The Influence of Micronutrients and Environmental Factors on Thyroid DNA Integrity
Abstract
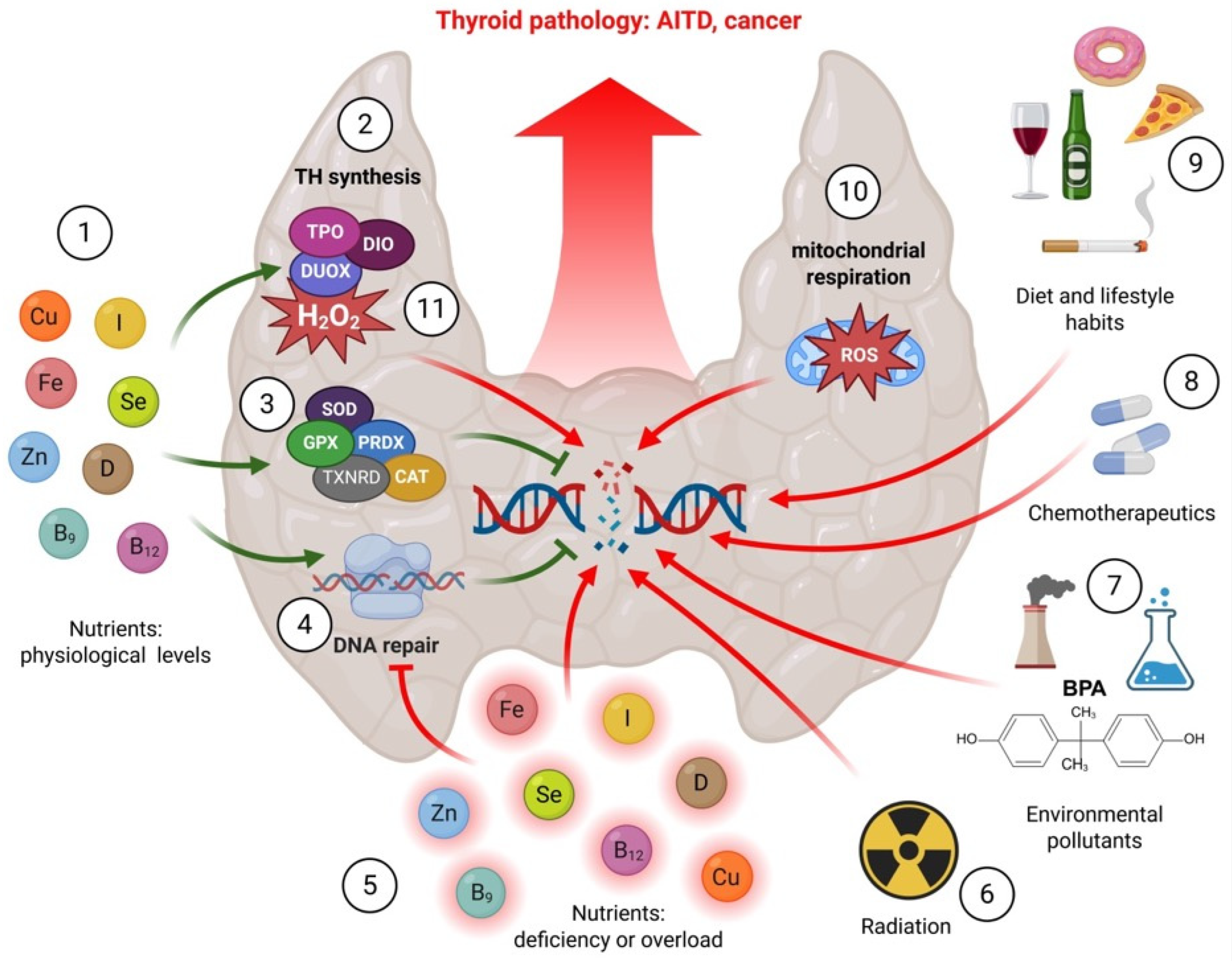
1. Introduction
2. Methodology
3. Micronutrients
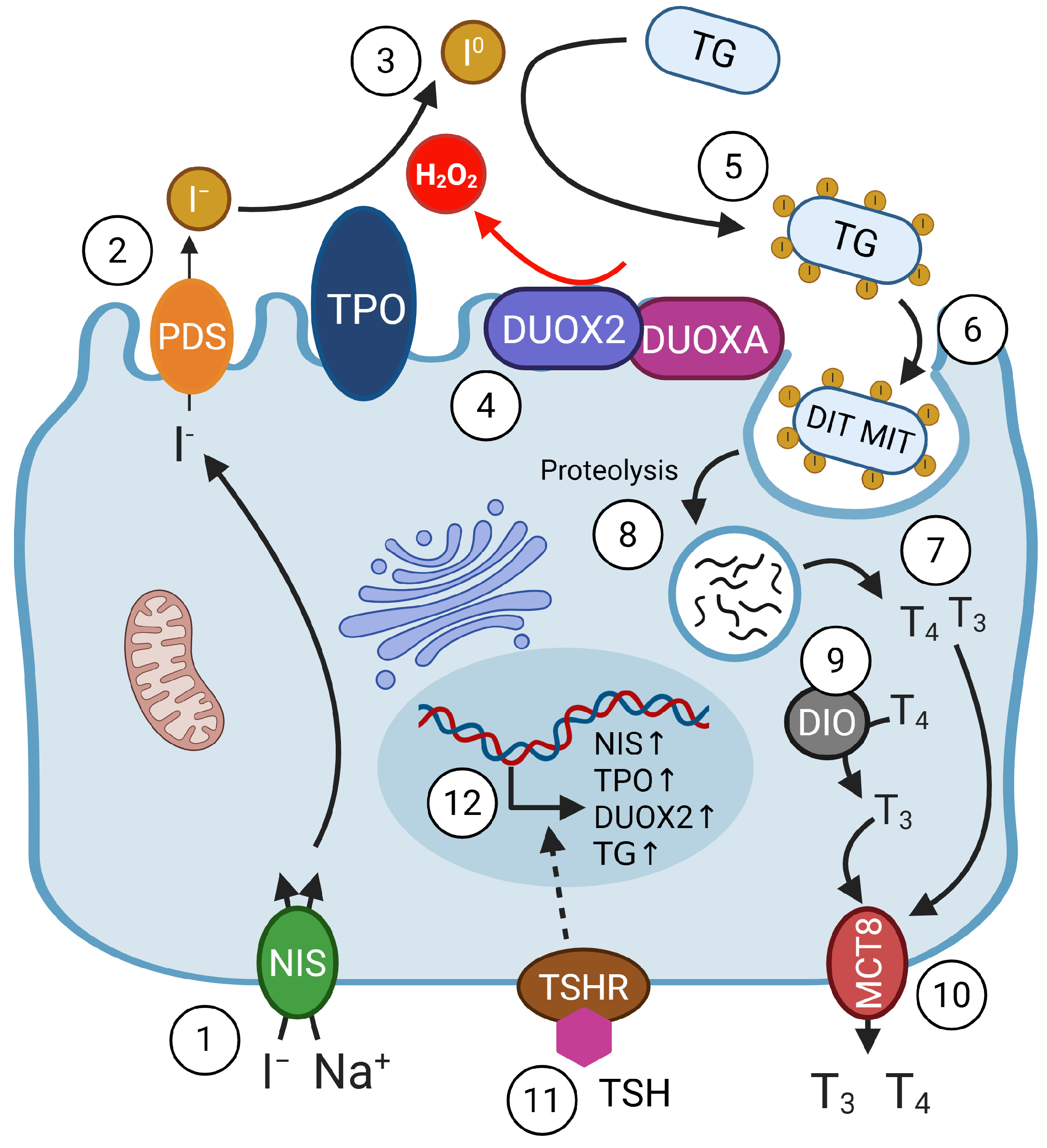
3.1. Iodide
3.2. Selenium
3.3. Iron
3.4. Zinc
3.5. Copper
3.6. Vitamin D
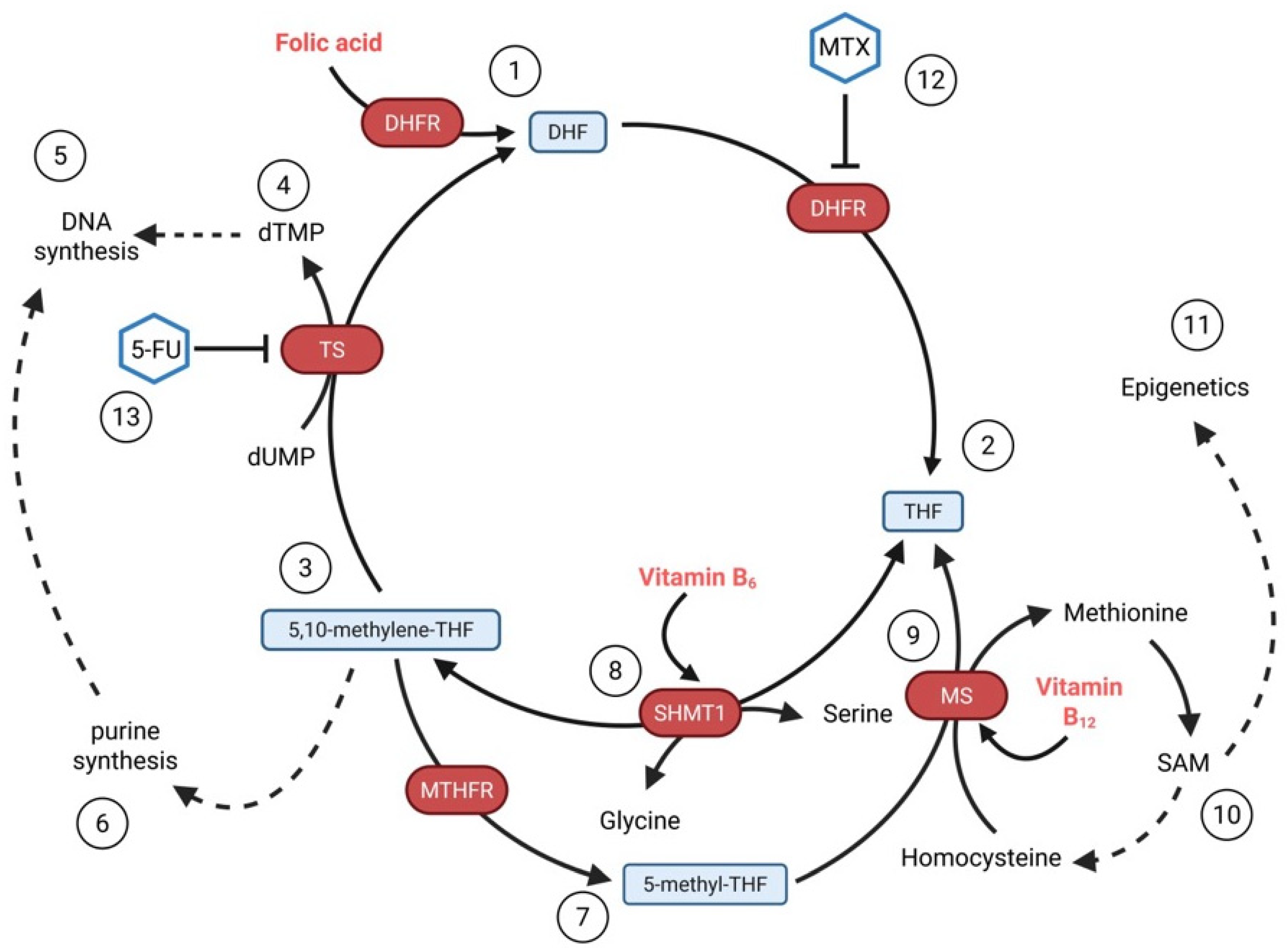
3.7. Folate (Vitamin B9) and Vitamin B12
4. Environmental Factors
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brent, G.A. Mechanisms of Thyroid Hormone Action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global Epidemiology of Hyperthyroidism and Hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Bogusławska, J.; Godlewska, M.; Gajda, E.; Piekiełko-Witkowska, A. Cellular and Molecular Basis of Thyroid Autoimmunity. Eur. Thyroid. J. 2022, 11, e210024. [Google Scholar] [CrossRef]
- Taylor, P.N.; Medici, M.M.; Hubalewska-Dydejczyk, A.; Boelaert, K. Hypothyroidism. Lancet 2024, 404, 1347–1364. [Google Scholar] [CrossRef]
- Chaker, L.; Cooper, D.S.; Walsh, J.P.; Peeters, R.P. Hyperthyroidism. Lancet 2024, 403, 768–780. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E. Consequences of Excess Iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.M. Thyroid Emergencies. J. Infus. Nurs. 2016, 39, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Soetedjo, N.N.M.; Agustini, D.; Permana, H. The Impact of Thyroid Disorder on Cardiovascular Disease: Unraveling the Connection and Implications for Patient Care. Int. J. Cardiol. Heart Vasc. 2024, 55, 101536. [Google Scholar] [CrossRef]
- Duntas, L.H.; Brenta, G. A Renewed Focus on the Association Between Thyroid Hormones and Lipid Metabolism. Front. Endocrinol. 2018, 9, 511. [Google Scholar] [CrossRef]
- Puthiyachirakal, M.A.; Hopkins, M.; AlNatsheh, T.; Das, A. Overview of Thyroid Disorders in Pregnancy. Matern. Health Neonatol. Perinatol. 2025, 11, 9. [Google Scholar] [CrossRef]
- Limaiem, F.; Kashyap, S.; Naing, P.T.; Mathias, P.M.; Giwa, A.O. Anaplastic Thyroid Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Carvalho, D.P.; Dupuy, C. Thyroid Hormone Biosynthesis and Release. Mol. Cell. Endocrinol. 2017, 458, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, M.; Banga, P.J. Thyroid Peroxidase as a Dual Active Site Enzyme: Focus on Biosynthesis, Hormonogenesis and Thyroid Disorders of Autoimmunity and Cancer. Biochimie 2019, 160, 34–45. [Google Scholar] [CrossRef]
- Szanto, I.; Pusztaszeri, M.; Mavromati, M. H2O2 Metabolism in Normal Thyroid Cells and in Thyroid Tumorigenesis: Focus on NADPH Oxidases. Antioxidants 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Driessens, N.; Costa, M.; De Deken, X.; Detours, V.; Corvilain, B.; Maenhaut, C.; Miot, F.; Van Sande, J.; Many, M.C.; et al. Roles of Hydrogen Peroxide in Thyroid Physiology and Disease. J. Clin. Endocrinol. Metab. 2007, 92, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Gielecińska, A.; Kciuk, M.; Kołat, D.; Kruczkowska, W.; Kontek, R. Polymorphisms of DNA Repair Genes in Thyroid Cancer. Int. J. Mol. Sci. 2024, 25, 5995. [Google Scholar] [CrossRef]
- Qin, N.; Wang, Z.; Liu, Q.; Song, N.; Wilson, C.L.; Ehrhardt, M.J.; Shelton, K.; Easton, J.; Mulder, H.; Kennetz, D.; et al. Pathogenic Germline Mutations in DNA Repair Genes in Combination With Cancer Treatment Exposures and Risk of Subsequent Neoplasms Among Long-Term Survivors of Childhood Cancer. J. Clin. Oncol. 2020, 38, 2728–2740. [Google Scholar] [CrossRef]
- Fenech, M. The Role of Nutrition in DNA Replication, DNA Damage Prevention and DNA Repair. In Principles of Nutrigenetics and Nutrigenomics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 27–32. ISBN 978-0-12-804572-5. [Google Scholar]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Shulhai, A.-M.; Rotondo, R.; Petraroli, M.; Patianna, V.; Predieri, B.; Iughetti, L.; Esposito, S.; Street, M.E. The Role of Nutrition on Thyroid Function. Nutrients 2024, 16, 2496. [Google Scholar] [CrossRef]
- Babić Leko, M.; Jureško, I.; Rozić, I.; Pleić, N.; Gunjača, I.; Zemunik, T. Vitamin D and the Thyroid: A Critical Review of the Current Evidence. Int. J. Mol. Sci. 2023, 24, 3586. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Feldt-Rasmussen, U.; Piekielko-Witkowska, A.; Gaspar da Rocha, A.; Badiu, C.; Koehrle, J.; Duntas, L. The ETA-ESE Statement on the European Chemicals Agency Opinion on Iodine as an Endocrine Disruptor. Eur. Thyroid. J. 2024, 13, e230244. [Google Scholar] [CrossRef]
- Fisher, D.A.; Oddie, T.H. Thyroid Iodine Content and Turnover in Euthyroid Subjects: Validity of Estimation of Thyroid Iodine Accumulation from Short-Term Clearance Studies. J. Clin. Endocrinol. Metab. 1969, 29, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Venturi, S. Evolutionary Significance of Iodine. Curr. Chem. Biol. 2011, 5, 155–162. [Google Scholar] [CrossRef]
- Aceves, C.; Mendieta, I.; Anguiano, B.; Delgado-Gonzalez, E. Molecular Iodine Has Extrathyroidal Effects as an Antioxidant, Differentiator, and Immunomodulator. Int. J. Mol. Sci. 2021, 22, 1228. [Google Scholar] [CrossRef] [PubMed]
- Karbownik-Lewinska, M.; Stepniak, J.; Milczarek, M.; Lewinski, A. Protective Effect of KI in mtDNA in Porcine Thyroid: Comparison with KIO3 and nDNA. Eur. J. Nutr. 2015, 54, 319–323. [Google Scholar] [CrossRef]
- Denef, J.F.; Many, M.C.; van den Hove, M.F. Iodine-Induced Thyroid Inhibition and Cell Necrosis: Two Consequences of the Same Free-Radical Mediated Mechanism? Mol. Cell. Endocrinol. 1996, 121, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Poncin, S.; Gerard, A.C.; Boucquey, M.; Senou, M.; Calderon, P.B.; Knoops, B.; Lengele, B.; Many, M.C.; Colin, I.M. Oxidative Stress in the Thyroid Gland: From Harmlessness to Hazard Depending on the Iodine Content. Endocrinology 2008, 149, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Leoni, S.G.; Kimura, E.T.; Santisteban, P.; De la Vieja, A. Regulation of Thyroid Oxidative State by Thioredoxin Reductase Has a Crucial Role in Thyroid Responses to Iodide Excess. Mol. Endocrinol. 2011, 25, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Galetti, V. Iodine Intake as a Risk Factor for Thyroid Cancer: A Comprehensive Review of Animal and Human Studies. Thyroid. Res. 2015, 8, 8. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Trimarchi, F. Iodine Nutrition Optimization: Are There Risks for Thyroid Autoimmunity? J. Endocrinol. Investig. 2021, 44, 1827–1835. [Google Scholar] [CrossRef]
- Krohn, K.; Maier, J.; Paschke, R. Mechanisms of Disease: Hydrogen Peroxide, DNA Damage and Mutagenesis in the Development of Thyroid Tumors. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 713–720. [Google Scholar] [CrossRef]
- Maier, J.; van Steeg, H.; van Oostrom, C.; Paschke, R.; Weiss, R.E.; Krohn, K. Iodine Deficiency Activates Antioxidant Genes and Causes DNA Damage in the Thyroid Gland of Rats and Mice. Biochim. Biophys. Acta 2007, 1773, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Lin, Y.; Liang, J. Radioactive Iodine-Refractory Differentiated Thyroid Cancer and Redifferentiation Therapy. Endocrinol. Metab. 2019, 34, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Kolypetri, P.; Carayanniotis, G. Apoptosis of NOD.H2 H4 Thyrocytes by Low Concentrations of Iodide Is Associated with Impaired Control of Oxidative Stress. Thyroid 2014, 24, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Di Matola, T.; D’Ascoli, F.; Salzano, S.; Bogazzi, F.; Fenzi, G.; Martino, E.; Rossi, G. Iodide Excess Induces Apoptosis in Thyroid Cells through a P53-Independent Mechanism Involving Oxidative Stress. Endocrinology 2000, 141, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, C.; Dong, L.; Kang, P.; Ding, C.; Zheng, T.; Wang, X.; Xiao, Y. Excessive Iodine Promotes Pyroptosis of Thyroid Follicular Epithelial Cells in Hashimoto’s Thyroiditis Through the ROS-NF-κB-NLRP3 Pathway. Front. Endocrinol. 2019, 10, 778. [Google Scholar] [CrossRef]
- Colin, I.M.; Denef, J.F.; Lengele, B.; Many, M.C.; Gerard, A.C. Recent Insights into the Cell Biology of Thyroid Angiofollicular Units. Endocr. Rev. 2013, 34, 209–238. [Google Scholar] [CrossRef]
- De la Vieja, A.; Santisteban, P. Role of Iodide Metabolism in Physiology and Cancer. Endocr. Relat. Cancer 2018, 25, R225–R245. [Google Scholar] [CrossRef]
- De la Vieja, A.; Riesco-Eizaguirre, G. Radio-Iodide Treatment: From Molecular Aspects to the Clinical View. Cancers 2021, 13, 995. [Google Scholar] [CrossRef]
- Morand, S.; Chaaraoui, M.; Kaniewski, J.; Deme, D.; Ohayon, R.; Noel-Hudson, M.S.; Virion, A.; Dupuy, C. Effect of Iodide on Nicotinamide Adenine Dinucleotide Phosphate Oxidase Activity and Duox2 Protein Expression in Isolated Porcine Thyroid Follicles. Endocrinology 2003, 144, 1241–1248. [Google Scholar] [CrossRef]
- Lyckesvard, M.N.; Kapoor, N.; Ingeson-Carlsson, C.; Carlsson, T.; Karlsson, J.O.; Postgard, P.; Himmelman, J.; Forssell-Aronsson, E.; Hammarsten, O.; Nilsson, M. Linking Loss of Sodium-Iodide Symporter Expression to DNA Damage. Exp. Cell Res. 2016, 344, 120–131. [Google Scholar] [CrossRef]
- Stasiolek, M.; Adamczewski, Z.; Sliwka, P.W.; Pula, B.; Karwowski, B.; Merecz-Sadowska, A.; Dedecjus, M.; Lewinski, A. The Molecular Effect of Diagnostic Absorbed Doses from (131)I on Papillary Thyroid Cancer Cells In Vitro. Molecules 2017, 22, 993. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, Y.; Chen, L.; Hu, L.; Zhu, F.; He, Q. High Iodine Induces DNA Damage in Autoimmune Thyroiditis Partially by Inhibiting the DNA Repair Protein MTH1. Cell. Immunol. 2019, 344, 103948. [Google Scholar] [CrossRef] [PubMed]
- Arczewska, K.D.; Stachurska, A.; Wojewodzka, M.; Karpinska, K.; Kruszewski, M.; Nilsen, H.; Czarnocka, B. hMTH1 Is Required for Maintaining Migration and Invasion Potential of Human Thyroid Cancer Cells. DNA Repair 2018, 69, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Arczewska, K.D.; Krasuska, W.; Stachurska, A.; Karpinska, K.; Sikorska, J.; Kiedrowski, M.; Lange, D.; Stepien, T.; Czarnocka, B. hMTH1 and GPX1 Expression in Human Thyroid Tissue Is Interrelated to Prevent Oxidative DNA Damage. DNA Repair 2020, 95, 102954. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.C.; Tomlinson, R.H. Selenium in Blood and Human Tissues. Clin. Chim. Acta 1967, 16, 311–321. [Google Scholar] [CrossRef]
- Behne, D.; Hilmert, H.; Scheid, S.; Gessner, H.; Elger, W. Evidence for Specific Selenium Target Tissues and New Biologically Important Selenoproteins. Biochim. Biophys. Acta 1988, 966, 12–21. [Google Scholar] [CrossRef]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedus, L. Selenium in Thyroid Disorders—Essential Knowledge for Clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef]
- Ekholm, R.; Bjorkman, U. Glutathione Peroxidase Degrades Intracellular Hydrogen Peroxide and Thereby Inhibits Intracellular Protein Iodination in Thyroid Epithelium. Endocrinology 1997, 138, 2871–2878. [Google Scholar] [CrossRef]
- Cammarota, F.; Fiscardi, F.; Esposito, T.; de Vita, G.; Salvatore, M.; Laukkanen, M.O. Clinical Relevance of Thyroid Cell Models in Redox Research. Cancer Cell Int. 2015, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Peng, F.; Xu, J.; Wang, G.; Zhao, Y. Increased Expression of GPX4 Promotes the Tumorigenesis of Thyroid Cancer by Inhibiting Ferroptosis and Predicts Poor Clinical Outcomes. Aging 2023, 15, 230–245. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.M.; Lee, R.T. Thioredoxin and Thioredoxin Target Proteins: From Molecular Mechanisms to Functional Significance. Antioxid. Redox Signal. 2013, 18, 1165–1207. [Google Scholar] [CrossRef] [PubMed]
- Leoni, S.G.; Sastre-Perona, A.; De la Vieja, A.; Santisteban, P. Selenium Increases Thyroid-Stimulating Hormone-Induced Sodium/Iodide Symporter Expression Through Thioredoxin/Apurinic/Apyrimidinic Endonuclease 1-Dependent Regulation of Paired Box 8 Binding Activity. Antioxid. Redox Signal. 2016, 24, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, S.; Vlantis, A.C.; Liu, S.Y.; Ng, E.K.; Chan, A.B.; Wu, J.; Du, J.; Wei, W.; Liu, X.; et al. Expression of Antioxidant Molecules and Heat Shock Protein 27 in Thyroid Tumors. J. Cell Biochem. 2016, 117, 2473–2481. [Google Scholar] [CrossRef]
- Metere, A.; Frezzotti, F.; Graves, C.E.; Vergine, M.; De Luca, A.; Pietraforte, D.; Giacomelli, L. A Possible Role for Selenoprotein Glutathione Peroxidase (GPx1) and Thioredoxin Reductases (TrxR1) in Thyroid Cancer: Our Experience in Thyroid Surgery. Cancer Cell Int. 2018, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, P.; Lv, H.-J.; Wu, Y.; Liu, S.; Deng, X.; Shi, B.; Fu, J. Comprehensive Analysis of Expression and Prognostic Value of Selenoprotein Genes in Thyroid Cancer. Genet. Test. Mol. Biomark. 2022, 26, 159–173. [Google Scholar] [CrossRef]
- Elhodaky, M.; Diamond, A.M. Selenium-Binding Protein 1 in Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3437. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Xu, J.; Lai, J.; Zhao, J.; Ma, L.; Sun, X. PRDM1 Promotes the Ferroptosis and Immune Escape of Thyroid Cancer by Regulating USP15-Mediated SELENBP1 Deubiquitination. J. Endocrinol. Investig. 2024, 47, 2981–2997. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, H.; Cheng, W.-H. Beneficial and Paradoxical Roles of Selenium at Nutritional Levels of Intake in Healthspan and Longevity. Free Radic. Biol. Med. 2018, 127, 3–13. [Google Scholar] [CrossRef]
- Serinkan Cinemre, F.B.; Bahtiyar, N.; Serinkan Cinemre, F.B.; Aydemir, B.; Değirmencioglu, S.; Cinemre, D.A.; Cinemre, G.C. The Role of Selenium, Selenoproteins and Oxidative DNA in Etiopathogenesis of Hashimoto Thyroiditis. J. Elementol. 2022, 27, 755–764. [Google Scholar] [CrossRef]
- Beckett, G.J.; Arthur, J.R. Selenium and Endocrine Systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Arnaldi, L.a.T.; Borra, R.C.; Maciel, R.M.B.; Cerutti, J.M. Gene Expression Profiles Reveal That DCN, DIO1, and DIO2 Are Underexpressed in Benign and Malignant Thyroid Tumors. Thyroid 2005, 15, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Romitti, M.; Wajner, S.M.; Zennig, N.; Goemann, I.M.; Bueno, A.L.; Meyer, E.L.S.; Maia, A.L. Increased Type 3 Deiodinase Expression in Papillary Thyroid Carcinoma. Thyroid 2012, 22, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Romitti, M.; Wajner, S.M.; Ceolin, L.; Ferreira, C.V.; Ribeiro, R.V.P.; Rohenkohl, H.C.; Weber, S.d.S.; Lopez, P.L.d.C.; Fuziwara, C.S.; Kimura, E.T.; et al. MAPK and SHH Pathways Modulate Type 3 Deiodinase Expression in Papillary Thyroid Carcinoma. Endocr. Relat. Cancer 2016, 23, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Piekiełko-Witkowska, A.; Nauman, A. Iodothyronine Deiodinases and Cancer. J. Endocrinol. Investig. 2011, 34, 716–728. [Google Scholar] [CrossRef]
- Angela De Stefano, M.; Porcelli, T.; Ambrosio, R.; Luongo, C.; Raia, M.; Schlumberger, M.; Salvatore, D. Type 2 Deiodinase Is Expressed in Anaplastic Thyroid Carcinoma and Its Inhibition Causes Cell Senescence. Endocr. Relat. Cancer 2023, 30, e230016. [Google Scholar] [CrossRef]
- Visser, W.E.; Bombardieri, C.R.; Zevenbergen, C.; Barnhoorn, S.; Ottaviani, A.; van der Pluijm, I.; Brandt, R.; Kaptein, E.; van Heerebeek, R.; van Toor, H.; et al. Tissue-Specific Suppression of Thyroid Hormone Signaling in Various Mouse Models of Aging. PLoS ONE 2016, 11, e0149941. [Google Scholar] [CrossRef]
- Nettore, I.C.; De Nisco, E.; Desiderio, S.; Passaro, C.; Maione, L.; Negri, M.; Albano, L.; Pivonello, R.; Pivonello, C.; Portella, G.; et al. Selenium Supplementation Modulates Apoptotic Processes in Thyroid Follicular Cells. Biofactors 2017, 43, 415–423. [Google Scholar] [CrossRef]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1297658. [Google Scholar] [CrossRef]
- Kohrle, J.; Gartner, R. Selenium and Thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 815–827. [Google Scholar] [CrossRef]
- Shetty, S.P.; Copeland, P.R. Selenocysteine Incorporation: A Trump Card in the Game of mRNA Decay. Biochimie 2015, 114, 97–101. [Google Scholar] [CrossRef]
- Wang, W.; Xue, H.; Li, Y.; Hou, X.; Fan, C.; Wang, H.; Zhang, H.; Shan, Z.; Teng, W. Effects of Selenium Supplementation on Spontaneous Autoimmune Thyroiditis in NOD.H-2h4 Mice. Thyroid 2015, 25, 1137–1144. [Google Scholar] [CrossRef]
- Ghaddhab, C.; Kyrilli, A.; Driessens, N.; Van Den Eeckhaute, E.; Hancisse, O.; De Deken, X.; Dumont, J.E.; Detours, V.; Miot, F.; Corvilain, B. Factors Contributing to the Resistance of the Thyrocyte to Hydrogen Peroxide. Mol. Cell. Endocrinol. 2019, 481, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; D’Ascola, A.; Vicchio, T.M.; Campo, S.; Gianì, F.; Giovinazzo, S.; Frasca, F.; Cannavò, S.; Campennì, A.; Trimarchi, F. Selenium Exerts Protective Effects against Oxidative Stress and Cell Damage in Human Thyrocytes and Fibroblasts. Endocrine 2020, 68, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.L.; Mihelc, E.M.; Pollok, K.E.; Smith, M.L. Chemotherapeutic Selectivity Conferred by Selenium: A Role for P53-Dependent DNA Repair. Mol. Cancer Ther. 2007, 6, 355–361. [Google Scholar] [CrossRef]
- Gupta, S.; Jaworska-Bieniek, K.; Lubinski, J.; Jakubowska, A. Can Selenium Be a Modifier of Cancer Risk in CHEK2 Mutation Carriers? Mutagenesis 2013, 28, 625–629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Songe-Moller, L.; van den Born, E.; Leihne, V.; Vagbo, C.B.; Kristoffersen, T.; Krokan, H.E.; Kirpekar, F.; Falnes, P.O.; Klungland, A. Mammalian ALKBH8 Possesses tRNA Methyltransferase Activity Required for the Biogenesis of Multiple Wobble Uridine Modifications Implicated in Translational Decoding. Mol. Cell. Biol. 2010, 30, 1814–1827. [Google Scholar] [CrossRef] [PubMed]
- Endres, L.; Begley, U.; Clark, R.; Gu, C.; Dziergowska, A.; Malkiewicz, A.; Melendez, J.A.; Dedon, P.C.; Begley, T.J. Alkbh8 Regulates Selenocysteine-Protein Expression to Protect against Reactive Oxygen Species Damage. PLoS ONE 2015, 10, e0131335. [Google Scholar] [CrossRef] [PubMed]
- Monies, D.; Vagbo, C.B.; Al-Owain, M.; Alhomaidi, S.; Alkuraya, F.S. Recessive Truncating Mutations in ALKBH8 Cause Intellectual Disability and Severe Impairment of Wobble Uridine Modification. Am. J. Hum. Genet. 2019, 104, 1202–1209. [Google Scholar] [CrossRef]
- Chiu-Ugalde, J.; Wirth, E.K.; Klein, M.O.; Sapin, R.; Fradejas-Villar, N.; Renko, K.; Schomburg, L.; Kohrle, J.; Schweizer, U. Thyroid Function Is Maintained despite Increased Oxidative Stress in Mice Lacking Selenoprotein Biosynthesis in Thyroid Epithelial Cells. Antioxid. Redox Signal. 2012, 17, 902–913. [Google Scholar] [CrossRef]
- Schoenmakers, E.; Chatterjee, K. Human Genetic Disorders Resulting in Systemic Selenoprotein Deficiency. Int. J. Mol. Sci. 2021, 22, 12927. [Google Scholar] [CrossRef]
- Kipp, A.P. Selenium in Colorectal and Differentiated Thyroid Cancer. Hormones 2020, 19, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yu, S.; He, W.; Li, J.; Xu, T.; Xue, J.; Shi, P.; Chen, S.; Li, Y.; Hong, S.; et al. Selenite Induces Cell Cycle Arrest and Apoptosis via Reactive Oxygen Species-Dependent Inhibition of the AKT/mTOR Pathway in Thyroid Cancer. Front. Oncol. 2021, 11, 668424. [Google Scholar] [CrossRef]
- Kohrle, J. Selenium in Endocrinology-Selenoprotein-Related Diseases, Population Studies, and Epidemiological Evidence. Endocrinology 2021, 162, bqaa228. [Google Scholar] [CrossRef]
- Kato, M.A.; Finley, D.J.; Lubitz, C.C.; Zhu, B.; Moo, T.-A.; Loeven, M.R.; Ricci, J.A.; Zarnegar, R.; Katdare, M.; Fahey, T.J. Selenium Decreases Thyroid Cancer Cell Growth by Increasing Expression of GADD153 and GADD34. Nutr. Cancer 2010, 62, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rostami, R.; Beiranvand, A.; Nourooz-Zadeh, S.; Rostami, M.; Mohammadi, A.; Nourooz-Zadeh, J. Association Between Essential Trace Elements and Thyroid Antibodies in the Blood of Women with Newly Diagnosed Hashimoto’s Thyroiditis. Int. J. Endocrinol. Metab. 2024, 22, e145599. [Google Scholar] [CrossRef]
- Lanzolla, G.; Marino, M.; Marcocci, C. Selenium in the Treatment of Graves’ Hyperthyroidism and Eye Disease. Front. Endocrinol. 2020, 11, 608428. [Google Scholar] [CrossRef]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marino, M.; Vaidya, B.; Wiersinga, W.M. Eugogo dagger The 2021 European Group on Graves’ Orbitopathy (EUGOGO) Clinical Practice Guidelines for the Medical Management of Graves’ Orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Hou, S.; Yuan, Z.; Li, C.; Zhang, W.; Zheng, L.; Li, X. The Role of Iron in Cancer Progression. Front. Oncol. 2021, 11, 778492. [Google Scholar] [CrossRef]
- Teh, M.R.; Armitage, A.E.; Drakesmith, H. Why Cells Need Iron: A Compendium of Iron Utilisation. Trends Endocrinol. Metab. 2024, 35, 1026–1049. [Google Scholar] [CrossRef]
- Karbownik-Lewinska, M.; Stepniak, J.; Lewinski, A. High Level of Oxidized Nucleosides in Thyroid Mitochondrial DNA.; Damaging Effects of Fenton Reaction Substrates. Thyroid. Res. 2012, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Stepniak, J.; Lewinski, A.; Karbownik-Lewinska, M. Membrane Lipids and Nuclear DNA Are Differently Susceptive to Fenton Reaction Substrates in Porcine Thyroid. Toxicol. Vitro 2013, 27, 71–78. [Google Scholar] [CrossRef]
- Dietz, J.V.; Fox, J.L.; Khalimonchuk, O. Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond. Cells 2021, 10, 2198. [Google Scholar] [CrossRef] [PubMed]
- Petronek, M.S.; Spitz, D.R.; Allen, B.G. Iron-Sulfur Cluster Biogenesis as a Critical Target in Cancer. Antioxidants 2021, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Stehling, O.; Vashisht, A.A.; Mascarenhas, J.; Jonsson, Z.O.; Sharma, T.; Netz, D.J.A.; Pierik, A.J.; Wohlschlegel, J.A.; Lill, R. MMS19 Assembles Iron-Sulfur Proteins Required for DNA Metabolism and Genomic Integrity. Science 2012, 337, 195–199. [Google Scholar] [CrossRef]
- Gari, K.; León Ortiz, A.M.; Borel, V.; Flynn, H.; Skehel, J.M.; Boulton, S.J. MMS19 Links Cytoplasmic Iron-Sulfur Cluster Assembly to DNA Metabolism. Science 2012, 337, 243–245. [Google Scholar] [CrossRef]
- Eng, Z.H.; Abdul Aziz, A.; Ng, K.L.; Mat Junit, S. Changes in Antioxidant Status and DNA Repair Capacity Are Corroborated with Molecular Alterations in Malignant Thyroid Tissue of Patients with Papillary Thyroid Cancer. Front. Mol. Biosci. 2023, 10, 1237548. [Google Scholar] [CrossRef]
- Mano, T.; Shinohara, R.; Iwase, K.; Kotake, M.; Hamada, M.; Uchimuro, K.; Hayakawa, N.; Hayashi, R.; Nakai, A.; Ishizuki, Y.; et al. Changes in Free Radical Scavengers and Lipid Peroxide in Thyroid Glands of Various Thyroid Disorders. Horm. Metab. Res. 1997, 29, 351–354. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Takano, T.; Miyauchi, A.; Matsuzuka, F.; Yoshida, H.; Kuma, K.; Amino, N. Decreased Expression of Catalase mRNA in Thyroid Anaplastic Carcinoma. Jpn. J. Clin. Oncol. 2003, 33, 6–9. [Google Scholar] [CrossRef]
- Hepp, M.; Werion, A.; De Greef, A.; de Ville de Goyet, C.; de Bournonville, M.; Behets, C.; Lengele, B.; Daumerie, C.; Mourad, M.; Ludgate, M.; et al. Oxidative Stress-Induced Sirtuin1 Downregulation Correlates to HIF-1alpha, GLUT-1, and VEGF-A Upregulation in Th1 Autoimmune Hashimoto’s Thyroiditis. Int. J. Mol. Sci. 2021, 22, 3806. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Song, R.; Gong, C.; Zhang, X.; Ren, G.; Li, J.; Chen, Y.; Qiu, L.; Mei, L.; Zhang, R.; et al. Ribonucleotide Reductase Large Subunit M1 Plays a Different Role in the Invasion and Metastasis of Papillary Thyroid Carcinoma and Undifferentiated Thyroid Carcinoma. Tumour Biol. 2016, 37, 3515–3526. [Google Scholar] [CrossRef] [PubMed]
- Castelblanco, E.; Zafon, C.; Maravall, J.; Gallel, P.; Martinez, M.; Capel, I.; Bella, M.R.; Halperin, I.; Temprana, J.; Iglesias, C.; et al. APLP2, RRM2, and PRC1: New Putative Markers for the Differential Diagnosis of Thyroid Follicular Lesions. Thyroid. 2017, 27, 59–66. [Google Scholar] [CrossRef]
- Ding, Y.-G.; Ren, Y.-L.; Xu, Y.-S.; Wei, C.-S.; Zhang, Y.-B.; Zhang, S.-K.; Guo, C.-A. Identification of Key Candidate Genes and Pathways in Anaplastic Thyroid Cancer by Bioinformatics Analysis. Am. J. Otolaryngol. 2020, 41, 102434. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Pluim, T.; Helms, A.; Bauer, A.; Tuttle, R.M.; Francis, G.L. Enzyme Expression Profiles Suggest the Novel Tumor-Activated Fluoropyrimidine Carbamate Capecitabine (Xeloda) Might Be Effective against Papillary Thyroid Cancers of Children and Young Adults. Cancer Chemother. Pharmacol. 2004, 53, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Song, M.; Qin, H.; Zhang, B.; Liu, Y.; Sun, Y.; Ma, Y.; Shi, T. Phosphoribosyl Pyrophosphate Amidotransferase Promotes the Progression of Thyroid Cancer via Regulating Pyruvate Kinase M2. OncoTargets Ther. 2020, 13, 7629–7639. [Google Scholar] [CrossRef]
- Chen, M.-M.; Meng, L.-H. The Double Faced Role of Xanthine Oxidoreductase in Cancer. Acta Pharmacol. Sin. 2022, 43, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Starokadomskyy, P.; Escala Perez-Reyes, A.; Burstein, E. Immune Dysfunction in Mendelian Disorders of POLA1 Deficiency. J. Clin. Immunol. 2021, 41, 285–293. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An Update to the Integrated Cancer Data Analysis Platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Siraj, A.K.; Bu, R.; Arshad, M.; Iqbal, K.; Parvathareddy, S.K.; Masoodi, T.; Ghazwani, L.O.; Al-Sobhi, S.S.; Al-Dayel, F.; Al-Kuraya, K.S. POLE and POLD1 Pathogenic Variants in the Proofreading Domain in Papillary Thyroid Cancer. Endocr. Connect. 2020, 9, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Eng, Z.H.; Abdullah, M.I.; Ng, K.L.; Abdul Aziz, A.; Arba’ie, N.H.; Mat Rashid, N.; Mat Junit, S. Whole-Exome Sequencing and Bioinformatic Analyses Revealed Differences in Gene Mutation Profiles in Papillary Thyroid Cancer Patients with and without Benign Thyroid Goitre Background. Front. Endocrinol. 2022, 13, 1039494. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.E.; David, C. Inborn Errors of Immunity and Autoimmune Disease. J. Allergy Clin. Immunol. Pract. 2023, 11, 1602–1622. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, S.; Yu, K.; Chen, K.; Zhao, L.; Zhang, J.; Dai, K.; Zhao, P. The Promoting Effect and Mechanism of MAD2L2 on Stemness Maintenance and Malignant Progression in Glioma. J. Transl. Med. 2023, 21, 863. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, L.; Gong, Z.; Zhou, K. Pan-Cancer Analysis Reveals PRIM2 as a Potential Biomarker for Diagnosis, Prognosis, and Immunomodulatory. Int. J. Genom. 2024, 2024, 8834415. [Google Scholar] [CrossRef]
- Silva, S.N.; Gil, O.M.; Oliveira, V.C.; Cabral, M.N.; Azevedo, A.P.; Faber, A.; Manita, I.; Ferreira, T.C.; Limbert, E.; Pina, J.E.; et al. Association of Polymorphisms in ERCC2 Gene with Non-Familial Thyroid Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2407–2412. [Google Scholar] [CrossRef]
- Shkarupa, V.M.; Mishcheniuk, O.Y.; Henyk-Berezovska, S.O.; Palamarchuk, V.O.; Klymenko, S.V. Polymorphism of DNA Repair Gene XPD Lys751Gln and Chromosome Aberrations in Lymphocytes of Thyroid Cancer Patients Exposed to Ionizing Radiation Due to the Chornobyl Accident. Exp. Oncol. 2016, 38, 257–260. [Google Scholar] [CrossRef]
- Lonjou, C.; Damiola, F.; Moissonnier, M.; Durand, G.; Malakhova, I.; Masyakin, V.; Le Calvez-Kelm, F.; Cardis, E.; Byrnes, G.; Kesminiene, A.; et al. Investigation of DNA Repair-Related SNPs Underlying Susceptibility to Papillary Thyroid Carcinoma Reveals MGMT as a Novel Candidate Gene in Belarusian Children Exposed to Radiation. BMC Cancer 2017, 17, 328. [Google Scholar] [CrossRef]
- Lutz, B.S.; Leguisamo, N.M.; Cabral, N.K.; Gloria, H.C.; Reiter, K.C.; Agnes, G.; Zanella, V.; Meyer, E.L.S.; Saffi, J. Imbalance in DNA Repair Machinery Is Associated with BRAF(V600E) Mutation and Tumor Aggressiveness in Papillary Thyroid Carcinoma. Mol. Cell. Endocrinol. 2018, 472, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.; Marques, I.J.; Saramago, A.; Moura, M.M.; Pojo, M.; Cabrera, R.; Santos, C.; Rosario, F.; Lousa, D.; Vicente, J.B.; et al. Identification of Novel Candidate Predisposing Genes in Familial Nonmedullary Thyroid Carcinoma Implicating DNA Damage Repair Pathways. Int. J. Cancer 2024, 156, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Shkarupa, V.; Henyk-Berezovska, S.; Palamarchuk, V.; Talko, V.; Klymenko, S. Research of DNA Repair Genes Polymorphism XRCC1 and XPD and the Risks of Thyroid Cancer Development in Persons Exposed to Ionizing Radiation after Chornobyl Disaster. Probl. Radiac. Med. Radiobiol. 2015, 20, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, A.J.; Land, C.E.; Bhatti, P.; Pineda, M.; Brenner, A.; Carr, Z.; Gusev, B.I.; Zhumadilov, Z.; Simon, S.L.; Bouville, A.; et al. Thyroid Nodules, Polymorphic Variants in DNA Repair and RET-Related Genes, and Interaction with Ionizing Radiation Exposure from Nuclear Tests in Kazakhstan. Radiat. Res. 2009, 171, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Sandler, J.E.; Huang, H.; Zhao, N.; Wu, W.; Liu, F.; Ma, S.; Udelsman, R.; Zhang, Y. Germline Variants in DNA Repair Genes, Diagnostic Radiation, and Risk of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.-P.; Junaid, M.; Ma, Y.-B.; Ahmad, F.; Jia, Y.-F.; Li, W.-G.; Pei, D.-S. Role of Human DNA2 (hDNA2) as a Potential Target for Cancer and Other Diseases: A Systematic Review. DNA Repair. 2017, 59, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, S.; Gao, G.; Xu, P.; Qian, M.; Yin, Y.; Yao, S.; Huang, Z.; Bian, Z. RTEL1 Is Upregulated in Gastric Cancer and Promotes Tumor Growth. J. Cancer Res. Clin. Oncol. 2024, 151, 23. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, D.; Li, K.; Cai, Y.; Xu, P.; Li, R.; Li, J.; Chen, X.; Chen, P.; Cui, G. E2F1 Mediated DDX11 Transcriptional Activation Promotes Hepatocellular Carcinoma Progression through PI3K/AKT/mTOR Pathway. Cell Death Dis. 2020, 11, 273. [Google Scholar] [CrossRef]
- Hambarde, S.; Tsai, C.-L.; Pandita, R.K.; Bacolla, A.; Maitra, A.; Charaka, V.; Hunt, C.R.; Kumar, R.; Limbo, O.; Le Meur, R.; et al. EXO5-DNA Structure and BLM Interactions Direct DNA Resection Critical for ATR-Dependent Replication Restart. Mol. Cell 2021, 81, 2989–3006.e9. [Google Scholar] [CrossRef]
- Nurmi, A.K.; Pelttari, L.M.; Kiiski, J.I.; Khan, S.; Nurmikolu, M.; Suvanto, M.; Aho, N.; Tasmuth, T.; Kalso, E.; Schleutker, J.; et al. NTHL1 Is a Recessive Cancer Susceptibility Gene. Sci. Rep. 2023, 13, 21127. [Google Scholar] [CrossRef]
- Moscatello, C.; Di Marcantonio, M.C.; Savino, L.; D’Amico, E.; Spacco, G.; Simeone, P.; Lanuti, P.; Muraro, R.; Mincione, G.; Cotellese, R.; et al. Emerging Role of Oxidative Stress on EGFR and OGG1-BER Cross-Regulation: Implications in Thyroid Physiopathology. Cells 2022, 11, 822. [Google Scholar] [CrossRef]
- Curia, M.C.; Catalano, T.; Aceto, G.M. MUTYH: Not Just Polyposis. World J. Clin. Oncol. 2020, 11, 428–449. [Google Scholar] [CrossRef]
- Magrin, L.; Fanale, D.; Brando, C.; Corsini, L.R.; Randazzo, U.; Di Piazza, M.; Gurrera, V.; Pedone, E.; Bazan Russo, T.D.; Vieni, S.; et al. MUTYH-Associated Tumor Syndrome: The Other Face of MAP. Oncogene 2022, 41, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Neta, G.; Brenner, A.V.; Sturgis, E.M.; Pfeiffer, R.M.; Hutchinson, A.A.; Aschebrook-Kilfoy, B.; Yeager, M.; Xu, L.; Wheeler, W.; Abend, M.; et al. Common Genetic Variants Related to Genomic Integrity and Risk of Papillary Thyroid Cancer. Carcinogenesis 2011, 32, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.; Racey, S.; Veuger, S. The Role of Iron in DNA and Genomic Instability in Cancer, a Target for Iron Chelators That Can Induce ROS. Appl. Sci. 2022, 12, 10161. [Google Scholar] [CrossRef]
- Veatch, J.R.; McMurray, M.A.; Nelson, Z.W.; Gottschling, D.E. Mitochondrial Dysfunction Leads to Nuclear Genome Instability via an Iron-Sulfur Cluster Defect. Cell 2009, 137, 1247–1258. [Google Scholar] [CrossRef]
- Paul, V.D.; Lill, R. Biogenesis of Cytosolic and Nuclear Iron–Sulfur Proteins and Their Role in Genome Stability. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2015, 1853, 1528–1539. [Google Scholar] [CrossRef]
- Sirokmany, G.; Geiszt, M. The Relationship of NADPH Oxidases and Heme Peroxidases: Fallin’ in and Out. Front. Immunol. 2019, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Multiple Nutritional Factors and Thyroid Disease, with Particular Reference to Autoimmune Thyroid Disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Babic Leko, M.; Gunjaca, I.; Pleic, N.; Zemunik, T. Environmental Factors Affecting Thyroid-Stimulating Hormone and Thyroid Hormone Levels. Int. J. Mol. Sci. 2021, 22, 6521. [Google Scholar] [CrossRef]
- Szczepanek-Parulska, E.; Hernik, A.; Ruchala, M. Anemia in Thyroid Diseases. Pol. Arch. Intern. Med. 2017, 127, 352–360. [Google Scholar] [CrossRef]
- Pastori, V.; Pozzi, S.; Labedz, A.; Ahmed, S.; Ronchi, A.E. Role of Nuclear Receptors in Controlling Erythropoiesis. Int. J. Mol. Sci. 2022, 23, 2800. [Google Scholar] [CrossRef]
- Garofalo, V.; Condorelli, R.A.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Relationship between Iron Deficiency and Thyroid Function: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4790. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, G.; De Carlo, E.; Murialdo, G.; Scandellari, C. A Possible Link between Genetic Hemochromatosis and Autoimmune Thyroiditis. Minerva Med. 2007, 98, 769–772. [Google Scholar]
- De Sanctis, V.; Soliman, A.T.; Canatan, D.; Yassin, M.A.; Daar, S.; Elsedfy, H.; Di Maio, S.; Raiola, G.; Corrons, J.-L.V.; Kattamis, C. Thyroid Disorders in Homozygous β-Thalassemia: Current Knowledge, Emerging Issues and Open Problems. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019029. [Google Scholar] [CrossRef] [PubMed]
- Rishi, G.; Huang, G.; Subramaniam, V.N. Cancer: The Role of Iron and Ferroptosis. Int. J. Biochem. Cell Biol. 2021, 141, 106094. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Sanchez, G.S.; Pita-Grisanti, V.; Quinones-Diaz, B.; Gumpper, K.; Cruz-Monserrate, Z.; Vivas-Mejia, P.E. Biological Functions and Therapeutic Potential of Lipocalin 2 in Cancer. Int. J. Mol. Sci. 2020, 21, 4365. [Google Scholar] [CrossRef] [PubMed]
- Campisi, A.; Bonfanti, R.; Raciti, G.; Bonaventura, G.; Legnani, L.; Magro, G.; Pennisi, M.; Russo, G.; Chiacchio, M.A.; Pappalardo, F.; et al. Gene Silencing of Transferrin-1 Receptor as a Potential Therapeutic Target for Human Follicular and Anaplastic Thyroid Cancer. Mol. Ther. Oncolytics 2020, 16, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Iannetti, A.; Pacifico, F.; Acquaviva, R.; Lavorgna, A.; Crescenzi, E.; Vascotto, C.; Tell, G.; Salzano, A.M.; Scaloni, A.; Vuttariello, E.; et al. The Neutrophil Gelatinase-Associated Lipocalin (NGAL), a NF-kappaB-Regulated Gene, Is a Survival Factor for Thyroid Neoplastic Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 14058–14063. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, J.; Feng, J.; Wang, J. E4BP4 Promotes Thyroid Cancer Proliferation by Modulating Iron Homeostasis through Repression of Hepcidin. Cell Death Dis. 2018, 9, 987. [Google Scholar] [CrossRef]
- Mollet, I.G.; Patel, D.; Govani, F.S.; Giess, A.; Paschalaki, K.; Periyasamy, M.; Lidington, E.C.; Mason, J.C.; Jones, M.D.; Game, L.; et al. Low Dose Iron Treatments Induce a DNA Damage Response in Human Endothelial Cells within Minutes. PLoS ONE 2016, 11, e0147990. [Google Scholar] [CrossRef]
- Shigeta, S.; Toyoshima, M.; Kitatani, K.; Ishibashi, M.; Usui, T.; Yaegashi, N. Transferrin Facilitates the Formation of DNA Double-Strand Breaks via Transferrin Receptor 1: The Possible Involvement of Transferrin in Carcinogenesis of High-Grade Serous Ovarian Cancer. Oncogene 2016, 35, 3577–3586. [Google Scholar] [CrossRef]
- Deng, Z.; Manz, D.H.; Torti, S.V.; Torti, F.M. Effects of Ferroportin-Mediated Iron Depletion in Cells Representative of Different Histological Subtypes of Prostate Cancer. Antioxid. Redox Signal. 2019, 30, 1043–1061. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Tang, T.; Wang, J.; Zhao, H.; Yang, H.Y.; Xi, J.; Zhang, B.; Fang, J.; Gao, K.; Wu, Y. Fusaricide Is a Novel Iron Chelator That Induces Apoptosis through Activating Caspase-3. J. Nat. Prod. 2021, 84, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Tseng, W.H.; Chi, J.T. The Intersection of DNA Damage Response and Ferroptosis-A Rationale for Combination Therapeutics. Biology 2020, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Niu, J.; Hu, P.; Tong, A.; Dai, Y.; Xu, F.; Li, F. A Ferroptosis-Related Signature Robustly Predicts Clinical Outcomes and Associates With Immune Microenvironment for Thyroid Cancer. Front. Med. 2021, 8, 637743. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wu, P.; Sheng, L.; Sun, W.; Zhang, H. Ferroptosis-Related Gene Signature Predicts the Prognosis of Papillary Thyroid Carcinoma. Cancer Cell Int. 2021, 21, 669. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Karwowski, B.T. Nutrition Can Help DNA Repair in the Case of Aging. Nutrients 2020, 12, 3364. [Google Scholar] [CrossRef]
- Severo, J.S.; Morais, J.B.S.; de Freitas, T.E.C.; Andrade, A.L.P.; Feitosa, M.M.; Fontenelle, L.C.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. The Role of Zinc in Thyroid Hormones Metabolism. Int. J. Vitam. Nutr. Res. 2019, 89, 80–88. [Google Scholar] [CrossRef]
- Kang, H.S.; Grimm, S.A.; Jothi, R.; Santisteban, P.; Jetten, A.M. GLIS3 Regulates Transcription of Thyroid Hormone Biosynthetic Genes in Coordination with Other Thyroid Transcription Factors. Cell Biosci. 2023, 13, 32. [Google Scholar] [CrossRef]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef]
- Kamaliyan, Z.; Clarke, T.L. Zinc Finger Proteins: Guardians of Genome Stability. Front. Cell Dev. Biol. 2024, 12, 1448789. [Google Scholar] [CrossRef]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-Finger Proteins in Health and Disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef]
- Brito, S.; Lee, M.-G.; Bin, B.-H.; Lee, J.-S. Zinc and Its Transporters in Epigenetics. Mol. Cells 2020, 43, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, J.; Tian, M.; Kang, N.; Xu, G.; Zhi, J.; Ruan, X.; Hou, X.; Zhang, W.; Yi, J.; et al. Pharmacological Inhibition of Ref-1 Enhances the Therapeutic Sensitivity of Papillary Thyroid Carcinoma to Vemurafenib. Cell Death Dis. 2022, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Celano, M.; Bulotta, S.; Bruno, R.; Arturi, F.; Giannasio, P.; Filetti, S.; Damante, G.; Tell, G. APE/Ref-1 Is Increased in Nuclear Fractions of Human Thyroid Hyperfunctioning Nodules. Mol. Cell. Endocrinol. 2002, 194, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Tell, G.; Pines, A.; Paron, I.; D’Elia, A.; Bisca, A.; Kelley, M.R.; Manzini, G.; Damante, G. Redox Effector Factor-1 Regulates the Activity of Thyroid Transcription Factor 1 by Controlling the Redox State of the N Transcriptional Activation Domain. J. Biol. Chem. 2002, 277, 14564–14574. [Google Scholar] [CrossRef] [PubMed]
- Prodosmo, A.; Giglio, S.; Moretti, S.; Mancini, F.; Barbi, F.; Avenia, N.; Di Conza, G.; Schunemann, H.J.; Pistola, L.; Ludovini, V.; et al. Analysis of Human MDM4 Variants in Papillary Thyroid Carcinomas Reveals New Potential Markers of Cancer Properties. J. Mol. Med. 2008, 86, 585–596. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, L.; Wei, Q.; Song, X.; Sturgis, E.M.; Li, G. Significance of MDM2 and P14 ARF Polymorphisms in Susceptibility to Differentiated Thyroid Carcinoma. Surgery 2013, 153, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, M.; Figlioli, G.; Maccari, G.; Garritano, S.; De Santi, C.; Melaiu, O.; Barone, E.; Bambi, F.; Ermini, S.; Pellegrini, G.; et al. Polymorphisms within Base and Nucleotide Excision Repair Pathways and Risk of Differentiated Thyroid Carcinoma. DNA Repair 2016, 41, 27–31. [Google Scholar] [CrossRef][Green Version]
- Tanrikulu, S.; Dogru-Abbasoglu, S.; Ozderya, A.; Ademoglu, E.; Karadag, B.; Erbil, Y.; Uysal, M. The 8-Oxoguanine DNA N-Glycosylase 1 (hOGG1) Ser326Cys Variant Affects the Susceptibility to Graves’ Disease. Cell Biochem. Funct. 2011, 29, 244–248. [Google Scholar] [CrossRef]
- Garcia-Quispes, W.A.; Perez-Machado, G.; Akdi, A.; Pastor, S.; Galofre, P.; Biarnes, F.; Castell, J.; Velazquez, A.; Marcos, R. Association Studies of OGG1, XRCC1, XRCC2 and XRCC3 Polymorphisms with Differentiated Thyroid Cancer. Mutat. Res. 2011, 709–710, 67–72. [Google Scholar] [CrossRef]
- Hou, X.; Tian, M.; Ning, J.; Wang, Z.; Guo, F.; Zhang, W.; Hu, L.; Wei, S.; Hu, C.; Yun, X.; et al. PARP Inhibitor Shuts down the Global Translation of Thyroid Cancer through Promoting Pol II Binding to DIMT1 Pause. Int. J. Biol. Sci. 2023, 19, 3970–3986. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.R.; Feng, H.; Zuniga, O.; Rodrigues, H.; Eldridge, D.E.; Yang, L.; Shen, C.; Williams, T.M. RAS-RAF-miR-296-3p Signaling Axis Increases Rad18 Expression to Augment Radioresistance in Pancreatic and Thyroid Cancers. Cancer Lett. 2024, 591, 216873. [Google Scholar] [CrossRef]
- Qin, J.; Fan, J.; Li, G.; Liu, S.; Liu, Z.; Wu, Y. DNA Double-Strand Break Repair Gene Mutation and the Risk of Papillary Thyroid Microcarcinoma: A Case-Control Study. Cancer Cell Int. 2021, 21, 334. [Google Scholar] [CrossRef] [PubMed]
- Zidane, M.; Truong, T.; Lesueur, F.; Xhaard, C.; Cordina-Duverger, E.; Boland, A.; Blanche, H.; Ory, C.; Chevillard, S.; Deleuze, J.F.; et al. Role of DNA Repair Variants and Diagnostic Radiology Exams in Differentiated Thyroid Cancer Risk: A Pooled Analysis of Two Case-Control Studies. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, J.O.; Backman, S.; Wang, N.; Stenman, A.; Crona, J.; Thutkawkorapin, J.; Ghaderi, M.; Tham, E.; Stalberg, P.; Zedenius, J.; et al. Whole-Genome Sequencing of Synchronous Thyroid Carcinomas Identifies Aberrant DNA Repair in Thyroid Cancer Dedifferentiation. J. Pathol. 2020, 250, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Stigliano, A.; Belcastro, E.; Giorda, E.; Rosado, M.M.; Grossi, A.; Assenza, M.R.; Moretti, F.; Fierabracci, A. P53 Activation Effect in the Balance of T Regulatory and Effector Cell Subsets in Patients with Thyroid Cancer and Autoimmunity. Front. Immunol. 2021, 12, 728381. [Google Scholar] [CrossRef]
- Yan, M.; Song, Y.; Wong, C.P.; Hardin, K.; Ho, E. Zinc Deficiency Alters DNA Damage Response Genes in Normal Human Prostate Epithelial Cells. J. Nutr. 2008, 138, 667–673. [Google Scholar] [CrossRef]
- Ho, E.; Ames, B.N. Low Intracellular Zinc Induces Oxidative DNA Damage, Disrupts P53, NFkappa B, and AP1 DNA Binding, and Affects DNA Repair in a Rat Glioma Cell Line. Proc. Natl. Acad. Sci. USA 2002, 99, 16770–16775. [Google Scholar] [CrossRef] [PubMed]
- Schoofs, H.; Schmit, J.; Rink, L. Zinc Toxicity: Understanding the Limits. Molecules 2024, 29, 3130. [Google Scholar] [CrossRef]
- Song, Y.; Leonard, S.W.; Traber, M.G.; Ho, E. Zinc Deficiency Affects DNA Damage, Oxidative Stress, Antioxidant Defenses, and DNA Repair in Rats. J. Nutr. 2009, 139, 1626–1631. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Urrego-Noguera, K.; Vargas-Sierra, H.; Pinzón-Fernández, M. Zinc and Ferritin Levels and Their Associations with Functional Disorders and/or Thyroid Autoimmunity: A Population-Based Case-Control Study. Int. J. Mol. Sci. 2024, 25, 10217. [Google Scholar] [CrossRef] [PubMed]
- Brylinski, L.; Kostelecka, K.; Wolinski, F.; Komar, O.; Milosz, A.; Michalczyk, J.; Bilogras, J.; Machrowska, A.; Karpinski, R.; Maciejewski, M.; et al. Effects of Trace Elements on Endocrine Function and Pathogenesis of Thyroid Diseases-A Literature Review. Nutrients 2025, 17, 398. [Google Scholar] [CrossRef] [PubMed]
- Beserra, J.B.; Morais, J.B.S.; Severo, J.S.; Cruz, K.J.C.; de Oliveira, A.R.S.; Henriques, G.S.; do Nascimento Marreiro, D. Relation Between Zinc and Thyroid Hormones in Humans: A Systematic Review. Biol. Trace Elem. Res. 2021, 199, 4092–4100. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, H.; Jiang, H.; Lin, H.; He, J.; Fan, S.; Yu, D.; Yang, L.; Tang, H.; Lin, E.; et al. The Effect of Micronutrient on Thyroid Cancer Risk: A Mendelian Randomization Study. Front. Nutr. 2024, 11, 1331172. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper Metabolism in Cell Death and Autophagy. Autophagy 2023, 19, 2175–2195. [Google Scholar] [CrossRef]
- Linder, M.C. The Relationship of Copper to DNA Damage and Damage Prevention in Humans. Mutat. Res. 2012, 733, 83–91. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper Toxicity, Oxidative Stress, and Antioxidant Nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Husain, N.; Mahmood, R. Copper(II) Generates ROS and RNS, Impairs Antioxidant System and Damages Membrane and DNA in Human Blood Cells. Environ. Sci. Pollut. Res. Int. 2019, 26, 20654–20668. [Google Scholar] [CrossRef]
- Schwerdtle, T.; Hamann, I.; Jahnke, G.; Walter, I.; Richter, C.; Parsons, J.L.; Dianov, G.L.; Hartwig, A. Impact of Copper on the Induction and Repair of Oxidative DNA Damage, Poly(ADP-Ribosyl)Ation and PARP-1 Activity. Mol. Nutr. Food Res. 2007, 51, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Bian, K.; Chen, F.; Humulock, Z.T.; Tang, Q.; Li, D. Copper Inhibits the AlkB Family DNA Repair Enzymes under Wilson’s Disease Condition. Chem. Res. Toxicol. 2017, 30, 1794–1796. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.L.; Hegde, P.M.; Holthauzen, L.M.F.; Hazra, T.K.; Rao, K.S.J.; Mitra, S. Specific Inhibition of NEIL-Initiated Repair of Oxidized Base Damage in Human Genome by Copper and Iron: Potential Etiological Linkage to Neurodegenerative Diseases. J. Biol. Chem. 2010, 285, 28812–28825. [Google Scholar] [CrossRef]
- Whiteside, J.R.; Box, C.L.; McMillan, T.J.; Allinson, S.L. Cadmium and Copper Inhibit Both DNA Repair Activities of Polynucleotide Kinase. DNA Repair 2010, 9, 83–89. [Google Scholar] [CrossRef]
- Kasprzak, K.S.; Bialkowski, K. Inhibition of Antimutagenic Enzymes, 8-Oxo-dGTPases, by Carcinogenic Metals. Recent Developments. J. Inorg. Biochem. 2000, 79, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.C.; Chung, S.; Kim, S.; Yoon, J.W.; Park, Y.J. Exploring the Role of Copper and Selenium in the Maintenance of Normal Thyroid Function among Healthy Koreans. J. Trace Elem. Med. Biol. 2020, 61, 126558. [Google Scholar] [CrossRef] [PubMed]
- Błażewicz, A.; Wiśniewska, P.; Skórzyńska-Dziduszko, K. Selected Essential and Toxic Chemical Elements in Hypothyroidism-A Literature Review (2001–2021). Int. J. Mol. Sci. 2021, 22, 10147. [Google Scholar] [CrossRef]
- Kucharzewski, M.; Braziewicz, J.; Majewska, U.; Gózdz, S. Copper, Zinc, and Selenium in Whole Blood and Thyroid Tissue of People with Various Thyroid Diseases. Biol. Trace Elem. Res. 2003, 93, 9–18. [Google Scholar] [CrossRef]
- Yu, W.; Liu, H.; Zhang, Y.; Liu, M.; Li, W.; Wang, L.; Li, D. Identification of 10 Differentially Expressed and Cuproptosis-Related Genes in Immune Infiltration and Prognosis of Thyroid Carcinoma. Cell. Mol. Biol. 2024, 70, 89–94. [Google Scholar] [CrossRef]
- Huang, J.; Shi, J.; Wu, P.; Sun, W.; Zhang, D.; Wang, Z.; Ji, X.; Lv, C.; Zhang, T.; Zhang, P.; et al. Identification of a Novel Cuproptosis-Related Gene Signature and Integrative Analyses in Thyroid Cancer. J. Clin. Med. 2023, 12, 2014. [Google Scholar] [CrossRef]
- Brady, D.C.; Crowe, M.S.; Turski, M.L.; Hobbs, G.A.; Yao, X.; Chaikuad, A.; Knapp, S.; Xiao, K.; Campbell, S.L.; Thiele, D.J.; et al. Copper Is Required for Oncogenic BRAF Signalling and Tumorigenesis. Nature 2014, 509, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Casio, M.; Range, D.E.; Sosa, J.A.; Counter, C.M. Copper Chelation as Targeted Therapy in a Mouse Model of Oncogenic BRAF-Driven Papillary Thyroid Cancer. Clin. Cancer Res. 2018, 24, 4271–4281. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, X.; Dong, J.; Li, L.; Meng, X.; Gao, L. In Vitro Study of the Pro-Apoptotic Mechanism of Amino Acid Schiff Base Copper Complexes on Anaplastic Thyroid Cancer. Eur. J. Pharm. Sci. 2025, 206, 107005. [Google Scholar] [CrossRef]
- El Nachef, L.; Al-Choboq, J.; Bourguignon, M.; Foray, N. Response of Fibroblasts from Menkes’ and Wilson’s Copper Metabolism-Related Disorders to Ionizing Radiation: Influence of the Nucleo-Shuttling of the ATM Protein Kinase. Biomolecules 2023, 13, 1746. [Google Scholar] [CrossRef] [PubMed]
- Dackiw, A.P.B.; Ezzat, S.; Huang, P.; Liu, W.; Asa, S.L. Vitamin D3 Administration Induces Nuclear P27 Accumulation, Restores Differentiation, and Reduces Tumor Burden in a Mouse Model of Metastatic Follicular Thyroid Cancer. Endocrinology 2004, 145, 5840–5846. [Google Scholar] [CrossRef]
- Peng, W.; Wang, K.; Zheng, R.; Derwahl, M. 1,25 Dihydroxyvitamin D3 Inhibits the Proliferation of Thyroid Cancer Stem-like Cells via Cell Cycle Arrest. Endocr. Res. 2016, 41, 71–80. [Google Scholar] [CrossRef]
- Graziano, S.; Johnston, R.; Deng, O.; Zhang, J.; Gonzalo, S. Vitamin D/Vitamin D Receptor Axis Regulates DNA Repair during Oncogene-Induced Senescence. Oncogene 2016, 35, 5362–5376. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Elkafas, H.; Ali, M.; Elmorsy, E.; Kamel, R.; Thompson, W.E.; Badary, O.; Al-Hendy, A.; Yang, Q. Vitamin D3 Ameliorates DNA Damage Caused by Developmental Exposure to Endocrine Disruptors in the Uterine Myometrial Stem Cells of Eker Rats. Cells 2020, 9, 1459. [Google Scholar] [CrossRef]
- Amirinejad, R.; Shirvani-Farsani, Z.; Naghavi Gargari, B.; Sahraian, M.A.; Mohammad Soltani, B.; Behmanesh, M. Vitamin D Changes Expression of DNA Repair Genes in the Patients with Multiple Sclerosis. Gene 2021, 781, 145488. [Google Scholar] [CrossRef] [PubMed]
- Petrova, B.; Maynard, A.G.; Wang, P.; Kanarek, N. Regulatory Mechanisms of One-Carbon Metabolism Enzymes. J. Biol. Chem. 2023, 299, 105457. [Google Scholar] [CrossRef] [PubMed]
- Chon, J.; Field, M.S.; Stover, P.J. Deoxyuracil in DNA and Disease: Genomic Signal or Managed Situation? DNA Repair 2019, 77, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Folate (Vitamin B9) and Vitamin B12 and Their Function in the Maintenance of Nuclear and Mitochondrial Genome Integrity. Mutat. Res. 2012, 733, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A.; Esmo Guidelines Committee. Electronic address: Clinicalguidelines@esmo. org Thyroid Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Updagger. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Mishra, P.; Laha, D.; Grant, R.; Nilubol, N. Advances in Biomarker-Driven Targeted Therapies in Thyroid Cancer. Cancers 2021, 13, 6194. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, R.; Huang, L.; Qin, Y.; Liu, W.; Huang, S.; Zhang, J. Comprehensive Comparisons of Different Treatments for Active Graves Orbitopathy: A Systematic Review and Bayesian Model–Based Network Meta-Analysis. J. Clin. Endocrinol. Metab. 2024, 110, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Benites-Zapata, V.A.; Ignacio-Cconchoy, F.L.; Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcón-Braga, E.A.; Al-Kassab-Córdova, A.; Herrera-Añazco, P. Vitamin B12 Levels in Thyroid Disorders: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 14, 1070592. [Google Scholar] [CrossRef]
- Yu, Y.; Tong, K.; Deng, J.; Wu, J.; Yu, R.; Xiang, Q. Unveiling the Connection Between Micronutrients and Autoimmune Thyroiditis: Are They True Friends? Biol. Trace Elem. Res. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Hatch, M.; Cardis, E. Somatic Health Effects of Chernobyl: 30 Years On. Eur. J. Epidemiol. 2017, 32, 1047–1054. [Google Scholar] [CrossRef]
- Larsson, M.; Rudqvist, N.; Spetz, J.; Shubbar, E.; Parris, T.Z.; Langen, B.; Helou, K.; Forssell-Aronsson, E. Long-Term Transcriptomic and Proteomic Effects in Sprague Dawley Rat Thyroid and Plasma after Internal Low Dose 131I Exposure. PLoS ONE 2020, 15, e0244098. [Google Scholar] [CrossRef]
- Morton, L.M.; Karyadi, D.M.; Stewart, C.; Bogdanova, T.I.; Dawson, E.T.; Steinberg, M.K.; Dai, J.; Hartley, S.W.; Schonfeld, S.J.; Sampson, J.N.; et al. Radiation-Related Genomic Profile of Papillary Thyroid Carcinoma after the Chernobyl Accident. Science 2021, 372, eabg2538. [Google Scholar] [CrossRef] [PubMed]
- Klubo-Gwiezdzinska, J. Childhood Exposure to Excess Ionizing Radiation Is Associated with Dose-Dependent Fusions as Molecular Drivers of Papillary Thyroid Cancer. Clin. Thyroidol. 2022, 34, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Saenko, V.; Mitsutake, N. Radiation-Related Thyroid Cancer. Endocr. Rev. 2024, 45, 1–29. [Google Scholar] [CrossRef]
- Lyckesvard, M.N.; Delle, U.; Kahu, H.; Lindegren, S.; Jensen, H.; Back, T.; Swanpalmer, J.; Elmroth, K. Alpha Particle Induced DNA Damage and Repair in Normal Cultured Thyrocytes of Different Proliferation Status. Mutat. Res. 2014, 765, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Huang, H.; Sandler, J.; Dai, M.; Ma, S.; Udelsman, R. Diagnostic Radiography Exposure Increases the Risk for Thyroid Microcarcinoma: A Population-Based Case-Control Study. Eur. J. Cancer Prev. 2015, 24, 439–446. [Google Scholar] [CrossRef]
- Memon, A.; Rogers, I.; Paudyal, P.; Sundin, J. Dental X-Rays and the Risk of Thyroid Cancer and Meningioma: A Systematic Review and Meta-Analysis of Current Epidemiological Evidence. Thyroid. 2019, 29, 1572–1593. [Google Scholar] [CrossRef]
- Han, M.A.; Kim, J.H. Diagnostic X-Ray Exposure and Thyroid Cancer Risk: Systematic Review and Meta-Analysis. Thyroid. 2018, 28, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Benavides, E.; Krecioch, J.R.; Connolly, R.T.; Allareddy, T.; Buchanan, A.; Spelic, D.; O’Brien, K.K.; Keels, M.A.; Mascarenhas, A.K.; Duong, M.-L.; et al. Optimizing Radiation Safety in Dentistry: Clinical Recommendations and Regulatory Considerations. J. Am. Dent. Assoc. 2024, 155, 280–293.e4. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Gutaj, N.; Gutaj, P.; Sowinski, J.; Wender-Ozegowska, E.; Czarnywojtek, A.; Brazert, J.; Ruchala, M. Influence of Cigarette Smoking on Thyroid Gland--an Update. Endokrynol. Pol. 2014, 65, 54–62. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, K.; Liu, X.; Liu, B.; Wang, Z. Association between XRCC1 and XRCC3 Gene Polymorphisms and Risk of Thyroid Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 3160–3167. [Google Scholar]
- Yuan, K.; Huo, M.; Sun, Y.; Wu, H.; Chen, H.; Wang, Y.; Fu, R. Association between X-Ray Repair Cross-Complementing Group 3 (XRCC3) Genetic Polymorphisms and Papillary Thyroid Cancer Susceptibility in a Chinese Han Population. Tumour Biol. 2016, 37, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q. Smoking, Genetic Polymorphisms in Dna Repair Genes and Risk of Thyroid Cancer. Public Health Theses, School of Public Health, Pokfulam, Hong Kong, 2016. [Google Scholar]
- Jalal, N.; Surendranath, A.R.; Pathak, J.L.; Yu, S.; Chung, C.Y. Bisphenol A (BPA) the Mighty and the Mutagenic. Toxicol. Rep. 2018, 5, 76–84. [Google Scholar] [CrossRef]
- Alsen, M.; Sinclair, C.; Cooke, P.; Ziadkhanpour, K.; Genden, E.; van Gerwen, M. Endocrine Disrupting Chemicals and Thyroid Cancer: An Overview. Toxics 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Goyal, K.; Goel, H.; Baranwal, P.; Dixit, A.; Khan, F.; Jha, N.K.; Kesari, K.K.; Pandey, P.; Pandey, A.; Benjamin, M.; et al. Unravelling the Molecular Mechanism of Mutagenic Factors Impacting Human Health. Environ. Sci. Pollut. Res. Int. 2021, 29, 61993–62013. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Liu, W.; Schioth, H.B. Can Exposure to Environmental Pollutants Be Associated with Less Effective Chemotherapy in Cancer Patients? Int. J. Environ. Res. Public Health 2022, 19, 2064. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Asadikaram, G.; Ashrafi, M.R.; Zeynali Nejad, H.; Abolhassani, M.; Abbasi-Jorjandi, M.; Sanjari, M. Organochlorine Pesticides and Epigenetic Alterations in Thyroid Tumors. Front. Endocrinol. 2023, 14, 1130794. [Google Scholar] [CrossRef]
- Pitto, L.; Gorini, F.; Bianchi, F.; Guzzolino, E. New Insights into Mechanisms of Endocrine-Disrupting Chemicals in Thyroid Diseases: The Epigenetic Way. Int. J. Environ. Res. Public Health 2020, 17, 7787. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.G.; Correia, J.; Adiga, D.; Rai, P.S.; Dsouza, H.S.; Chakrabarty, S.; Kabekkodu, S.P. A Comprehensive Review on the Carcinogenic Potential of Bisphenol A: Clues and Evidence. Environ. Sci. Pollut. Res. Int. 2021, 28, 19643–19663. [Google Scholar] [CrossRef] [PubMed]
- Karbownik-Lewinska, M.; Stepniak, J.; Iwan, P.; Lewinski, A. Iodine as a Potential Endocrine Disruptor-a Role of Oxidative Stress. Endocrine 2022, 78, 219–240. [Google Scholar] [CrossRef]
- Gassman, N.R. Induction of Oxidative Stress by Bisphenol A and Its Pleiotropic Effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef]
- Kose, O.; Rachidi, W.; Beal, D.; Erkekoglu, P.; Fayyad-Kazan, H.; Kocer Gumusel, B. The Effects of Different Bisphenol Derivatives on Oxidative Stress, DNA Damage and DNA Repair in RWPE-1 Cells: A Comparative Study. J. Appl. Toxicol. 2020, 40, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Ramirez, S.A.; Salazar, A.M.; Sordo, M.; Ostrosky-Wegman, P.; Morales-Pacheco, M.; Cruz-Burgos, M.; Losada-Garcia, A.; Rodriguez-Martinez, G.; Gonzalez-Ramirez, I.; Vazquez-Santillan, K.; et al. Transcriptome-Wide Analysis of Low-Concentration Exposure to Bisphenol A, S, and F in Prostate Cancer Cells. Int. J. Mol. Sci. 2023, 24, 9462. [Google Scholar] [CrossRef] [PubMed]
- Porreca, I.; Ulloa Severino, L.; D’Angelo, F.; Cuomo, D.; Ceccarelli, M.; Altucci, L.; Amendola, E.; Nebbioso, A.; Mallardo, M.; De Felice, M.; et al. “Stockpile” of Slight Transcriptomic Changes Determines the Indirect Genotoxicity of Low-Dose BPA in Thyroid Cells. PLoS ONE 2016, 11, e0151618. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.D.; Xavier, L.L.F.; Goncalves, C.F.L.; Santos-Silva, A.P.; Paiva-Melo, F.D.; Freitas, M.L.; Fortunato, R.S.; Alves, L.M.; Ferreira, A.C.F. Bisphenol A Increases Hydrogen Peroxide Generation by Thyrocytes Both in Vivo and in Vitro. Endocr. Connect. 2018, 7, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Croce, L.; Ricci, G.; Magri, F.; Rotondi, M.; Imbriani, M.; Chiovato, L. Thyroid Disrupting Effects of Old and New Generation PFAS. Front. Endocrinol. 2020, 11, 612320. [Google Scholar] [CrossRef]
- van Gerwen, M.; Colicino, E.; Guan, H.; Dolios, G.; Nadkarni, G.N.; Vermeulen, R.C.H.; Wolff, M.S.; Arora, M.; Genden, E.M.; Petrick, L.M. Per- and Polyfluoroalkyl Substances (PFAS) Exposure and Thyroid Cancer Risk. EBioMedicine 2023, 97, 104831. [Google Scholar] [CrossRef] [PubMed]
- Temkin, A.M.; Hocevar, B.A.; Andrews, D.Q.; Naidenko, O.V.; Kamendulis, L.M. Application of the Key Characteristics of Carcinogens to Per and Polyfluoroalkyl Substances. Int. J. Environ. Res. Public Health 2020, 17, 1668. [Google Scholar] [CrossRef]
- Boyd, R.I.; Ahmad, S.; Singh, R.; Fazal, Z.; Prins, G.S.; Madak Erdogan, Z.; Irudayaraj, J.; Spinella, M.J. Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer. Cancers 2022, 14, 2919. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, X.; Cui, Z.; Chen, W.; Xu, S.; Chen, K.; Wang, F.; Zheng, F.; Ni, H.; Shen, H.-M.; et al. Low Dose PFDA Induces DNA Damage and DNA Repair Inhibition by Promoting Nuclear cGAS Accumulation in Ovarian Epithelial Cells. Ecotoxicol. Environ. Saf. 2023, 265, 115503. [Google Scholar] [CrossRef]
| Name of Enzyme/Gene | Primary Function | Iron Cofactor | Involvement in Thyroid Disease | References |
|---|---|---|---|---|
| Catalase | Removes H2O2 by its hydrolysis to water and oxygen | Heme iron centre | Downregulated in TC, GD, HT, and follicular adenoma (FA) tissues. | [55,99,100,101,102] |
| Ribonucleotide reductase (RRM2 subunit) | Synthesis of deoxyribonucleotides (dNTPs) | Di-iron centre | RRM1 subunit is overexpressed in PTC; RRM2 subunit is overexpressed in PTC, TC, and ATC tissues. RRM1 and RRM2 overexpression positively correlates with markers of aggression, and disease progression. | [103,104,105] |
| Dihydropyrimidine dehydrogenase (DPYD) | Pyrimidine catabolism. Brakes down uracil, thymine and 5-FU | Fe-S cluster | DPYD expression is more pronounced in PTC than in normal tissues, which may suggest a poor response to 5-FU-based therapies. | [106] |
| Phosphoribosyl pyrophosphate amidotransferase (PPAT) | De novo purine synthesis pathway | Fe-S cluster | PPAT is overexpressed in TC and supports malignant traits. | [107] |
| Xanthine dehydrogenase/oxidase | Purine catabolism. The enzyme exists in two forms: xanthine dehydrogenase (XDH) and xanthine oxidase (XO). | Fe-S cluster | XDH/XO levels are in general low in thyroid tissue but upregulated in TC. | [108] |
| DNA polymerase α (POLA) | Initiation of DNA replication. Modulation of interferon responses. | Fe-S cluster | POLA1 catalytic subunit defect may lead to developmental delay without the thyroid phenotype. Another medical condition associated with POLA1 deficiency is autoinflammatory interferonopathy, with a lack of signs of autoimmune disease including lack of AITD. The TCGA dataset analysed using the UALCAN cancer database (https://ualcan.path.uab.edu, accessed on 23 April 2025) suggests the downregulation of POLA1 in TC tissues. | [109,110,111,112] |
| DNA polymerase δ (POLD) | DNA replication and repair. Involved in multiple DNA repair pathways. | Fe-S cluster | Catalytic subunit POLD1 is downregulated in PTC and associated with poor clinical course. POLD1 mutations in benign thyroid goitre and PTC imply involvement in cancer progression. | [113,114] |
| DNA polymerase ε (POLE) | DNA replication and repair. Involved in multiple DNA repair pathways. | Fe-S cluster | POLE catalytic subunit is downregulated in PTC tissues. Deficiency of accessory POLE subunit (POLE2) induces hypothyroidism. | [113,115] |
| DNA polymerase ζ (POLZ) | Translesion DNA synthesis | Fe-S cluster | TCGA dataset analysed using the UALCAN cancer database (https://ualcan.path.uab.edu) suggests the downregulation of POLZ catalytic subunit REV3L and the upregulation of accessory subunit REV7/FANCV/MAD2L2 in TC tissues | [110,111,112,116] |
| DNA primase (PRIM) | Initiation of RNA primers for DNA replication | Fe-S cluster | TCGA dataset analysed using the UALCAN cancer database (https://ualcan.path.uab.edu) suggests a slight downregulation of catalytic, PRIM1, and regulatory PRIM2 subunits in TC tissue. | [110,111,112,117] |
| Helicase XPD/ERCC2 | Nucleotide excision repair, transcription initiation | Fe-S cluster | XPD polymorphisms may increase TC susceptibility in general, as well as increase radiation-related TC risk. Low XPD expression is associated with BRAFV600E mutation and markers of aggressive disease. | [118,119,120,121,122,123] |
| Helicase FANCJ/BRIP1/BACH1 | Double-strand break repair, interstrand crosslink repair (Fanconi anaemia pathway) | Fe-S cluster | FANCJ polymorphisms are not associated with TC risk. | [120,124,125] |
| Helicase DNA2 | Okazaki fragment processing, DNA repair, telomere maintenance | Fe-S cluster | DNA2 is frequently deleted and overexpressed in TC. | [126] |
| Helicase RTEL1 | Telomere maintenance | Fe-S cluster | RTEL1 is upregulated in TC. | [127] |
| Helicase DDX11/ChlR1 | Involved in homologous recombination and tolerance of replication stress | Fe-S cluster | DDX11 shows a low expression level in thyroid tissue without expression changes in TC. | [128] |
| Exonuclease EXO5 | UV-induced DNA damage and interstrand crosslink repair | Fe-S cluster | EXO5 is downregulated in TC tissue. | [129] |
| Glycosylase NTH1/NTHL1 | Base excision repair; repairs oxidised pyrimidines, mainly Tg, 5-OHC, 5-OH | Fe-S cluster | Increased incidence of TC in NTH1 mutation carriers. NTH1 polymorphisms have no influence on radiation related TC risk. | [125,130] |
| Glycosylase MUTYH | Base excision repair (repairs A:G and A:8-oxoG mismatches) | Fe-S cluster | MUTYH mutation increases the thyroid nodule frequency and PTC risk. In thyroid cells, MUTYH is upregulated upon oxidative stress. | [131,132,133] |
| Demethylase ALKBH2/3 | Direct repair of alkylated DNA bases | Non-heme iron centre | ALKBH3 polymorphisms may increase TC susceptibility in general as well as increase the radiation-related TC risk. | [125,134] |
| Name Enzyme/Gene | Primary Function | Role of Zinc | Involvement in Thyroid Disease | References |
|---|---|---|---|---|
| Endonuclease APE1/APEX1/Ref-1 | Single-strand DNA break repair; involved in redox signalling | May act as a cofactor; modulates activity | APE1/Ref-1, in addition to its involvement in DNA repair, also acts as a redox regulator modulating DNA-binding and the transcriptional activity of thyroid-specific transcription factors Pax8 and TTF-1 in thyroid cells. APE1 expression is elevated in thyroid cancer tissues and in the nuclear fractions of hyperfunctioning thyroid nodules. Inhibiting the redox domain of APE1 has shown promise in overcoming resistance to certain cancer therapies in preclinical models. | [165,166,167] |
| Endonuclease APE2/APEX2 | Single-strand DNA break repair | Cofactor in zinc finger domain | TCGA dataset analysed using the UALCAN cancer database (https://ualcan.path.uab.edu) suggests the APE2 upregulation in TC tissue. | [110,111,112] |
| APTX | Single-strand DNA break repair | Cofactor in zinc finger domain | TCGA dataset analysed using the UALCAN cancer database (https://ualcan.path.uab.edu) suggests the downregulation of APTX in TC tissue. | [110,111,112] |
| BARD1 | Double-strand DNA break repair | Cofactor in zinc finger domain | TCGA dataset analysed using the UALCAN cancer database (https://ualcan.path.uab.edu) suggests the downregulation of BARD1 in TC tissue. | [110,111,112] |
| BRCA1/FANCS | Double-strand DNA break repair, interstrand crosslink repair (Fanconi anaemia pathway) | Cofactor in zinc finger domain | BRCA1 genetic variation may modulate TC risk. | [16] |
| Endonuclease CtIP/RBBP8 | Double-strand DNA break repair | Cofactor in zinc finger domain | TCGA dataset analysed using the UALCAN cancer database (https://ualcan.path.uab.edu) suggests the downregulation of CtIP in TC tissue. | [110,111,112] |
| Ligase LIG3α | Base excision repair; double-strand DNA break repair | Cofactor in zinc finger domain | TCGA dataset analysed using the UALCAN cancer database (https://ualcan.path.uab.edu) suggests the downregulation of LIG3α in TC tissue. | [110,111,112] |
| MDM2 | DNA damage response | Cofactor in zinc finger domain | MDM2 is overexpressed in TC tissues. MDM2 genetic variation increases TC risk. | [168,169] |
| MDM4 | DNA damage response | Cofactor in zinc finger domain | MDM4 is often downregulated in TC tissue. | [168] |
| Glycosylase MUTYH | Base excision repair | Cofactor in Zinc Linchpin Motif | MUTYH mutation increases thyroid nodule frequency and PTC risk. In thyroid cells, MUTYH is upregulated upon oxidative stress. | [131,132,133] |
| Glycosylase NEIL2 | Base excision repair | Cofactor in zinc finger domain | TCGA dataset analysed using UALCAN cancer database (https://ualcan.path.uab.edu) suggest downregulation of NEIL2 in TC tissue. | [110,111,112] |
| Glycosylase NEIL3 | Base excision repair | Cofactor in zinc finger domain | NEIL3 polymorphisms may modulate TC risk | [170] |
| Glycosylase OGG1 | Base excision repair | Cofactor in zinc finger domain | OGG1 polymorphisms are associated with increased GD but not TC risk. OGG1 is overexpressed in PTC tissues. | [99,171,172] |
| PARP1 | Single-strand DNA break repair; double-strand DNA break repair; DNA damage response | Cofactor in zinc finger domain | Application of PARP1 inhibitors was suggested in TC management. | [173] |
| Polymerase POLE | DNA replication and repair. Involved in multiple DNA repair pathways. | Cofactor in ubiquitin-binding zinc finger | POLE catalytic subunit is downregulated in PTC tissues. Deficiency of accessory POLE subunit (POLE2) induces hypothyroidism. | [113,115] |
| RAD18 | Translesion synthesis | Cofactor in zinc finger domain | RAD18 gene expression is elevated in PTC tissues harbouring the BRAFV600E mutation. Increased RAD18 levels are positively associated with poor prognosis in these cases. | [174] |
| RAD50 | Double-strand DNA break repair | Cofactor in zinc hook domain | Germline RAD50 variants may increase TC risk. | [175] |
| RPA | RPA protects binds single-stranded DNA from nucleolytic degradation. Involved in multiple DNA repair pathways | Cofactor in zinc finger domain | Germline variants of RPA subunits may increase susceptibility to TC. TCGA dataset analysed using UALCAN cancer database (https://ualcan.path.uab.edu) suggest downregulation of RPA subunits in TC tissue. | [110,111,112,176] |
| TP53 | DNA damage response | Cofactor in DNA-binding core domain | TP53 mutations are frequent in ATC tumours and are associated with dedifferentiation and disease progression. TP53 polymorphisms may increase TC risk. TP53 reactivation may support anti-tumour immune responses in TC patients and suppress autoimmunity observed in AITD. | [16,177,178] |
| XPA | Nucleotide excision repair | Cofactor in zinc finger domain | TCGA dataset analysed using the UALCAN cancer database (https://ualcan.path.uab.edu) suggests the downregulation of XPA in TC tissue. | [110,111,112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arczewska, K.D.; Piekiełko-Witkowska, A. The Influence of Micronutrients and Environmental Factors on Thyroid DNA Integrity. Nutrients 2025, 17, 2065. https://doi.org/10.3390/nu17132065
Arczewska KD, Piekiełko-Witkowska A. The Influence of Micronutrients and Environmental Factors on Thyroid DNA Integrity. Nutrients. 2025; 17(13):2065. https://doi.org/10.3390/nu17132065
Chicago/Turabian StyleArczewska, Katarzyna D., and Agnieszka Piekiełko-Witkowska. 2025. "The Influence of Micronutrients and Environmental Factors on Thyroid DNA Integrity" Nutrients 17, no. 13: 2065. https://doi.org/10.3390/nu17132065
APA StyleArczewska, K. D., & Piekiełko-Witkowska, A. (2025). The Influence of Micronutrients and Environmental Factors on Thyroid DNA Integrity. Nutrients, 17(13), 2065. https://doi.org/10.3390/nu17132065






