An Adapted Cardioprotective Diet with or Without Phytosterol and/or Krill Oil Supplementation in Familial Hypercholesterolemia: Results of a Pilot Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Participants
2.3. Randomization and Blinding
2.4. Study Interventions
2.5. Outcomes
- -
- Primary outcomes after the 120-day follow-up. (1) For phytosterol vs. placebo groups: mean LDL-c level. For krill oil vs. placebo groups: mean Lp[a] level. (2) Adherence to treatment assessed by presence in appointments, diet quality according to the Cardiovascular Health Diet Index (CHDI) [26], proportion of consumed investigational products, and analysis of plasma phytosterol and erythrocyte fatty acid levels [18].
- -
- Secondary outcomes after the 120-day follow-up. (1) Means of total cholesterol, high density lipoprotein cholesterol (HDL-c), serum triglycerides, non-HDL cholesterol, very-low density lipoprotein cholesterol (VLDL-c), total cholesterol/HDL-c ratio, LDL-c/HDL-c ratio, atherogenic index, triglycerides/HDL-c ratio, oxidized LDL (ox-LDL), and apolipoproteins (Apo[s]) A-I and B. (2) Frequency of adverse events throughout the study. (3) Components of protocol implementation evaluated based on loss-to-follow-up rates, recruitment rates within the expected time frame, and the selection and adjustments of remote platforms/media for site training and study participants.
2.6. Study Procedures
2.7. Sample Size
2.8. Statistical Analysis
3. Results
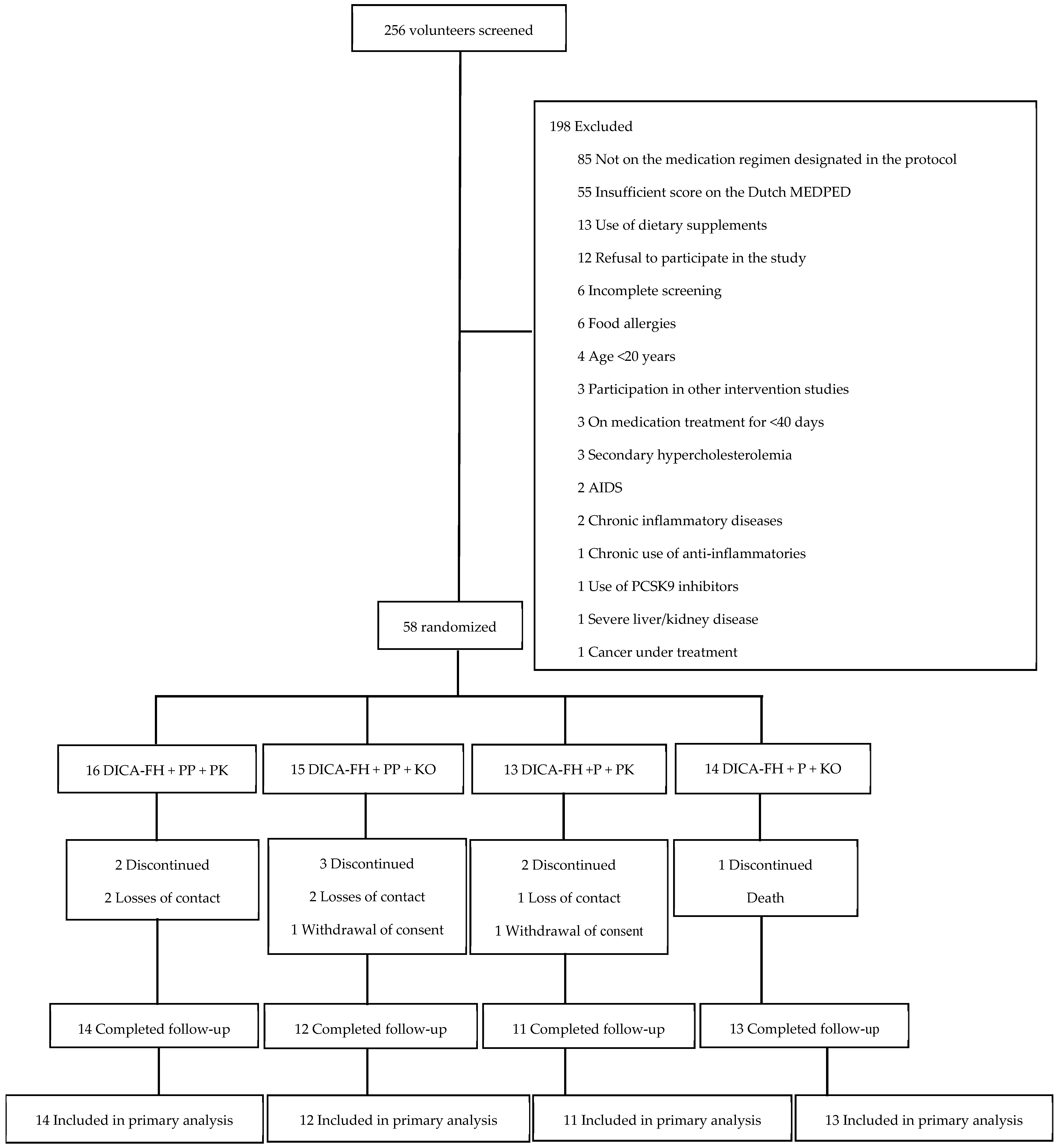
3.1. Recruitment and Participant Characteristics
3.2. Retention
3.3. Primary Outcomes
3.3.1. LDL-c and Lp(a)
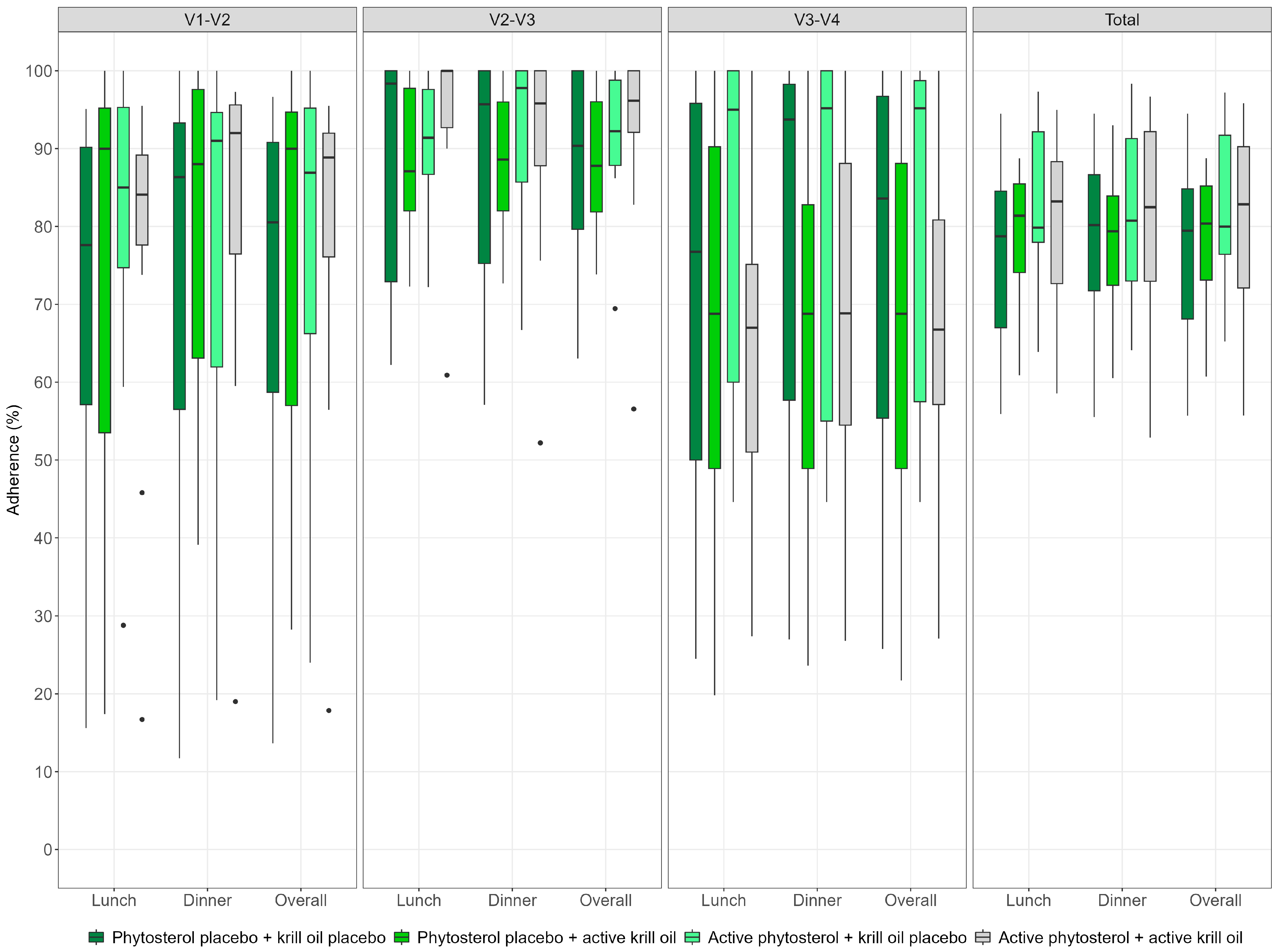
3.3.2. Adherence
- Attendance at visits and investigational product consumption:
- Diet quality:
- Plasma phytosterol analysis:
- Erythrocyte fatty acid analysis:
3.4. Secondary Outcomes
3.4.1. Clinical Lipid Profile and Anthropometric Markers
3.4.2. LDL-ox and Apolipoproteins
3.4.3. Adverse Events
3.4.4. Protocol Implementation Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCG8 | ATP binding cassette subfamily G member 8 gene |
| Apo(s) | Apolipoproteins |
| APOB | Apolipoprotein B gene |
| APOE | Apolipoprotein E gene |
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CHDI | Cardiovascular Health Diet Index |
| CI | Confidence interval |
| CONSORT | Consolidated Standards of Reporting Trials |
| DHA | Docosahexaenoic acid |
| DICA Br | DIeta CArdioprotetora Brasileira |
| DICA-FH | DIeta CArdioprotetora Brasileira adapted to familial hypercholesterolemia |
| Dutch MEDPED | Dutch Lipid Clinic Network |
| eCRF | Electronic case report form |
| EPA | Eicosapentaenoic acid |
| FH | Familial hypercholesterolemia |
| HDL-c | High-density lipoprotein cholesterol |
| IQR | Interquartile range |
| LDL-c | Low-density lipoprotein cholesterol |
| LDLR | LDL receptor gene |
| Lp(a) | Lipoprotein(a) |
| n-3 PUFA | omega-3 polyunsaturated fatty acid |
| ox-LDL | Oxidized LDL |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 gene |
| R24h | 24-h dietary recall |
| SD | Standard deviation |
| STAP1 | Signal transducing adaptor family member 1 gene |
| VLDL-c | Very-low density lipoprotein cholesterol |
| WHO-UTN | World Health Organization Universal Trial Number |
References
- Beheshti, S.O.; Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J. Am. Coll. Cardiol. 2020, 75, 2553–2566. [Google Scholar] [CrossRef] [PubMed]
- Di Taranto, M.D.; Giacobbe, C.; Fortunato, G. Familial hypercholesterolemia: A complex genetic disease with variable phenotypes. Eur. J. Med. Genet. 2020, 63, 103831. [Google Scholar] [CrossRef] [PubMed]
- Ferrières, J.; Banks, V.; Pillas, D.; Giorgianni, F.; Gantzer, L.; Lekens, B.; Désaméricq, G. Screening and treatment of familial hypercholesterolemia in a French sample of ambulatory care patients: A retrospective longitudinal cohort study. PLoS ONE 2021, 16, e0255345. [Google Scholar] [CrossRef]
- McGowan, M.P.; Hosseini Dehkordi, S.H.; Moriarty, P.M.; Duell, P.B. Diagnosis and treatment of heterozygous familial hypercholesterolemia. J. Am. Heart Assoc. 2019, 8, e013225. [Google Scholar] [CrossRef]
- Vuorio, A.; Watts, G.F.; Schneider, W.J.; Tsimikas, S.; Kovanen, P.T. Familial hypercholesterolemia and elevated lipoprotein(a): Double heritable risk and new therapeutic opportunities. J. Intern. Med. 2020, 287, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Roy, G.; Drouin-Chartier, J.P. Cardiovascular disease prevention in heterozygous familial hypercholesterolemia: How important is a healthy diet in the era of long-lasting cholesterol-lowering drug therapies? Curr. Opin. Lipidol. 2024, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Arora, A.; Kumar, R.; Singh, M.; Malhotra, S.; Shafiq, N. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia. Cochrane Database Syst. Rev. 2014, 2014, CD001918. [Google Scholar] [CrossRef]
- Barkas, F.; Nomikos, T.; Liberopoulos, E.; Panagiotakos, D. Diet and Cardiovascular Disease Risk Among Individuals with Familial Hypercholesterolemia: Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2436. [Google Scholar] [CrossRef]
- Izar, M.C.D.O.; Giraldez, V.Z.R.; Bertolami, A.; Santos Filho, R.D.D.; Lottenberg, A.M.; Assad, M.H.V.; Salgado Filho, W. Update of the Brazilian guideline for familial hypercholesterolemia—2021. Arq. Bras. Cardiol. 2021, 117, 782–844. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Antoniazzi, L.; Arroyo-Olivares, R.; Bittencourt, M.S.; Tada, M.T.; Lima, I.; Jannes, C.E.; Krieger, J.E.; Pereira, A.C.; Quintana-Navarro, G.; Muñiz-Grijalvo, O.; et al. Adherence to a Mediterranean diet, dyslipidemia and inflammation in familial hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2014–2022. [Google Scholar] [CrossRef] [PubMed]
- Brasil Ministry of Health. Cardioprotective Nutrition: Guidance Manual for Healthcare Professionals in Primary Care/Ministry of Health, Hospital do Coração; Ministry of Health: Rio de Janeiro, Brazil, 2018; p. 138. Available online: http://189.28.128.100/dab/docs/portaldab/publicacoes/alimentacao_cardioprotetora_orien_pro_saude_ab.pdf (accessed on 26 January 2020).
- Weber, B.; Bersch-Ferreira, Â.C.; Torreglosa, C.R.; Marcadenti, A.; Lara, E.S.; da Silva, J.T.; de Souza, C.V. Implementation of a Brazilian cardioprotective nutritional (BALANCE) program for improvement on quality of diet and secondary prevention of cardiovascular events: A randomized, multicenter trial. Am. Heart J. 2019, 215, 187–197. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Familial Hypercholesterolaemia: Identification and Management. Available online: www.nice.org.uk/guidance/cg71 (accessed on 7 October 2021).
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 2006, 189, 19–30. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, Y.; Hua, T.; Liu, X.L.; Liu, T.; Yuan, R.Y.; Zhang, X. Omega-3 polyunsaturated fatty acid supplementation improves lipid metabolism and endothelial function by providing a beneficial eicosanoid-pattern in patients with acute myocardial infarction: A randomized, controlled trial. Clin. Nutr. 2021, 40, 445–459. [Google Scholar] [CrossRef]
- Ulven, S.M.; Holven, K.B. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc. Health Risk Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef]
- de Abreu-Silva, E.O.; Machado, R.H.V.; dos Santos, B.R.; Kojima, F.S.; Santos, R.H.N.; Murizine, G.S.; Marcadenti, A. An adapted cardioprotective diet with or without phytosterol and/or krill oil supplement in familial hypercholesterolemia: A pilot study protocol. Clin. Nutr. Open. Sci. 2004, 24, 127–139. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A.; PAFS consensus group. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- Williams, R.R.; Hunt, S.C.; Schumacher, M.C.; Hegele, R.A.; Leppert, M.F.; Ludwig, E.H.; Hopkins, P.N. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am. J. Cardiol. 1993, 72, 171–176. [Google Scholar] [CrossRef]
- Weber, B.; Bersch-Ferreira, Â.C.; Torreglosa, C.R.; Ross-Fernandes, M.B.; da Silva, J.T.; Galante, A.P.; dos Santos, K.G. The Brazilian Cardioprotective Nutritional Program to reduce events and risk factors in secondary prevention for cardiovascular disease: Study protocol (The BALANCE Program Trial). Am. Heart J. 2016, 171, 73–81.e1-2. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Faulkner, D.; Vidgen, E.; Trautwein, E.A.; Parker, T.L.; Connelly, P.W. A dietary portfolio approach to cholesterol reduction: Combined effects of plant sterols, vegetable proteins, and viscous fibers in hypercholesterolemia. Metabolism 2002, 51, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Faulkner, D.A.; Wong, J.M.; de Souza, R.; Connelly, P.W. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA 2003, 290, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Chiavaroli, L.; Wong, J.M.; Kendall, C.; Lewis, G.F.; Vidgen, E.; Lamarche, B. Adding monounsaturated fatty acids to a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. CMAJ 2010, 182, 1961–1967. [Google Scholar] [CrossRef]
- Cacau, L.T.; Marcadenti, A.; Bersch-Ferreira, A.C.; Weber, B.; Almeida, J.C.D.; Rodrigues, C.C.R.; Marchioni, D. The AHA Recommendations for a Healthy Diet and Ultra-Processed Foods: Building a New Diet Quality Index. Front. Nutr. 2022, 9, 804121. [Google Scholar] [CrossRef] [PubMed]
- Harada, P.H.; Miname, M.H.; Benseñor, I.M.; Santos, R.D.; Lotufo, P.A. Familial hypercholesterolemia prevalence in an admixed racial society: Sex and race matter. The ELSA-Brasil. Atherosclerosis 2018, 277, 273–277. [Google Scholar] [CrossRef]
- Civeira, F.; Jarauta, E.; Cenarro, A.; García-Otín, A.L.; Tejedor, D.; Zambón, D.; Mallen, M.; Ros, E.; Pocoví, M. Frequency of low-density lipoprotein receptor gene mutations in patients with a clinical diagnosis of familial combined hyperlipidemia in a clinical setting. J. Am. Coll. Cardiol. 2008, 52, 1546–1553. [Google Scholar] [CrossRef]
- Jannes, C.E.; Santos, R.D.; de Souza Silva, P.R.; Turolla, L.; Gagliardi, A.C.; Marsiglia, J.D.; Chacra, A.P.; Miname, M.H.; Rocha, V.Z.; Filho, W.S.; et al. Familial hypercholesterolemia in Brazil: Cascade screening program, clinical and genetic aspects. Atherosclerosis 2015, 238, 101–107. [Google Scholar] [CrossRef]
- Roy, G.; Boucher, A.; Couture, P.; Drouin-Chartier, J.P. Impact of Diet on Plasma Lipids in Individuals with Heterozygous Familial Hypercholesterolemia: A Systematic Review of Randomized Controlled Nutritional Studies. Nutrients 2021, 13, 235. [Google Scholar] [CrossRef]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. Consumption of a defined, plant-based diet reduces lipoprotein(a), inflammation, and other atherogenic lipoproteins and particles within 4 weeks. Clin. Cardiol. 2018, 41, 1062–1068. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Petersen, K.S.; Kris-Etherton, P.M.; Berglund, L. Diet and Lp(a): Does Dietary Change Modify Residual Cardiovascular Risk Conferred by Lp(a)? Nutrients 2020, 12, 2024. [Google Scholar] [CrossRef]
- Riley, T.M.; Sapp, P.A.; Kris-Etherton, P.M.; Petersen, K.S. Effects of saturated fatty acid consumption on lipoprotein (a): A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2024, 120, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Estruch, R.; Salas-Salvadó, J.; Martínez-Gonzalez, M.A.; Arós, F.; Vila, J.; Corella, D.; Díaz, O.; Sáez, G.; de la Torre, R.; et al. Effect of the Mediterranean diet on heart failure biomarkers: A randomized sample from the PREDIMED trial. Eur. J. Heart Fail. 2014, 16, 543–550. [Google Scholar] [CrossRef]
- Scholz, M.; Horn, K.; Pott, J.; Gross, A.; Kleber, M.E.; Delgado, G.E.; Ceglarek, U. Genome-wide meta-analysis of phytosterols reveals five novel loci and a detrimental effect on coronary atherosclerosis. Nat. Commun. 2022, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.; Kalsbeek, A.; Westra, J.; Disselkoen, C.; ESmith, C.; Tintle, N. Genome-Wide Interaction Study of Omega-3 PUFAs and Other Fatty Acids on Inflammatory Biomarkers of Cardiovascular Health in the Framingham Heart Study. Nutrients 2017, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.M.; Bourbon, M. Polygenic contribution for familial hypercholesterolemia (FH). Curr. Opin. Lipidol. 2021, 32, 392–395. [Google Scholar] [CrossRef]
- Nunes, V.S.; Ilha, A.O.G.; Ferreira, G.D.S.; Bombo, R.P.A.; Afonso, M.S.; Lavrador, M.S.F.; Machado, R.M.; Nakandakare, E.R.; Quintão, E.C.R.; Lottenberg, A.M. Plasma lathosterol measures rates of cholesterol synthesis and efficiency of dietary phytosterols in reducing the plasma cholesterol concentration. Clinics 2022, 77, 100028. [Google Scholar] [CrossRef]
- Martins, C.M.; Fonseca, F.A.; Ballus, C.A.; Figueiredo-Neto, A.M.; Meinhart, A.D.; de Godoy, H.T.; Izar, M.C. Common sources and composition of phytosterols and their estimated intake by the population in the city of São Paulo, Brazil. Nutrition 2013, 29, 865–871. [Google Scholar] [CrossRef]
- Gonçalinho, G.H.F.; Sampaio, G.R.; Soares-Freitas, R.A.M.; Damasceno, N.R.T. Omega-3 Fatty Acids in Erythrocyte Membranes as Predictors of Lower Cardiovascular Risk in Adults without Previous Cardiovascular Events. Nutrients 2021, 13, 1919. [Google Scholar] [CrossRef]
- Dai, X.W.; Zhang, B.; Wang, P.; Chen, C.G.; Chen, Y.M.; Su, Y.X. Erythrocyte membrane n-3 fatty acid levels and carotid atherosclerosis in Chinese men and women. Atherosclerosis 2014, 232, 79–85. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; Domellöf, M.; Hojsak, I.; Hulst, J.M.; Kennedy, K.; Koletzko, B.; Mihatsh, W.; Stijnen, T. Attrition in Long-Term Nutrition Research Studies: A Commentary by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Early Nutrition Research Working Group. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 180–182. [Google Scholar] [CrossRef] [PubMed]
| (a) | ||
|---|---|---|
| Phytosterol Placebo (n = 31) | Active Phytosterol (n = 27) | |
| Sex, no./total no. (%) | ||
| Female | 18/31 (58.1%) | 16/27 (59.3%) |
| Male | 13/31 (41.9%) | 11/27 (40.7%) |
| Age, in years, mean (SD) | 53.8 (14.1) | 55.3 (13.4) |
| Race | ||
| White | 17/31 (54.8%) | 17/27 (63%) |
| Pardo | 8/31 (25.8%) | 5/27 (18.5%) |
| Other | 6/31 (19.4%) | 5/27 (18.5%) |
| Marital status, no./total no. (%) | ||
| Married | 19/31 (61.3%) | 15/27 (55.6%) |
| Other | 12/31 (38.7%) | 12/27 (44.4%) |
| Average monthly household income, in USD 1, no./total no. (%) | ||
| ≥1231.17 | 20/31 (64.5%) | 17/27 (63%) |
| <1231.17 | 11/31 (34.5%) | 10/27 (37%) |
| Education level, in years of schooling, no./total no. (%) | ||
| ≤9 years | 9/31 (29%) | 9/27 (33%) |
| >9 years | 22/31 (71%) | 18/27 (67%) |
| Physical activity, no./total no. (%) | ||
| Sedentary/Low | 18/31 (58.1%) | 15/27 (55.6%) |
| Moderate | 10/31 (32.3%) | 7/27 (25.9%) |
| High | 3/31 (9.7%) | 5/27 (18.5%) |
| Family history of FH, no./total no. (%) | ||
| Unknown | 3/31 (9.7%) | 5/27 (18.5%) |
| No | 5/31 (16.1%) | 4/27 (14.8%) |
| Yes | 23/31 (74.2%) | 18/27 (66.7%) |
| Family history of premature CAD, no./total no. (%) | ||
| Unknown | 2/31 (6.5%) | 1/27 (3.7%) |
| No | 11/31 (35.5%) | 8/27 (29.6%) |
| Yes | 18/31 (58.1%) | 18/27 (66.7%) |
| Premature CAD in a first-degree relative, no./total no. (%) | ||
| Male < 55 years | 14/18 (77.8%) | 12/18 (66.7%) |
| Female < 65 years | 4/18 (22.2%) | 6/18 (33.3%) |
| Time since FH diagnosis, in years, median [quartiles] | 2 [1–7.8] | 7 [0.5–12.5] |
| Current smoking, no./total no. (%) | 1/31 (3.2%) | 4/27 (14.8%) |
| Alcohol consumption, no./total no. (%) | 10/31 (32.3%) | 16/27 (59.3%) |
| Estimated daily ethanol consumption, in g, median [quartiles] | 2.3 [2.1–7] (n = 10) | 2.4 [1.2–3.9] (n = 16) |
| Type 2 Diabetes, no./total no. (%) | 9/31 (29%) | 10/27 (37%) |
| Hypertension, no./total no. (%) | 22/31 (71%) | 22/27 (81.5%) |
| Previous cardiovascular events, no./total no. (%) | ||
| No | 15/31 (48.4%) | 13/27 (48.1%) |
| Yes | 16/31 (51.6%) | 14/27 (51.9%) |
| Myocardial Infarction, no./total no. (%) | 10/16 (62.5%) | 10/14 (71.4%) |
| Stroke, no./total no. (%) | 2/16 (12.5%) | 0/14 (0%) |
| Other, no./total no. (%) | 4/16 (25%) | 4/14 (28.6%) |
| Body weight, in kg, mean (SD) | 79.9 (16) | 77.6 (14.8) |
| Body mass index, in kg/m2, mean (SD) | 29.3 (4.1) | 29.5 (4.3) |
| Waist circumference, in cm, mean (SD) | 96.8 (11.2) | 96.1 (13.9) |
| Dutch MEDPED, in points, mean (SD) | 9.3 (3.6) (n = 31) | 8.4 (3.6) (n = 26) |
| FH diagnosis according to Dutch MEDPED, no./total no. (%) | ||
| Probable (6–8 points) | 17/31 (54.8%) | 19/26 (73.1%) |
| Definitive (>8 points) | 14/31 (45.2%) | 7/26 (26.9%) |
| (b) | ||
| Krill Oil Placebo (n = 29) | Krill Oil (n = 29) | |
| Sex, no./total no. (%) | ||
| Female | 13/29 (44.8%) | 21/29 (72.4%) |
| Male | 16/29 (55.2%) | 8/29 (27.6%) |
| Age, in years, mean (SD) | 52.6 (13.4) | 56.4 (14) |
| Race | ||
| White | 19/29 (65.5%) | 15/29 (51.7%) |
| Pardo | 5/29 (17.2%) | 8/29 (27.6%) |
| Other | 5/29 (17.3%) | 6/29 (20.7%) |
| Marital status, no./total no. (%) | ||
| Married | 18/29 (62.1%) | 16/29 (55.2%) |
| Other | 11/29 (37.9%) | 13/29 (44.8%) |
| Average monthly household income, in USD 1, no./total no. (%) | ||
| ≥1231.17 | 17/29 (58.6%) | 20/29 (69%) |
| <1231.17 | 12/29 (41.4%) | 9/29 (31%) |
| Education level, in years of schooling, no./total no. (%) | ||
| ≤9 years | 9/29 (69%) | 9/29 (69%) |
| >9 years | 20/29 (31%) | 20/29 (31%) |
| Physical activity, no./total no. (%) | ||
| Sedentary/Low | 15/29 (51.7%) | 18/29 (62.1%) |
| Moderate | 11/29 (37.9%) | 6/29 (20.7%) |
| High | 3/29 (10.3%) | 5/29 (17.2%) |
| Family history of FH, no./total no. (%) | ||
| Unknown | 3/29 (10.3%) | 5/29 (17.2%) |
| No | 4/29 (13.8%) | 5/29 (17.2%) |
| Yes | 22/29 (75.9%) | 19/29 (65.5%) |
| Family history of premature CAD, no./total no. (%) | ||
| Unknown | 1/29 (3.4%) | 2/29 (6.9%) |
| No | 9/29 (31%) | 10/29 (34.5%) |
| Yes | 19/29 (65.5%) | 17/29 (58.6%) |
| Premature CAD in a first-degree relative, no./total no. (%) | ||
| Male < 55 years | 13/19 (68.4%) | 13/17 (76.5%) |
| Female < 65 years | 6/19 (31.6%) | 4/17 (23.5%) |
| Time since FH diagnosis, in years, median [quartiles] | 6 [1–11] | 2 [0–14] |
| Current smoking, no./total no. (%) | 3/29 (10.3%) | 2/29 (6.9%) |
| Alcohol consumption, no./total no. (%) | 15/29 (51.7%) | 11/29 (37.9%) |
| Estimated daily ethanol consumption, in g, median [quartiles] | 2.5 [1.9–6.7] (n = 15) | 2.1 [1.6–3.5] (n = 11) |
| Type 2 Diabetes, no./total no. (%) | 12/29 (41.4%) | 7/29 (24.1%) |
| Hypertension, no./total no. (%) | 23/29 (79.3%) | 21/29 (72.4%) |
| Previous cardiovascular events, no./total no. (%) | ||
| No | 13/29 (44.8%) | 15/29 (51.7%) |
| Yes | 16/29 (55.2%) | 14/29 (48.3%) |
| Myocardial Infarction, no./total no. (%) | 11/16 (68.8%) | 9/14 (64.3%) |
| Stroke, no./total no. (%) | 1/16 (6.2%) | 1/14 (7.1%) |
| Other, no./total no. (%) | 4/16 (25%) | 4/14 (28.6%) |
| Body weight, in kg, mean (SD) | 78 (16.1) | 79.7 (14.9) |
| Body mass index, in kg/m2, mean (SD) | 28.5 (4.5) | 30.3 (3.5) |
| Waist circumference, in cm, mean (SD) | 95.6 (12.8) | 97.4 (12.1) |
| Dutch MEDPED, in points, mean (SD) | 8.9 (3.2) (n = 28) | 8.9 (2.8) (n = 29) |
| FH diagnosis according to Dutch MEDPED, no./total no. (%) | ||
| Probable (6–8 points) | 18/29 (62.1%) | 18/28 (64.3%) |
| Definitive (>8 points) | 11/29 (37.9%) | 10/28 (35.7%) |
| Phytosterol | Krill Oil | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 26) | Active (n = 23) | Difference 95%CI 1 | Placebo (n = 25) | Active (n = 24) | Difference 95% CI 1 | |
| LDL-c, mg/dL | ||||||
| Baseline | 132.5 [108.9–151.6] | 119.6 [108.8–151.6] | −7.6 (−32.4; 21.1) | 114.4 [81.4–141.1] | 137.2 [114.9–158] | 27.4 (3.1; 54) |
| Final | 119.2 [92.9–148.7] | 133.3 [90.8–162.8] | 0.1 (−36.6; 32.9) | 112.6 [77.94–161.93] | 120.9 [100.7–164.5] | 15 (−18.2; 49.2) |
| Difference, in mg/dL (95%CI) 2 | −8.2 (−18.3; 6.3) | 0.6 (−14.4; 20.1) | 6.2 (−10.6; 26.3) | 1 (−10.3; 14.6) | −11.9 (−23.2; 15.6) | −12.1 (−28; 4.5) |
| Difference, in % (IQR) 3 | −5.9 [−17.4–1.7] | 0 [−18.31–22.85] | 3.3 (−12.9; 20.9) | −1.9 [−10.7–19.6] | −11.3 [−21.7–1.4] | −8.1 (−22.3; 6.8) |
| Lp(a), mg/dL | ||||||
| Baseline | 27.7 [20.3–55.5] | 38.1 [15.2–64] | 1.2 (−16; 21.7) | 35.3 [16.1–57.1] | 30.6 [18.9–63.6] | 1.2 (−17; 17.8) |
| Final | 27.4 [19.2–50.8] | 25.8 [13.5–74.6] | −0.9 (−15.8; 17.1) | 23.4 [14.4–51.3] | 31.2 [17.6–56.9] | 3.3 (−10; 19.3) |
| Difference, in mg (95%CI) 2 | −3 (−7.2; −0.3) * | −5.2 (−14.6; 0.2) | −1.00 (−7.00; 3.30) | −5.8 (−10.5; −1.5) ¶ | −2.3 (−7.9; 2.2) | 1.7 (−2.5; 8.2) |
| Difference, in % (IQR) 3 | −10.9 [−21.6–1.9] * | −8.9 [−25.2–0] | −1.6 (−12.4; 12.4) | −16.1 [−25.2–−0.8] ¶ | −8.1 [−18.4–5.8] | 8.2 (−3.4; 21.6) |
| Phytosterol | Krill Oil | |||||
|---|---|---|---|---|---|---|
| Placebo | Active | Difference 95%CI 1 | Placebo | Active | Difference 95%CI 1 | |
| Fruits, points | ||||||
| Baseline | 6.2 ± 3.9 (n = 31) | 6.7 ± 3.7 (n = 27) | 0.47 (−1.50; 2.50) | 6.5 ± 3.9 (n = 29) | 6.4 ± 3.8 (n = 29) | −0.07 (−2.10; 1.90) |
| Final | 7.7 ± 3.2 (n = 26) | 7.1 ± 3.9 (n = 24) | −0.61 (−2.65; 1.43) | 7.6 ± 3.6 (n = 25) | 7.2 ± 3.6 (n = 25) | −0.40 (−2.43; 1.63) |
| Vegetables, points | ||||||
| Baseline | 2.2 ± 1.6 (n = 31) | 2.9 ± 1.9 (n = 27) | 0.72 (−0.20; 1.60) | 2.5 ± 1.7 (n = 29) | 2.5 ± 1.9 (n = 29) | −0.03 (−1.00; 0.90) |
| Final | 2.6 ± 1.9 (n = 26) | 3.1 ± 2 (n = 24) | 0.56 (−0.55; 1.67) | 2.9 ± 1.9 (n = 25) | 2.8 ± 2 (n = 25) | −0.07 (−1.18; 1.05) |
| Whole grains, points | ||||||
| Baseline | 2 ± 2.9 (n = 31) | 3.1 ± 3.3 (n = 27) | 1.04 (−0.60; 2.70) | 3 ± 3.3 (n = 29) | 2.1 ± 3 (n = 29) | −0.92 (−2.60; 0.70) |
| Final | 3.5 ± 3.4 (n = 26) | 3.5 ± 3.3 (n = 24) | −0.04 (−1.96; 1.88) | 3.8 ± 3.5 (n = 25) | 3.2 ± 3.3 (n = 25) | −0.59 (−2.50; 1.32) |
| Legumes, points | ||||||
| Baseline | 8.4 ± 3.7 (n = 31) | 8.5 ± 3.6 (n = 27) | 0.13 (−1.80; 2.10) | 8.6 ± 3.5 (n = 29) | 8.3 ± 3.8 (n = 29) | −0.34 (−2.30; 1.60) |
| Final | 9.2 ± 2.7 (n = 26) | 9.6 ± 2 (n = 24) | 0.35 (−1.01; 1.71) | 9.6 ± 2 (n = 25) | 9.2 ± 2.8 (n = 25) | −0.40 (−1.78; 0.98) |
| Nuts, points | ||||||
| Baseline | 3.1 ± 4.4 (n = 31) | 4.4 ± 4.4 (n = 27) | 1.33 (−1.00; 3.70) | 3.2 ± 4.4 (n = 29) | 4.3 ± 4.4 (n = 29) | 1.03 (−1.30; 3.40) |
| Final | 4.2 ± 4.1 (n = 26) | 4.5 ± 4.4 (n = 24) | 0.28 (−2.14; 2.70) | 4.2 ± 3.9 (n = 25) | 4.5 ± 4.5 (n = 25) | 0.33 (−2.07; 2.74) |
| Dairy, points | ||||||
| Baseline | 4.8 ± 5.1 (n = 31) | 4.1 ± 5 (n = 27) | −0.76 (−3.40; 1.90) | 4.5 ± 5.1 (n = 29) | 4.5 ± 5.1 (n = 29) | 0.00 (−2.70; 2.70) |
| Final | 5.8 ± 5 (n = 26) | 5.4 ± 5.1 (n = 24) | −0.35 (−3.24; 2.53) | 6 ± 5 (n = 25) | 5.2 ± 5.1 (n = 25) | −0.80 (−3.67; 2.07) |
| Fish and seafood, points | ||||||
| Baseline | 1.8 ± 2.8 (n = 31) | 3.5 ± 3.6 (n = 27) | 1.74 (0.00; 3.50) | 3.3 ± 3.6 (n = 29) | 1.9 ± 2.8 (n = 29) | −1.38 (−3.10; 0.30) |
| Final | 3.1 ± 3.5 (n = 26) | 5.4 ± 4.4 (n = 24) | 2.34 (0.06; 4.62) * | 4.2 ± 4.5 (n = 25) | 4.2 ± 3.7 (n = 25) | 0.00 (−2.35; 2.35) |
| Red meat, points | ||||||
| Baseline | 3.2 ± 4.8 (n = 31) | 1.9 ± 4 (n = 27) | −1.37 (−3.70; 0.90) | 2.4 ± 4.4 (n = 29) | 2.8 ± 4.5 (n = 29) | 0.34 (−2.00; 2.70) |
| Final | 2.7 ± 4.5 (n = 26) | 2.1 ± 4.1 (n = 24) | −0.61 (−3.07; 1.86) | 2 ± 4.1 (n = 25) | 2.8 ± 4.6 (n = 25) | 0.80 (−1.67; 3.27) |
| Processed meat, points | ||||||
| Baseline | 9.4 ± 2.5 (n = 31) | 9.3 ± 2.7 (n = 27) | −0.10 (−1.50; 1.30) | 9.3 ± 2.6 (n = 29) | 9.3 ± 2.6 (n = 29) | 0.00 (−1.40; 1.40) |
| Final | 10 ± 0 (n = 26) | 8.8 ± 3.4 (n = 24) | −1.25 (−2.68; 0.18) | 9.2 ± 2.8 (n = 25) | 9.6 ± 2 (n = 25) | 0.40 (−0.98; 1.78) |
| Sweet sugar beverages, points | ||||||
| Baseline | 6.5 ± 4.9 (n = 31) | 7.8 ± 4.2 (n = 27) | 1.33 (−1.10; 3.70) | 6.2 ± 4.9 (n = 29) | 7.9 ± 4.1 (n = 29) | 1.72 (−0.70; 4.10) |
| Final | 7.7 ± 4.3 (n = 26) | 7.1 ± 4.6 (n = 24) | −0.61 (−3.16; 1.94) | 8 ± 4.1 (n = 25) | 6.8 ± 4.8 (n = 25) | −1.20 (−3.72; 1.32) |
| Ultra-processed food, points | ||||||
| Baseline | 10 ± 0 (n = 31) | 10 ± 0 (n = 27) | - | 10 ± 0 (n = 29) | 10 ± 0 (n = 29) | - |
| Final | 10 ± 0 (n = 26) | 10 ± 0 (n = 24) | - | 10 ± 0 (n = 25) | 10 ± 0 (n = 25) | - |
| CHDI, total points | ||||||
| Baseline | 57.5 ± 16.5 (n = 31) | 62.1 ± 15.6 (n = 27) | 4.53 (−3.90; 13.00) | 59.5 ± 16.2 (n = 29) | 59.8 ± 16.3 (n = 29) | 0.36 (−8.20; 8.90) |
| Final | 66.5 ± 14.7 (n = 26) | 66.5 ± 15.3 (n = 24) | 0.06 (−8.49; 8.62) | 67.5 ± 16 (n = 25) | 65.5 ± 13.9 (n = 25) | −1.92 (−10.44; 6.60) |
| Phytosterol | Krill Oil | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 20) | Active (n = 20) | Difference 95%CI 1 | Placebo (n = 20) | Active (n = 20) | Difference 95%CI 1 | |
| Campesterol, µg/mL | ||||||
| Baseline | 0.44 [0.13–0.71] | 0.36 [0.18–0.52] | −0.03 (−0.31; 0.17) | 0.36 [0.18–0.65] | 0.41 [0.14–0.71] | 0.01 (−0.21; 0.25) |
| Final | 0.14 [0.09–0.57] | 0.43 [0.13–0.79] | 0.09 (−0.03; 0.49) | 0.21 [0.09–0.61] | 0.25 [0.12–0.74] | 0.04 (−0.09; 0.34) |
| Difference, in µg/mL (95%CI) 2 | −0.17 (−0.59; 0.09) | 0.09 (−0.2; 0.52) | 0.32 (−0.15; 0.76) | −0.12 (−0.4; 0.14) | 0.02 (−0.33; 0.47) | 0.15 (−0.32; 0.62) |
| Campesterol, µg/mg TC 3 | ||||||
| Baseline | 0.21 [0.05–0.39] | 0.17 [0.08–0.31] | 0.00 (−0.15; 0.08) | 0.17 [0.08–0.36] | 0.21 [0.07–0.34] | −0.01 (−0.14; 0.10) |
| Final | 0.08 [0.05–0.32] | 0.12 [0.07–0.44] | 0.05 (−0.02; 0.19) | 0.12 [0.05–0.31] | 0.11 [0.06–0.35] | 0.01 (−0.07; 0.11) |
| Difference, in µg/mg TC (95%CI) 2 | −0.08 (−0.23; 0.03) | 0.04 (−0.11; 0.28) | 0.14 (−0.08; 0.36) | −0.05 (−0.21; 0.07) | 0.02 (−0.14; 0.25) | 0.09 (−0.14; 0.31) |
| Campesterol, µg/mg LDL-c 3 | ||||||
| Baseline | 0.34 [0.07–0.61] | 0.25 [0.12–0.48] | 0.03 (−0.25; 0.17) | 0.28 [0.11–0.64] | 0.27 [0.09–0.5] | −0.04 (−0.27; 0.13) |
| Final | 0.14 [0.08–0.48] | 0.33 [0.12–0.7] | 0.07 (−0.05; 0.32) | 0.24 [0.09–0.48] | 0.18 [0.09–0.58] | 0.01 (−0.19; 0.18) |
| Difference, in µg/mg LDL-c (95%CI) 2 | −0.12 (−0.33; 0.07) | 0.10 (−0.19; 0.53) | 0.20 (−0.12; 0.61) | −0.08 (−0.34; 0.1) | 0.04 (−0.2; 0.38) | 0.16 (−0.21; 0.54) |
| Stigmasterol, µg/mL | ||||||
| Baseline | 0.28 [0.14–0.52] | 0.1 [0.03–0.19] | −0.16 (−0.32; −0.05) * | 0.24 [0.1–0.36] | 0.15 [0.06–0.26] | −0.06 (−0.20; 0.06) |
| Final | 0.05 [0.02–0.15] | 0.13 [0.05–0.27] | 0.05 (−0.01; 0.13) | 0.09 [0.04–0.18] | 0.08 [0.02–0.3] | 0.00 (−0.06; 0.11) |
| Difference, in µg/mL (95%CI) 2 | −0.24 (−0.6; −0.1) * | 0.03 (−0.06; 0.15) | 0.28 (0.10; 0.48) * | −0.16 (−0.41; −0.03) * | −0.01 (−0.16; 0.11) | 0.14 (−0.04; 0.35) |
| Stigmasterol, µg/mg TC 3 | ||||||
| Baseline | 0.13 [0.07–0.25] | 0.06 [0.02–0.09] | −0.07 (−0.17; −0.01) * | 0.08 [0.05–0.22] | 0.07 [0.03–0.12] | −0.03 (−0.09; 0.02) |
| Final | 0.03 [0.01–0.1] | 0.08 [0.03–0.11] | 0.02 (−0.01; 0.06) | 0.06 [0.02–0.1] | 0.04 [0.01–0.11] | −0.01 (−0.04; 0.03) |
| Difference, in µg/mg TC (95%CI) 2 | −0.11 (−0.3; −0.05) * | 0.01 (−0.03; 0.07) | 0.12 (0.05; 0.22) * | −0.08 (−0.2; −0.01) ¶ | −0.01 (−0.08; 0.05) | 0.05 (−0.03; 0.15) |
| Stigmasterol, µg/mg LDL-c 3 | ||||||
| Baseline | 0.23 [0.09–0.39] | 0.1 [0.03–0.16] | −0.11 (−0.27; −0.01) 2 | 0.15 [0.09–0.34] | 0.12 [0.03–0.23] | −0.05 (−0.16; 0.04) |
| Final | 0.05 [0.02–0.15] | 0.14 [0.06–0.19] | 0.05 (−0.02; 0.12) | 0.1 [0.04–0.17] | 0.06 [0.02–0.17] | −0.01 (−0.08; 0.04) |
| Difference, in µg/mg LDL-c (95%CI) 2 | −0.18 (−0.53; −0.07) * | 0.04 (−0.05; 0.13) | 0.20 (0.08; 0.39) * | −0.11 (−0.3; −0.004) ≠ | −0.02 (−0.13; 0.09) | 0.10 (−0.05; 0.26) |
| Beta-sitosterol, µg/mL | ||||||
| Baseline | 0.2 [0.15–1.84] | 0.37 [0.1–1.7] | −0.04 (−0.19; 0.69) | 0.23 [0.12–1.89] | 0.2 [0.12–1.65] | −0.01 (−0.65; 0.14) |
| Final | 0.04 [0.02–0.1] | 0.07 [0.02–0.15] | 0.01 (−0.03; 0.07) | 0.07 [0.02–0.12] | 0.06 [0.02–0.14] | 0.00 (−0.04; 0.05) |
| Difference, in µg/mL (95%CI) 2 | −0.81 (−1.25; −0.13) * | −0.79 (−1.47; −0.13) * | 0.04 (−0.33; 0.31) | −0.89 (−1.67; −0.1) * | −0.64 (−1.23; −0.13) * | −0.01 (−0.21; 0.63) |
| Beta-sitosterol, µg/mg TC 3 | ||||||
| Baseline | 0.1 [0.06–0.84] | 0.12 [0.05–0.81] | −0.01 (−0.08; 0.40) | 0.1 [0.06–0.99] | 0.11 [0.06–0.71] | 0.00 (−0.42; 0.06) |
| Final | 0.02 [0.01–0.05] | 0.04 [0.01–0.07] | 0.00 (−0.01; 0.03) | 0.03 [0.01–0.07] | 0.03 [0.01–0.05] | 0.00 (−0.03; 0.02) |
| Difference, in µg/mg TC (95%CI) 2 | −0.35 (−0.65; −0.05) * | −0.37 (−0.76; −0.05) * | 0.00 (−0.16; 0.11) | −0.44 (−0.78; −0.04) * | −0.24 (−0.55; −0.06) * | −0.01 (−0.08; 0.37) |
| Beta-sitosterol, µg/mg LDL-c 3 | ||||||
| Baseline | 0.16 [0.09–1.23] | 0.24 [0.1–1.47] | 0.01 (−0.11; 0.81) | 0.2 [0.09–1.68] | 0.16 [0.1–1.27] | −0.02 (−0.58; 0.10) |
| Final | 0.04 [0.02–0.08] | 0.06 [0.03–0.12] | 0.01 (−0.02; 0.06) | 0.06 [0.02–0.12] | 0.05 [0.02–0.09] | 0.00 (−0.05; 0.03) |
| Difference, in µg/mg LDL-c (95%CI) 2 | −0.49 (−1.04; −0.08) * | −0.71 (−1.13; −0.08) * | −0.02 (−0.48; 0.25) | −0.74 (−1.46; −0.08) | −0.42 (−0.92; −0.09) | −0.01 (−0.20; 0.62) |
| Phytosterol | Krill Oil | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 24) | Active (n = 23) | Difference 95%CI 1 | Placebo (n = 24) | Active (n = 23) | Difference 95%CI 1 | |
| (EPA) Eicosapentaenoic (C20:5 n3), % | ||||||
| Baseline | 0.46 [0.39–0.62] | 0.49 [0.41–0.6] | 0.02 (−0.09; 0.12) | 0.46 [0.39–0.6] | 0.49 [0.36–0.65] | 0.00 (−0.11; 0.10) |
| Final | 0.3 [0.02–0.57] | 0.34 [0.12–0.66] | 0.06 (−0.13; 0.27) | 0.25 [0.03–0.37] | 0.47 [0.08–0.85] | 0.21 (−0.01; 0.47) |
| Difference (95%CI) 2 | −0.17 (−0.39; 0.05) | −0.09 (−0.27; 0.12) | 0.10 (−0.20; 0.34) | −0.24 (−0.40; −0.06) * | 0.02 (−0.2; 0.24) | 0.24 (−0.04; 0.52) |
| (DHA) Docosahexaenoic (C22:6 n3), % | ||||||
| Baseline | 3.54 ± 0.95 | 3.34 ± 0.97 | −0.20 (−0.77; 0.36) | 3.57 ± 0.77 | 3.32 ± 1.12 | −0.25 (−0.82; 0.32) |
| Final | 4.33 ± 1.3 | 4.46 ± 1.35 | 0.13 (−0.65; 0.91) | 4.13 ± 1.33 | 4.67 ± 1.25 | 0.54 (−0.22; 1.30) |
| Difference (95%CI) 2 | 0.78 (0.2; 1.37) ● | 1.12 (0.54; 1.69) * | 0.33 (−0.46; 1.13) | 0.56 (0.07; 1.05) ¶ | 1.35 (0.72; 1.97) * | 0.79 (0.02; 1.56) ● |
| Omega−3 Index, % 3 | ||||||
| Baseline | 4.07 [3.36–4.69] | 3.6 [3.29–4.25] | −0.24 (−0.85; 0.32) | 3.83 [3.35–4.85] | 3.68 [3.36–4.25] | −0.15 (−0.91; 0.38) |
| Final | 4.54 [3.64–5.37] | 4.63 [3.87–5.88] | 0.15 (−0.64; 1.21) | 4.23 [3.07–5.07] | 4.81 [4.46–5.93] | 0.85 (−0.04; 1.67) |
| Difference (95%CI) 2 | 0.53 (−0.30; 1.40) | 1.10 (0.31; 1.92) * | 0.50 (−0.41; 1.54) | 0.27 (−0.35; 1.03) | 1.38 (0.44; 2.17) * | 0.95 (0.04; 1.87) |
| Omega-3/Omega-6 ratio 4 | ||||||
| Baseline | 0.15 [0.13–0.19] | 0.13 [0.12–0.17] | −0.01 (−0.04; 0.01) | 0.15 [0.12–0.2] | 0.13 [0.12–0.16] | −0.02 (−0.05; 0.01) |
| Final | 0.18 [0.15–0.23] | 0.17 [0.15–0.24] | 0.01 (−0.02; 0.05) | 0.16 [0.12–0.2] | 0.18 [0.17–0.24] | 0.03 (−0.01; 0.07) |
| Difference (95%CI) 2 | 0.02 (−0.013; 0.051) | 0.05 (0.03; 0.08) * | 0.03 (−0.01; 0.07) | 0.01 (−0.023; 0.04) | 0.06 (0.03; 0.08) | 0.05 (0.01; 0.09) ● |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Abreu-Silva, E.O.; Machado, R.H.V.; dos Santos, B.R.; Kojima, F.C.S.; Santos, R.H.N.; Negrelli, K.d.L.; Rodrigues, L.B.; de Barros e Silva, P.G.M.; de Lima, A.G.; Sanchez, J.G.; et al. An Adapted Cardioprotective Diet with or Without Phytosterol and/or Krill Oil Supplementation in Familial Hypercholesterolemia: Results of a Pilot Randomized Clinical Trial. Nutrients 2025, 17, 2008. https://doi.org/10.3390/nu17122008
de Abreu-Silva EO, Machado RHV, dos Santos BR, Kojima FCS, Santos RHN, Negrelli KdL, Rodrigues LB, de Barros e Silva PGM, de Lima AG, Sanchez JG, et al. An Adapted Cardioprotective Diet with or Without Phytosterol and/or Krill Oil Supplementation in Familial Hypercholesterolemia: Results of a Pilot Randomized Clinical Trial. Nutrients. 2025; 17(12):2008. https://doi.org/10.3390/nu17122008
Chicago/Turabian Stylede Abreu-Silva, Erlon Oliveira, Rachel Helena Vieira Machado, Bianca Rodrigues dos Santos, Flávia Cristina Soares Kojima, Renato Hideo Nakagawa Santos, Karina do Lago Negrelli, Letícia Barbante Rodrigues, Pedro Gabriel Melo de Barros e Silva, Andressa Gusmão de Lima, João Gabriel Sanchez, and et al. 2025. "An Adapted Cardioprotective Diet with or Without Phytosterol and/or Krill Oil Supplementation in Familial Hypercholesterolemia: Results of a Pilot Randomized Clinical Trial" Nutrients 17, no. 12: 2008. https://doi.org/10.3390/nu17122008
APA Stylede Abreu-Silva, E. O., Machado, R. H. V., dos Santos, B. R., Kojima, F. C. S., Santos, R. H. N., Negrelli, K. d. L., Rodrigues, L. B., de Barros e Silva, P. G. M., de Lima, A. G., Sanchez, J. G., El Khouri, F. J., Bersch-Ferreira, Â. C., Carvalho, A. B., de Oliveira, T. M., Izar, M. C., Sampaio, G. R., Damasceno, N. R. T., Rogero, M. M., Torres, E. A. F. d. S., ... Marcadenti, A. (2025). An Adapted Cardioprotective Diet with or Without Phytosterol and/or Krill Oil Supplementation in Familial Hypercholesterolemia: Results of a Pilot Randomized Clinical Trial. Nutrients, 17(12), 2008. https://doi.org/10.3390/nu17122008







