, Y.X.; Zheng, X.-J.; Wang, W.; Liao, K.; Mo, H.Y.; Lin, J.; Yang, W.; Piao, H.L.; et al. Methionine Deficiency Facilitates Antitumour Immunity by Altering m6A Methylation of Immune Checkpoint Transcripts. Gut 2023, 72, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, R.; Zhang, L.; Rifky, M.; Sui, W.; Zhu, Q.; Zhang, J.; Yin, J.; Zhang, M. Dietary Intervention in Depression—A Review. Food Funct. 2022, 13, 12475–12486. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A Randomised Controlled Trial of Dietary Improvement for Adults with Major Depression (the ‘SMILES’ Trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Vaghef Mehrabany, E.; Ranjbar, F.; Asghari Jafarabadi, M.; Hosseinpour Arjmand, S.; Ebrahimi Mameghani, M. Calorie Restriction in Combination with Prebiotic Supplementation in Obese Women with Depression: Effects on Metabolic and Clinical Response. Nutr. Neurosci. 2021, 24, 339–353. [Google Scholar] [CrossRef]
- Yary, T.; Aazami, S. Dietary Intake of Zinc Was Inversely Associated with Depression. Biol. Trace Elem. Res. 2012, 145, 286–290. [Google Scholar] [CrossRef]
- Park, M.; Choi, J.; Lee, H.J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef]
- Gumus, H.; Ilgin, R.; Koc, B.; Yuksel, O.; Kizildag, S.; Guvendi, G.; Karakilic, A.; Kandis, S.; Hosgorler, F.; Ates, M.; et al. A Combination of Ketogenic Diet and Voluntary Exercise Ameliorates Anxiety and Depression-like Behaviors in Balb/c Mice. Neurosci. Lett. 2022, 770, 136443. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Faghfouri, A.H.; Keramati, M.; Jamilian, P.; Jamilian, P.; Mohagheghi, A.; Farnam, A. Probiotics as an Effective Therapeutic Approach in Alleviating Depression Symptoms: An Umbrella Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 8292–8300. [Google Scholar] [CrossRef]
- Agah, S.; Alizadeh, A.M.; Mosavi, M.; Ranji, P.; Khavari Daneshvar, H.; Ghasemian, F.; Bahmani, S.; Tavassoli, A. More Protection of Lactobacillus Acidophilus than Bifidobacterium Bifidum Probiotics on Azoxymethane-Induced Mouse Colon Cancer. Probiotics Antimicrob. Proteins 2019, 11, 857–864. [Google Scholar] [CrossRef]
- Gurbatri, C.; Lia, I.; Vincent, R.; Coker, C.; Castro, S.; Treuting, P.; Hinchliffe, T.; Arpaia, N.; Danino, T. Engineered Probiotics for Local Delivery of Checkpoint Blockade Nanobodies. Cancer Res. 2020, 80, eaax0876. [Google Scholar] [CrossRef]
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for Cancer Alternative Prevention and Treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, J.; Lauber, C.L.; Walters, W.A.; Parfrey, L.W.; Clemente, J.C.; Gevers, D.; Knight, R. Experimental and Analytical Tools for Studying the Human Microbiome. Nat. Rev. Genet. 2012, 13, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Matsuda, K.; Nomoto, K. Counting the Countless: Bacterial Quantification by Targeting Rrna Molecules to Explore the Human Gut Microbiota in Health and Disease. Front. Microbiol. 2018, 9, 1417. [Google Scholar] [CrossRef]
- Moludi, J.; Saber, A.; Zozani, M.A.; Moradi, S.; Azamian, Y.; Hajiahmadi, S.; Pasdar, Y.; Moradi, F. The Efficacy of Probiotics Supplementation on the Quality of Life of Patients with Gastrointestinal Disease: A Systematic Review of Clinical Studies. Prev. Nutr. Food Sci. 2024, 29, 237–255. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A Defined Commensal Consortium Elicits CD8 T Cells and Anti-Cancer Immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A Randomized Controlled Trial to Test the Effect of Multispecies Probiotics on Cognitive Reactivity to Sad Mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, C.; Li, W.; Wei, J.; Hong, H.; Li, J.; Feng, L.; Wei, H.; Xin, H.; Chen, T. A Phase Ii Randomized Clinical Trial and Mechanistic Studies Using Improved Probiotics to Prevent Oral Mucositis Induced by Concurrent Radiotherapy and Chemotherapy in Nasopharyngeal Carcinoma. Front. Immunol. 2021, 12, 618150. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani Poor, Z.; Tajabadi Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of Psychotropic-like Properties of a Probiotic Formulation (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) in Rats and Human Subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Miqdady, M.; Al Mistarihi, J.; Azaz, A.; Rawat, D. Prebiotics in the Infant Microbiome: The Past, Present, and Future. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Hou, C.; Gao, Y.; Xue, Y.; Yan, Y.; Guo, X. Metagenomic Analysis of Gut Microbiota Modulatory Effects of Jujube (Ziziphus Jujuba Mill.) Polysaccharides in a Colorectal Cancer Mouse Model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Su, S.; Dai, G.; He, L. Prebiotics Modulate Gut Microbiota-Mediated T-Cell Immunity to Enhance Theinhibitory Effect of Sintilimab in Lewis Lung Adenocarcinoma Model Mice. Anti-Cancer Agents Med. Chem. 2023, 23, 1966–1973. [Google Scholar] [CrossRef]
- Li, Y.; Elmén, L.; Segota, I.; Xian, Y.; Tinoco, R.; Feng, Y.; Fujita, Y.; Segura Muñoz, R.R.; Schmaltz, R.; Bradley, L.M.; et al. Prebiotic-Induced Anti-Tumor Immunity Attenuates Tumor Growth. Cell Rep. 2020, 30, 1753–1766.e6. [Google Scholar] [CrossRef]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Floel, A. Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef]
- Ganesan, K.; Jayachandran, M.; Xu, B. Diet-Derived Phytochemicals Targeting Colon Cancer Stem Cells and Microbiota in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 3976. [Google Scholar] [CrossRef]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. [Google Scholar] [CrossRef]
- Luo, B.; Wen, Y.; Ye, F.; Wu, Y.; Li, N.; Farid, M.S.; Chen, Z.; El Seedi, H.R.; Zhao, C. Bioactive Phytochemicals and Their Potential Roles in Modulating Gut Microbiota. J. Agric. Food Res. 2023, 12, 100583. [Google Scholar] [CrossRef]
- Pérez Burillo, S.; Navajas Porras, B.; López Maldonado, A.; Hinojosa Nogueira, D.; Pastoriza, S.; Rufián Henares, J.Á. Green Tea and Its Relation to Human Gut Microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.M.; Hintze, K.J.; Rompato, G.; Wettere, A.J.V.; Ward, R.E.; Phatak, S.; Neal, C.; Armbrust, T.; Stewart, E.C.; Thomas, A.J.; et al. Dietary Supplementation with Black Raspberries Altered the Gut Microbiome Composition in a Mouse Model of Colitis-Associated Colorectal Cancer, Although with Differing Effects for a Healthy versus a Western Basal Diet. Nutrients 2022, 14, 5270. [Google Scholar] [CrossRef]
- Zhu, X.H.; Lang, H.D.; Wang, X.L.; Hui, S.C.; Zhou, M.; Kang, C.; Yi, L.; Mi, M.T.; Zhang, Y. Synergy between Dihydromyricetin Intervention and Irinotecan Chemotherapy Delays the Progression of Colon Cancer in Mouse Models. Food Funct. 2019, 10, 2040–2049. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, C.; Wang, X.L.; Zhou, M.; Chen, M.T.; Zhu, X.H.; Liu, K.; Wang, B.; Zhang, Q.Y.; Zhu, J.D.; et al. Dietary Factors Modulate Colonic Tumorigenesis through the Interaction of Gut Microbiota and Host Chloride Channels. Mol. Nutr. Food Res. 2018, 62, 1700554. [Google Scholar] [CrossRef]
- Gong, Y.; Dong, R.; Gao, X.; Li, J.; Jiang, L.; Zheng, J.; Cui, S.; Ying, M.; Yang, B.; Cao, J.; et al. Neohesperidin Prevents Colorectal Tumorigenesis by Altering the Gut Microbiota. Pharmacol. Res. 2019, 148, 104460. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Xiong, Y.; Huang, J.; Liu, Z. An Analysis of the Intestinal Microbiome Combined with Metabolomics to Explore the Mechanism of How Jasmine Tea Improves Depression in Cums-Treated Rats. Foods 2024, 13, 2636. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Y.; Chen, H.; Jiang, H.; Zhou, F.; Lv, B.; Xu, M. Curcumin Alleviates Dss-Induced Anxiety-like Behaviors via the Microbial-Brain-Gut Axis. Oxidative Med. Cell. Longev. 2022, 2022, 6244757. [Google Scholar] [CrossRef]
- Wang, A.; Liu, Y.; Zeng, S.; Liu, Y.; Li, W.; Wu, D.; Wu, X.; Zou, L.; Chen, H. Dietary Plant Polysaccharides for Cancer Prevention: Role of Immune Cells and Gut Microbiota, Challenges and Perspectives. Nutrients 2023, 15, 3019. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng Polysaccharides Alter the Gut Microbiota and Kynurenine/Tryptophan Ratio, Potentiating the Antitumour Effect of Antiprogrammed Cell Death 1/Programmed Cell Death Ligand 1 (Anti-PD-1/PD-L1) Immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma Lucidum Polysaccharide Modulates Gut Microbiota and Immune Cell Function to Inhibit Inflammation and Tumorigenesis in Colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, M.; Jin, H.; Yang, J.; Kang, S.; Liu, Y.; Yang, S.; Ma, S.; Ni, J. Effects of Lycium Barbarum Polysaccharides on Immunity and the Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice. Front. Microbiol. 2021, 12, 701566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.P.; Cheng, J.; Liu, Q.; Xu, G.H.; Li, C.F.; Yi, L.T. Dendrobium Officinale Polysaccharides Alleviate Depression-like Symptoms via Regulating Gut Microbiota-Neuroinflammation in Perimenopausal Mice. J. Funct. Foods 2022, 88, 104912. [Google Scholar] [CrossRef]
- Wang, M.; Sun, P.; Li, Z.; Li, J.; Lv, X.; Chen, S.; Zhu, X.; Chai, X.; Zhao, S. Eucommiae Cortex Polysaccharides Attenuate Gut Microbiota Dysbiosis and Neuroinflammation in Mice Exposed to Chronic Unpredictable Mild Stress: Beneficial in Ameliorating Depressive-like Behaviors. J. Affect. Disord. 2023, 334, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shi, A.; Wang, Q.; Zhou, J. High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota. Nutrients 2019, 11, 3005. [Google Scholar] [CrossRef]
- Gao, J.; Ma, L.; Yin, J.; Li, T.; Yin, Y.; Chen, Y. Canola Oil Ameliorates Obesity by Suppressing Lipogenesis and Reprogramming the Gut Microbiota in Mice via the Ampk Pathway. Nutrients 2024, 16, 3379. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurised Bacterium Blunts Colitis Associated Tumourigenesis by Modulation of CD8+ T Cells in Mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Pu, S.; Khazanehei, H.; Jones, P.J.; Khafipour, E. Interactions between Obesity Status and Dietary Intake of Monounsaturated and Polyunsaturated Oils on Human Gut Microbiome Profiles in the Canola Oil Multicenter Intervention Trial (Comit). Front. Microbiol. 2016, 7, 1612. [Google Scholar] [CrossRef]
- Na, G.; He, C.; Zhang, S.; Tian, S.; Bao, Y.; Shan, Y. Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 1962. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, B.; Li, Y.; Yuan, Q. Potential Mechanisms of Cancer Prevention and Treatment by Sulforaphane, a Natural Small Molecule Compound of Plant-Derived. Mol. Med. 2024, 30, 94. [Google Scholar] [CrossRef]
- Melim, C.; Lauro, M.R.; Pires, I.M.; Oliveira, P.J.; Cabral, C. The Role of Glucosinolates from Cruciferous Vegetables (Brassicaceae) in Gastrointestinal Cancers: From Prevention to Therapeutics. Pharmaceutics 2022, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Holman, J.; McKinstry, D.; Trindade, B.C.; Eaton, K.A.; Mendoza-Castrejon, J.; Ho, S.; Wells, E.; Yuan, H.; Wen, B.; et al. A Steamed Broccoli Sprout Diet Preparation That Reduces Colitis via the Gut Microbiota. J. Nutr. Biochem. 2023, 112, 109215. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tian, K.; Zhang, T.; Fan, C.H.; Zhou, P.; Zeng, D.; Zhao, S.; Li, L.S.; Smith, H.S.; Li, J.; et al. Cyanate Induces Oxidative Stress Injury and Abnormal Lipid Metabolism in Liver through Nrf2/Ho-1. Molecules 2019, 24, 3231. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Singh, D.; Singh, G.; Attri, S.; Singh, D.; Sharma, M.; Buttar, H.S.; Bedi, N.; Singh, B.; Arora, S. Pharmacokinetics and Toxicity Profiling of 4-(Methylthio)Butyl Isothiocyanate with Special Reference to Pre-Clinical Safety Assessment Studies. Toxicon 2022, 212, 19–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Chadaideh, K.S.; Li, Y.; Li, Y.; Gu, X.; Liu, Y.; Guasch-Ferré, M.; Rimm, E.B.; Hu, F.B.; Willett, W.C.; et al. Butter and Plant-Based Oils Intake and Mortality. JAMA Intern. Med. 2025, e250205. [Google Scholar] [CrossRef]
- Sarvizadeh, M.; Hasanpour, O.; Naderi Ghale Noie, Z.; Mollazadeh, S.; Rezaei, M.; Pourghadamyari, H.; Masoud Khooy, M.; Aschner, M.; Khan, H.; Rezaei, N.; et al. Allicin and Digestive System Cancers: From Chemical Structure to Its Therapeutic Opportunities. Front. Oncol. 2021, 11, 650256. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Zhang, X.; Du, X.; Guan, Y.; Li, M.; Chang, A.K.; He, X.; Li, X.; Bi, X. Antidepressant Effects of Sulforaphane (SFN) and Its Derivatives SLL-III-9 and SLL-III-120 and Their Potential Underlying Mechanisms Based on the Microbiota–Gut–Brain Axis. Food Funct. 2024, 15, 10539–10552. [Google Scholar] [CrossRef]
- Yang, Y.; Eguchi, A.; Mori, C.; Hashimoto, K. Dietary Sulforaphane Glucosinolate Mitigates Depression-like Behaviors in Mice with Hepatic Ischemia/Reperfusion Injury: A Role of the Gut-Liver-Brain Axis. J. Psychiatr. Res. 2024, 176, 129–139. [Google Scholar] [CrossRef]
- Tsenkova, M.; Brauer, M.; Pozdeev, V.I.; Kasakin, M.; Busi, S.B.; Schmoetten, M.; Cheung, D.; Meyers, M.; Rodriguez, F.; Gaigneaux, A.; et al. Ketogenic Diet Suppresses Colorectal Cancer through the Gut Microbiome Long Chain Fatty Acid Stearate. Nat. Commun. 2025, 16, 1792. [Google Scholar] [CrossRef]
- McLeod, A.; Wolf, P.; Chapkin, R.S.; Davidson, L.A.; Ivanov, I.; Berbaum, M.; Williams, L.R.; Gaskins, H.R.; Ridlon, J.; Sanchez Flack, J.; et al. Design of the Building Research in Crc Prevention (Bridge-Crc) Trial: A 6-Month, Parallel Group Mediterranean Diet and Weight Loss Randomized Controlled Lifestyle Intervention Targeting the Bile Acid-Gut Microbiome Axis to Reduce Colorectal Cancer Risk among African American/Black Adults with Obesity. Trials 2023, 24, 113. [Google Scholar] [CrossRef]
- Piazzi, G.; Prossomariti, A.; Baldassarre, M.; Montagna, C.; Vitaglione, P.; Fogliano, V.; Biagi, E.; Candela, M.; Brigidi, P.; Balbi, T.; et al. A Mediterranean Diet Mix Has Chemopreventive Effects in a Murine Model of Colorectal Cancer Modulating Apoptosis and the Gut Microbiota. Front. Oncol. 2019, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, L.; Dai, X.; Huang, Y.; Gao, Y.; Wang, F. Modulation of Intestinal Metabolites by Calorie Restriction and Its Association with Gut Microbiota in a Xenograft Model of Colorectal Cancer. Discov. Oncol. 2024, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, H.; Xia, C.; Dong, Q.; Chen, E.; Qiu, Y.; Su, Y.; Xie, H.; Zeng, L.; Kuang, J.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Probiotics to Reduce the Severity of Oral Mucositis Induced by Chemoradiotherapy for Patients with Nasopharyngeal Carcinoma. Cancer 2019, 125, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Hsu, J.; Bergerot, P.G.; Gillece, J.D.; Folkerts, M.; Reining, L.; Trent, J.; Highlander, S.K.; Pal, S.K. Randomized Trial Assessing Impact of Probiotic Supplementation on Gut Microbiome and Clinical Outcome from Targeted Therapy in Metastatic Renal Cell Carcinoma. Cancer Med. 2021, 10, 79–86. [Google Scholar] [CrossRef]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic Supplement Attenuates Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer: A Randomised, Double-Blind, and Placebo-Controlled Trial. Eur. J. Cancer 2022, 161, 10–22. [Google Scholar] [CrossRef]
- Sugimoto, T.; Atobe, S.; Kado, Y.; Takahashi, A.; Motoori, M.; Sugimura, K.; Miyata, H.; Yano, M.; Tanaka, K.; Doki, Y.; et al. Gut Microbiota Associated with the Mitigation Effect of Synbiotics on Adverse Events of Neoadjuvant Chemotherapy in Patients with Esophageal Cancer: A Retrospective Exploratory Study. J. Med. Microbiol. 2023, 72, 1723. [Google Scholar] [CrossRef]
- Huo, R.; Yang, W.J.; Liu, Y.; Liu, T.; Li, T.; Wang, C.Y.; Pan, B.S.; Wang, B.L.; Guo, W. Stigmasterol: Remodeling Gut Microbiota and Suppressing Tumor Growth through Treg and CD8+ T Cells in Hepatocellular Carcinoma. Phytomedicine 2024, 129, 155225. [Google Scholar] [CrossRef]
- Zhu, Q.; Han, Y.; He, Y.; Meng, P.; Fu, Y.; Yang, H.; He, G.; Long, M.; Shi, Y. Quercetin Inhibits Neuronal Ferroptosis and Promotes Immune Response by Targeting Lipid Metabolism-Related Gene PTGS2 to Alleviate Breast Cancer-Related Depression. Phytomedicine 2024, 130, 155560. [Google Scholar] [CrossRef]
- Tajasuwan, L.; Kettawan, A.; Rungruang, T.; Wunjuntuk, K.; Prombutara, P. Role of Dietary Defatted Rice Bran in the Modulation of Gut Microbiota in Aom/Dss-Induced Colitis-Associated Colorectal Cancer Rat Model. Nutrients 2023, 15, 1528. [Google Scholar] [CrossRef]
- Lee, P.S.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Inhibitory Effect of Garcinol on Obesity-Exacerbated, Colitis-Mediated Colon Carcinogenesis. Mol. Nutr. Food Res. 2021, 65, e2100410. [Google Scholar] [CrossRef]


| Cancer Type | Microbes Enhanced in the Gut | Microbes Inhibited in the Gut | References |
|---|---|---|---|
| Gastric cancer | Achromobacter, Citrobacter, Phyllobacter, Clostridium, Rhodococcus, and Lactobacillus. | Helicobacter, Blautia producta, Butyricoccus pullicaecorum, and R. faecis. | [64,65] |
| Breast cancer | Escherichia coli, Klebsiella sp_1_1_55, Prevotella amnii, Enterococcus gallinarum, Actinomyces sp. HPA0247, Shewanella putrefaciens, and Erwinia amylovora. | Eubacterium eligens, Lactobacillus vaginalis, Acinetobacter radioresistens, and Enterococcus gallinarum. | [66] |
| Prostate cancer | Bacteroides, Streptococcus, Rikenellaceae, Alistipes, and Lachnospira spp. | - | [67,68] |
| Liver cancer | Streptococcus, Klebsiella, Proteobacteria, Stenotrophomonas, Proteobacteria, and Veillonella. | Ruminococcus, Faecalibafcterium, Firmicutes, Ruminococcaceae, Butyricicoccus, and Lachnospiraceae. | [69,70] |
| Lung cancer | Bacteroides, Veillonella, and Fusobacterium. | Escherichia-Shigella, Kluyvera, Fecalibacterium, Enterobacter, and Dialister. | [71] |
| Colorectal cancer | Malassezia, Talaromyces, Trametes, Bacteroides fragilis, Akkermansia muciniphila, Clostridium hathewayi, and Alistipes finegoldii. | Pleosporaceae, Alemnaria, Blautia producta, and Roseburia faecis. | [72,73,74] |
| Pancreatic cancer | Proteobacteria, Synergistetes, Euryarchaeota, Bacteroides, and Verrucomicrobia. | Firmicutes, Actinobacteria, and Proteobacteria. | [75] |
| Pancreatic cancer | Streptococcus, Bifidobacterium, Subdoligranulum, Blautia, Romboutsia, Collinsella, Paeniclostridium, Dorea, and Atopobium. | Lachnospira, Bacteroides, Agathobacter, Fusobacterium, Parabacteroides, Paraprevotella, Butyricicoccus, Tyzzerella, Fusicatenibacter, and Sutterella. | [65,76] |
| Esophageal cancer | Bacteroidetes and Prevotella. | Faecalibacterium, Roseburia, and Blautia obeum. | [77,78] |
| Cervical cancer | Bacteroides and Parabacteroides. | Anaerostipes, Bifidobacterium, Blautia, Enterococcus faecalis, Dorea, Eubacterium, Ruminococcus, and Streptococci. | [79] |
| Types | Sample | Intervention | Key Findings and Conclusions | Reference |
|---|---|---|---|---|
| Dietary patterns | ||||
| KD | Humanized microbiome CRC mouse model; germ-free mice | KD consumption (dose not specified); microbiome transplantation |
| [179] |
| MD-MIX, AOM, LFD | A/J male mice (AOM-treated; healthy LFD controls) | MD-MIX supplementation in LFD-fed mice; AOM injection (dose not specified) |
| [180] |
| CR, IF | Female mice with subcutaneous MC38 tumors | Six groups: Ad libitum, CR, IF, antibiotics+ad libitum, antibiotics+CR, antibiotics+IF. |
| [181] |
| CR | CRC xenograft mice | CR: Initiated 12 days post-inoc. (100 mm³), 3 weeks duration. |
| [182] |
| Probiotics and prebiotics | ||||
| Probiotic combination (Bifidobacterium longum, Lactobacillus lactis, Enterococcus faecium) | Adults (18–70 years) with locally advanced nasopharyngeal carcinoma (n = 99) | Three capsules twice daily; 7 week |
| [183] |
| Bifidobacterium animalis-containing probiotic yogurt | 20 randomized metastatic renal cell carcinoma (mRCC) patients initiating VEGF-TKI therapy. | Two 4 oz servings of probiotic yogurt daily, continued for ≥12 weeks (stool sampling until week 12) |
| [184] |
| Bifidobacterium longum, Lactobacillus acidophilus, Enterococcus faecalis | 159 breast cancer patients; Sprague–Dawley rats (hippocampal damage model) | Three capsules (0.84 g each) twice daily during chemotherapy (4–6 cycles, 21 day/cycle) |
| [185] |
| Lacticaseibacillus paracasei Shirota, Bifidobacterium breve Yakult, galacto-oligosaccharides | 73 esophageal cancer patients undergoing NAC | LBG+EN: 600 mL EN, 3 g probiotics, 15 mL GOS daily (pre-NAC to end) |
| [186] |
| Diet-derived phytochemicals | ||||
| Stigmasterol | Balb/c mice bearing subcutaneous hepatocellular carcinoma | Oral administration of stigmasterol at doses of 0 (control), 50, 100, or 200 mg/kg every 2 days for 3 weeks |
| [187] |
| Quercetin | BALB/c mice (BCRD model induced by 4T1 cells + CORT); primary hippocampal neurons (induced by LPS + CORT) | In vivo: quercetin treatment in BCRD mice. In vitro: hippocampal neurons treated with quercetin. PTGS2 overexpression to validate mechanism |
| [188] |
| Defatted rice bran | AOM/DSS-induced colitis-associated CRC rat model | Defatted rice bran supplementation |
| [189] |
| Garcinol | HFD-induced obese mice with AOM/DSS colitis-associated colon cancer | 0.05% dietary garcinol supplementation |
| [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Yang, H.; Wang, R.; Wang, X.; Zhang, X. Modulating Gut Microbiota with Dietary Components: A Novel Strategy for Cancer–Depression Comorbidity Management. Nutrients 2025, 17, 1505. https://doi.org/10.3390/nu17091505
Dai H, Yang H, Wang R, Wang X, Zhang X. Modulating Gut Microbiota with Dietary Components: A Novel Strategy for Cancer–Depression Comorbidity Management. Nutrients. 2025; 17(9):1505. https://doi.org/10.3390/nu17091505
Chicago/Turabian StyleDai, Haochen, Haiyi Yang, Rui Wang, Xuanpeng Wang, and Xin Zhang. 2025. "Modulating Gut Microbiota with Dietary Components: A Novel Strategy for Cancer–Depression Comorbidity Management" Nutrients 17, no. 9: 1505. https://doi.org/10.3390/nu17091505
APA StyleDai, H., Yang, H., Wang, R., Wang, X., & Zhang, X. (2025). Modulating Gut Microbiota with Dietary Components: A Novel Strategy for Cancer–Depression Comorbidity Management. Nutrients, 17(9), 1505. https://doi.org/10.3390/nu17091505









