Elemental Influence: The Emerging Role of Zinc, Copper, and Selenium in Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
3. The Role of Essential Trace Elements in Osteoarthritis: Biological Mechanisms and Clinical Evidence
3.1. Zinc (Zn): Cartilage Regeneration and Inflammatory Modulation
Clinical and Experimental Evidence
3.2. Copper (Cu): Structural, Antioxidant, and Immunomodulatory Roles in OA
Clinical and Experimental Evidence
3.3. Selenium (Se): Redox and Immune-Modulatory Roles in Osteoarthritis
Mechanistic, Therapeutic, and Population-Level Evidence
4. Combined Effects and Interactions
5. Challenges and Controversies
5.1. Conflicting Clinical Evidence
5.2. Bioavailability and Absorption Factors
5.3. Safety and Toxicity Concerns
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tang, S.; Zhang, C.; Oo, W.M.; Fu, K.; Risberg, M.A.; Bierma-Zeinstra, S.M.; Neogi, T.; Atukorala, I.; Malfait, A.M.; Ding, C.; et al. Osteoarthritis. Nat. Rev. Dis. Primers 2025, 11, 10. [Google Scholar] [CrossRef]
- Tuncay Duruöz, M.; Öz, N.; Gürsoy, D.E.; Hande Gezer, H. Clinical aspects and outcomes in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101855. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, Z.; Sun, C.; Xu, G.; He, J. Global, regional and national burden of osteoarthritis in 1990-2021: A systematic analysis of the global burden of disease study 2021. BMC Musculoskelet. Disord. 2024, 25, 1021. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697. [Google Scholar] [CrossRef]
- Kapoor, M. Pathogenesis of osteoarthritis. In Osteoarthritis: Pathogenesis, Diagnosis, Available Treatments, Drug Safety, Regenerative and Precision Medicine; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–28. [Google Scholar]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Li, G.; Cheng, T.; Yu, X. The Impact of Trace Elements on Osteoarthritis. Front. Med. 2021, 8, 771297. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yao, X.; Li, Y.; Li, J.; Zhang, J.; Zou, Z.; Kang, F.; Dong, S. Injectable hydrogel with selenium nanoparticles delivery for sustained glutathione peroxidase activation and enhanced osteoarthritis therapeutics. Mater. Today Bio 2023, 23, 100864. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68. [Google Scholar] [CrossRef]
- Cuffaro, D.; Gimeno, A.; Bernardoni, B.L.; Di Leo, R.; Pujadas, G.; Garcia-Vallvé, S.; Nencetti, S.; Rossello, A.; Nuti, E. Identification of N-Acyl Hydrazones as New Non-Zinc-Binding MMP-13 Inhibitors by Structure-Based Virtual Screening Studies and Chemical Optimization. Int. J. Mol. Sci. 2023, 24, 11098. [Google Scholar] [CrossRef]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: From enzyme activity to extracellular matrix cross-links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef]
- Handy, D.E.; Joseph, J.; Loscalzo, J. Selenium, a Micronutrient That Modulates Cardiovascular Health via Redox Enzymology. Nutrients 2021, 13, 3238. [Google Scholar] [CrossRef] [PubMed]
- Gamble, S.C.; Wiseman, A.; Goldfarb, P.S. Selenium-dependent glutathione peroxidase and other selenoproteins: Their synthesis and biochemical roles. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1997, 68, 123–134. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Tian, T.; Wang, B.; Pan, Y. Meta-analysis of the Relationship Between Zinc and Copper in Patients with Osteoarthritis. Biol. Trace Elem. Res. 2025, 203, 635–645. [Google Scholar] [CrossRef]
- Yang, W.M.; Lv, J.F.; Wang, Y.Y.; Xu, Y.M.; Lin, J.; Liu, J.; Chen, J.J.; Wang, X.Z. The Daily Intake Levels of Copper, Selenium, and Zinc Are Associated with Osteoarthritis but Not with Rheumatoid Arthritis in a Cross-sectional Study. Biol. Trace Elem. Res. 2023, 201, 5662–5670. [Google Scholar] [CrossRef]
- McCall, K.A.; Huang, C.-C.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef]
- Zastrow, M.L.; Pecoraro, V.L. Designing Hydrolytic Zinc Metalloenzymes. Biochemistry 2014, 53, 957–978. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Yao, G.; Wang, Z.; Xie, R.; Zhanghuang, C.; Yan, B. Trace element zinc metabolism and its relation to tumors. Front. Endocrinol. 2024, 15, 1457943. [Google Scholar] [CrossRef] [PubMed]
- Kiouri, D.P.; Chasapis, C.T.; Mavromoustakos, T.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and its binding proteins: Essential roles and therapeutic potential. Arch. Toxicol. 2025, 99, 23–41. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, S.; Hanson, S.R.; Miyaki, S.; Grogan, S.P.; Kinoshita, M.; Asahara, H.; Wong, C.H.; Lotz, M.K. Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 10202–10207. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Das, A.K.; Ghosh, N.; Sil, P.C. Chapter 2.7—Superoxide dismutase. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–166. [Google Scholar] [CrossRef]
- Valentine, J.S.; Ellerby, L.M.; Graden, J.A.; Nishida, C.R.; Gralla, E.B. Copper-Zinc Superoxide Dismutase: Mechanistic and Biological Studies. In Bioinorganic Chemistry: An Inorganic Perspective of Life; Springer: Berlin/Heidelberg, Germany, 1995; pp. 77–91. [Google Scholar]
- Marreiro, D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Kloubert, V.; Rink, L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015, 6, 3195–3204. [Google Scholar] [CrossRef]
- Bravo, A.; Araujo, S.; Vargas, M.E.; Mesa, J.; Souki, A.; Bermúdez, V.; Cano, C. Actividad de la enzima antioxidante superóxido dismutasa y niveles de cobre y zinc en pacientes con diabetes mellitus tipo 2. Arch. Venez. Farmacol. Ter. 2007, 26, 37–41. [Google Scholar]
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef]
- Huang, T.C.; Chang, W.T.; Hu, Y.C.; Hsieh, B.S.; Cheng, H.L.; Yen, J.H.; Chiu, P.R.; Chang, K.L. Zinc Protects Articular Chondrocytes through Changes in Nrf2-Mediated Antioxidants, Cytokines and Matrix Metalloproteinases. Nutrients 2018, 10, 471. [Google Scholar] [CrossRef]
- Kosik-Bogacka, D.I.; Lanocha-Arendarczyk, N.; Kot, K.; Zietek, P.; Karaczun, M.; Prokopowicz, A.; Kupnicka, P.; Ciosek, Z. Calcium, magnesium, zinc and lead concentrations in the structures forming knee joint in patients with osteoarthritis. J. Trace Elem. Med. Biol. 2018, 50, 409–414. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Huang, C.; Huang, H.; Lan, N.; Liu, S.; Luo, Y.; Zheng, L.; Liu, G.; Qin, Z.; Zhao, J. Zn2+-driven metformin conjugated with siRNA attenuates osteoarthritis progression by inhibiting NF-κB signaling and activating autophagy. Biomaterials 2025, 319, 123210. [Google Scholar] [CrossRef]
- Lee, M.; Won, Y.; Shin, Y.; Kim, J.H.; Chun, J.S. Reciprocal activation of hypoxia-inducible factor (HIF)-2α and the zinc-ZIP8-MTF1 axis amplifies catabolic signaling in osteoarthritis. Osteoarthr. Cartil. 2016, 24, 134–145. [Google Scholar] [CrossRef]
- Wei, W.; Qi, X.; Cheng, B.; He, D.; Qin, X.; Zhang, N.; Zhao, Y.; Chu, X.; Shi, S.; Cai, Q.; et al. An atlas of causal association between micronutrients and osteoarthritis. Prev. Med. 2024, 185, 108063. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Hong, E.; Ye, W.; Ma, L.; Cun, D.; Huang, F.; Jiang, Z. Mendelian randomization of serum micronutrients and osteoarthritis risk: Focus on zinc. Nutr. J. 2025, 24, 38. [Google Scholar] [CrossRef]
- Ajileye, A.S.; Ajileye, A.B.; Emokpae, A.M. Serum Levels of Some Essential Trace Elements in Patients with Osteoarthritis. Afr. J. Biomed. Res. 2024, 27, 359–366. [Google Scholar]
- Dąbrowski, M.; Zioła-Frankowska, A.; Frankowski, M.; Kaczmarczyk, J.; Kubaszewski, Ł. Comparison of Bone Tissue Trace Element Content in the Different Radiological Stages of Hip Osteoarthritis. Int. J. Environ. Res. Public Health 2021, 18, 3260. [Google Scholar] [CrossRef] [PubMed]
- Rushton, M.D.; Young, D.A.; Loughlin, J.; Reynard, L.N. Differential DNA methylation and expression of inflammatory and zinc transporter genes defines subgroups of osteoarthritic hip patients. Ann. Rheum. Dis. 2015, 74, 1778–1782. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Lee, S.H.; Jhun, J.; Choi, J.; Jung, K.; Cho, K.H.; Kim, S.J.; Yang, C.W.; Park, S.H.; Cho, M.L. The Combination of Probiotic Complex, Rosavin, and Zinc Improves Pain and Cartilage Destruction in an Osteoarthritis Rat Model. J. Med. Food 2018, 21, 364–371. [Google Scholar] [CrossRef]
- Asensio, G.; Benito-Garzón, L.; Ramírez-Jiménez, R.A.; Guadilla, Y.; Gonzalez-Rubio, J.; Abradelo, C.; Parra, J.; Martín-López, M.R.; Aguilar, M.R.; Vázquez-Lasa, B.; et al. Biomimetic Gradient Scaffolds Containing Hyaluronic Acid and Sr/Zn Folates for Osteochondral Tissue Engineering. Polymers 2021, 14, 12. [Google Scholar] [CrossRef]
- Khader, A.; Arinzeh, T.L. Biodegradable zinc oxide composite scaffolds promote osteochondral differentiation of mesenchymal stem cells. Biotechnol. Bioeng. 2020, 117, 194–209. [Google Scholar] [CrossRef]
- You, X.; Ye, Y.; Lin, S.; Zhang, Z.; Guo, H.; Ye, H. Identification of key genes and immune infiltration in osteoarthritis through analysis of zinc metabolism-related genes. BMC Musculoskelet. Disord. 2024, 25, 227. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of copper on mitochondrial function and metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Medeiros, D.M. Copper, iron, and selenium dietary deficiencies negatively impact skeletal integrity: A review. Exp. Biol. Med. 2016, 241, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Li, G. Molecular insights into lysyl oxidases in cartilage regeneration and rejuvenation. Front. Bioeng. Biotechnol. 2020, 8, 359. [Google Scholar] [CrossRef]
- Kagan, H.M.; Li, W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J. Cell Biochem. 2003, 88, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Xiao, Y.; Guan, M.; Zhang, X.; Yu, J.; Han, M.; Li, Z. Copper metabolism in osteoarthritis and its relation to oxidative stress and ferroptosis in chondrocytes. Front. Mol. Biosci. 2024, 11, 1472492. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.S. Copper and immunity. Am. J. Clin. Nutr. 1998, 67, 1064S–1068S. [Google Scholar] [CrossRef]
- Lukasewycz, O.A.; Prohaska, J.R. The immune response in copper deficiency. Ann. N. Y. Acad. Sci. 1990, 587, 147–159. [Google Scholar] [CrossRef]
- Guan, T.; Wu, Z.; Xu, C.; Su, G. The association of trace elements with arthritis in US adults: NHANES 2013–2016. J. Trace Elem. Med. Biol. 2023, 76, 127122. [Google Scholar] [CrossRef]
- Amerikanou, C.; Valsamidou, E.; Karavoltsos, S.; Tagkouli, D.; Sakellari, A.; Kontou, M.; Houhoula, D.; Kalogeropoulos, N.; Zoumpoulakis, P.; Kaliora, A.C. Circulating Copper Is Associated with Inflammatory Biomarkers in Greek Older Adults with Osteoarthritis. Biol. Trace Elem. Res. 2024, 202, 1866–1877. [Google Scholar] [CrossRef]
- Roczniak, W.; Brodziak-Dopierała, B.; Cipora, E.; Jakóbik-Kolon, A.; Kluczka, J.; Babuśka-Roczniak, M. Factors that Affect the Content of Cadmium, Nickel, Copper and Zinc in Tissues of the Knee Joint. Biol. Trace Elem. Res. 2017, 178, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Anderson, K.B.; Williams, L.J.; Stuart, A.L.; Hyde, N.K.; Holloway-Kew, K.L. Dietary intakes of copper and selenium in association with bone mineral density. Nutrients 2024, 16, 2777. [Google Scholar] [CrossRef]
- Saati, A.A.; Adly, H.M. Assessing the correlation between blood trace element concentrations, picky eating habits, and intelligence quotient in school-aged children. Children 2023, 10, 1249. [Google Scholar] [CrossRef]
- Ha, J.-H.; Doguer, C.; Wang, X.; Flores, S.R.; Collins, J.F. High-iron consumption impairs growth and causes copper-deficiency anemia in weanling Sprague-Dawley rats. PLoS ONE 2016, 11, e0161033. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Y.; Wu, C.; Liang, J.; Tang, K.; Lin, Z.; Chen, L.; Lu, Y.; Wang, Q. Conversion of senescent cartilage into a pro-chondrogenic microenvironment with antibody-functionalized copper sulfate nanoparticles for efficient osteoarthritis therapy. J. Nanobiotechnol. 2023, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jiang, H.; Wu, C.; Lin, Y.; Tan, G.; Zhan, J.; Han, L.; Zhu, Y.; Shang, P.; Liu, L.; et al. Copper silicate nanoparticle-mediated delivery of astragaloside-IV for osteoarthritis treatment by remodeling the articular cartilage microenvironment. J. Control. Release 2025, 381, 113583. [Google Scholar] [CrossRef]
- Yassin, N.Z.; El-Shenawy, S.M.; Abdel-Rahman, R.F.; Yakoot, M.; Hassan, M.; Helmy, S. Effect of a topical copper indomethacin gel on inflammatory parameters in a rat model of osteoarthritis. Drug Des. Dev. Ther. 2015, 9, 1491–1498. [Google Scholar] [CrossRef]
- Gao, H.; Ning, E.; Zhang, X.; Shao, Z.; Hu, D.; Bai, L.; Che, H.; Hao, Y. Injectable microspheres filled with copper-containing bioactive glass improve articular cartilage healing by regulating inflammation and recruiting stem cells. Regen. Biomater. 2025, 12, rbae142. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liu, H.; Wang, S.; Hu, Y.; Huang, B.; Li, M.; Gao, J.; Wang, X.; Su, J. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/ anti-inflammatory therapy of osteoarthritis. Compos. Part B Eng. 2022, 237, 109855. [Google Scholar] [CrossRef]
- Liu, H.; Ji, M.; Yang, T.; Zou, S.; Qiu, X.; Zhan, F.; Chen, J.; Yan, F.; Ding, F.; Li, P. Regulation of fibroblast phenotype in osteoarthritis using CDKN1A-loaded copper sulfide nanoparticles delivered by mesenchymal stem cells. Am. J. Physiol.—Cell Physiol. 2025, 328, C679–C698. [Google Scholar] [CrossRef]
- Geng, D.; Lin, R.; Wei, P.; Tang, C.; Xu, Y.; Wang, L. The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in Osteoarthritis. Med. Sci. Monit. 2024, 30, e943738. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Luo, J.; Wang, C.; Kapilevich, L.; Zhang, X.A. Roles and mechanisms of copper homeostasis and cuproptosis in osteoarticular diseases. Biomed. Pharmacother. 2024, 174, 116570. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.; Meng, C.; Li, C.; Jia, D.; Xu, Y. The effect of copper and vitamin D on osteoarthritis outcomes: A Mendelian randomization study. Medicine 2024, 103, e39828. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, C.; Sun, Y.; Francis, M.; Ryu, M.S.; Grider, A.; Ye, K. Genetically predicted circulating levels of copper and zinc are associated with osteoarthritis but not with rheumatoid arthritis. Osteoarthr. Cartil. 2021, 29, 1029–1035. [Google Scholar] [CrossRef]
- Sies, H. The Concept of Oxidative Stress After 30 Years. In Biochemistry of Oxidative Stress: Physiopathology and Clinical Aspects; Gelpi, R.J., Boveris, A., Poderoso, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–11. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Prasad, B.; Akanksha, A.; Kaur, P.S.; Gupta, S. Understanding selenoproteins: Structural insights, biological functions and transformative applications in therapeutics. Process Biochem. 2025, 150, 148–160. [Google Scholar] [CrossRef]
- Zhang, Y.; Roh, Y.J.; Han, S.J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.R. Role of Selenoproteins in Redox Regulation of Signaling and the Antioxidant System: A Review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Yen, C.C.; Huang, L.W.; Hu, Y.C.; Huang, T.C.; Hsieh, B.S.; Chang, K.L. Selenium Lessens Osteoarthritis by Protecting Articular Chondrocytes from Oxidative Damage through Nrf2 and NF-κB Pathways. Int. J. Mol. Sci. 2024, 25, 2511. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Luo, J.; Tong, Y.; Zheng, Y.; Ji, L.; He, Z.; Jing, Q.; Huang, J.; Zhang, Y.; et al. The Protective Effect of Selenium Nanoparticles in Osteoarthritis: In vitro and in vivo Studies. Drug Des. Dev. Ther. 2023, 17, 1515–1529. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, H.; Yang, Z.; Bao, M.; Lin, X.; Han, J.; Qu, C. Progress of Selenium Deficiency in the Pathogenesis of Arthropathies and Selenium Supplement for Their Treatment. Biol. Trace Elem. Res. 2022, 200, 4238–4249. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xie, M.; Lei, G.; Zeng, C.; Yang, T.; Yang, Z.; Wang, Y.; Li, J.; Wei, J.; Tian, J.; et al. A Cross-Sectional Study of Association between Plasma Selenium Levels and the Prevalence of Osteoarthritis: Data from the Xiangya Osteoarthritis Study. J. Nutr. Health Aging 2022, 26, 197–202. [Google Scholar] [CrossRef]
- Wahl, L.; Chillon, T.S.; Seemann, P.; Ohrndorf, S.; Ochwadt, R.; Becker, W.; Schomburg, L.; Hoff, P. AB0522 Selenium Status in Patients with Rheumatoid Arthritis, Psoriatic Arthritis and Juvenile Idiopathic Arthritis. Ann. Rheum. Dis. 2024, 83, 1535. [Google Scholar] [CrossRef]
- Wahl, L.; Samson Chillon, T.; Seemann, P.; Ohrndorf, S.; Ochwadt, R.; Becker, W.; Schomburg, L.; Hoff, P. Serum selenium, selenoprotein P and glutathione peroxidase 3 in rheumatoid, psoriatic, juvenile idiopathic arthritis, and osteoarthritis. J. Nutr. Biochem. 2025, 135, 109776. [Google Scholar] [CrossRef]
- Ning, Y.; Hu, M.; Diao, J.; Gong, Y.; Huang, R.; Chen, S.; Zhang, F.; Liu, Y.; Chen, F.; Zhang, P.; et al. Genetic Variants and Protein Alterations of Selenium- and T-2 Toxin-Responsive Genes Are Associated With Chondrocytic Damage in Endemic Osteoarthropathy. Front. Genet. 2022, 12, 773534. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.S.; Kim, J.H. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Wang, S.; Geng, L.; Zhao, G.; Meng, P.; Yuan, L.; Guo, X. Effectiveness of Selenium on Chondrocyte Glycoprotein Glycosylation Which Play Important Roles in the Pathogenesis of an Endemic Osteoarthritis, Kashin–Beck Disease. Biol. Trace Elem. Res. 2022, 200, 1531–1537. [Google Scholar] [CrossRef]
- Park, K.C.; Choi, J.; Choi, S.; Lee, G.; An, H.J.; Yun, H.; Lee, S. Therapeutic potential of Polydopamine-Coated selenium nanoparticles in Osteoarthritis treatment. Int. J. Pharm. 2025, 675, 125568. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Liu, S.; Xiao, P.; Du, C.; Zhan, J.; Chen, Z.; Chen, L.; Li, K.; Huang, W.; et al. Cascade targeting selenium nanoparticles-loaded hydrogel microspheres for multifaceted antioxidant defense in osteoarthritis. Biomaterials 2025, 318, 123195. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Yang, F.; Hong, J.; Wang, W.; Li, S.; Jiang, G.; Yan, S. Causal relationship of serum nutritional factors with osteoarthritis: A Mendelian randomization study. Rheumatology 2021, 60, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Chandrapalan, T.; Kwong, R.W.M. Functional significance and physiological regulation of essential trace metals in fish. J. Exp. Biol. 2021, 224, 238790. [Google Scholar] [CrossRef]
- Kwong, R.W.M. Trace metals in the teleost fish gill: Biological roles, uptake regulation, and detoxification mechanisms. J. Comp. Physiol. B 2024, 194, 749–763. [Google Scholar] [CrossRef]
- Levy, M.A.; Tsai, Y.H.; Reaume, A.; Bray, T.M. Cellular response of antioxidant metalloproteins in Cu/Zn SOD transgenic mice exposed to hyperoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L172–L182. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.T.; Zhen, J.; Leng, J.Y.; Cai, L.; Ji, H.L.; Keller, B.B. Zinc as a countermeasure for cadmium toxicity. Acta Pharmacol. Sin. 2021, 42, 340–346. [Google Scholar] [CrossRef]
- Quilliot, D.; Dousset, B.; Guerci, B.; Dubois, F.; Drouin, P.; Ziegler, O. Evidence that diabetes mellitus favors impaired metabolism of zinc, copper, and selenium in chronic pancreatitis. Pancreas 2001, 22, 299–306. [Google Scholar] [CrossRef]
- Hajhashemy, Z.; Foshati, S.; Bagherniya, M.; Askari, G. The association between blood selenium and metabolic syndrome in adults: A systematic review and dose–response meta-analysis of epidemiologic studies. Front. Nutr. 2025, 11, 1451342. [Google Scholar] [CrossRef]
- Tamura, Y. The role of zinc homeostasis in the prevention of diabetes mellitus and cardiovascular diseases. J. Atheroscler. Thromb. 2021, 28, 1109–1122. [Google Scholar] [CrossRef]
- Kraus, V.B. The zinc link. Nature 2014, 507, 441–442. [Google Scholar] [CrossRef]
- Buneaux, F.; Buneaux, J.J.; Fabiani, P.; Galmiche, P. Zinc and enzymes in the synovial fluid and blood in various types of rheumatism. Rev. Rhum. Mal. Osteoartic. 1978, 45, 699–701. [Google Scholar] [PubMed]
- Yazar, M.; Sarban, S.; Kocyigit, A.; Isikan, U.E. Synovial fluid and plasma selenium, copper, zinc, and iron concentrations in patients with rheumatoid arthritis and osteoarthritis. Biol. Trace Elem. Res. 2005, 106, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Neve, J.; Vertongen, F.; Famaey, J.P. Synovial Fluid Copper, Zinc and Selenium in Relation to Inflammatory Parameters in Rheumatic Diseases. In Biology of Copper Complexes; Sorenson, J.R.J., Ed.; Humana Press: Totowa, NJ, USA, 1987; pp. 583–589. [Google Scholar] [CrossRef]
- Struniolo, G.C.; D’Inca, R.; Lecis, P.E.; Naccarato, R. Factors influencing the absorption of trace elements in healthy conditions and in gastrointestinal diseases. Ital. J. Gastroenterol. 1994, 26, 247–260. [Google Scholar] [PubMed]
- Cousins, R.J.; Liuzzi, J.P. Chapter 61—Trace Metal Absorption and Transport. In Physiology of the Gastrointestinal Tract, 6th ed.; Said, H.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1485–1498. [Google Scholar] [CrossRef]
- do Nascimento da Silva, E.; Cadore, S. Bioavailability Assessment of Copper, Iron, Manganese, Molybdenum, Selenium, and Zinc from Selenium-Enriched Lettuce. J. Food Sci. 2019, 84, 2840–2846. [Google Scholar] [CrossRef]
- Pattan, V.; Chang Villacreses, M.M.; Karnchanasorn, R.; Chiu, K.C.; Samoa, R. Daily Intake and Serum Levels of Copper, Selenium and Zinc According to Glucose Metabolism: Cross-Sectional and Comparative Study. Nutrients 2021, 13, 4044. [Google Scholar] [CrossRef]
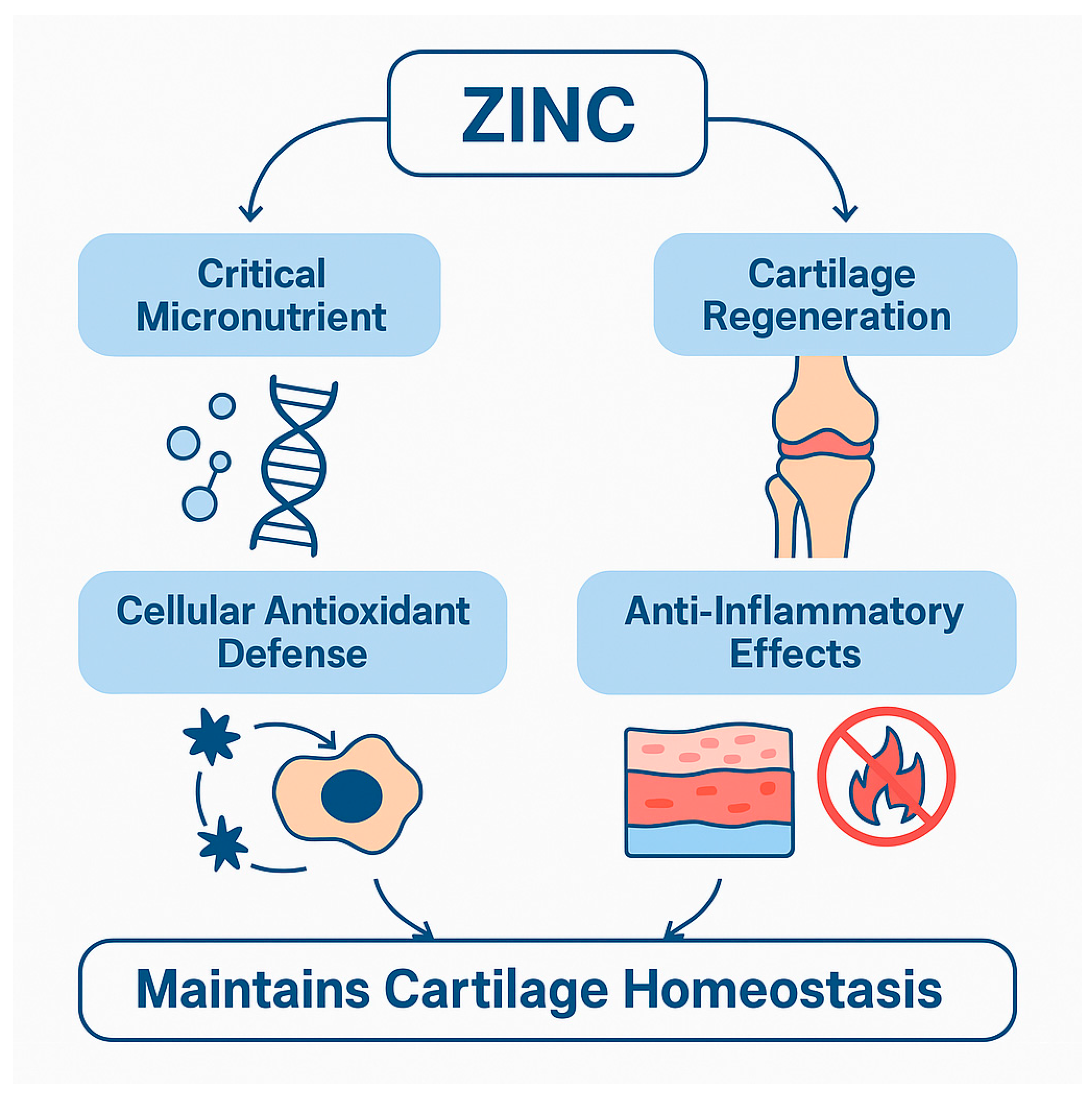
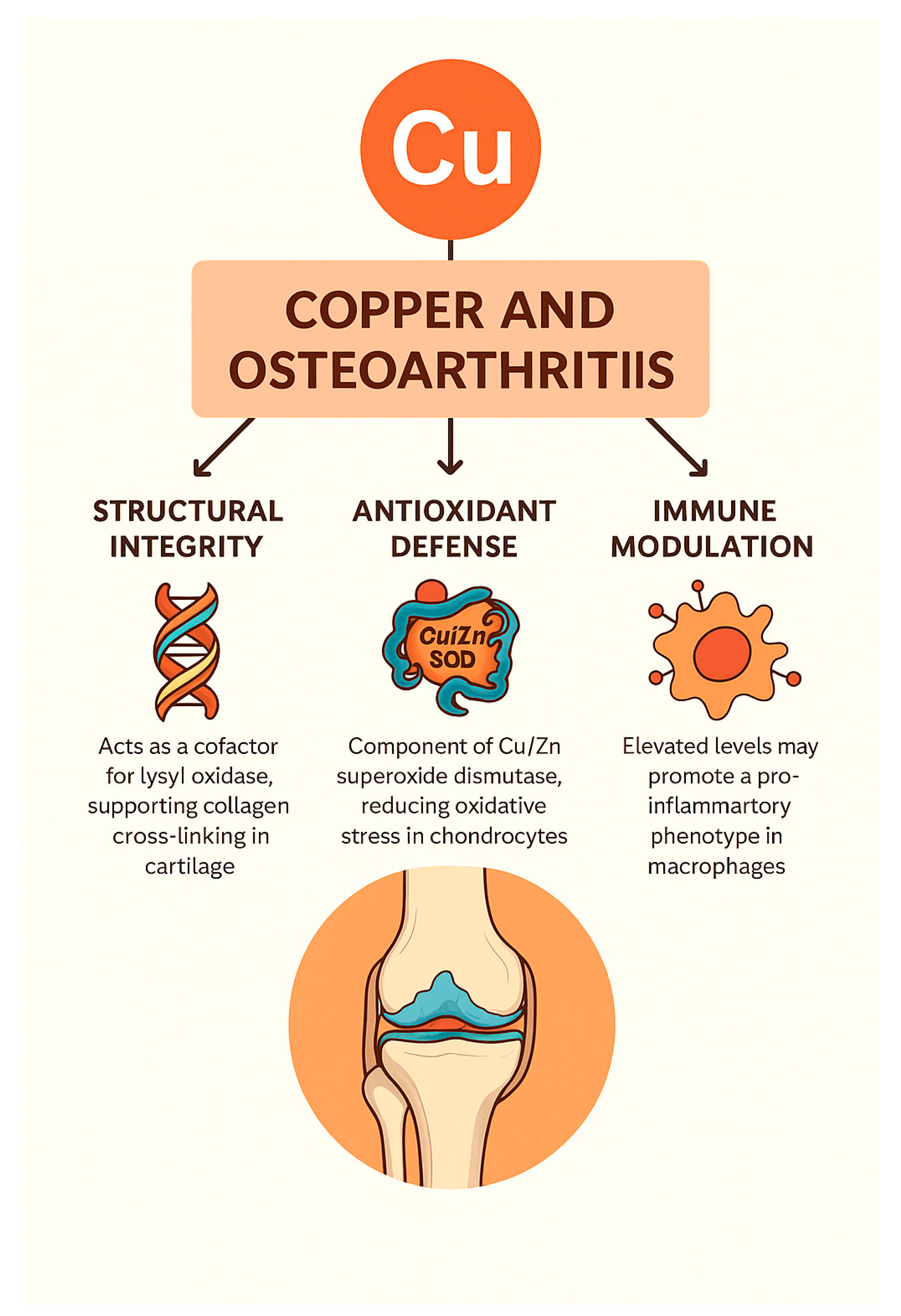
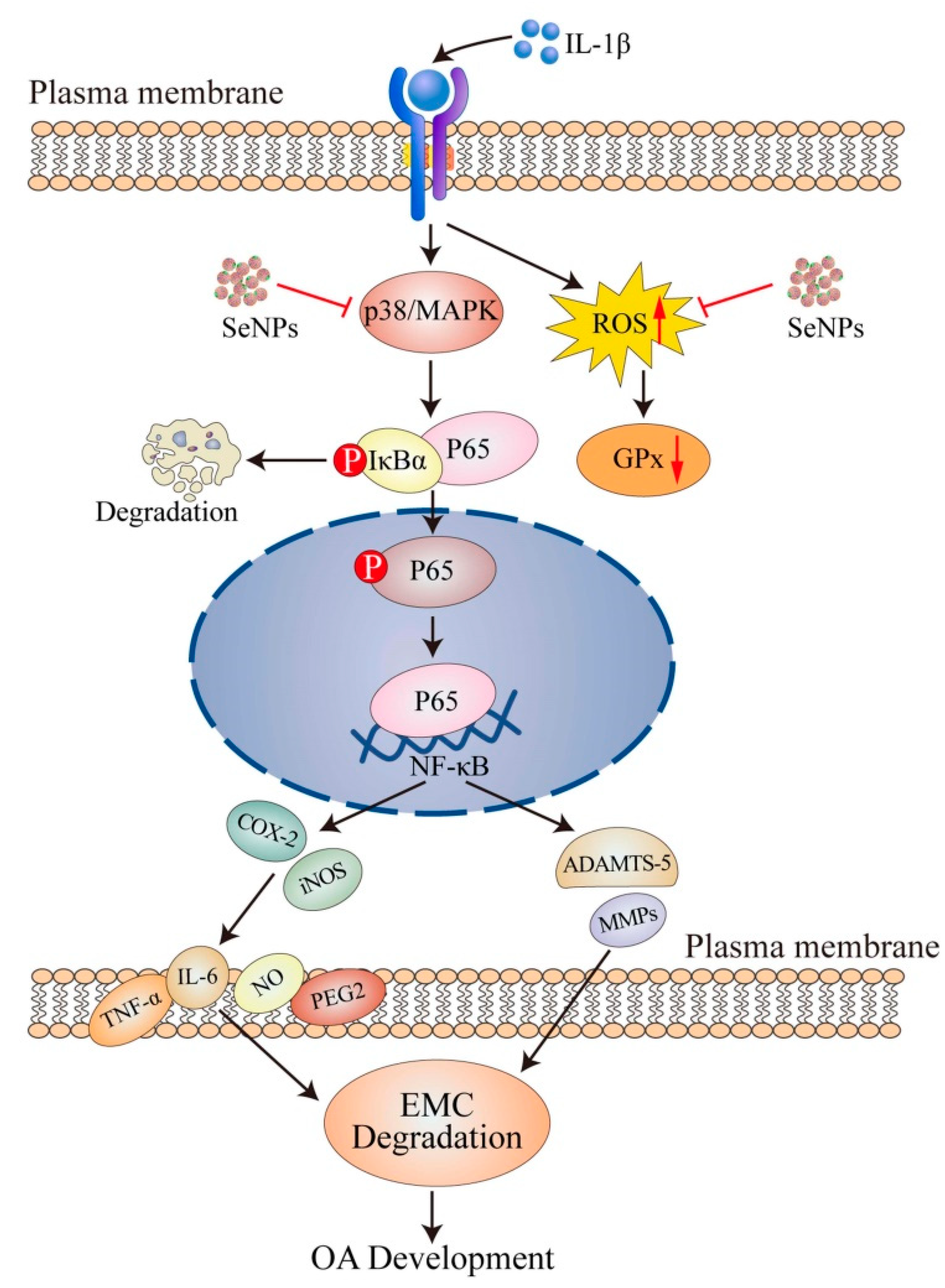
| Functional Role | Mechanism/Target | Outcome in OA | Reference(s) |
|---|---|---|---|
| Antioxidant Defense | Cofactor in Cu/Zn-SOD | Reduces oxidative stress and cell damage | [24,25,26,27] |
| Anti-Inflammatory Modulation | Inhibits the NF-κB pathway and reduces cytokines (TNF-α, IL-1β) | Decreases inflammation, slows progression | [18,29] |
| Chondrocyte Protection | Activates the p-Akt/Nrf2 pathway | Enhances viability, reduces apoptosis | [30,31] |
| Matrix Degradation Control | Modulates MMP13, ADAMTS5 via ZIP8-MTF1 axis | Slows ECM breakdown | [33,37,38] |
| Genetic/Transcriptomic Regulation | Upregulation of MMPs (MMP2, MMP3, MMP9, MMP13) | Associated with OA risk and progression | [42] |
| Regenerative Therapy | Zinc-loaded scaffolds (ZnFO, ZnO NPs) | Promotes cartilage/bone regeneration | [40,41] |
| Dietary Influence | Excess zinc intake, high serum Zn levels | Linked to increased OA risk | [15,34] |
| Combination Therapy | Zinc + probiotics/rosavin | Reduces cytokines, protects cartilage | [39] |
| Bone Zinc Accumulation | Increased Zn in femoral bone | Correlates with disease severity | [37] |
| Functional Domain | Role of Copper | OA-Relevant Outcomes | Reference(s) |
|---|---|---|---|
| ECM Integrity | Cofactor for lysyl oxidase (LOX) | Maintains collagen cross-linking and cartilage structure | [43,44,45,46,47] |
| Antioxidant Defense | Component of Cu/Zn-SOD | Reduces ROS and protects chondrocytes from oxidative stress | [48] |
| Ferroptosis Regulation | Regulates redox homeostasis and prevents lipid peroxidation | Prevents chondrocyte death and degeneration | [48] |
| Inflammation Modulation | Influences cytokine production, macrophage polarization | Controls joint inflammation and immune balance | [49,50,64] |
| Serum and Tissue Levels | Elevated or deficient levels linked with OA severity | Indicates copper’s role in OA risk and progression | [14,15,36,37,52,53,54] |
| Nanotherapeutic Delivery | B2M-CuS, CSP@AS-IV, Cu-Indo gel, PMs@CuBG, etc. | Enhances targeted therapy, ECM synthesis, and cartilage repair | [58,59,60,61,62,63] |
| Genetic Associations | Transporter gene variants, cuproptosis-related genes | Contribute to susceptibility and disease mechanisms | [48,65,67,68] |
| Domain | Key Functions | References |
|---|---|---|
| Antioxidant Signaling and Genetic Regulation | Modulates selenoproteins (GPX1, SELENOP); regulates NF-κB, PI3K/Akt, and TGF-β pathways; SNPs in GPX1, SELENOS, DIO2, PPARG, SMAD3, and ADAM12 influence redox and ECM homeostasis. | [80,81,82] |
| Chondrogenesis and DNA Repair | Enhances SOX9, COL2A1, and aggrecan expression; supports cartilage development and DNA repair mechanisms; protects against oxidative genomic instability. | [7,82] |
| Cellular Stress Response and Matrix Preservation | Restores mitochondrial function; reduces ROS, MMP13, IL-1β, and COX-2; supports glycosylation changes; attenuates oxidative stress and apoptosis. | [73,77,83] |
| Innovative Nanomedicine Strategies | SeNPs, PDA-SeNPs, and HA-SeNPs modulate inflammation and ECM degradation; enhance antioxidant activity and cartilage repair; regulate NF-κB, MAPK, and Wnt pathways; and enable targeted delivery. | [8,75,84,85] |
| Epidemiologic Risk Modification | Inverse association of Se status with OA risk; genetic MR studies; low Se in OA populations; sex-specific protective effects. | [15,36,86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amhare, A.F.; Liu, H.; Qiao, L.; Deng, H.; Han, J. Elemental Influence: The Emerging Role of Zinc, Copper, and Selenium in Osteoarthritis. Nutrients 2025, 17, 2069. https://doi.org/10.3390/nu17132069
Amhare AF, Liu H, Qiao L, Deng H, Han J. Elemental Influence: The Emerging Role of Zinc, Copper, and Selenium in Osteoarthritis. Nutrients. 2025; 17(13):2069. https://doi.org/10.3390/nu17132069
Chicago/Turabian StyleAmhare, Abebe Feyissa, Haobiao Liu, Lichun Qiao, Huan Deng, and Jing Han. 2025. "Elemental Influence: The Emerging Role of Zinc, Copper, and Selenium in Osteoarthritis" Nutrients 17, no. 13: 2069. https://doi.org/10.3390/nu17132069
APA StyleAmhare, A. F., Liu, H., Qiao, L., Deng, H., & Han, J. (2025). Elemental Influence: The Emerging Role of Zinc, Copper, and Selenium in Osteoarthritis. Nutrients, 17(13), 2069. https://doi.org/10.3390/nu17132069









