Highlights
- Dual-Action Probiotics: L. plantarum strains LTJ1 and LTJ48, derived from Baijiu grains, degrade purines, lower uric acid in cells/mice, and restore gut flora.
- Multi-Target Therapy: These strains suppress ADA, inhibit URAT1/GLUT9, and enhance ABCG2, offering safer hyperuricemia treatment.
- Microbiome Regulation: LTJ1 and LTJ48 enrich beneficial bacteria and reduce harmful taxa, improving gut health in HUA mice.
Abstract
Objectives: Hyperuricemia (HUA) is a metabolic disorder linked to serious complications, yet current treatments face safety limitations. This study aimed to identify novel probiotic strains from Chinese Baijiu fermentation grains with dual-action mechanisms for HUA management—direct uric acid (UA) reduction and gut microbiota restoration. Methods: Two Lactiplantibacillus plantarum strains (LTJ1/LTJ48) were screened for purine/nucleoside degradation using HPLC. Their efficacy was evaluated in HepG2 cells and HUA mice. Key assessments included UA levels, renal/hepatic markers (AST, CRE, BUN), ADA/XOD activity, UA transporter expression (URAT1, GLUT9, ABCG2), and 16S rRNA-based microbiota analysis. Results: LTJ1/LTJ48 degraded >97% of purines/nucleosides in vitro. In HUA mice, they reduced serum UA by 31.0% (LTJ1) and 51.5% (LTJ48), improved renal/hepatic function, and suppressed ADA activity. They modulated UA transporters and restored gut microbiota. Conclusions: LTJ1/LTJ48 exhibit multi-target HUA alleviation via purine degradation, ADA inhibition, UA transporter regulation, and microbiota remodeling, offering a safer probiotic-based alternative to conventional therapies. Their translational potential warrants further clinical exploration.
1. Introduction
Hyperuricemia (HUA), defined as a serum uric acid (UA) level exceeding 420 μmol/L on two consecutive measurements, results from purine metabolism dysregulation and/or renal-intestinal excretion impairment [1,2]. This condition is clinically linked to multi-organ complications such as cardiovascular diseases [3], chronic kidney disease [4], and metabolic syndrome [5], with its global prevalence rising markedly in recent decades, especially in younger cohorts. Pathogenetically, HUA originates from two distinct mechanisms: disordered purine metabolism causing UA overproduction, and underexcretion primarily through renal or intestinal pathways [6]. Sustained HUA induces urate crystal deposition in joints and soft tissues, triggering subsequent sterile inflammation and gout onset, with approximately 20% of cases progressing to clinical gout [7]. Critically, emerging evidence identifies the gut–kidney axis as a master regulator of systemic urate homeostasis, establishing the intestine as both an immunometabolic interface and an alternative compensatory excretion route [8].
Current therapeutic strategies for HUA management integrate pharmacotherapy with nutritional approaches [9,10]. Clinically endorsed agents comprise three mechanistic classes: xanthine oxidase (XOD) inhibitors (such as allopurinol and febuxostat) that suppress de novo urate synthesis through competitive inhibition of hypoxanthine oxidation [11]; uricosuric drugs (predominantly benzbromarone) targeting urate transporter 1 (URAT1) to enhance renal clearance [12]; and recombinant uricolytic enzymes (notably rasburicase) mediating the enzymatic conversion of UA to water-soluble allantoin [13]. Nevertheless, significant safety concerns constrain their application. Allopurinol carries risks of severe cutaneous adverse reactions [14], febuxostat demonstrates elevated cardiovascular event rates [15], and benzbromarone potentiates drug-induced liver injury [16], whereas probenecid–aspirin polytherapy compromises both urate-lowering efficacy and antimicrobial safety profiles [17,18]. These limitations underscore the urgent need for safer alternatives.
Accumulating evidence positions gut microbiota dysbiosis as a pathogenic driver of HUA [19]. High-purine diets exert dual pathological effects: inducing structural damage to the intestinal barrier and triggering taxonomic shifts in microbial communities—mechanistically linked to impaired UA excretion and breakdown of immune tolerance [19,20,21]. Notably, purine-metabolizing probiotics derived from traditional fermented foods have garnered research attention as multi-mechanistic therapeutic candidates, exhibiting enteric purine sequestration, microbiota restitution, and immunometabolic modulation capabilities [22,23]. Of particular interest, Lactiplantibacillus plantarum (L. plantarum)—a phylogenetically diverse species enriched in fermented substrates—has demonstrated translational potential in metabolic disorders through xanthine oxidoreductase activity and microbiota-derived metabolite production [24,25].
In order to develop and explore the ability of potential probiotics to improve hyperuricemia, in this study, we identified and characterized two L. plantarum strains (designated LTJ1 and LTJ48) from Chinese baijiu fermentation grains. We hypothesized their therapeutic potential for HUA intervention through three mechanistic axes: purine/nucleoside degradation capacity, urate-lowering efficacy in cellular and murine models, and microbiome-modulating metabolic regulation of UA homeostasis.
2. Materials and Methods
2.1. In Vitro Screening of Lactiplantibacillus with High Purine and Nucleoside Degradation Capability
Lactiplantibacillus strains were previously isolated and screened from the fermented grains of soy sauce-flavored baijiu produced in northern China. Primary cultures were established by inoculating single colonies into 1 mL sterile MRS broth (AOBOXING), followed by 12 h incubation at 37 °C. Secondary cultures were prepared through 2% (v/v) inoculation of fresh MRS medium with primary culture under identical incubation conditions. Following centrifugation at 4000× g for 3 min, bacterial pellets from secondary cultures underwent triple washing with sterile saline. Washed cell suspensions were then incubated at 37 °C for 8 h in the prepared 0.2 g/L of inosine and adenosine (Meilunbio, Dalian, China) or 0.2 g/L of guanine and hypoxanthine (Macklin, Shanghai, China) mixture buffer. Reactions were terminated with 0.1 mol/L HClO4 (Century Aokebio, Wuhan, China).
Subsequent HPLC analysis (20 μL injection volume) employed a Tri-Sal Plus C18 column (SALA2-5046250, Agilent Technologies, Santa Clara, CA, USA) with the following parameters: Mobile phase: 20 mmol/L KH2PO4 (pH 3.0, Jiangtian Chemical, Nantong, China) containing 10% methanol (Concord Technology, Hong Kong, China), Flow rate: 1 mL/min, Column temperature: 25 °C, Detection: 254 nm (hypoxanthine, inosine, adenosine); 273 nm (guanine). Quantification was performed using external calibration curves established for each analyte. Substrate degradation rates were calculated based on peak area reduction compared to abiotic controls.
2.2. Strain Identification
Genomic DNA of L. plantarum strains were isolated using the Tianamp Bacteria DNA Kit. A 16S rRNA gene amplification was performed through PCR with universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The PCR reaction mixture (25 μL total volume) contained 17.0 μL sterile ddH2O, 1.0 μL each of forward and reverse primers (10 μmol/L), 1.0 μL dNTPs (2.5 mmol/L), 0.5 μL Taq DNA polymerase (5 U/μL), 2.5 μL 10× PCR buffer, and 2.0 μL DNA template. Thermal cycling parameters consisted of an initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation (95 °C, 45 s), annealing (56 °C, 1 min), and extension (72 °C, 1 min), with a final extension at 72 °C for 10 min. PCR products were purified and subjected to bidirectional sequencing through a commercial sequencing service (Genewiz, Suzhou, China). Sequence alignment and species identification were performed using the BLASTn algorithm against the NCBI GenBank database (https://blast.ncbi.nlm.nih.gov, accessed on 10 April 2023).
2.3. Growth Kinetics Determination
Bacterial growth kinetics were monitored through optical density measurements at 600 nm (OD600) using a spectrophotometric method. The strain was inoculated into MRS broth at 2% (v/v) inoculum size and incubated at 37 °C for 36 h. To establish the growth curve, aliquots were aseptically sampled at 4 h intervals for OD600 determination and plotting cultivation time against corresponding OD600 values.
2.4. Induction of HUA Cell Model
HepG2 cells were maintained in DMEM (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA) under 5% CO2 at 37 °C. At 80–90% confluence during the logarithmic growth phase, cells were trypsinized and seeded in 6-well plates at a density of 1 × 106 cells/well. Following a 24 h adhesion period, cultures were washed twice with PBS (pH 7.4) before experimental interventions. For HUA induction, three experimental groups were established: NC group: received 2 mL fresh basal medium; model group: treated with 2 mL PBS-supplemented medium; and treatment group: exposed to 15% (v/v) filter-sterilized L. plantarum metabolites in complete medium. After 24 h pre-treatment, all groups (except NC) received adenosine solution (0.25 mmol/L final concentration) for purine nucleotide loading. Following subsequent 24 h incubation, xanthine oxidase (XOD, Nanjing Jiancheng, Nanjing, China) was introduced at 0.005 U/mL concentration to initiate UA biosynthesis. The enzymatic reaction was terminated after 12 h by immediate supernatant collection. The dosage and time used were verified by a CCK-8 experiment.
2.5. Quantitative UA Analysis
The supernatant from the wells was collected. UA concentrations were determined using a commercial enzymatic colorimetric assay kit (Nanjing Jiancheng) following the manufacturer’s protocol. Absorbance measurements at 293 nm were performed in triplicate using a microplate reader, with UA standards establishing the calibration curve. Data normalization was performed against total cellular protein content quantified by a BCA assay.
2.6. Animal Experiment Design
All experimental protocols were approved by the Animal Ethics Committee of Tianjin University of Science and Technology and conducted in compliance with institutional guidelines for laboratory animal care. Fifty male SPF Kunming mice (4 weeks old, 18–22 g) purchased from SPF Biotechnology Co., Ltd. (Beijing, China) were acclimatized for 7 days under controlled conditions (24 ± 2 °C, 52 ± 5% humidity, 12 h light/dark cycle).
Mice were randomly divided into five groups (n = 10/group): negative control (NC); HUA model (M); LTJ1 intervention (1 × 108 CFU L. plantarum LTJ1); LTJ48 intervention (1 × 108 CFU L. plantarum LTJ48); and allopurinol control (AP). HUA was induced via daily oral gavage of 10 g/kg yeast extract combined with intraperitoneal injection of 300 mg/kg potassium oxonate (all groups except NC). Intervention groups received corresponding bacterial solutions 1 h post-modeling. Treatments continued for 21 d with daily dose adjustment based on body weight. After final administration, blood samples were collected via orbital puncture. Mice were then euthanized by cervical dislocation for tissue harvesting (liver, kidney, small intestine, colon/cecum contents), and the tissues were stored at −80 °C for subsequent analyses.
2.7. RT-qPCR Analysis
Total RNA was isolated from cellular samples and murine tissues (kidney/small intestine) using Trizol reagent (Sigma, St. Louis, MO, USA) following standard extraction protocol. RNA integrity was confirmed by spectrophotometry prior to reverse transcription. cDNA was synthesized using the GoScript™ Reverse Transcription System (Promega, Fitchburg, WI, USA) according to the manufacturer’s instructions. qPCR was performed using SYBR® Green Master Mix (DBI® Bioscience, Shanghai, China) on a StepOnePlus™ Real-Time PCR System. Each 20 μL reaction contained 10 μL 2 × SYBR Green Mix, 0.4 μL ROX passive reference dye, 1 μL gene-specific primers (10 μM), 1 μL cDNA template, and 7.6 μL nuclease-free water (Solarbio, Beijing, China). Reactions were performed in triplicate with the following thermal profile: 95 °C for 10 min, 40 cycles of 95 °C (15 s), 60 °C (30 s), and 72 °C (30 s). Gene expression quantification was calculated using the 2−ΔΔCT method normalized to GAPDH as an endogenous control. Primer sequences are listed in Table 1.

Table 1.
Sequences of RT-qPCR specific primer.
2.8. DNA Extraction and 16S rRNA Gene Amplification
Mouse colonic contents were homogenized and subjected to genomic DNA extraction using a commercial kit (Solarbio, Beijing, China) following the manufacturer’s protocol. The hypervariable V3–V4 region of the bacterial 16S rRNA gene was amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). PCR reactions (25 μL) contained Fast Pfu DNA polymerase (TransGen, Beijing, China) and were performed under the following conditions: 95 °C for 3 min; 35 cycles of 95 °C (30 s), 55 °C (30 s), and 72 °C (45 s); and final extension at 72 °C for 10 min. Amplicons were verified by 1.0% agarose gel electrophoresis, purified using a gel extraction kit, and subjected to a second round of PCR for index addition. Sequencing libraries were constructed by ligating Illumina adapters and quantified by Qubit fluorometry. Paired-end sequencing (2 × 250 bp) was performed on an Illumina HiSeq 2500 platform. Raw sequencing data were processed using bioinformatic pipelines (QIIME2 and DADA2) for quality filtering, ASV clustering, and taxonomic classification against the SILVA database to characterize microbial community composition and diversity.
2.9. Statistical Analysis
All statistical analyses were performed using GraphPad Prism 8 software. For comparisons between two independent groups, two-tailed Student’s t-tests were applied to assess statistical significance. The significance thresholds were defined as follows: not significant (ns) for p > 0.05; * p < 0.05; ** p < 0.01; *** p < 0.001. Quantitative data are presented as mean ± standard deviation (SD) from three independent biological replicates.
3. Results
3.1. Solation and Characterization of Nucleoside and Purine-Degrading L. plantarum LTJ1 and LTJ48
To identify bacterial candidates with nucleoside and purine-degrading capabilities, 36 Lactobacillus strains were rigorously screened by incubating them for 8 h in PBS containing a nucleoside or purine mixture (0.2 g/L). Metabolic profiles were analyzed via HPLC, with characteristic absorption peaks identified for key substrates: inosine (8.197 min), adenosine (12.716 min), guanine (8.954 min), hypoxanthine (5.301 min), and xanthine (4.820 min) (Figure 1A–F). HPLC analysis revealed a consistent metabolic pattern: Enzymatic conversion by Lactobacillus strains generated xanthine and hypoxanthine as terminal products, accompanied by substrate depletion. Among all candidates, L. plantarum LTJ1 and LTJ48—previously isolated from Chinese baijiu fermentation grains—exhibited exceptional degradation efficiency (Figure 1G,H). LTJ1 completely degraded adenosine (100%) and inosine (99.5%), with 98.01% guanine and 12.0% hypoxanthine degradation, yielding a total purine reduction of 35.2% within 8 h (Figure 1B,E,G,H). Similarly, LTJ48 degraded 100% of adenosine and 99.2% of inosine, along with 97.7% of guanine and 27.6% of hypoxanthine, achieving a total purine degradation rate of 42.2% within 8 h (Figure 1C,F–H). These findings suggest that L. plantarum LTJ1 and LTJ48 may serve as promising candidates for the intervention of HUA.
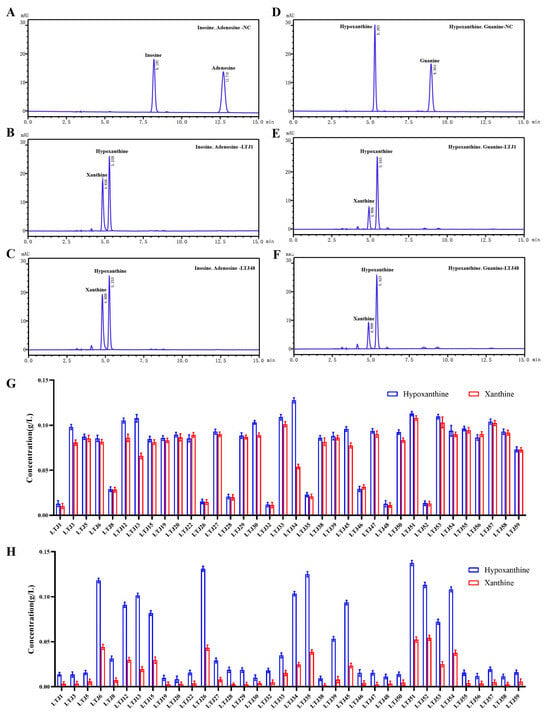
Figure 1.
HPLC analysis of nucleoside and purine degradation by L. plantarum LTJ1 and LTJ48. (A) Chromatogram of nucleoside mixture (0.2 g/L adenosine and 0.2 g/L inosine); (B) degradation profiles of adenosine and inosine by LTJ1 and LTJ48; (C,D) chromatogram of purine mixture (0.2 g/L guanine and 0.2 g/L hypoxanthine); (E) degradation profiles of guanine and hypoxanthine by LTJ1 and LTJ48; and (F) screening results of 36 Lactobacillus strains for nucleoside (G) and purine (H) degradation. Characteristic retention times for key metabolites: inosine (8.197 min), adenosine (12.716 min), guanine (8.954 min), hypoxanthine (5.301 min), and xanthine (4.820 min).
3.2. Molecular Identification and Growth Characterization of L. plantarum LTJ1 and LTJ48
Following isolation and purification via three-zone streaking, both LTJ1 and LTJ48 formed opaque, white circular colonies on agar plates (Figure 2A). Growth curve analysis revealed comparable proliferation patterns, with both strains entering the stationary phase after 20 h of cultivation (Figure 2B). Molecular identification was performed by amplifying 16S rRNA genes through PCR, followed by sequencing and comparative analysis against the GenBank bacterial database. Phylogenetic reconstruction (neighbor-joining method) demonstrated 97.0% (LTJ1) and 98% (LTJ48) sequence similarity to L. plantarum type strains, confirming their taxonomic classification (Figure 2C). The strains have been deposited in the China General Microbiological Culture Collection Center (CGMCC) under accession numbers CGMCC No. 25123 (LTJ1) and CGMCC No. 25124 (LTJ48).
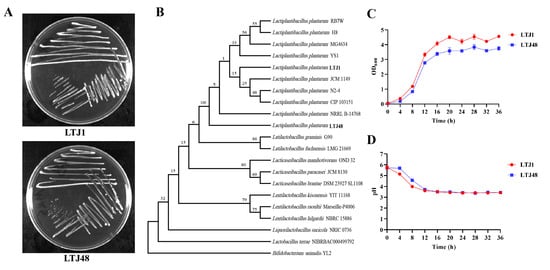
Figure 2.
Molecular and phenotypic characterization of L. plantarum LTJ1 and LTJ48. (A) Colony morphology on MRS agar after 48 h incubation (37 °C); (B) phylogenetic tree reconstructed by neighbor-joining method based on 16S rRNA gene sequences; (C) the growth curve of LTJ1 and LTJ48; and (D) the acid production capacity of LTJ1 and LTJ48.
3.3. L. plantarum LTJ1 and LTJ48 Suppress UA Biosynthesis in HUA HepG2 Cells
To evaluate the anti-HUA potential of LTJ1 and LTJ48 metabolites (postbiotics), we established a HUA cell model using HepG2 hepatocytes. Treatment with 15% (v/v) postbiotics from LTJ1 and LTJ48 significantly reduced intracellular UA levels by 40.0% (p < 0.001) and 43.6% (p < 0.001), respectively, compared to untreated HUA controls (Figure 3A). We further investigated its mechanistic basis through multi-omics analysis. Targeted enzymatic assays revealed marked suppression of purine nucleoside phosphorylase (PNP) activity and adenosine deaminase (ADA) activity (p < 0.001 vs. model group), paralleled by downregulation of PNP and ADA gene expression (Figure 3B–D).
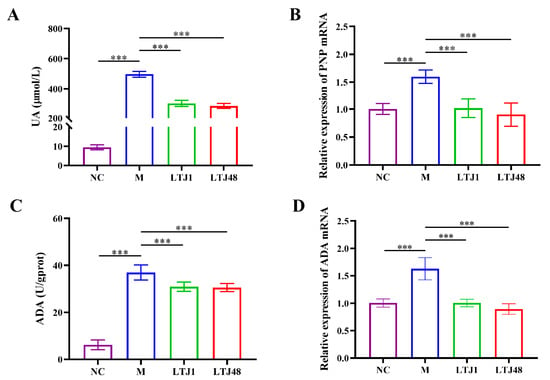
Figure 3.
L. plantarum LTJ1 and LTJ48 attenuate HUA in HepG2 hepatocytes. (A) Intracellular UA levels after 24 h co-culture; (B) RT-qPCR analysis of PNP mRNA expression; (C) ADA enzymatic activity; and (D) ADA gene expression quantified by RT-qPCR. NC was the control group. M was the HUA cell model group. The LTJ1 group is the postbiotics of L. plantarum LTJ1 (15% v/v). The LTJ48 group is the postbiotics of L. plantarum LTJ48 (15% v/v). Data expressed as mean ± SD (n = 4 biological replicates), *** p < 0.001.
3.4. L. plantarum LTJ1 and LTJ48 Ameliorate HUA in Mice Through ADA Suppression
To validate the anti-HUA effects in vivo, HUA mouse model was established through daily administration of yeast extract and potassium oxonate for 21 days, simulating a long-term high-purine dietary intake. Mice were orally supplemented with L. plantarum LTJ1 or LTJ48 (1 × 109 CFU/day). Serum analysis demonstrated successful model induction, with the HUA group exhibiting a 3.8-fold increase in UA compared to controls (p < 0.001) (Figure 4A). Both strains significantly reduced serum UA levels: LTJ1 by 31.0% and LTJ48 by 51.5% (p < 0.001 vs. HUA group) (Figure 4A). Concomitantly, LTJ1/LTJ48 ameliorated hepatic and renal dysfunction, as evidenced: Serum creatinine (CRE) was reduced by 40.6% (LTJ1, p < 0.01) and 47.9% (LTJ48, p < 0.001), blood urea nitrogen (BUN) was lowered by 21.1% (LTJ1, p < 0.05) and 30.0% (LTJ48, p < 0.01), and aspartate aminotransferase (AST) decreased by 17.4% (LTJ1, p < 0.05) and 25.9% (LTJ48, p < 0.01). However, no significant changes in alanine aminotransferase (ALT) were observed (p > 0.05) (Figure 4B–E). Mechanistically, while neither strain inhibited xanthine oxidase (XOD) activity, both significantly suppressed adenosine deaminase (ADA) activity (LTJ1: 40.0%, p < 0.001; LTJ48: 47.5% reduction, p < 0.001) (Figure 4F,G), aligning with cellular findings. These findings align with in vitro results, highlighting ADA suppression—rather than XOD inhibition—as the primary mechanism underpinning the anti-HUA effects of both strains.
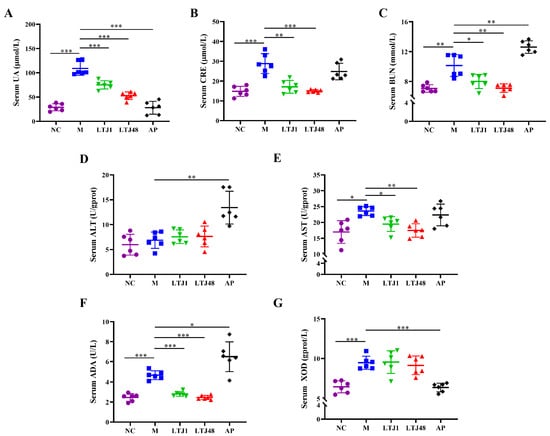
Figure 4.
Influence of L. plantarum LTJ1 and LTJ48 on physiological and biochemical indices of HUA mice. (A) Serum UA levels; (B) serum CRE levels; (C) serum BUN levels; (D) serum ALT levels; (E) serum AST levels; (F) serum AST levels; and (G) serum XOD levels. NC was the control group. M was the HUA model group. AP was the group treated with the positive drug allopurinol. The LTJ1 group was given live L. plantarum LTJ1. The LTJ48 group was given inactivate L. plantarum LTJ48. Data represent mean ± SD (n = 6/group). Statistical significance vs. HUA model group: * p < 0.05, ** p < 0.01, *** p < 0.001.
3.5. L. plantarum LTJ1 and LTJ48 Modulate UA Transporters in the Liver and Kidney Tissue of HUA Mice
To elucidate the molecular mechanisms underlying UA reduction, we analyzed key urate transporters: renal URAT1, intestinal ATP-binding cassette sub-family G member 2 (ABCG2), and dual-localized glucose transporter 9 (GLUT9). Both strains significantly suppressed UA reabsorption by downregulating GLUT9 (LTJ1, 22.9% reduction, p < 0.001 and LTJ48, 57.0% reduction, p < 0.001) and URAT1 (LTJ1, 48.4% reduction, p < 0.001 and LTJ48, 36.7% reduction, p < 0.001) expression compared to the HUA model group in the kidney (Figure 5A,B). Concurrent inhibition of GLUT9 (LTJ1, 69.6% reduction, p < 0.001 and LTJ48, 38.1% reduction, p < 0.001) and was accompanied by a 1.4-fold upregulation (LTJ1, p < 0.05 and LTJ48, p < 0.01) of the excretion transporter ABCG2 (Figure 5C,D) in the small intestine. This dual-tissue, multi-transporter modulation—inhibiting reabsorption (URAT1/GLUT9) while enhancing excretion (ABCG2)—demonstrates a novel mechanism for UA homeostasis, distinct from single-target pharmacological approaches.
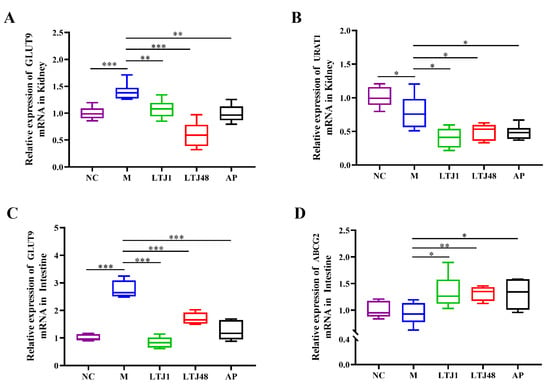
Figure 5.
Multi-target regulation of uric acid transporters by L. plantarum LTJ1 and LTJ48 in HUA mice. (A) Renal GLUT9 expression; (B) renal URAT1 expression; (C) intestinal GLUT9 expression; and (D) intestinal ABCG2 expression. NC was the control group. M was the HUA model group. AP was the group treated with the positive drug allopurinol. The LTJ1 group was given live L. plantarum LTJ1. The LTJ48 group was given inactivate L. plantarum LTJ48. Data represent mean ± SD (n = 6/group). Statistical significance vs. HUA model group: * p < 0.05, ** p < 0.01, *** p < 0.001.
3.6. L. plantarum LTJ1 and LTJ48 Restore Gut Microbiota Homeostasis in HUA Mice
Through 16S rRNA sequencing analysis, we comprehensively explored the dynamic regulation of intestinal microbiota in HUA mice by L. plantarum LTJ1 and LTJ48 (Figure 6). At the phylum level, the dominant taxa were Bacteroidetes, Firmicutes, Actinobacteria, and Verrucomicrobia (Figure 6A). Comparative analysis revealed that the relative abundance of Bacteroidetes decreased in the model group, while Firmicutes and Verrucomicrobia increased. Treatment with LTJ1 further reduced Bacteroidetes and Verrucomicrobia, accompanied by a significant increase in Firmicutes and Actinobacteria compared to the model group. LTJ48 interventions, conversely, reduced Verrucomicrobia abundance while enhancing Bacteroidetes compared to the model group. At the genus level, Bacteroides dominated in the control group, with limited genus diversity (Figure 6B). In the model group, the relative abundance of Lactobacillus, Odoribacter, and Lachnospiraceae exceeded that of the control group. The LTJ1 group exhibited higher abundance of Firmicutes, particularly Limosilactobacillus and Dubosiella, whereas the LTJ48 group showed reduced Lachnospiraceae but increased Lactobacillus and Alloprevotella, distinguishing it from the control, model, and positive drug groups. The AP group displayed decreased Oscillibacter and Lachnospiraceae alongside elevated Lactobacillus compared to the model group.
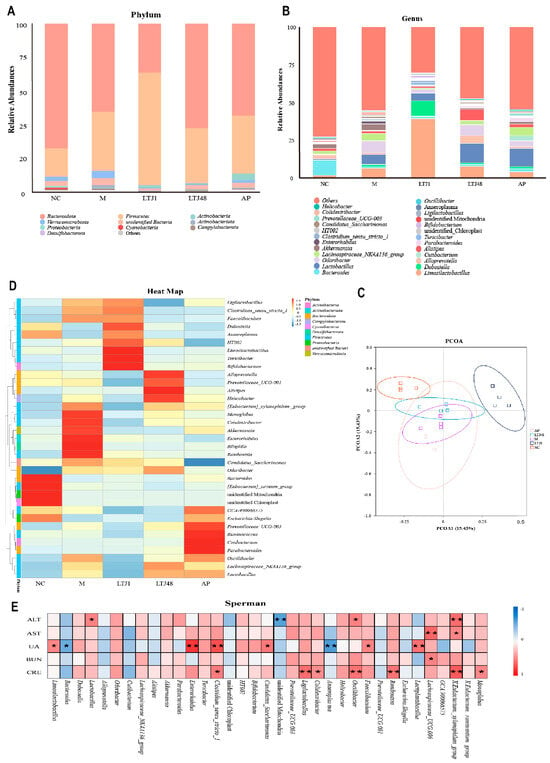
Figure 6.
Functional and structural modulation of gut microbiota by L. plantarum LTJ1 and LTJ48 in HUA models. (A) Phylum-level microbiota composition among different groups; (B) genus-level abundance of dominant taxa; (C) principal coordinate analysis (PCoA) based on Bray−Curtis di−similarity; and (D) heatmap of taxonomic clustering patterns. NC was the control group. M was the HUA model group. (E) Spearman correlations between biomarkers (ALT, AST, UA, BUN, and CRE) and microbiota genera. AP was the group treated with the positive drug allopurinol. The LTJ1 group was given live L. plantarum LTJ1. The LTJ48 group was given inactivate L. plantarum LTJ48. Data represent mean ± SD (n = 6/group). Statistical significance vs. HUA model group: * p < 0.05, ** p < 0.01.
Principal coordinates analysis (PCoA) (Figure 6C) highlighted distinct clustering between the control and model groups, reflecting dietary impacts on microbiota composition. Notably, the LTJ1 group formed a unique cluster, differing from the model, LTJ48, and drug groups, illustrating strain-specific microbial restructuring. Heatmap analysis (Figure 6D) further delineated taxon correlations. The NC group was associated with Bacteroides, while the model group was enriched in Romboutsia, Monoglobus, Colidextribacter, Enterorhabdus, and Bilophilla; the LTJ1 group was linked to Dubosiella, Limosilactobacillus, Ligilactobacillus, Turicibacter, Bifidobacterium, and Clostridium; the LTJ48 group was enriched in Alloprevotella, Alistipes, Helicobacter, and Prevotellaceae; and the AP group was correlated with Ruminococcus, Oscillibacter, and Cutibacteroides. Spearman correlation analysis (Figure 6E) identified significant associations. UA levels were positively correlated with L. plantarum, Lactobacillus, and Clostridium, but negatively correlated with Bacteroides and Anaeroplasma. Collectively, these findings demonstrate that LTJ1 and LTJ48 interventions induce significant intestinal microbiota remodeling in HUA mice.
4. Discussion
Probiotic supplementation offers a safer and more accessible alternative to conventional pharmaceuticals for HUA management [22,26]. While Lactobacillus strains are recognized for their therapeutic potential in metabolic disorders such as HUA and inflammation [27,28], the mechanisms underlying their UA-lowering effects—beyond direct purine degradation—remain poorly understood. This study pioneers a holistic investigation of L. plantarum LTJ1/LTJ48, shifting focus from isolated purine metabolism to systemic impacts on hepatic/renal function and gut microbiota ecology.
Previous studies have identified Lactobacillus strains with variable purine and UA degradation capacities, highlighting their strain-specific metabolic potential [29,30,31,32]. Building on this foundation, we screened and characterized L. plantarum LTJ1 and LTJ48, which achieved near-complete nucleoside degradation and reduced total purines by 35.2% and 42.2%, respectively, within 8 h—surpassing the performance of Lactobacillus reuteri TSR332 (90% inosine assimilation in 30 min) [29] and Limosilactobacillus fermentum JL-3 (40.9% UA reduction over 24 h) [30]. Critically, cross-study comparisons of degradation efficacy are confounded by methodological heterogeneity, including variations in substrate composition, incubation duration, and analytical protocols.
In the study of purine metabolism, the impact of the substances on ADA enzyme activity emerged as a key consideration. Expanding beyond nucleoside degradation, we evaluated LTJ1/LTJ48 metabolites in a hyperuricemic HepG2 cell model, observing a 24 h reduction in UA levels accompanied by downregulated PNP and ADA gene expression and ADA activity. These findings reveal a multi-target mechanism extending beyond purine catabolism. In vivo, a 21-day intervention with LTJ1/LTJ48 in HUA mice reduced serum UA levels. Parallel research endeavors yielded similar findings: Pediococcus acidilactici GQ01 and Priestia megaterium ASC-1 exhibited reductions of 40.87% [23] and 67.24% [33], respectively, in comparison to their corresponding model groups. Elevated CRE, BUN, and AST—markers of renal and hepatic dysfunction [34,35,36]—were also ameliorated by LTJ1/LTJ48. Notably, while high-purine diets increased XOD activity, LTJ1/LTJ48 selectively suppressed ADA without affecting XOD, consistent with cellular findings. This concordance between in vitro and in vivo results underscores the robustness of their anti-HUA effects.
Impaired UA excretion, driven by excessive renal reabsorption (~90% of filtered UA), is a central mechanism in HUA pathogenesis. This process is regulated by UA transporters: reabsorption mediators GLUT9 and URAT1, and the excretion pump ABCG2 [37,38]. In HUA, dysregulation of these transporters—characterized by upregulated GLUT9/URAT1 and downregulated ABCG2—promotes UA retention [39,40]. Our study demonstrates that LTJ1/LTJ48 restores UA homeostasis by downregulating GLUT9/URAT1 and upregulating ABCG2 in the kidney or small intestine. This dual-action mechanism simultaneously reduces UA reabsorption and enhances excretion, offering a novel therapeutic strategy for HUA management.
Under high-purine dietary conditions, the model group exhibited a significant microbial dysbiosis characterized by elevated abundances of Odoribacter, Lachnospiraceae, and Bacteroides compared to the NC group—a pattern consistent with HUA-associated enrichment of opportunistic pathogens. Notably, Lactobacillus proliferation observed in untreated HUA models may reflect compensatory responses to dietary stress rather than therapeutic effects. In contrast, targeted supplementation with L. plantarum strains LTJ1 and LTJ48 induced substantial structural reorganization of gut microbiota, driving differential probiotic enrichment. The LTJ1 intervention uniquely elevated Limosilactobacillus (relieves HUA [41]), Dubosiella (intestinal anti-inflammatory modulator [42]), Faecalibacterium (typically depleted in HUA patients [43]), and Bifidobacterium, accompanied by Firmicutes dominance and distinct PCoA clustering. Parallel effects emerged in LTJ48-treated mice showing reduced Lachnospiraceae and Enterobacter alongside increased Alistipes (cholesterol regulator [44]) and Alloprevotella (SCFA producer countering metabolic inflammation [45]). Crucially, both probiotics suppressed disease-exacerbating taxa while amplifying commensals with documented roles in barrier protection and UA metabolism regulation.
This study provides the necessary theoretical support for the commercial development of new probiotics. New probiotics can be used to develop new commercial products for improving hyperuricemia, such as functional foods and beverages, including probiotic yogurt/fermented milk, probiotic solid beverages/granules, and probiotic energy bars/snacks; dietary supplements, including probiotic capsules for high uric acid and intestinal-uric acid metabolism joint regulation products; and medical-grade products, which are mainly auxiliary treatment preparations used in conjunction with low-purine diets.
5. Conclusions
In summary, this investigation elucidates the dual probiotic potential of L. plantarum strains LTJ1 and LTJ48 in mitigating HUA through nucleoside and purine degradation. Systematic evaluation across cellular and murine HUA models revealed their significant urate-lowering efficacy coupled with modulatory effects on gut microbiota composition and metabolic functionality. Notably, these strains offer a targeted microbial therapeutic approach distinct from conventional HUA interventions, demonstrating direct enzymatic catabolism of purine precursors, restorative microbiota remodeling, and synergistic reduction of serum UA biomarkers. This evidence positions microbiome-based modulation as an innovative prophylactic strategy for chronic HUA management and its associated comorbidities.
Author Contributions
Conceptualization, X.L. and F.Z.; methodology, X.F., X.W. and J.C.; software, J.C.; validation, X.L., F.Z., J.C., M.L., J.T. and J.W.; formal analysis, F.Z.; investigation, J.C., M.L., J.T. and J.W.; resources, X.L.; data curation, X.L., F.Z., J.C. and M.L.; writing—original draft preparation, F.Z., X.F. and J.C.; writing—review and editing, X.L. and F.Z.; visualization, J.C. and M.L.; supervision, X.L. and F.Z.; project administration, X.L. and F.Z.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Tianjin Science and Technology Achievement Transfer and Transformation Project (24ZYCGSY00390, to X.L.), the National Key Research and Development Program Project (2018YFA0901700; 2017YFD0400303, to X.L.), and the Chongqing Special Project for Technology Innovation and Application Development in Rural Revitalization (CSTB2024TIAD-ZXX0007, to F.Z.).
Institutional Review Board Statement
The study was approved by the Academic Committee of Tianjin University of Science and Technology (approval code: KJDA20220921, approval date: 21 September 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript.
| HUA | Hyperuricemia |
| XOD | Xanthine oxidase |
| URAT1 | Urate transporter 1 |
| ADA | Adenosine deaminase |
| CRE | Serum creatinine |
| BUN | Blood urea nitrogen |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| ABCG2 | ATP-binding cassette sub-family G member 2 |
| GLUT9 | Glucose transporter 9 |
References
- Pang, S.; Jiang, Q.; Sun, P.; Li, Y.; Zhu, Y.; Liu, J.; Ye, X.; Chen, T.; Zhao, F.; Yang, W. Hyperuricemia prevalence and its association with metabolic disorders: A multicenter retrospective real-world study in China. Ann. Transl. Med. 2021, 9, 1550. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Iida, S.; Katsuyama, H. A possible therapeutic application of the selective inhibitor of Urate Transporter 1, Dotinurad, for metabolic syndrome, chronic kidney Disease, and Cardiovascular Disease. Cells 2024, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, M.; Huang, S.; Lan, X.; Zheng, J.; Luo, H.; He, Y.; Lei, W. Hyperuricemia: A key contributor to endothelial dysfunction in cardiovascular diseases. FASEB J. 2023, 37, e23012. [Google Scholar] [CrossRef]
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z.; Gao, J. Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef]
- Vareldzis, R.; Perez, A.; Reisin, E. Hyperuricemia: An intriguing connection to metabolic syndrome, diabetes, kidney disease, and hypertension. Curr. Hypertens. Rep. 2024, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Ae, R.; Kosami, K.; Kanbay, M.; Andres-Hernando, A.; Hisatome, I.; Lanaspa, M.A. Current updates and future perspectives in uric acid research 2024. Hypertens. Res. 2024, 48, 1–7. [Google Scholar] [CrossRef]
- Yang, S.; Liu, H.; Fang, X.-M.; Yan, F.; Zhang, Y. Signaling pathways in uric acid homeostasis and gout: From pathogenesis to therapeutic interventions. Int. Immunopharmacol. 2024, 132, 111932. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, J.; Zou, Q.; Wang, W.; Yan, G.; Guo, R.; Yuan, T.; Wang, Y.; Liu, X.; Liu, Z. Tryptophan Metabolism-Regulating Probiotics Alleviate Hyperuricemia by Protecting the Gut Barrier Integrity and Enhancing Colonic Uric Acid Excretion. J. Agric. Food. Chem. 2024, 72, 26746–26761. [Google Scholar] [CrossRef]
- Zhang, W. Hyperuricemia: Current State and Prospects. Explor. Res. Hypothesis. Med. 2025, 10, 49–55. [Google Scholar] [CrossRef]
- Al-Worafi, Y.M. Gout and Hyperuricemia Management in Developing Countries. In Handbook of Medical and Health Sciences in Developing Countries: Education, Practice, and Research; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–42. [Google Scholar]
- Ullah, Z.; Yue, P.; Mao, G.; Zhang, M.; Liu, P.; Wu, X.; Zhao, T.; Yang, L. A comprehensive review on recent xanthine oxidase inhibitors of dietary based bioactive substances for the treatment of hyperuricemia and gout: Molecular mechanisms and perspective. Int. J. Biol. Macromol. 2024, 278, 134832. [Google Scholar] [CrossRef]
- Guo, W.; Wei, M.; Li, Y.; Xu, J.; Zang, J.; Chen, Y.; Chen, L. Mechanisms of urate transport and uricosuric drugs inhibition in human URAT1. Nat. Commun. 2025, 16, 1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y. Anti-hyperuricemia bioactive peptides: A review on obtaining, activity, and mechanism of action. Food Funct. 2024, 15, 5714–5736. [Google Scholar]
- Hung, S.-I.; Mockenhaupt, M.; Blumenthal, K.G.; Abe, R.; Ueta, M.; Ingen-Housz-Oro, S.; Phillips, E.J.; Chung, W.-H. Severe cutaneous adverse reactions. Nat. Rev. Dis. Primers 2024, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Ghossan, R.; Tabesh, O.A.; Fayad, F.; Richette, P.; Bardin, T. Cardiovascular Safety of Febuxostat in Patients with Gout or Hyperuricemia: A Systematic Review of Randomized Controlled Trials. JCR J. Clin. Rheumatol. 2024, 30, e46–e53. [Google Scholar] [CrossRef]
- Li, G.; Hu, Y.; Zhao, H.; Peng, Z.; Shang, X.; Zhang, J.; Xie, K.; Li, M.; Zhou, X.; Zhou, Q. Slow Metabolism–Driven Amplification of Hepatic PPARγ Agonism Mediates Benzbromarone-Induced Obesity-Specific Liver Injury. Adv. Sci. 2025, 12, 2409126. [Google Scholar] [CrossRef]
- Kaufmann, D.; Chaiyakunapruk, N.; Schlesinger, N. Optimizing Gout Treatment: A Comprehensive Review of Current and Emerging Uricosurics. Jt. Bone Spine 2024, 92, 105826. [Google Scholar] [CrossRef]
- Soraci, L.; Cherubini, A.; Paoletti, L.; Filippelli, G.; Luciani, F.; Laganà, P.; Gambuzza, M.E.; Filicetti, E.; Corsonello, A.; Lattanzio, F. Safety and tolerability of antimicrobial agents in the older patient. Drugs Aging 2023, 40, 499–526. [Google Scholar] [CrossRef]
- Lv, Q.; Zhou, J.; Wang, C.; Yang, X.; Han, Y.; Zhou, Q.; Yao, R.; Sui, A. A dynamics association study of gut barrier and microbiota in hyperuricemia. Front. Microbiol. 2023, 14, 1287468. [Google Scholar] [CrossRef]
- Sun, X.; Wen, J.; Guan, B.; Li, J.; Luo, J.; Li, J.; Wei, M.; Qiu, H. Folic acid and zinc improve hyperuricemia by altering the gut microbiota of rats with high-purine diet-induced hyperuricemia. Front. Microbiol. 2022, 13, 907952. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Liao, W.; Huang, J.; Liu, Y.; Li, Z.; Tang, J. Gut microbiota remodeling: A promising therapeutic strategy to confront hyperuricemia and gout. Front. Cell. Infect. Microbiol. 2022, 12, 935723. [Google Scholar] [CrossRef]
- Sun, L.; Ni, C.; Zhao, J.; Wang, G.; Chen, W. Probiotics, bioactive compounds and dietary patterns for the effective management of hyperuricemia: A review. Crit. Rev. Food Sci. 2024, 64, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, S.; Liu, S.; Prasanthi, H.A.C.; Li, Y.; Cao, J.; Zhong, F.; Guo, L.; Lu, F.; Luo, X. Postbiotic of Pediococcus acidilactici GQ01, a Novel Probiotic Strain Isolated from Natural Fermented Wolfberry, Attenuates Hyperuricaemia in Mice through Modulating Uric Acid Metabolism and Gut Microbiota. Foods 2024, 13, 923. [Google Scholar] [CrossRef]
- Ro, K.-S.; Zhao, L.; Hu, Y.; Ge, M.; Du, L.; Xie, J. Anti-hyperuricemic properties and mechanism of Lactiplantibacillus plantarum X7023. Process. Biochem. 2024, 136, 26–37. [Google Scholar] [CrossRef]
- Zou, Y.; Ro, K.-S.; Jiang, C.; Yin, D.; Zhao, L.; Zhang, D.; Du, L.; Xie, J. The anti-hyperuricemic and gut microbiota regulatory effects of a novel purine assimilatory strain, Lactiplantibacillus plantarum X7022. Eur. J. Nutr. 2024, 63, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Zhong, H.; Chen, F.; Regenstein, J.; Hu, X.; Cai, L.; Feng, F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. 2022, 62, 3979–3989. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, Z.; Lu, Y. The potential of probiotics in the amelioration of hyperuricemia. Food Funct. 2022, 13, 2394–2414. [Google Scholar] [CrossRef]
- Xu, J.; Tu, M.; Fan, X.; Guo, Y.; Zhang, T.; Zeng, X.; Cai, Z.; Wu, Z.; Pan, D. A novel strain of Levilactobacillus brevis PDD-5 isolated from salty vegetables has beneficial effects on hyperuricemia through anti-inflammation and improvement of kidney damage. Food Sci. Hum. Wellness 2024, 13, 898–908. [Google Scholar] [CrossRef]
- Kuo, Y.-W.; Hsieh, S.-H.; Chen, J.-F.; Liu, C.-R.; Chen, C.-W.; Huang, Y.-F.; Ho, H.-H. Lactobacillus reuteri TSR332 and Lactobacillus fermentum TSF331 stabilize serum uric acid levels and prevent hyperuricemia in rats. PeerJ 2021, 9, e11209. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, Z.; Feng, P.; Li, R.; Chen, X.; Tian, X.; Han, R.; Kakade, A.; Liu, P.; Li, X. Limosilactobacillus fermentum JL-3 isolated from “Jiangshui” ameliorates hyperuricemia by degrading uric acid. Gut Microbes 2021, 13, 1897211. [Google Scholar] [CrossRef]
- Wu, J.; Aga, L.; Tang, L.; Li, H.; Wang, N.; Yang, L.; Zhang, N.; Wang, X.; Wang, X. Lacticaseibacillus paracasei JS-3 Isolated from “Jiangshui” Ameliorates Hyperuricemia by Regulating Gut Microbiota and iTS Metabolism. Foods 2024, 13, 1371. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, P.; Xiong, S.; Wei, B.; Du, T.; Huang, T.; Yu, Q.; Xie, M.; Xiong, T. Lacticaseibacillus rhamnosus NCUH061012 alleviates hyperuricemia via modulating gut microbiota and intestinal metabolites in mice. Food Biosci. 2024, 58, 103699. [Google Scholar] [CrossRef]
- Zhu, W.; Bi, S.; Fang, Z.; Iddrisu, L.; Deng, Q.; Sun, L.; Gooneratne, R. Priestia megaterium ASC-1 Isolated from Pickled Cabbage Ameliorates Hyperuricemia by Degrading Uric Acid in Rats. Microorganisms 2024, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zeng, T.; Tian, J.; Yan, J.; Lan, Z.; Chen, J.; Xin, X.; Lei, B.; Cai, Z. Long-term environmental cadmium exposure induced serum metabolic changes related to renal and liver dysfunctions in a female cohort from Southwest China. Sci. Total Environ. 2021, 798, 149379. [Google Scholar] [CrossRef]
- Shen, S.; Yan, X.; Xu, B. The blood urea nitrogen/creatinine (BUN/cre) ratio was U-shaped associated with all-cause mortality in general population. Renal Failure 2022, 44, 184–190. [Google Scholar] [CrossRef]
- Kalas, M.A.; Chavez, L.; Leon, M.; Taweesedt, P.T.; Surani, S. Abnormal liver enzymes: A review for clinicians. World. J. Hepatol. 2021, 13, 1688. [Google Scholar] [CrossRef]
- Nian, Y.-L.; You, C.-G. Susceptibility genes of hyperuricemia and gout. Hereditas 2022, 159, 30. [Google Scholar] [CrossRef]
- Song, Y.; March, J. Hyperuricemia and the small intestine: Transport mechanisms and co-morbidities. Biotechnol. Notes. 2022, 3, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Q.; Sun, K.-X.; Guo, X.; Chen, Y.-Y.; Feng, C.-Y.; Chen, J.-S.; Barreira, J.C.; Prieto, M.A.; Sun, J.-Y.; Zhang, J.-D. The antihyperuricemia activity of Astragali Radix through regulating the expression of uric acid transporters via PI3K/Akt signalling pathway. J. Ethnopharmacol. 2023, 317, 116770. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Liu, Y.; Huang, C.; Feng, W.; Cui, H.; Li, M. Xanthoceras sorbifolium leaves alleviate hyperuricemic nephropathy by inhibiting the PI3K/AKT signaling pathway to regulate uric acid transport. J. Ethnopharmacol. 2024, 327, 117946. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, P.; Hu, X.; Cao, W.; Liu, P.; Han, H.; Jin, W.; Li, X. Probiotic Limosilactobacillus fermentum GR-3 ameliorates human hyperuricemia via degrading and promoting excretion of uric acid. Iscience 2022, 25, 105198. [Google Scholar] [CrossRef]
- Li, R.; Yang, P.; Liu, B.; Ye, Z.; Zhang, P.; Li, M.; Gong, Y.; Huang, Y.; Yang, L.; Li, M. Lycium barbarum polysaccharide remodels colon inflammatory microenvironment and improves gut health. Heliyon 2024, 10, e30594. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Zhang, N.; Gao, H.; Wang, G.; Liang, H.; Xue, M. Influence of intestinal microecology in the development of gout or hyperuricemia and the potential therapeutic targets. Int. J. Rheum. Dis. 2023, 26, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, Y.; Zhang, Y.; Yang, Y.; Su, W.; Yang, Y.; Sun, L.; Zhang, F.; Yu, J.; Wang, Y. Gut microbiota specifically mediates the anti-hypercholesterolemic effect of berberine (BBR) and facilitates to predict BBR’s cholesterol-decreasing efficacy in patients. J. Adv. Res. 2022, 37, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, Y.; Zhang, Y.; Dong, J.; Jiang, S.; Tang, Y. DHA-enriched phosphatidylserine ameliorates cyclophosphamide-induced liver injury via regulating the gut-liver axis. Int. Immunopharmacol. 2024, 140, 112895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).