Gryllus bimaculatus Hydrolysate Ameliorates Obesity-Induced Muscle Atrophy by Activating Skeletal Muscle AMPK in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrolysis of GB Powder Using Enzyme
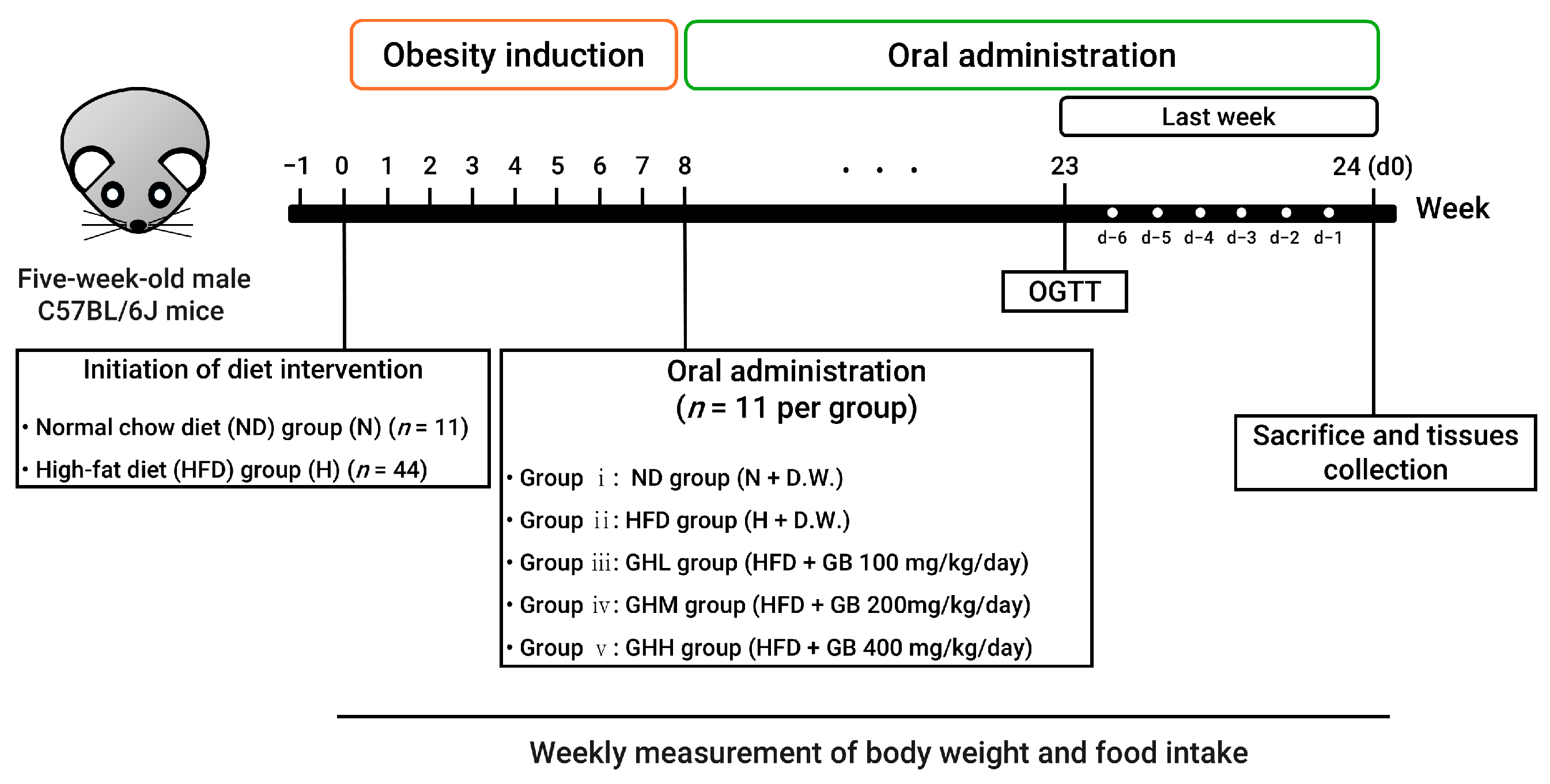
2.2. Animal Experiments
2.3. Serum Biochemistry
2.4. Grip Strength Test
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. Histological Analysis
2.7. Muscle Lipid Contents
2.8. Total RNA Extraction and Quantitative Real Time PCR (qRT-PCR)
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
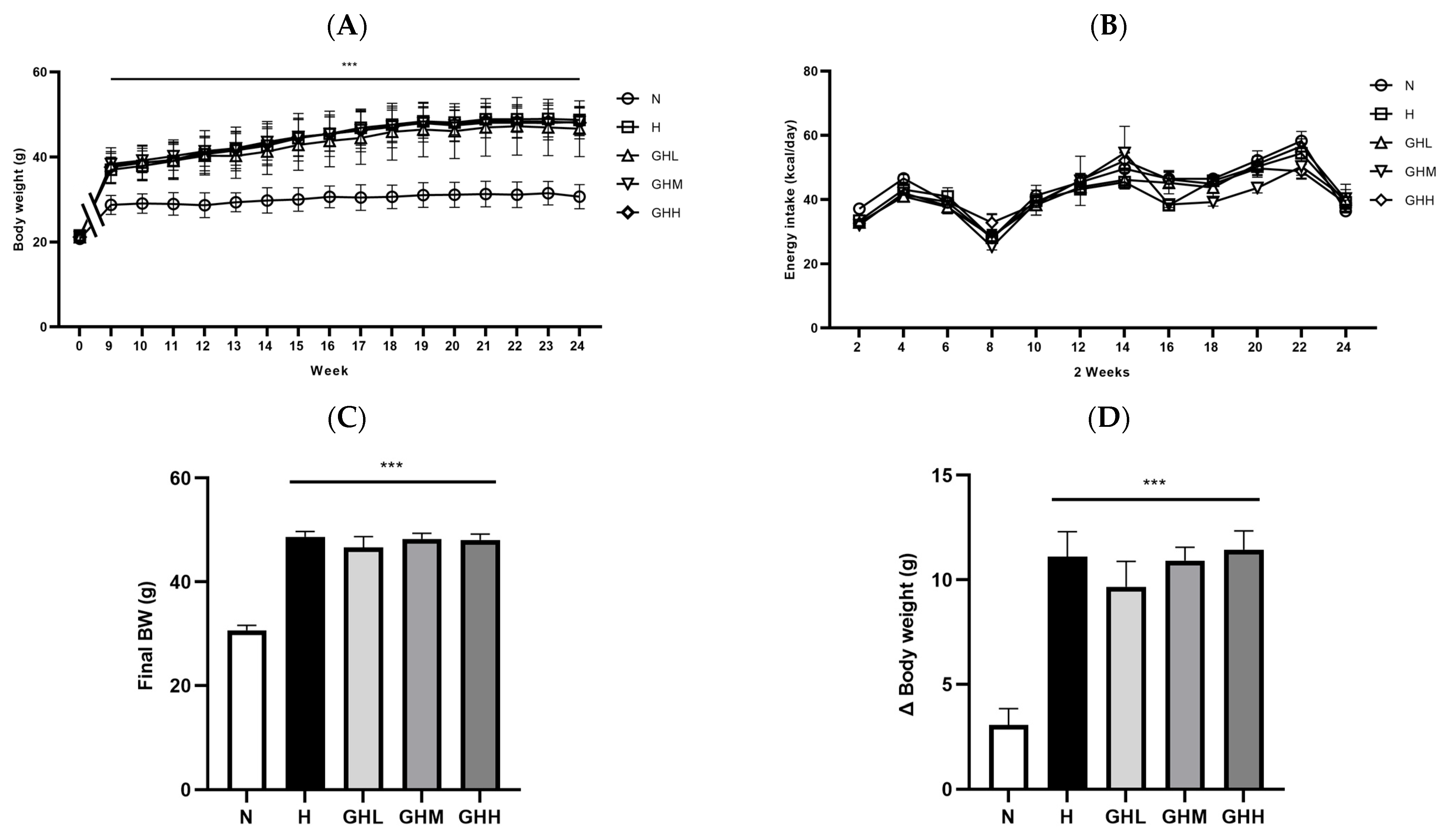
3.1. Effect of GB Supplementation on Body Weight Changes, Energy Intake, and Fat Mass
3.2. Effect of GB Supplementation on Glucose Homeostasis
3.3. Effects of GB Supplementation on Muscle Function and Muscular Ectopic Fat Accumulation
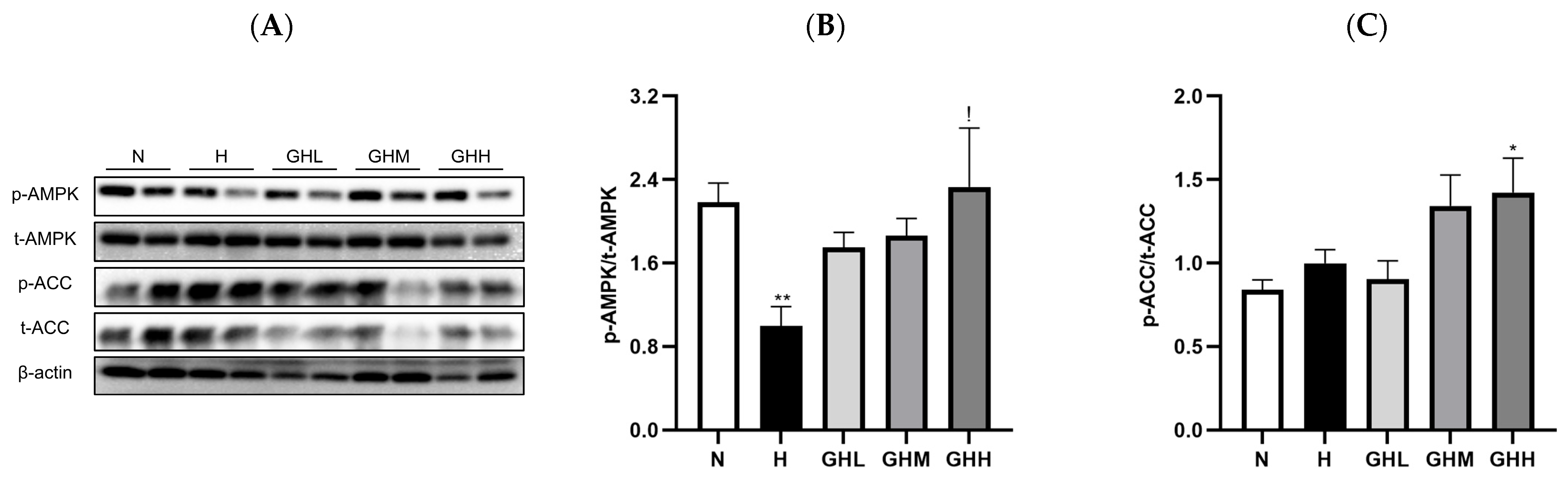
3.4. Effects of GB Supplementation on Muscular Ampk Activation
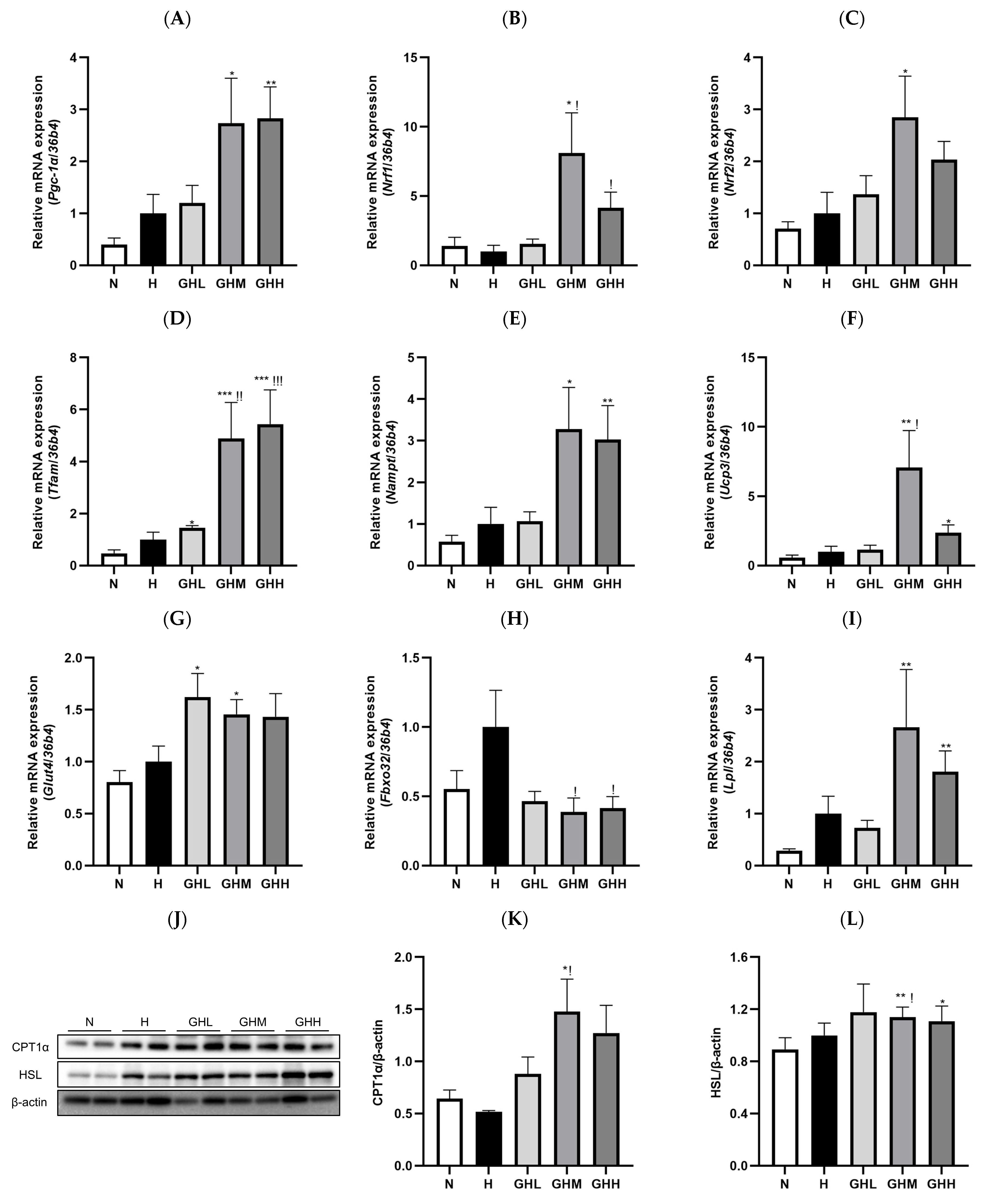
3.5. Effects of GB Supplementation on Gene and Protein Expression in Skeletal Muscle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 36B4 | Ribosomal protein lateral stalk subunit P0 |
| AICAR | 5-Aminoimidazole-4-carboxamide ribonucleotide |
| ACC | Acetyl-CoA carboxylase |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| ANOVA | One-way analysis of variance |
| ART | Aligned rank transform |
| AST | Aspartate aminotransferase |
| AUC | Area under the curve |
| CPT1α | Carnitine palmitoyltransferase 1 alpha |
| CSA | Cross-sectional area |
| DIO | Diet-induced obesity |
| EAT | epididymal white adipose tissue |
| ELISA | Enzyme-linked immunosorbent assay |
| ERRα | Estrogen-related receptor alpha |
| Fbxo32 | F-box protein 32 |
| FAO | Fatty acid oxidation |
| FFAs | Free fatty acids |
| GA | gastrocnemius |
| GB | Gryllus bimaculatus |
| GHL | HFD + 100 mg/kg/day GB group |
| GHM | HFD + 200 mg/kg/day GB group |
| GHH | HFD + 400 mg/kg/day GB group |
| GLUT4 | Glucose transporter type 4 |
| H | High-fat diet group |
| HDL-C | High-density lipoprotein cholesterol |
| H&E | Hematoxylin and eosin |
| HFD | High-fat diet |
| HOMA-IR | Serum homeostasis model assessment-estimated IR |
| HSL | Hormone sensitive lipase |
| IR | Insulin resistance |
| LDL-C | Low-density lipoprotein cholesterol |
| LPL | Lipoprotein lipase |
| mTOR | Mammalian target of rapamycin |
| N | Normal diet group |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NBF | Neutral-buffered formalin |
| ND | Normal diet |
| NRF1 | Nuclear respiratory factor 1 |
| NRF2 | Nuclear respiratory factor 2 |
| OGTT | Oral glucose tolerance test |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator-1 alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| RAT | Retroperitoneal white adipose tissue |
| SEM | Standard error of the mean |
| SIRT1 | Sirtuin 1 |
| SQT | Subcutaneous white adipose tissue |
| T2D | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TG | Triglycerides |
| TFAM | Mitochondrial transcription factor A |
| UCP3 | Uncoupling protein 3 |
| WAT | White adipose tissue |
References
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the obesity epidemic: After the sudden rise, is the upward trajectory beginning to flatten? Curr. Obes. Rep. 2023, 12, 514–527. [Google Scholar] [CrossRef]
- Mahase, E. Global cost of overweight and obesity will hit $4.32 tn a year by 2035, report warns. BMJ 2023, 380, 523. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D. Role of body fat distribution and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 2008, 93, S57–S63. [Google Scholar] [CrossRef] [PubMed]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Trouwborst, I.; Bowser, S.M.; Goossens, G.H.; Blaak, E.E. Ectopic fat accumulation in distinct insulin resistant phenotypes; targets for personalized nutritional interventions. Front. Nutr. 2018, 5, 77. [Google Scholar] [CrossRef]
- Yao, S.; Yuan, Y.; Zhang, H.; Meng, X.; Jin, L.; Yang, J.; Wang, W.; Ning, G.; Zhang, Y.; Zhang, Z. Berberine attenuates the abnormal ectopic lipid deposition in skeletal muscle. Free Radic. Biol. Med. 2020, 159, 66–75. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, T.; Gomez, N.A.; Wang, B.; Zhu, M.J.; Du, M. Raspberry alleviates obesity-induced inflammation and insulin resistance in skeletal muscle through activation of AMP-activated protein kinase (AMPK) α1. Nutr. Diabetes 2018, 8, 39. [Google Scholar] [CrossRef]
- Jang, H.; Joung, H.; Chu, J.; Cho, M.; Kim, Y.W.; Kim, K.H.; Shin, C.H.; Lee, J.; Ha, J.H. Lactobacillus delbrueckii subsp. lactis CKDB001 ameliorates metabolic complications in high-fat diet-induced obese mice. Nutrients 2024, 16, 4260. [Google Scholar]
- Bai, J.; Zhang, S.; Cao, J.; Sun, H.; Mang, Z.; Shen, W.L.; Li, H. Hernandezine, a natural herbal alkaloid, ameliorates type 2 diabetes by activating AMPK in two mouse models. Phytomedicine 2022, 105, 154366. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol. Life Sci. 2010, 67, 3407–3423. [Google Scholar] [CrossRef] [PubMed]
- Samant, V.; Prabhu, A. Exercise, exerkines and exercise mimetic drugs: Molecular mechanisms and therapeutics. Life Sci. 2024, 359, 123225. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- Viollet, B.; Lantier, L.; Devin-Leclerc, J.; Hebrard, S.; Amouyal, C.; Mounier, R.; Foretz, M.; Andreelli, F. Targeting the AMPK pathway for the treatment of Type 2 diabetes. Front. Biosci. (Landmark Ed.) 2009, 14, 3380–3400. [Google Scholar] [CrossRef]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef]
- Lyu, Q.; Wen, Y.; He, B.; Zhang, X.; Chen, J.; Sun, Y.; Zhao, Y.; Xu, L.; Xiao, Q.; Deng, H. The ameliorating effects of metformin on disarrangement ongoing in gastrocnemius muscle of sarcopenic and obese sarcopenic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166508. [Google Scholar] [CrossRef]
- Kang, M.J.; Moon, J.W.; Lee, J.O.; Kim, J.H.; Jung, E.J.; Kim, S.J.; Oh, J.Y.; Wu, S.W.; Lee, P.R.; Park, S.H.; et al. Metformin induces muscle atrophy by transcriptional regulation of myostatin via HDAC6 and FoxO3a. J. Cachexia Sarcopenia Muscle 2022, 13, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Domise, M.; Sauvé, F.; Didier, S.; Caillerez, R.; Bégard, S.; Carrier, S.; Colin, M.; Marinangeli, C.; Buée, L.; Vingtdeux, V. Neuronal AMP-activated protein kinase hyper-activation induces synaptic loss by an autophagy-mediated process. Cell Death Dis. 2019, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. Regulation of AMP-activated protein kinase by natural and synthetic activators. Acta Pharm. Sin. B 2016, 6, 1–19. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Feher, M.; Schmidt, J.M. Property distributions: Differences between drugs, natural products, and molecules from combinatorial chemistry. J. Chem. Inf. 2003, 43, 218–227. [Google Scholar]
- Higashida, K.; Kim, S.H.; Jung, S.R.; Asaka, M.; Holloszy, J.O.; Han, D.H. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013, 11, e1001603. [Google Scholar] [CrossRef]
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.; Hayes, D.; Milic, M.; Padulo, J. Herbal medicine for sports: A review. J. Int. Soc. Sports Nutr. 2018, 15, 14. [Google Scholar] [CrossRef]
- Mongioì, L.M.; La Vignera, S.; Cannarella, R.; Cimino, L.; Compagnone, M.; Condorelli, R.A.; Calogero, A.E. The role of resveratrol administration in human obesity. Int. J. Mol. Sci. 2021, 22, 4362. [Google Scholar] [CrossRef]
- Liceaga, A.M. Edible insects, a valuable protein source from ancient to modern times. Adv. Food Nutr. Res. 2022, 101, 129–152. [Google Scholar]
- Van Huis, A. Prospects of insects as food and feed. Org. Agr. 2021, 11, 301–308. [Google Scholar] [CrossRef]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Delaune, T.; Silva, J.V.; Descheemaeker, K.; Van De Ven, G.; Schut, A.G.; van Wijk, M.; Hammond, J.; Hochman, Z.; Taulya, G.; et al. The future of farming: Who will produce our food? Food Secur. 2021, 13, 1073–1099. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y. Edible insects as future food: Chances and challenges. J. Future Foods 2021, 1, 38–46. [Google Scholar] [CrossRef]
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Reverberi, M. Edible insects: Cricket farming and processing as an emerging market. J. Insects Food Feed 2019, 6, 211–220. [Google Scholar] [CrossRef]
- Aiello, D.; Barbera, M.; Bongiorno, D.; Cammarata, M.; Censi, V.; Indelicato, S.; Mazzotti, F.; Napoli, A.; Piazzese, D.; Saiano, F. Edible insects an alternative nutritional source of bioactive compounds: A review. Molecules 2023, 28, 699. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Machì, M.; Cianciosi, D.; Navarro-Hortal, M.D.; Battino, M. Edible insects: A novel nutritious, functional, and safe food alternative. Food Front. 2022, 3, 358–365. [Google Scholar] [CrossRef]
- Devi, W.D.; Bonysana, R.; Kapesa, K.; Rai, A.K.; Mukherjee, P.K.; Rajashekar, Y. Potential of edible insects as source of functional foods: Biotechnological approaches for improving functionality. Syst. Microbiol. Biomanuf. 2022, 2, 461–472. [Google Scholar] [CrossRef]
- Hwang, B.B.; Chang, M.H.; Lee, J.H.; Heo, W.; Kim, J.K.; Pan, J.H.; Kim, Y.J.; Kim, J.H. The edible insect Gryllus bimaculatus protects against gut-derived inflammatory responses and liver damage in mice after acute alcohol exposure. Nutrients 2019, 11, 857. [Google Scholar] [CrossRef]
- Nam, H.K.; Kang, T.W.; Kim, I.W.; Choi, R.Y.; Kim, H.W.; Park, H.J. Physicochemical properties of cricket (Gryllus bimaculatus) gel fraction with soy protein isolate for 3D printing-based meat analogue. Food Biosci. 2023, 53, 102772. [Google Scholar] [CrossRef]
- Arama, D.; Kinyuru, J.; Kiage-Mokua, B.; Ochieng, B.O.; Tanga, C.M. Unraveling the physicochemical attributes of three cricket (Gryllus bimaculatus)-enriched biscuit products and implications on consumers’ preference and willingness to pay. LWT 2023, 185, 115171. [Google Scholar] [CrossRef] [PubMed]
- Murugu, D.K.; Onyango, A.N.; Ndiritu, A.K.; Osuga, I.M.; Xavier, C.; Nakimbugwe, D.; Tanga, C.M. From farm to fork: Crickets as alternative source of protein, minerals, and vitamins. Front. Nutr. 2021, 8, 704002. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, E.Y.; Baek, D.J.; Oh, Y.S. Gryllus bimaculatus extract ameliorates high-fat diet-induced hyperglycemia and hyperlipidemia by inhibiting hepatic lipogenesis through AMPK activation. Food Sci. Biotechnol. 2022, 31, 1289–1297. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, S.J.; Kim, S.H.; Park, W.J.; Han, J.S. Gryllus bimaculatus-containing diets protect against dexamethasone-induced muscle atrophy, but not high-fat diet-induced obesity. Food Sci. Nutr. 2023, 11, 2787–2797. [Google Scholar] [CrossRef]
- Kim, K.; Park, E.Y.; Baek, D.J.; Jang, S.E.; Oh, Y.S. Gryllus bimaculatus extract protects against lipopolysaccharide-derived inflammatory response in human colon epithelial Caco-2 cells. Insects 2021, 12, 873. [Google Scholar] [CrossRef]
- Kim, N.; Jung, S.; Lee, E.; Jo, E.B.; Yoon, S.; Jeong, Y. Gryllus bimaculatus De Geer hydrolysates alleviate lipid accumulation, inflammation, and endoplasmic reticulum stress in palmitic acid-treated human hepatoma G2 cells. J. Ethnopharmacol. 2022, 291, 115117. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Han, J.S. Gryllus bimaculatus extract protects against lipopolysaccharide and palmitate-induced production of proinflammatory cytokines and inflammasome formation. Mol. Med. Rep. 2021, 23, 206. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, G.H.; Lee, H.Y.; Hoang, T.H.; Chae, H.J. Glucose-lowering effect of Gryllus bimaculatus powder on streptozotocin-induced diabetes through the AKT/mTOR pathway. Food Sci. Nutr. 2019, 8, 402–409. [Google Scholar] [CrossRef]
- Park, K.; Jung, S.; Ha, J.H.; Jeong, Y. Protaetia brevitarsis hydrolysate mitigates muscle dysfunction and ectopic fat deposition triggered by a high-fat diet in mice. Nutrients 2025, 17, 213. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Jung, J.; Lee, M.; Park, S.H.; Cho, W.; Kim, J.; Eun, S.; Lee, J. Rose Petal Extract Ameliorates Obesity in High Fat Diet-Induced Obese Mice. Prev. Nutr. Food Sci. 2024, 29, 125. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, C.S.; Park, D.R. NAMPT Regulates Mitochondria Biogenesis via NAD Metabolism and Calcium Binding Proteins during Skeletal Muscle Contraction. J. Exerc. Nutrition Biochem. 2014, 18, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.J.; Lee, J.; Lee, J.K.; Byun, J.; Kim, I.; Ha, J.H. Lowering n-6/n-3 Ratio as an Important Dietary Intervention to Prevent LPS-Inducible Dyslipidemia and Hepatic Abnormalities in ob/ob Mice. Int. J. Mol. Sci. 2022, 23, 6384. [Google Scholar] [CrossRef]
- Son, H.K.; Xiang, H.; Park, S.; Lee, J.; Lee, J.J.; Jung, S.; Ha, J.H. Partial Replacement of Dietary Fat with Polyunsaturated Fatty Acids Attenuates the Lipopolysaccharide-Induced Hepatic Inflammation in Sprague-Dawley Rats Fed a High-Fat Diet. Int. J. Environ. Res. Public Health 2021, 18, 10986. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.K.; Lee, J.J.; Park, S.; Jung, S.; Lee, H.J.; Ha, J.H. Partial Replacement of High-Fat Diet with Beef Tallow Attenuates Dyslipidemia and Endoplasmic Reticulum Stress in db/db Mice. J. Med. Food 2022, 25, 660–674. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only ANOVA Procedures. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Vancouver, BC, Canada, 7–12 May 2011; pp. 143–146. [Google Scholar]
- Coen, P.M.; Goodpaster, B.H. Role of Intramyocellular Lipids in Human Health. Trends Endocrinol. Metab. 2012, 23, 391–398. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and Function of AMPK in Physiology and Diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Taillandier, D.; Polge, C. Skeletal Muscle Atrogenes: From Rodent Models to Human Pathologies. Biochimie 2019, 166, 251–269. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Unlocking the Biological Potential of Proteins from Edible Insects through Enzymatic Hydrolysis: A Review. Innov. Food Sci. Emerg. Technol. 2017, 43, 239–252. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are intact peptides absorbed from the healthy gut in the adult human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Karaś, M.; Baraniak, B.; Jakubczyk, A. Evaluation of ACE, α-glucosidase, and lipase inhibitory activities of peptides obtained by in vitro digestion of selected species of edible insects. Eur. Food Res. Technol. 2020, 246, 1361–1369. [Google Scholar] [CrossRef]
- Fashakin, O.O.; Tangjaidee, P.; Unban, K.; Klangpetch, W.; Khumsap, T.; Sringarm, K.; Rawdkuen, S.; Phongthai, S. Isolation and Identification of Antioxidant Peptides Derived from Cricket (Gryllus bimaculatus) Protein Fractions. Insects 2023, 14, 674. [Google Scholar] [CrossRef]
- Prado, C.M.; Wells, J.C.; Smith, S.R.; Stephan, B.C.; Siervo, M. Sarcopenic obesity: A critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Akerström, T.C.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Yang, M.; Sun, J.; Zhao, Y.; Tang, D. The effect of irisin as a metabolic regulator and its therapeutic potential for obesity. Int. J. Endocrinol. 2021, 2021, 6572342. [Google Scholar] [CrossRef]
- Kong, S.; Cai, B.; Nie, Q. PGC-1α affects skeletal muscle and adipose tissue development by regulating mitochondrial biogenesis. Mol. Genet. Genomics 2022, 297, 621–633. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARδ Agonists Are Exercise Mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects Against Metabolic Disease by Activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Ren, J.M.; Young, L.H.; Pypaert, M.; Mu, J.; Birnbaum, M.J.; Shulman, G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA 2002, 99, 15983–15987. [Google Scholar] [CrossRef]
- Julien, S.G.; Kim, S.Y.; Brunmeir, R.; Sinnakannu, J.R.; Ge, X.; Li, H.; Ma, W.; Yaligar, J.; Kn, B.P.; Velan, S.S.; et al. Narciclasine attenuates diet-induced obesity by promoting oxidative metabolism in skeletal muscle. PLoS Biol. 2017, 15, e1002597. [Google Scholar] [CrossRef]
- Sánchez-Estrada, M.L.; Aguirre-Becerra, H.; Feregrino-Pérez, A.A. Bioactive Compounds and Biological Activity in Edible Insects: A Review. Heliyon 2024, 10, e24045. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and Their Effects on Muscle Atrophy and Muscle Health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef] [PubMed]
- McKnight, J.R.; Satterfield, M.C.; Jobgen, W.S.; Smith, S.B.; Spencer, T.E.; Meininger, C.J.; McNeal, C.J.; Wu, G. Beneficial Effects of L-Arginine on Reducing Obesity: Potential Mechanisms and Important Implications for Human Health. Amino Acids 2010, 39, 349–357. [Google Scholar] [CrossRef]
- Niu, W.; Wang, H.; Wang, B.; Mao, X.; Du, M. Resveratrol Improves Muscle Regeneration in Obese Mice Through Enhancing Mitochondrial Biogenesis. J. Nutr. Biochem. 2021, 98, 108804. [Google Scholar] [CrossRef]
- Ganguly, K.; Dutta, S.D.; Jeong, M.S.; Patel, D.K.; Cho, S.J.; Lim, K.T. Naturally-derived protein extract from Gryllus bimaculatus improves antioxidant properties and promotes osteogenic differentiation of hBMSCs. PLoS ONE 2021, 16, e0249291. [Google Scholar] [CrossRef]



| Parameter | N | H | GHL | GHM | GHH |
|---|---|---|---|---|---|
| TG (mmol/L) | 3.22 ± 0.28 a | 3.79 ± 0.50 a | 3.34 ± 0.25 a | 2.99 ± 0.48 a | 3.49 ± 0.38 a |
| TC (mmol/L) | 2.22 ± 0.58 a | 3.83 ± 0.58 b | 5.24 ± 0.47 b | 5.31 ± 0.37 b | 5.74 ± 0.25 b |
| LDL-C (mmol/L) | 0.97 ± 0.35 a | 2.27 ± 0.35 b | 2.87 ± 0.25 b | 3.04 ± 0.23 b | 3.14 ± 0.21 b |
| HDL-C (mmol/L) | 0.60 ± 0.03 a | 1.23 ± 0.12 b | 1.71 ± 0.17 b | 1.37 ± 0.07 b | 1.71 ± 0.13 b |
| AST (IU/L) | 47.62 ± 3.9 a | 51.94 ± 1.03 a | 57.04 ± 4.54 a | 42.25 ± 2.47 a | 37.63 ± 2.27 a |
| ALT (IU/L) | 5.86 ± 0.76 a | 20.89 ± 4.80 b | 17.54 ± 4.36 ab | 18.87 ± 3.52 b | 15.14 ± 4.53 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Jung, S.; Li, C.; Ha, J.-H.; Jeong, Y. Gryllus bimaculatus Hydrolysate Ameliorates Obesity-Induced Muscle Atrophy by Activating Skeletal Muscle AMPK in Mice. Nutrients 2025, 17, 1990. https://doi.org/10.3390/nu17121990
Park K, Jung S, Li C, Ha J-H, Jeong Y. Gryllus bimaculatus Hydrolysate Ameliorates Obesity-Induced Muscle Atrophy by Activating Skeletal Muscle AMPK in Mice. Nutrients. 2025; 17(12):1990. https://doi.org/10.3390/nu17121990
Chicago/Turabian StylePark, Kyungeun, Sunyoon Jung, Chunmei Li, Jung-Heun Ha, and Yoonhwa Jeong. 2025. "Gryllus bimaculatus Hydrolysate Ameliorates Obesity-Induced Muscle Atrophy by Activating Skeletal Muscle AMPK in Mice" Nutrients 17, no. 12: 1990. https://doi.org/10.3390/nu17121990
APA StylePark, K., Jung, S., Li, C., Ha, J.-H., & Jeong, Y. (2025). Gryllus bimaculatus Hydrolysate Ameliorates Obesity-Induced Muscle Atrophy by Activating Skeletal Muscle AMPK in Mice. Nutrients, 17(12), 1990. https://doi.org/10.3390/nu17121990








