A Secondary Analysis of Caloric Restriction and Exercise Effects on Cognitive Function in Functionally Limited Postmenopausal Women with Overweight or Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Experimental Groups
2.3. Outcomes
2.3.1. Cognitive Measures
2.3.2. Physical Measures
2.4. Statistical Methods
3. Results
3.1. Participants
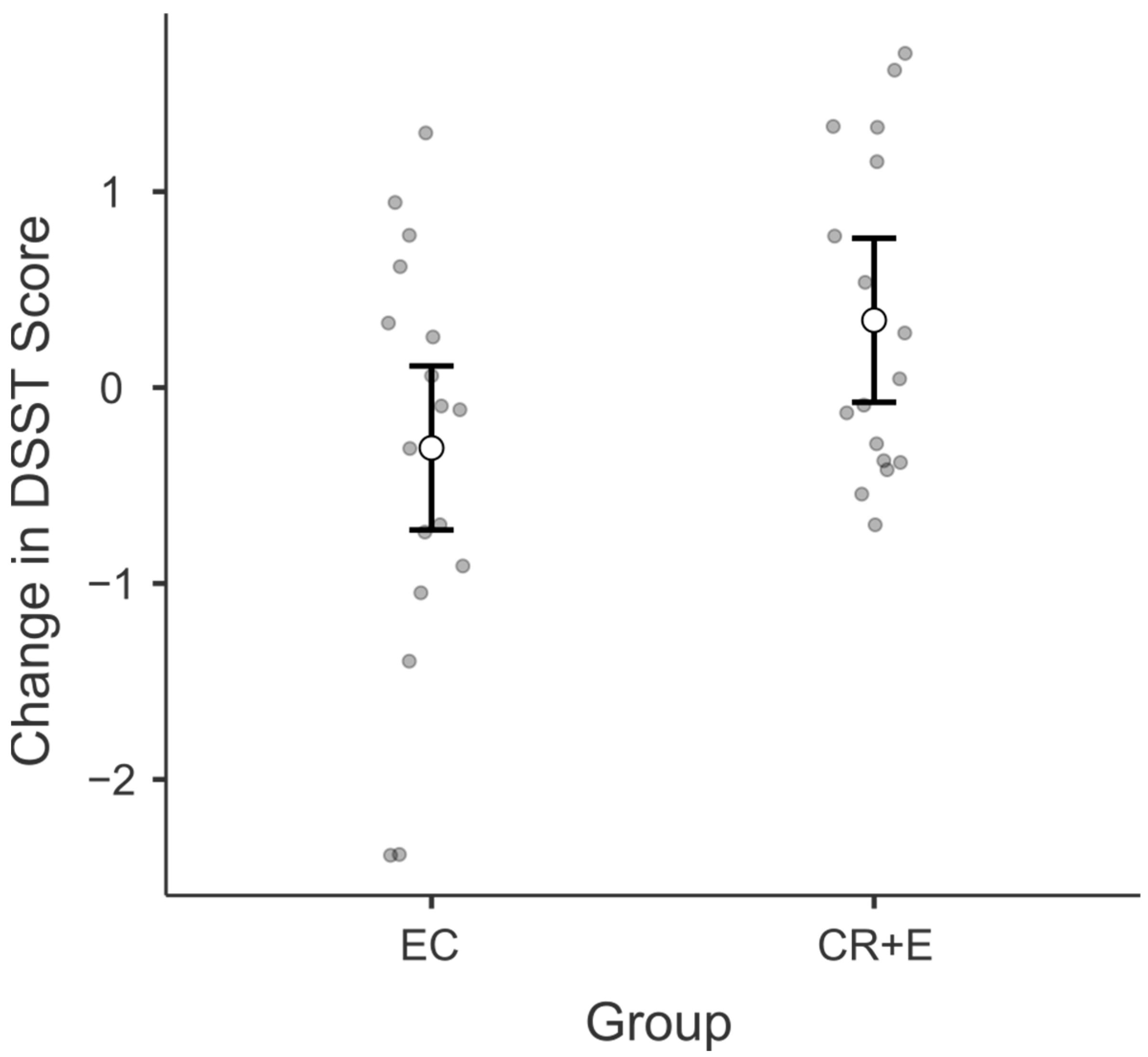
3.2. Digit Symbol Substitution Test (DSST)
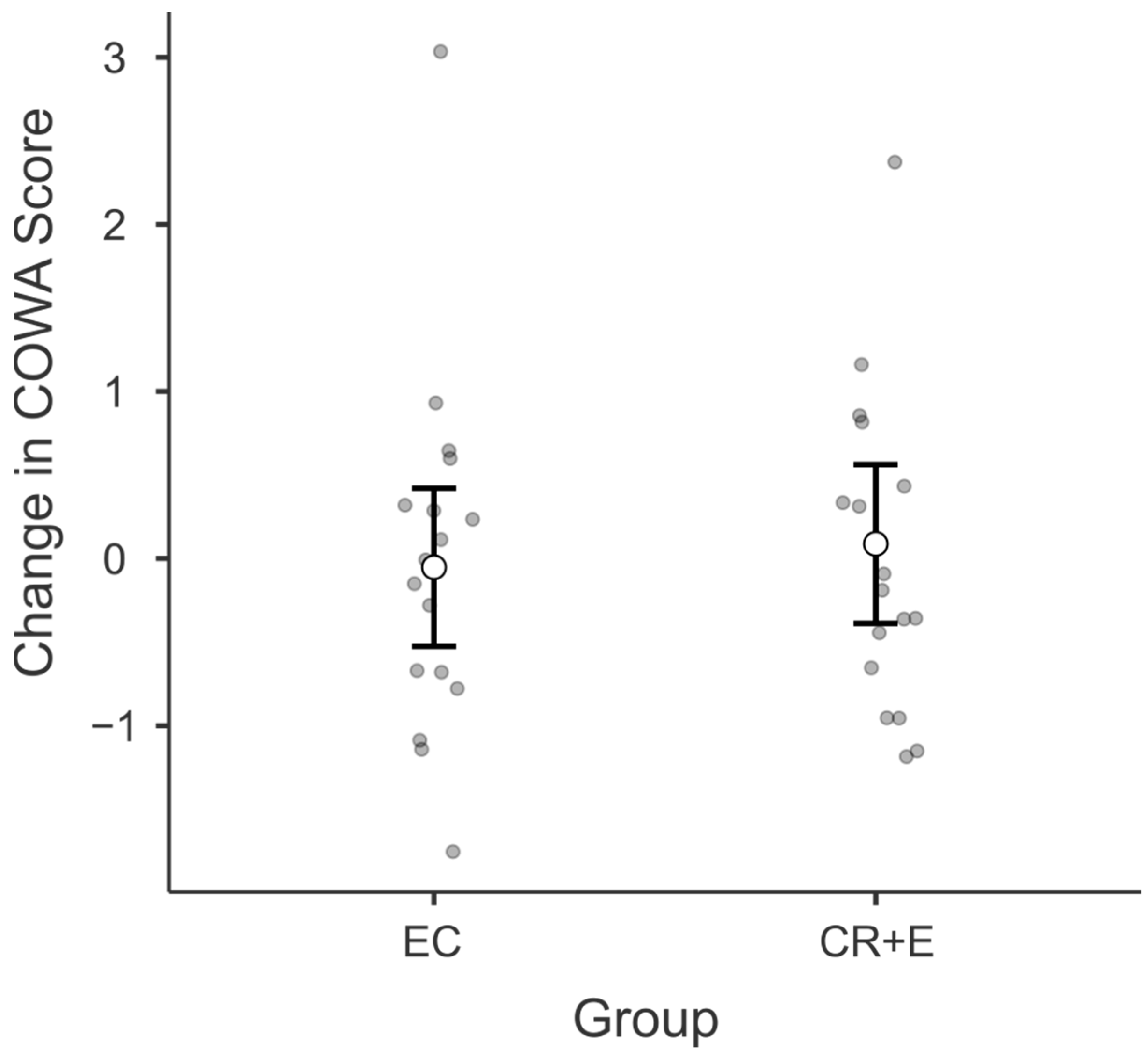
3.3. Controlled Oral Word Association (COWA)
3.4. Adherence, Weight Loss, and Physical Functioning
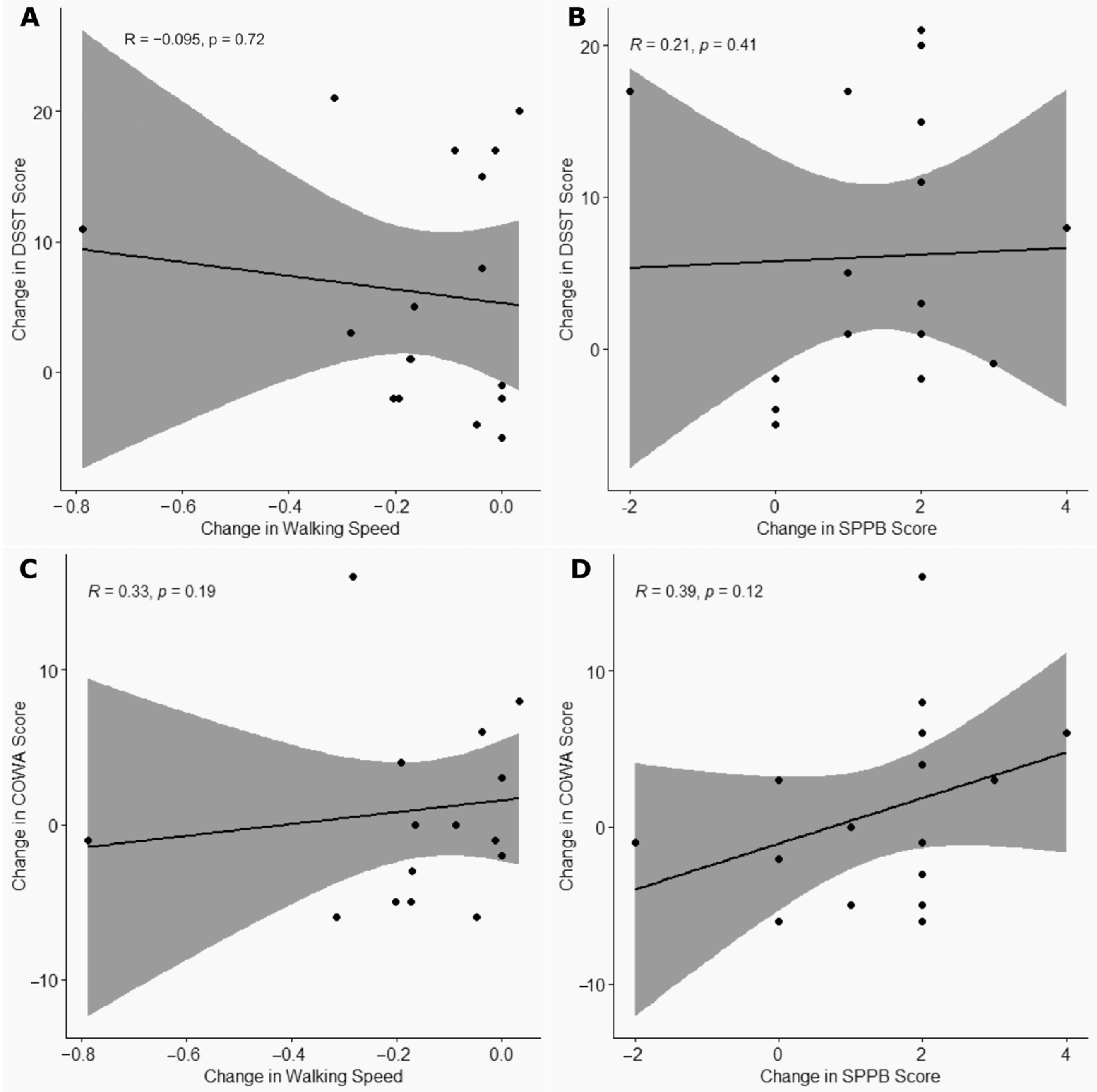
3.5. Correlation Between Changes in Cognitive and Physical Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortman, J.; Velkoff, V. An Aging Nation: The Older Population in the United States. Census.gov. Available online: https://www.census.gov/library/publications/2014/demo/p25-1140.html (accessed on 10 January 2025).
- Fakhouri, T.H.I.; Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of obesity among older adults in the United States, 2007–2010. NCHS Data Brief 2012, 106, 1–8. [Google Scholar]
- Hales, C.; Carroll, M.D.; Fryar, C.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. Available online: https://www.cdc.gov/nchs/products/databriefs/db360.htm (accessed on 14 December 2023).
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Kuller, L.H.; Lopez, O.L.; Diehr, P.; O’Meara, E.S.; Longstreth, W.T., Jr.; Luchsinger, J.A. Midlife and Late-Life Obesity and the Risk of Dementia: Cardiovascular Health Study. Arch. Neurol. 2009, 66, 336–342. [Google Scholar] [CrossRef]
- Qizilbash, N.; Gregson, J.; Johnson, M.E.; Pearce, N.; Douglas, I.; Wing, K.; Evans, S.J.W.; Pocock, S.J. BMI and risk of dementia in two million people over two decades: A retrospective cohort study. Lancet Diabetes Endocrinol. 2015, 3, 431–436. [Google Scholar] [CrossRef]
- Karlsson, I.K.; Gatz, M.; Arpawong, T.E.; Dahl Aslan, A.K.; Reynolds, C.A. The dynamic association between body mass index and cognition from midlife through late-life, and the effect of sex and genetic influences. Sci. Rep. 2021, 11, 7206. [Google Scholar] [CrossRef]
- Jenkins, K.R. Obesity’s effects on the onset of functional impairment among older adults. Gerontologist 2004, 44, 206–216. [Google Scholar] [CrossRef]
- Handing, E.P.; Leng, X.I.; Kritchevsky, S.B.; Craft, S. Association Between Physical Performance and Cognitive Function in Older Adults Across Multiple Studies: A Pooled Analysis Study. Innov. Aging 2020, 4, igaa050. [Google Scholar] [CrossRef]
- Iso-Markku, P.; Aaltonen, S.; Kujala, U.M.; Halme, H.-L.; Phipps, D.; Knittle, K.; Vuoksimaa, E.; Waller, K. Physical Activity and Cognitive Decline Among Older Adults: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2354285. [Google Scholar] [CrossRef]
- Pitrou, I.; Vasiliadis, H.-M.; Hudon, C. Body mass index and cognitive decline among community-living older adults: The modifying effect of physical activity. Eur. Rev. Aging Phys. Act. 2022, 19, 3. [Google Scholar] [CrossRef]
- Lee, K.M.; Hunger, J.M.; Tomiyama, A.J. Weight stigma and health behaviors: Evidence from the Eating in America Study. Int. J. Obes. 2021, 45, 1499–1509. [Google Scholar] [CrossRef]
- Puhl, R.M.; Himmelstein, M.S.; Pearl, R.L. Weight stigma as a psychosocial contributor to obesity. Am. Psychol. 2020, 75, 274–289. [Google Scholar] [CrossRef]
- Holwerda, T.J.; Deeg, D.J.H.; Beekman, A.T.F.; van Tilburg, T.G.; Stek, M.L.; Jonker, C.; Schoevers, R.A. Feelings of loneliness, but not social isolation, predict dementia onset: Results from the Amsterdam Study of the Elderly (AMSTEL). J. Neurol. Neurosurg. Psychiatry 2014, 85, 135–142. [Google Scholar] [CrossRef]
- Wilson, R.S.; Scherr, P.A.; Schneider, J.A.; Tang, Y.; Bennett, D.A. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology 2007, 69, 1911–1920. [Google Scholar] [CrossRef]
- Timkova, V.; Mikula, P.; Nagyova, I. Psychosocial distress in people with overweight and obesity: The role of weight stigma and social support. Front. Psychol. 2025, 15, 1474844. [Google Scholar] [CrossRef]
- Bielak, A.A.M. How can we not “lose it” if we still don’t understand how to “use it”? Unanswered questions about the influence of activity participation on cognitive performance in older age–A mini-review. Gerontology 2010, 56, 507–519. [Google Scholar] [CrossRef]
- Kelly, M.E.; Duff, H.; Kelly, S.; McHugh Power, J.E.; Brennan, S.; Lawlor, B.A.; Loughrey, D.G. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Syst. Rev. 2017, 6, 259. [Google Scholar] [CrossRef]
- Caristia, S.; De Vito, M.; Sarro, A.; Leone, A.; Pecere, A.; Zibetti, A.; Filigheddu, N.; Zeppegno, P.; Prodam, F.; Faggiano, F.; et al. Is Caloric Restriction Associated with Better Healthy Aging Outcomes? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2290. [Google Scholar] [CrossRef]
- Veronese, N.; Facchini, S.; Stubbs, B.; Luchini, C.; Solmi, M.; Manzato, E.; Sergi, G.; Maggi, S.; Cosco, T.; Fontana, L. Weight loss is associated with improvements in cognitive function among overweight and obese people: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017, 72, 87–94. [Google Scholar] [CrossRef]
- Anton, S.D.; Manini, T.M.; Milsom, V.A.; Dubyak, P.; Cesari, M.; Cheng, J.; Daniels, M.J.; Marsiske, M.; Pahor, M.; Leeuwenburgh, C.; et al. Effects of a weight loss plus exercise program on physical function in overweight, older women: A randomized controlled trial. Clin. Interv. Aging 2011, 6, 141–149. [Google Scholar] [CrossRef]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight Loss, Exercise, or Both and Physical Function in Obese Older Adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef]
- Napoli, N.; Shah, K.; Waters, D.L.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Effect of weight loss, exercise, or both on cognition and quality of life in obese older adults. Am. J. Clin. Nutr. 2014, 100, 189–198. [Google Scholar] [CrossRef]
- Peven, J.C.; Jakicic, J.M.; Rogers, R.J.; Lesnovskaya, A.; Erickson, K.I.; Kang, C.; Zhou, X.; Porter, A.; Donofry, S.D.; Watt, J.C.; et al. The Effects of a 12-Month Weight Loss Intervention on Cognitive Outcomes in Adults with Overweight and Obesity. Nutrients 2020, 12, 2988. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Babyak, M.A.; Craighead, L.; Welsh-Bohmer, K.A.; Browndyke, J.N.; Strauman, T.A.; Sherwood, A. Effects of the Dietary Approaches to Stop Hypertension Diet, Exercise, and Caloric Restriction on Neurocognition in Overweight Adults With High Blood Pressure. Hypertension 2010, 55, 1331–1338. [Google Scholar] [CrossRef]
- Krauss, R.M.; Eckel, R.H.; Howard, B.; Appel, L.J.; Daniels, S.R.; Deckelbaum, R.J.; Erdman, J.W.; Kris-Etherton, P.; Goldberg, I.J.; Kotchen, T.A.; et al. AHA Dietary Guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000, 102, 2284–2299. [Google Scholar] [CrossRef]
- Borg, G.A.V. Perceived Exertion Scale. 1970. Available online: https://psycnet.apa.org/doiLanding?doi=10.1037%2Ft58166-000 (accessed on 10 January 2025).
- Jaeger, J. Digit Symbol Substitution Test. J. Clin. Psychopharmacol. 2018, 38, 513–519. [Google Scholar] [CrossRef]
- Patterson, J. F-A-S Test. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 1024–1026. ISBN 978-0-387-79948-3. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Morris, T.P.; White, I.R.; Royston, P. Tuning multiple imputation by predictive mean matching and local residual draws. BMC Med. Res. Methodol. 2014, 14, 75. [Google Scholar] [CrossRef]
- Zhang, Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann. Transl. Med. 2016, 4, 30. [Google Scholar] [CrossRef]
- Handing, E.P.; Small, B.J.; Reynolds, S.L.; Kumar, N.B. Impact of Dietary Factors and Inflammation on Cognition among Older Adults. J. Prev. Alzheimers Dis. 2015, 2, 220–226. [Google Scholar] [CrossRef]
- Barnard, N.D.; Bush, A.I.; Ceccarelli, A.; Cooper, J.; de Jager, C.A.; Erickson, K.I.; Fraser, G.; Kesler, S.; Levin, S.M.; Lucey, B.; et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol. Aging 2014, 35 (Suppl. 2), S74–S78. [Google Scholar] [CrossRef]
- Dhana, K.; Evans, D.A.; Rajan, K.B.; Bennett, D.A.; Morris, M.C. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology 2020, 95, e374–e383. [Google Scholar] [CrossRef]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef]
- Nguyen, J.C.D.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef]
- Segal, Y.; Gunturu, S. Psychological Issues Associated with Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sutin, A.R.; Stephan, Y.; Robinson, E.; Daly, M.; Terracciano, A. Perceived weight discrimination and risk of incident dementia. Int. J. Obes. 2005 2019, 43, 1130–1134. [Google Scholar] [CrossRef]
- Sutin, A.R.; Stephan, Y.; Luchetti, M.; Terracciano, A. Loneliness and Risk of Dementia. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2020, 75, 1414–1422. [Google Scholar] [CrossRef]
- Liu, J.; Yi, P.; Liu, F. The Effect of Early Time-Restricted Eating vs Later Time-Restricted Eating on Weight Loss and Metabolic Health. J. Clin. Endocrinol. Metab. 2023, 108, 1824–1834. [Google Scholar] [CrossRef]
- Shapiro, A.L.B.; Tjaden, A.H.; Edelstein, S.L.; Kahn, S.E.; Srikanthan, P.; Knowler, W.C.; Venditti, E.M.; Golden, S.H.; Carmichael, O.; Luchsinger, J.A.; et al. The association of insulin responses and insulin sensitivity with cognition in adults with pre-diabetes: The Diabetes Prevention Program Outcomes Study. J. Diabetes Complicat. 2024, 38, 108764. [Google Scholar] [CrossRef]
- Song, L.; Li, H.; Fu, X.; Cen, M.; Wu, J. Association of the Oxidative Balance Score and Cognitive Function and the Mediating Role of Oxidative Stress: Evidence from the National Health and Nutrition Examination Survey (NHANES) 2011–2014. J. Nutr. 2023, 153, 1974–1983. [Google Scholar] [CrossRef]
- Samtani, S.; Mahalingam, G.; Lam, B.C.P.; Lipnicki, D.M.; Lima-Costa, M.F.; Blay, S.L.; Castro-Costa, E.; Shifu, X.; Guerchet, M.; Preux, P.-M.; et al. Associations between social connections and cognition: A global collaborative individual participant data meta-analysis. Lancet Healthy Longev. 2022, 3, e740–e753. [Google Scholar] [CrossRef]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Diet or Exercise Interventions vs Combined Behavioral Weight Management Programs: A Systematic Review and Meta-Analysis of Direct Comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef]
- Olateju, I.V.; Ogwu, D.; Owolabi, M.O.; Azode, U.; Osula, F.; Okeke, R.; Akabalu, I. Role of Behavioral Interventions in the Management of Obesity. Cureus 2021, 13, e18080. [Google Scholar] [CrossRef]
| Characteristics Mean [SD] | Total N = 34 | EC N = 17 | CR + E N = 17 |
|---|---|---|---|
| Age (years) | 63.7 [5.6] | 63.7 [6.7] | 63.7 [4.5] |
| Non-Hispanic Black | 53% | 53% | 53% |
| BMI (kg/m2) | 36.9 [6.2] | 35.9 [6.8] | 37.9 [5.5] |
| Education (years) | 14.4 [2.7] | 14.4 [3.1] | 14.5 [2.3] |
| COWA | 31.4 [9] | 29.9 [8] | 32.7 [9.9] |
| DSST | 41.3 [11.1] | 41.1 [10.2] | 41.5 [12.1] |
| Walking Speed (m/s) | 1 [0.3] | 1 [0.3] | 1 [0.3] |
| SPPB | 9.2 [1] | 9.1 [1.1] | 9.3 [0.9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLaren, C.W.; Pearl, R.L.; Smith, G.E.; Anton, S.D. A Secondary Analysis of Caloric Restriction and Exercise Effects on Cognitive Function in Functionally Limited Postmenopausal Women with Overweight or Obesity. Nutrients 2025, 17, 2075. https://doi.org/10.3390/nu17132075
McLaren CW, Pearl RL, Smith GE, Anton SD. A Secondary Analysis of Caloric Restriction and Exercise Effects on Cognitive Function in Functionally Limited Postmenopausal Women with Overweight or Obesity. Nutrients. 2025; 17(13):2075. https://doi.org/10.3390/nu17132075
Chicago/Turabian StyleMcLaren, Christian W., Rebecca L. Pearl, Glenn E. Smith, and Stephen D. Anton. 2025. "A Secondary Analysis of Caloric Restriction and Exercise Effects on Cognitive Function in Functionally Limited Postmenopausal Women with Overweight or Obesity" Nutrients 17, no. 13: 2075. https://doi.org/10.3390/nu17132075
APA StyleMcLaren, C. W., Pearl, R. L., Smith, G. E., & Anton, S. D. (2025). A Secondary Analysis of Caloric Restriction and Exercise Effects on Cognitive Function in Functionally Limited Postmenopausal Women with Overweight or Obesity. Nutrients, 17(13), 2075. https://doi.org/10.3390/nu17132075











