Abstract
Gut microbiota has become an area of increasing interest for its potential role in metabolic dysfunction-associated steatotic liver disease (MASLD) and its more advanced form, metabolic dysfunction-associated steatohepatitis (MASH)—now recognized as the most frequent liver disease worldwide. Research suggests that imbalances in the intestinal microbiota, including dysbiosis and increased intestinal permeability, may contribute to the pathogenesis of MASLD and progression to MASH. These changes affect insulin resistance and trigger inflammatory responses by disrupting the gut–liver axis. This review examined the current evidence connecting gut microbiota to MASLD and MASH, exploring how microbial shifts might influence liver health. Emerging strategies—such as probiotics, prebiotics, and targeted dietary changes—that may help prevent or manage these conditions are also discussed. Finally, key areas where further studies are required to understand the role of microbiota and its therapeutic potential are highlighted.
1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is currently the most common hepatic disease, affecting approximately 30–38% of the adult population [1,2,3]. In 2023, in a Delphi consensus, the term MASLD replaced the previous terminology, non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) [1]. In the new nomenclature, MASLD refers to a condition in which hepatic steatosis co-exists with at least one cardiovascular risk factor (high waist circumference, hypertension, impaired fasting glucose/glucose tolerance, hypertriglyceridemia, decreased levels of HDL cholesterol). The rationale behind the change in nomenclature is the need to abolish the terms “nonalcoholic” and “fatty” due to stigma. The terminology “steatotic liver disease” was approved because it included different etiologies of steatosis. Furthermore, a new category was added: metabolic and alcohol-related/associated liver disease (MetALD), in case of metabolic dysfunction-associated liver disease coexisting with significant alcohol consumption (140–350 g/week and 210–420 g/week for females and males, respectively) [1]. The growth in MASLD incidence and prevalence is related to the worldwide spread of obesity. However, in addition to the connection to obesity, MASLD presents a strong bond with type 2 diabetes (T2D), probably owing to the significant role of insulin resistance in the pathogenesis of both disorders [1].
Histologically, MASLD is characterized by abnormal fat storage in hepatocytes: steatosis is present in more than 5% of hepatocytes without significant ballooning, as other causes of liver disease are absent. MASLD may progress to metabolic-associated steatohepatitis (MASH), characterized by steatosis and inflammation with hepatocyte ballooning, an indicator of liver damage; the exacerbation of the disease includes hepatic fibrosis, cirrhosis, and hepatocellular carcinoma [4].
In March 2024, the Food and Drug Administration approved Resmetirom as the first drug for the treatment of MASH associated with fibrosis. However, since then, no pharmacological therapy for MASLD/MASH has been available. Lifestyle modification and weight loss >10% are the most effective strategies to reverse the disease [5]. Nevertheless, most patients with MASLD/MASH did not achieve such weight reduction [6]. Therefore, further research on other effective treatments is necessary.
Since altered gut microbiota (dysbiosis) plays a critical part in the pathogenesis of MASLD, it has started to gain attention as a possible target therapy for MASLD [7]. The gut–liver axis, a two-way connection involving the intestines, liver, and immune system, is disrupted in MASLD due to an imbalance in microbial composition. Dysbiosis results in intestinal permeability, allowing bacterial endotoxins to enter the portal circulation, provoking hepatic inflammation and insulin resistance. Additionally, altered microbiota can affect bile acid metabolism, short-chain fatty acid (SCFA) synthesis, and choline metabolism, all contributing to hepatic steatosis and fibrosis [8]. Alteration of gut microbiome has been connected not only with MASLD, but also with other metabolic dysfunctions like T2D and obesity [9,10,11]. Hence, modulation of the gut microbiota has been widely investigated as a target for treating the condition. Several therapeutic strategies for enhancing the heterogeneity and abundance of the gut microbiota have been investigated over the years, including probiotics, prebiotics, and tailored diet modifications. Understanding these microbial interactions offers potential therapeutic targets to mitigate MASLD progression, given that hepatocellular carcinoma may occur in a noncirrhotic liver [12].
This narrative review outlines the current evidence about dietary supplements that may modulate the microbiota and subsequently impact MASLD/MASH.
2. Data Sources and Searches
We conducted a literature search of English-language publications indexed in MEDLINE, the Cochrane Library, EMBASE, Web of Science, and PubMed, with coverage up to February 2025. The search strategy was tailored for narrative synthesis and employed combinations of the following keywords: non-alcoholic fatty liver disease, NAFLD, metabolic-associated steatotic liver disease, MASLD, non-alcoholic steatohepatitis, metabolic-associated steatohepatitis, MASH, liver disease, probiotics, prebiotics, microbiota, microbiome, gut, and gut–liver axis. Boolean operators (e.g., AND, OR) were used to combine terms where appropriate. No specific filters were applied for publication type or study design beyond language and relevance. The reference lists of relevant articles were also screened to identify additional sources. Inclusion criteria were (a) original research articles, clinical trials, meta-analyses, and narrative or systematic reviews; (b) studies directly addressing the role of microbiota, probiotics, or prebiotics in MASLD, NAFLD, MASH, or related hepatic conditions; (c) human and relevant preclinical studies. Exclusion criteria included (a) case reports, case series, brief communications, commentaries, editorials, and conference abstracts; (b) non-English language publications; (c) articles not directly relevant to the gut–liver axis or MASLD-related outcomes.
3. Gut Microbiota in MASLD/MASH
The ensemble of the organisms that inhabit the human body is called microbiota, whereas the term microbiome relates to the genomic constituent of microbiota [13]. It is composed of bacteria, fungi, archaea, protists, and viruses [14,15]. These micro-organisms lie not only in the gastrointestinal tract, but also in the epidermis, oral cavity, and respiratory and genitourinary tracts [16].
Gut microbiota is impacted by many aspects, such as early life microbiota composition; host DNA; and psychological, social, and geographic contexts. Diet is one of the major drivers of gut microbiota composition: in a real-world setting, thousands of different nutrients daily interact with trillions of microorganisms, in an intriguing multitude of possible pathways and interventions. In this field, probiotics are live microorganisms that, when dispensed in sufficient quantity, confer a potential benefit on the host [17]; conversely, prebiotics were described as a non-digestible food component that helpfully impacts the host by selectively stimulating the development and/or activity of one or a limited amount of bacteria and thus gives benefits to the host [18].
The intestinal microbiota has a fundamental role in metabolizing carbohydrates, proteins, polyphenols, vitamins, and bile [19,20]. Various processes by which the intestinal microbiota influences the worsening of MASLD/MASH have been described. Indeed, the increment of intestinal permeability, the translocation of dysbiotic microorganisms, and the synthesis of metabolites can be related to modifications in microbiota composition, and it can produce disordered inflammatory reactions that influence liver metabolism [21].
Studies have demonstrated that modifications in the quantity and quality of gut microbiota are connected to the development and progression of MASLD. Intriguingly, every stage of MASLD/MASH has a specific microbiota pattern [10], since the severity of the disease has been attributed to the depletion of commensal bacterial metabolic effects [11,22]. Actually, in MASLD, Bacteroides are reduced and Firmicutes and Proteobacteria are increased at the bacterial phylum grade [10]. Moreover, at the bacterial family grade, Enterobacteriaceae are enhanced, and, on the other hand, Rikenellaceae and Ruminococcaceae are reduced [10]. At the bacterial genera level, Escherichia, Dorea, and Peptoniphilus are demonstrated to be increased; and Anaerosporobacter, Coprococcus, Eubacterium, Faecalibacterium, and Prevotella are reduced [10]. Compared to MASLD, in MASH, the gut microbiome is characterized by a high representation of Bacteroides, whereas fibrosis presents elevated levels of Ruminococcus [11]. All of the above shows that in MASH, if compared with MASLD, gene expression implicated in lipopolysaccharide (LPS) synthesis in gut microbiota was increased [23]; this was recently confirmed by Barchetta et al. in a study in which LPS and its binding protein had been recognized as possible factors in the pathogenesis of MASLD [24]. Moreover, an increase in flagellar biosynthesis gene expression is linked with fibrosis [21]. Bacterial translocation, owing to enhanced gut permeability, and raised blood levels of LPS have been linked to MASLD [25].
The literature has so far demonstrated the critical role of gut microbiota in MASLD [10]. Strong evidence indicated that performing a fecal microbiome transplantation (FMT) on a germ-free murine model derived from a human with MASLD resulted in partial MASLD histological features [26]. Indeed, in 2023, Le Roy et al. highlighted that differences in microbiota composition may influence the answer to a high-fat diet (HFD) in mice [27]; such data confirmed the hypothesis that gut microbiota is implicated in the pathogenesis of MALSD, even independently of obesity [27]. Successively, Soderberg et al. performed FMT on germ-free mice from newborns of lean mothers and with obesity, respectively [28]. The mice with a transplanted fecal microbiome from mothers with obesity gave birth to mice that showed MASLD-like modifications, with periportal inflammation in addition to MASLD [28]. Moreover, experiments with FMTs from individuals with MASLD to germ-free murine models corroborated this pathogenetic thesis. Accordingly, Chiu et al. moved the gut microbiome from MASH subjects to germ-free murine models while feeding the mice with an HFD [29]. The authors showed that the mice increased fat weight, hepatic steatosis, inflammation, multifocal necrosis, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, along with pro-inflammatory cytokines such as endotoxin and interleukin-6 (IL-6) [29]. Conversely, germ-free rats fed with an HFD showed lower lipid storage and liver inflammation. In 2018, Hoyles et al. also reported that microbiome transplantation from MALSD subjects to germ-free murine models led to hepatic steatosis and a MASLD gut microbiota mark [30].
4. Modulators of Gut Microbiota in the MASLD Pathogenesis
One of the most relevant types of prebiotics are indigestible carbohydrates, among them resistant starch, inulin, lignin, pectin, cellulose, fructo-oligosaccharides (FOS), and galacto-oligosaccharides (GOS). These nutrients reach the colon undigested, where they are degraded by carbohydrate-active enzymes (CAZymes), abundantly expressed by the microbiome. Those fibers can be fermented in the colon, producing SCFAs, such as acetate, propionate, formate, butyrate, lactate, and succinate. SCFAs are critical for maintaining enterocyte health and differentiation, mucus secretion, and preventing bacterial translocation [9,31] (see Figure 1).
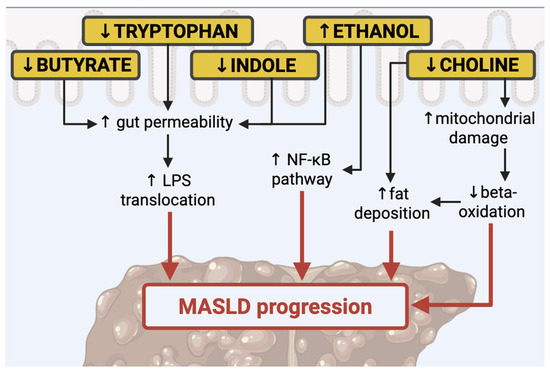
Figure 1.
Principal modulators of gut microbiota in the MASLD. Nuclear factor-κB (NF-κB), lipopolysaccharide (LPS).
4.1. SCFA
SCFAs are the results of the fermentation of nondigestible carbohydrates (NDC) that become available to the gut microbiota. The most important NDCs are acetate, propionate, and butyrate [32,33]. Bacteroides are the principal producers of propionate and acetate, and conversely, Firmicutes of butyrate [32]. Butyrate and propionate are reported to be remedies for bowel inflammation [34]. In rats, acetate and propionate supplementation reduced lipogenesis and fat storage, protecting them from HFD-induced weight gain [35].
Butyrate is an efficient anti-inflammatory mediator [36,37]. Since butyrate decreases the inflammation in the gut, studies have demonstrated that it may discourage the progression of inflammatory processes systemically. Indeed, butyrate can stimulate the activation of the regulatory T cells, which seem to be responsible for the inactivation of T-helper 17 (Th-17) cells and T cells; this results in the extinguishment of pro-inflammatory pathways [36,37]. Rationally, low levels of butyrate are responsible for low-grade inflammation and a less effective anti-inflammatory response. Butyrate is connected to the reduction in inflammatory pathways through different actions. Firstly, through the improvement in tight junction function. This effect is displayed by the induction of mucin production, which preserves the integrity of the intestinal barrier and prevents the translocation of bacteria and their products into the portal circulation. Secondly, through the inhibition of histone deacetylases. Lastly, it serves as an energy provider for colonocytes to maintain gut health [38,39,40].
In MASLD, reduced levels of butyrate may result in increased gut permeability, which is connected to the risk of LPS transfer in the bloodstream. LPS is engaged in the development of MASLD, as Fei et al. demonstrated, showing that endotoxin-producing bacteria were over-presented in people living with obesity [41]. In a paper published in 2020, it has been highlighted that LPS levels in the blood and liver are higher in individuals with MASLD than in healthy subjects [42].
On the other hand, butyrate may stimulate the expression of glucagon-like peptide-1 receptor (GLP-1r) to moderate the severity of MASLD [23]. In a paper, Svegliati-Baroni et al. showed that the expression of the GLP-1r is decreased in the hepatocytes of murine models fed HFD and patients with MASH [43]. Moreover, it was noticed that the activation of GLP-1r in the hepatocytes increased the oxidation of β-fatty acids and ameliorated insulin resistance [43]. Zhou et al. demonstrated that GLP-1 levels are equal in patients with MASLD and subjects without MASLD, but in people living with MASLD, the GLP-1r is low-expressed [23]. Intriguingly, in a rat fed a high-fat diet, supplementation with butyrate is connected to an enhanced expression of GLP-1r, which alleviates liver steatosis [23]. Moreover, in a recent paper, patients with MASLD had higher plasma levels of propionate, formate, valerate, and α-methylbutyrate but reduced acetate concentrations [44]. Consistently, in subjects with MASLD, marked fibrosis was positively connected with propionate, butyrate, valerate, and α-methylbutyrate [44]. On the other hand, in another study, it has been reported that butyrate supplementation prevents the progression from liver steatosis to steatohepatitis due to the protection from the induction of inducible nitric oxide synthase and lipid peroxidation in the liver [45]. In a paper published in Nature, authors showed that acetate is strongly connected with hepatic de novo lipogenesis, in particular, as a result of the microbial metabolism of fructose [46].
While SCFAs—particularly butyrate—play key roles in gut and liver homeostasis, conflicting evidence about their association with both protective and fibrogenic pathways in MASLD underscores the need for further mechanistic clarification.
4.2. Polyphenols
Polyphenols are a varied class of organic substances with prebiotic effects [47]. In nature, there are more than 8000 different compounds that, according to their chemical structure, can be categorized into flavonoids, lignans, phenolic acids, stilbenes, non-phenolic metabolites, and other polyphenols [48,49]. Like indigestible carbohydrates, most dietary polyphenols are not absorbed in the small intestine. When they arrive in the large bowel, they perform health benefits directly, stimulating the growth of advantageous microorganisms and inhibiting the spread of dangerous ones, and indirectly, by their metabolites, such as SCFAs [50]. Among polyphenols, flavonoids, particularly anthocyanins, have been shown to improve metabolic disorders such as MASLD. They increase the abundance of beneficial gut bacteria, including F. prausnitzii, Lactobacillus, and E. rectum, while reducing pathogens such as Desulfovibrio and Enterococcus [50,51]. Other polyphenols with a specific effect on the improvement of MASLD are stilbenes: 2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside has been shown to improve liver mitochondrial dysfunction and lipid accumulation [52]. Another group of pigmented biologically active phytochemicals is betacyanins. They are studied for their ability to display beneficial actions in animal models of MASLD through modulation of the intestinal microbiota, reducing the abundance of Firmicutes and increasing that of Bacteroides at the phylum level. Furthermore, betacyanins also dramatically increase the amount of Akkermansia at the genus level [53].
4.3. Bile Acids
Bile acids (BAs) are molecules synthesized in the liver from cholesterol and stored in the gallbladder. Besides helping the absorption of lipids, bile acids are also crucial in the metabolism of glucose. The intestinal microbiota transforms primary bile acids, cholic acid (CA), and chenodeoxycholic acid (CDCA) into secondary bile acids like deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA) [54,55]; this process occurs in the distal small intestine and large bowel. BA is indirectly engaged in antimicrobial defenses operated by the farnesoid X receptor (FXR). The activation of FXR decreases fatty acid and triglyceride production in the liver by reducing the expression of the liver X receptor (LXR) and Sterol Regulatory Element Binding Protein 1C (SREBP-1C) [56]. Accordingly, FXR-deficient mice show decreased insulin sensitivity and reduced glucose tolerance [57]. On the other hand, FXR activation by specific agonists suppresses bile acid and fatty acid synthesis and increases glucose and insulin sensitivity in murine models with obesity and diabetes. Furthermore, FXR stimulation seems to reduce primary biliary cirrhosis and MASH by diminishing the bile acid pool and liver fibrosis [58,59]. In a meta-analysis including 19 studies and 154,807 individuals, it emerged that total BA levels in patients with MASLD were more elevated than those in individuals without MASLD; in particular, UDCA, taurococholic acid (TCA), CDCA, taurochenodeoxycholic acid, and glycocholic acids were elevated. Remarkably, TCA, taurodeoxycholic acid, taurolithocholic acids, and glycolithocholic acids demonstrated a possible ability to discriminate MASH [60].
4.4. Tryptophan
Tryptophan is an essential amino acid, which means that it needs to be integrated with the diet to be available for the organism; this aromatic amino acid disposes of various pathways to be metabolized. When tryptophan is decomposed by tryptophan hydroxylase 1 and 2 (Thp1/Thp2), serotonin (5-HT) becomes available; moreover, in the indole pathway, tryptophan is transformed into indole, plus other metabolites [61].
Existing data have shown that reduced levels of tryptophan are connected to alteration of the intestinal barrier integrity [62,63]; consequently, it has been demonstrated that supplementation with tryptophan increases intestinal barrier integrity [64]. The positive effect of this amino acid on gut health leads to improvement in liver steatosis. Consequently, MASLD is characterized by poor levels of tryptophan, since in this condition, its metabolism is impaired [65]. In a paper by Ritze et al., the authors found that tryptophan supplementation improved liver steatosis induced by a fructose-based diet in rats [65].
Moreover, the indole metabolite is crucial in preserving intestinal wall integrity: in selected mice, depletion of indole was associated with impaired intestinal integrity [64]. Accordingly, supplementation with indole metabolite determines an increase in the expression of molecules implicated in the assembly of tight junctions. Furthermore, another study highlighted that indole levels in the bloodstream were inversely associated with obesity and liver steatosis [66]. In line, Ji et al. reported that indole-3-acetic, derived from indole, reduces lipogenesis and inflammation in the liver of mice nourished with HFD [67].
Finally, serotonin has also been connected with MASLD since high levels of serotonin are accountable for inhibiting the energy expenditure of brown adipose tissue [68]; this evidence is confirmed by Arto et al., who demonstrated in a cohort of 76 patients that alteration in tryptophan catabolism is related to MASLD, highlighting the potential utility of targeting these pathways in therapeutic approaches [69].
4.5. Choline and Trimethylamine
Choline is a fundamental component of cellular and mitochondrial membranes and serves as a precursor of acetylcholine, a key neurotransmitter. Among its derivatives, phosphatidylcholine plays a central role in the formation of very low-density lipoprotein (VLDL) from triglycerides and in the solubilization of bile acids for their elimination [70,71]. Reduced choline levels alter mitochondrial membrane composition, compromising their function and contributing to impaired adenosine triphosphate (ATP) synthesis and reduced beta-oxidation. This metabolic dysfunction promotes the progression of hepatic steatosis [72,73]. Accordingly, diets deficient in choline are commonly used in animal models to investigate the pathogenesis and progression of MASLD, as they replicate features observed in humans, such as hepatic triglyceride accumulation [74]. For instance, Arao et al. demonstrated that a choline/methionine-deficient diet in a murine model induced MASH and was associated with reduced mitochondrial DNA content [75]. Choline availability is also influenced by the gut microbiota. Choline is metabolized by intestinal microbes, which can reduce its systemic bioavailability and increase the risk of deficiency [76]. Furthermore, the microbiota facilitates the conversion of choline into trimethylamine (TMA), which is absorbed and converted by the liver into trimethylamine N-oxide (TMAO [77]. Excessive TMAO production contributes to further reductions in circulating choline levels, interfering with hepatic VLDL export and bile acid synthesis. These alterations may worsen hepatic fat accumulation, oxidative and inflammatory damage, and disrupt glucose metabolism [78,79]. Consistent with these mechanistic insights, a recent randomized controlled trial by Perva et al. showed that nutritional supplementation containing choline, along with other compounds (5-MTHF, betaine, alpha-linolenic acid, eicosapentaenoic acid, choline bitartrate, docosahexaenoic acid, and vitamin B12), had beneficial effects on liver fibrosis and steatosis parameters in individuals with metabolic syndrome and obesity [80]. However, the role of TMAO in MASLD remains controversial. In a recent study, TMAO was associated with hepatic lipid accumulation and accelerated disease progression in murine models [81]. Additionally, TMAO has been shown to impair intestinal barrier integrity at multiple levels, promoting liver endothelial dysfunction, capillarization of liver sinusoidal endothelial cells (LSECs), and alterations in macrophage polarization [81]. A systematic review and meta-analysis published in 2022 further supported TMAO’s involvement in hepatic triglyceride accumulation, impaired cholesterol transport, disrupted glucose and energy homeostasis, and alterations in bile acid metabolism [82].
Nonetheless, despite growing preclinical evidence, further research is required to clarify the causative role of TMAO in human MASLD and to determine whether it represents a therapeutic target or a secondary marker of disease.
4.6. Ethanol
Ethanol is a derivative of saccharolytic fermentation. In a paper published in 2000, researchers suggested a relationship between ethanol blood concentrations and changes in intestinal microbiota [83]. Additionally, research indicates that dysbiosis in patients with MASH is linked to ethanol-producing microorganisms, including Escherichia coli, Bacteroides, Bifidobacterium, and Clostridium [84,85]. In particular, one study pointed out an increase in ethanol levels in subjects with MASH compared to fit individuals or patients living with obesity but without MASH [84]. Ethanol produced by gut bacteria, along with its oxidized compound, acetaldehyde, may contribute to the worsening of MASLD This occurs through direct toxic action on liver cells, impairment of the intestinal barrier, elevated portal endotoxemia, and activation of the nuclear factor-κB (NF-κB) pathway, which heightens inflammatory pathways in peripheral areas’ cells [86,87].
5. Drugs and Microbiota Modulation
5.1. Metformin
Metformin is the first-line glucose-lowering therapy in type 2 diabetes mellitus (DM2), although its mechanism of action remains complex and not fully understood [88]. Recent studies have shown that metformin treatment is associated with notable changes in gut microbiota composition, including an increase in the abundance of Escherichia coli and a reduction in Intestinibacter [89,90]. Additionally, metformin influences the abundance of bacteria involved in the production of short-chain fatty acids (SCFAs), particularly enhancing the presence of Akkermansia muciniphila [89,90]. Akkermansia muciniphila plays a key role in maintaining intestinal barrier integrity and promoting SCFA production. These effects, in turn, exert beneficial actions on adipose tissue, skeletal muscle, and the liver by improving insulin sensitivity [91].
Supporting the relevance of microbiota in mediating the effects of metformin, a study demonstrated that transplantation of gut microbiota from metformin-treated individuals into germ-free mice led to improved blood glucose levels. This finding suggests that the glycemic benefits of metformin may be partially mediated through alterations in gut microbial composition [90].
Consistently, evidence from three meta-analyses indicates that metformin exerts beneficial effects on MASLD, including reductions in AST and ALT levels and decreased liver fat content [92,93,94]. However, these findings are not universally confirmed. Other studies, including a meta-analysis, reported neutral effects of metformin on hepatic fat accumulation and transaminase levels [95,96,97,98,99]. These discrepancies may be attributed to heterogeneity in study design, treatment duration, or patient populations.
Overall, while the interaction between metformin and the gut microbiota appears to contribute to its metabolic effects, further research is needed to clarify the extent and consistency of its potential in the management of MASLD.
5.2. Rifaximin
Rifaximin is an oral, non-systemic antibiotic that exerts its action locally in the gut without entering the bloodstream (it is not absorbed). It displays broad-spectrum antimicrobial activity against both aerobic and anaerobic and Gram-positive and Gram-negative bacteria, many of which are involved in the production of LPS in the intestinal lumen [100,101]. Rifaximin is widely used in clinical practice for the management of hepatic encephalopathy, traveler’s diarrhea, and irritable bowel syndrome [102,103,104]. Beyond its established antimicrobial applications, recent studies have explored its potential role in liver-related diseases, including MASLD and MASH. In a recently published RCT, the combination of rifaximin with a probiotic and prebiotic—administered alongside metformin—in patients with MASLD and T2D led to the resolution of subclinical inflammation and improved intestinal barrier integrity [105]. Notably, the intervention group also showed reductions in AST and ALT levels, as well as an improvement in the degree of liver steatosis [105]. Supporting these findings, a previous study by Gangarapu and colleagues reported that short-term rifaximin treatment in patients with MASH resulted in decreased LPS production, which was associated with lower levels of transaminases, ferritin, and LDL cholesterol [102]. However, these beneficial effects were not observed in patients with MASLD in the same study, suggesting that the therapeutic response to rifaximin may differ depending on disease stage or phenotype [102].
In another RCT, rifaximin therapy led to reductions in endotoxin levels, cytokeratin-18, transaminases, insulin resistance, and MASLD-liver fat score, with overall improvement in MASH parameters [106].
Taken together, the available evidence suggests that rifaximin may have a beneficial impact on MASH by targeting the inflammatory component of the disease, although further studies are needed to clarify its role across the MASLD spectrum.
6. Diet and Gut Microbiota Modulation
6.1. Fibers
Different studies have evaluated the impact of specific fibers on the intestinal microbiota: fermentable dietary fibers, such as inulin, oligofructose, FOS, or GOS, have a beneficial action on microbiota composition and derived metabolites, with individual differences in the expected increase of SCFAs production [107].
RCTs have shown that fiber-rich whole grains significantly reduce lipopolysaccharide binding protein (LBP) and inflammation, improving gut barrier function and microbiota diversity [31]. On the contrary, low-fiber diets increase mucus-degrading microorganisms (Akkermansia muciniphila and Bacteroides caccae) and decrease fiber-degrading species (Bacteroides ovatus and Eubacterium rectale), reducing SCFA production [108]. Since the mucus layer in the colon acts as a primary defense against pathogens, the weakening of this barrier has a significant impact on susceptibility to gastrointestinal infections [109].
Within different fermentable nutrients, resistant starch (RS) reaches the large intestine in far more significant amounts than other substrates, including non-starch polysaccharides, oligosaccharides, unabsorbed sugars, and dietary protein. This considerable quantity accessible for fermentation by intestinal microbiota leads to a corresponding production of SCFAs [110] and to benefits related to their production. RS is a prebiotic fiber that can be categorized into five categories. RS1 is physically unreachable because it is confined within cell walls or in whole or partially milled grains seeds; RS2 is finely textured native starch characterized by high crystallinity structure; RS3 is subjected to retrograde metamorphism or recrystallization starch; RS4 is starch that has been chemically modified or mixed with non-starch components bonds; and RS5 serves as a starch-lipid complex primarily associated with amylose–lipid complexes [111,112].
Concerning liver metabolism, RS supplementation (40 g/die) led to a 5.89% reduction in hepatic lipid content in a 4-month randomized placebo-controlled clinical trial in subjects with MASLD [113]. Moreover, RS reduced the presence of Bacteroides stercoris, which correlates with MASLD progression and improved liver inflammation and steatosis [113]. Omnivores may experience gut microbial signatures with other diet patterns, as long as they include a comparable variety of fiber-rich plant-based foods in their diets [114].
6.2. Lipids
Dietary lipids quality and quantity have beneficial or detrimental health effects, some of which are mediated by induced changes in the gut microbiome. An HFD induces an overrepresentation of LPS-expressing bacteria, increasing the risk of rising LPS circulating levels [115,116]. This condition, called “metabolic endotoxemia”, is associated with enhanced intestinal permeability, probably as a result of reduced tight junction protein expression. These negative effects are mainly caused by the overconsumption of saturated fats, while the consumption of unsaturated fats appears to be protective [117]. A diet rich in unsaturated fats produces an increase in the presence of beneficial taxa such as Akkermansia and Bifidobacterium, and a decrease in critical microorganisms such as Streptococcus and Escherichia [118]. Saturated fatty-acid-rich foods, for example, butter or lard, have been reported to augment the presence of Lachnospiraceae Blautia, Roseburia, Lachnospira, Agathobacter, Fusicatenibacter, Lachnoclostridium, Bacteroides, Turicibacter, and Bilophila spp., which promote inflammation [117,119]. An adequate intake of omega-3 polyunsaturated fatty acids (PUFAs) is connected with increased quantities of Bifidobacterium and Lactobacillus, as well as a remarkable increase in several SCFA (butyrate)-producing genera, including Blautia, Bacteroides, Roseburia, and Coprococcus [120]. This evidence was also demonstrated in a randomized intervention trial, where an omega-3 fatty acid supplementation exhibited a potential prebiotic effect, modifying the gut microbiome composition, leading to substantial increases in iso-butyrate and isovalerate [121]. Finally, omega-3 PUFA supplementation is related to a reversible enhancement in various SCFA-producing bacteria, no matter the route of administration. However, a connection between the gut microbiome and systemic omega-3 PUFA exposure has not yet been demonstrated [120].
6.3. Proteins
Only a few studies examined the impact of overall dietary protein intake on the intestinal microbiota in humans living with overweight or obesity. It is noteworthy that consumption of a high-protein diet (25 percent of energy intake) from various sources (meat, fish, dairy and plant) showed no effect on alpha or beta diversity, but when corrected for fat intake, a change in gut microbiota could be reported, largely due to the amount of protein consumed [122]. Specific amino acids may influence the gut microbiota composition, either directly or through their metabolites. L-carnitine is metabolized by the intestinal microbiota to trimethylamine (TMA), which in turn is transported from the portal circulation to the liver and converted to trimethylamine N-oxide (TMAO); the latter is a metabolite correlated with atherosclerotic cardiovascular disease and associated with the risk of MASLD [123]. In rats and humans, specific microorganisms of the gut microbiota have been linked with the potential to convert L-carnitine to TMA, with a common association with Prevotella [31].
6.4. Food Additives
The food industry has been increasingly utilizing food additives to enhance food and beverages’ preservation, freshness, taste, texture, or appearance. Studies in mice have illustrated that common dietary emulsifiers could modify intestinal microbiota diversity and cause gut microbiota dysbiosis. These modifications lead to bacterial incursion into the inner mucus layer and enhance inflammation, raising circulating LPS [124,125]. Low doses of two common emulsifiers, carboxymethyl cellulose and polysorbate-80, when tested in cultures with human intestinal microbiota, induced elevated levels of bioactive flagellin, likely due to dysbiosis or changes in bacterial gene expression [124]. A RCT aimed to explore these effects in humans through a short-term dietary intervention revealed that dietary emulsifiers reduced microbiota diversity and SCFA synthesis [126]. Non-caloric artificial sweeteners (NAS) are another extensively (https://synonyms.reverso.net/sinonimi/en/extensively, accessed on 27 January 2025) consumed group of food additives; some studies have linked NAS consumption to dysbiosis, but other data show heterogeneous results, and more research on humans is needed [127,128]. Besides artificial sweeteners, food additives such as emulsifiers, preservatives, flavor enhancers, and dyes can compromise the integrity of the intestinal wall and alter the structure of gut microbiota, which may trigger inflammation. These processes can result in oxidative stress that the body may not adequately counter, increasing the risk of impaired lipid metabolism in the liver [129]
Ultra-processed foods (UPFs) are industrially made and are primarily characterized by high levels of added sugars, saturated fats, salt, and additives, while lacking essential nutrients and fiber [130]. Because of their high calorie density and poor nutrient quality, UPFs lead to a higher overall caloric consumption, resulting in weight gain and obesity [131]. Furthermore, due to their relationship with insulin resistance, UPFs are central in the pathogenesis of MASLD. They typically have high fructose levels, metabolized in the liver, and are potentially responsible for increased fat storage [132]. The harmful impact of fructose contained in UPFs is intensified by ingredients like saturated fats, food additives, and the lack of fiber and vital nutrients, all of which promote de novo lipogenesis and worsen metabolic dysfunction [133]. Additionally, UPFs can trigger oxidative stress and inflammation, crucial steps in advancing MASLD to more severe forms such as steatohepatitis [134]. Moreover, the low nutritional quality of UPFs—characterized by a lack of fiber and antioxidants and an excess of additives—can disturb the gut microbiome, resulting in adverse health consequences [135].
The overall composition of a diet interacts with gut microbiota, affecting both the types and quantities of bacteria, as well as their growth and interactions with enterocytes. Therefore, employing specially structured diets could be an effective intervention for MASLD.
7. Diet Manipulation for Enriching Gut Microbiota in MASLD
Several foods can deliver different pre- and probiotics to the gastrointestinal tract, and the overall diet may have an effect on the composition of the microbiota. Hence, a growing body of evidence shows that dietary modulation can have a therapeutic effect on MASLD.
7.1. Probiotics
In a RCT published in 2017, Manzhalii et al. illustrated that in a cohort of subjects with MASLD, the group that received not only the prescription of a low-calorie/low-fat diet but also a mixture of probiotics (L. casei, L. rhamnosus, L. bulgaris, B. longum, and S. thermophilus) experienced an improvement in liver inflammation [136]. This evidence was anticipated in 2012 by Malaguarnera et al., [112] who recruited for a RCT of 66 patients with biopsy-proven MASH at baseline. After 24 weeks, the intervention group treated with Bifidobacterium Longum plus FOS plus lifestyle modifications experienced a reduction in TNF-α, CRP, AST levels, homeostatic model assessment for insulin resistance (HOMA-IR), serum endotoxin, steatosis, and the MASH activity index [137]. Furthermore, in a cohort of subjects with MASLD, Sepideh et al. [113] observed that supplementation with probiotics (Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, and Streptococcus thermophilus) for 8 weeks improved insulin resistance, TNF-α, and IL-6 decreased significantly compared to baseline [138] Adversely, another RCT evaluated the impact of the co-administration of probiotics and prebiotics (FOS plus Bifidobacterium animalis subspecies lactis BB-12) vs. placebo in patients with MASLD; data showed that after one year of supplementation with a symbiotic cocktail (probiotic and prebiotic), the fecal microbiome varied but did not ameliorate liver steatosis or markers of fibrosis [139].
7.2. Sulforaphane
In a RCT with 140 participants with MASLD enrolled, the intervention group, which took six broccoli seed tablets rich in sulforaphane—an isothiocyanate present in cruciferous—after 12 weeks of supplementation, showed an increase in GLP-1. On the other side, levels of glucose, insulin, and HOMA-IR were reduced [140].
7.3. Polyphenol
In a RCT, Agrinier et al. showed that a polyphenol-rich extract from the Amazonian berry camu-camu may reduce liver steatosis in adults with overweight, after 12 weeks of treatment [51]. Moreover, in a pilot paper, authors hypothesized that onion polyphenols could potentially be utilized to compose dietary supplements as potential multi-target-directed ligands in MASLD [141]. Finally, a scoping review recently published—aiming to organize the present data on bioactive-substance-based supplementation for individuals with MASLD—showed that curcumin, silymarin, resveratrol, coffee, green tea, and berberine were the most studied bioactive substances [142]. However, data are abundant just for curcumin and silymarin [142].
7.4. Inulin
Back in 2019, researchers found that inulin-propionate ester, projected to selectively carry propionate to the colon, inulin, and cellulose were effective in improving insulin sensitivity for twelve adults with overweight or obesity but without diabetes, after 42 days of supplementation [143]. In a small RCT, Reshef et al. [144]. evaluated 8 individuals with MASLD and metabolic syndrome who received inulin-type fructans compared with 11 who received a placebo. There were no significant changes in liver steatosis, liver function markers, and inflammatory panel (fibroblast growth factor-19 and LPS) from baseline to the end of the treatment in both the prebiotic and placebo groups [144].
In 2020, Lin Chong demonstrated in a RCT that a short-term treatment with metronidazole, followed by a probiotic inulin supplementation, may reduce ALT levels in patients with MASLD more effectively than a short-term very low-calorie diet (VLCD) [145]. The authors hypothesized that the positive metabolic effects of short-term VLCD in adults with MASLD could be accentuated by metronidazole to treat dysbiotic gut microbiota, followed by a period of inulin supplementation as a maintenance [145].
7.5. Diet
As expected, in a RCT published in 2022, the authors pointed out that a low glycemic index Mediterranean diet plus physical activity determines improvement in liver steatosis measured through controlled attenuation parameter (CAP, FibroscanTM, Echosens, Paris, France), an increase in the Firmicutes phylum and Ruminococcaceae, bacteria linked to liver protection [146]. On the other side, Jian et al. [122] evaluated the impact of an excess of 1000 kcal/day of diets rich in either saturated fat, unsaturated fat, or simple sugars for 3 weeks; the researchers reported that high intake of saturated fat enhanced Proteobacteria concentrations and sugar overfeeding increased Lactococcus and E. coli. On the other hand, unsaturated fat increased butyrate producers. In this study, the carriage of Bilophila was recognized as a possible risk factor for diet-determined hepatic steatosis in humans [147]. Moreover, in a RCT that enrolled 34 subjects with MASLD, it was shown that a freshwater fish-based diet may be beneficial to MASLD by regulating intestinal microbiota and its metabolites [148]; indeed, data highlighted that Faecalibacterium enhanced under the freshwater fish-based diet was positively associated with the levels of propionic acid, butyric acid, valeric acid, but negatively with acetic acid, isobutyric acid, isovaleric acid, and hexanoic acid levels [148]. Finally, Chen et al. reported that daily intake of yogurt was superior to milk in improving insulin resistance and liver fat in Chinese women living with obesity, MASLD, and metabolic syndrome [149]. Probiotic yogurt and its consumption are inversely associated with the prevalence of MASLD [150]. In a RCT that investigated the effects of symbiotic yogurt, with prebiotics (inulin) and probiotics (Bifidobacterium animalis), a significant reduction in hepatic steatosis and liver enzymes was observed in the intervention group [151]. Another double-blind RCT investigated the effects of probiotic yogurt consumption in 72 patients affected by MASLD. The study showed a reduction of 4.67 and 5.42% in concentrations of ALT and AST, respectively, compared with the control group [152]. A cross-sectional study, conducted among adults in the 2011–2016 National Health and Nutrition Examination Survey, linked the intake of probiotic yogurt with a beneficial action on liver steatosis [53] (see Figure 2).
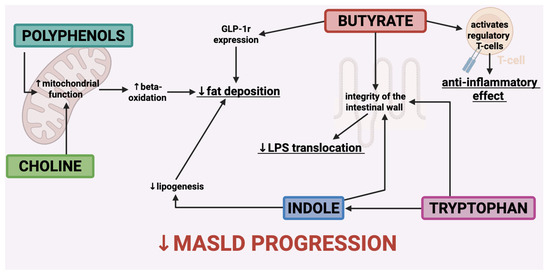
Figure 2.
The primary metabolites that contribute to the alteration of the microbiota exert a positive influence on the natural progression of MASLD. Receptor of Glucagon-Like Peptide—1 (GLP-1 r); lipopolysaccharide (LPS).
8. Conclusions
This narrative review examines the impact of microbiota on the pathogenesis of MASLD and the potential for microbiota manipulation to enhance metabolic liver disease management. Dysbiosis is highlighted as a significant factor contributing to enhanced intestinal permeability and systemic inflammation, which is why research has focused on various therapeutic strategies, such as the administration of probiotics, prebiotics, symbiotics, and specific dietary patterns. Promising results from preclinical studies indicate that targeted supplementation could impact the progression of MASLD by restoring microbial balance. However, further standardized studies involving humans are essential to clarify the microbiota’s effective role as a therapeutic target for metabolic liver disease (see Table 1).

Table 1.
Most relevant studies concerning microbiota manipulation through diet in humans. Randomized controlled trial (RCT); metabolic-associated steatohepatitis (MASH); body mass index (BMI); metabolic-associated steatotic liver disease (MASLD); alanine transaminase (ALT); aspartate transaminase (AST); fructo-oligosaccharides (FOS); low-density lipoprotein (LDL); C-reactive protein (CRP); homeostatic model for insulin-resistance (HOMA-IR); tumor necrosis factor α (TNF)-α; interleukin-6 (IL-6); lipopolysaccharides (LPS); glucagon-like peptide 1 (GLP-1); inulin-type fructans (ITFs); very-low-calorie diet (VLCD); controlled attenuation parameter (CAP); gamma glutamyl transferase (GGT); insulin resistance (IR).
Author Contributions
Conceptualization: F.P. (Federica Perazza), M.L.P., F.P. (Fabio Piscaglia), F.R. and M.S.; methodology: F.R.; resources, F.P. (Federica Perazza), L.L., B.S., F.G., A.B. (Alessia Bonaiumi), A.B. (Alice Beretta) and S.F.; writing-original draft preparation: F.P. (Federica Perazza), L.L., B.S., F.G., A.B. (Alessia Bonaiumi), A.B. (Alice Beretta) and S.F.; writing—review and editing: F.P. (Federica Perazza), L.L., B.S., F.G., A.B. (Alessia Bonaiumi), A.B. (Alice Beretta), S.F., M.L.P., F.P. (Fabio Piscaglia), F.R. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Alessia Bonalumi, Alice Beretta were employed by the company HandyDiet SRL. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest concerning this article.
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Ekstedt, M.; Wong, G.L.-H.; Hagström, H. Changing Epidemiology, Global Trends and Implications for Outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-Alcoholic Fatty Liver Disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Chitturi, S.; Wong, G.L.-H.; Yu, J.; Chan, H.L.-Y.; Farrell, G.C. Pathogenesis and Novel Treatment Options for Non-Alcoholic Steatohepatitis. Lancet Gastroenterol. Hepatol. 2016, 1, 56–67. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef]
- Do, A.; Zahrawi, F.; Mehal, W.Z. Therapeutic Landscape of Metabolic Dysfunction-Associated Steatohepatitis (MASH). Nat. Rev. Drug Discov. 2025, 24, 171–189. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Xie, R.; Dai, C.-S.; Gao, H.-W.; Zhou, G.; Qi, T.-T.; Wang, W.-Y.; Wang, H.; Cui, Y.-M. Thyroid Hormone Receptor-Beta Agonist HSK31679 Alleviates MASLD by Modulating Gut Microbial Sphingolipids. J. Hepatol. 2025, 82, 189–202. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. Bile Acids and Butyrate in the Effects of Probiotics/Synbiotics on Nonalcoholic Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1475–1476. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut Microbiota and Human NAFLD: Disentangling Microbial Signatures from Metabolic Disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The Severity of Nonalcoholic Fatty Liver Disease Is Associated with Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatology 2016, 63, 764. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Wong, V.W.-S.; Zhang, X.; Yu, J. Interplay between Gut Microbiome, Host Genetic and Epigenetic Modifications in MASLD and MASLD-Related Hepatocellular Carcinoma. Gut 2024, 74, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef]
- Matijašić, M.; Meštrović, T.; Čipčić Paljetak, H.; Perić, M.; Barešić, A.; Verbanac, D. Gut Microbiota beyond Bacteria—Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The Role of the Microbiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, E.T.; Palacios, T.; Thomsen, M.; Vitetta, L. Intestinal Microbiome Shifts, Dysbiosis, Inflammation, and Non-Alcoholic Fatty Liver Disease. Front. Microbiol. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Del Ben, M.; Pastori, D.; Carnevale, R.; Baratta, F.; Overi, D.; Francis, H.; Cardinale, V.; Onori, P.; Safarikia, S.; et al. Increased Liver Localization of Lipopolysaccharides in Human and Experimental NAFLD. Hepatology 2020, 72, 470. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Sentinelli, F.; Chiappetta, C.; Di Cristofano, C.; Silecchia, G.; Leonetti, F.; Baroni, M.G.; Cavallo, M.G. Reduced Lipopolysaccharide-Binding Protein (LBP) Levels Are Associated with Non-Alcoholic Fatty Liver Disease (NAFLD) and Adipose Inflammation in Human Obesity. Int. J. Mol. Sci. 2023, 24, 17174. [Google Scholar] [CrossRef]
- Nseir, W.; Artul, S.; Nasrallah, N.; Mahamid, M. The Association between Primary Bacteremia of Presumed Gastrointestinal Origin and Nonalcoholic Fatty Liver Disease. Dig. Liver Dis. 2016, 48, 343–344. [Google Scholar] [CrossRef]
- Burz, S.D.; Monnoye, M.; Philippe, C.; Farin, W.; Ratziu, V.; Strozzi, F.; Paillarse, J.-M.; Chêne, L.; Blottière, H.M.; Gérard, P. Fecal Microbiota Transplant from Human to Mice Gives Insights into the Role of the Gut Microbiota in Non-Alcoholic Fatty Liver Disease (NAFLD). Microorganisms 2021, 9, 199. [Google Scholar] [CrossRef]
- Roy, T.L.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal Microbiota Determines Development of Non-Alcoholic Fatty Liver Disease in Mice. Gut 2013, 62, 1787–1794. [Google Scholar] [CrossRef]
- Soderborg, T.K.; Clark, S.E.; Mulligan, C.E.; Janssen, R.C.; Babcock, L.; Ir, D.; Young, B.; Krebs, N.; Lemas, D.J.; Johnson, L.K.; et al. The Gut Microbiota in Infants of Obese Mothers Increases Inflammation and Susceptibility to NAFLD. Nat. Commun. 2018, 9, 4462. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Ching, Y.-H.; Li, Y.-P.; Liu, J.-Y.; Huang, Y.-T.; Huang, Y.-W.; Yang, S.-S.; Huang, W.-C.; Chuang, H.-L. Nonalcoholic Fatty Liver Disease Is Exacerbated in High-Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis. Nutrients 2017, 9, 1220. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernández-Real, J.-M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular Phenomics and Metagenomics of Hepatic Steatosis in Non-Diabetic Obese Women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Vieira, A.T.; Vinolo, M.A.R.; Oliveira, F.A.; Curi, R.; Martins, F.d.S. The Central Role of the Gut Microbiota in Chronic Inflammatory Diseases. J. Immunol. Res. 2014, 2014, 689492. [Google Scholar] [CrossRef]
- Weitkunat, K.; Stuhlmann, C.; Postel, A.; Rumberger, S.; Fankhänel, M.; Woting, A.; Petzke, K.J.; Gohlke, S.; Schulz, T.J.; Blaut, M.; et al. Short-Chain Fatty Acids and Inulin, but Not Guar Gum, Prevent Diet-Induced Obesity and Insulin Resistance through Differential Mechanisms in Mice. Sci. Rep. 2017, 7, 6109. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. Inflammation-Modulating Effect of Butyrate in the Prevention of Colon Cancer by Dietary Fiber. Clin. Color. Cancer 2018, 17, e541–e544. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.-N.; Vitetta, L. Effects of Intestinal Microbial–Elaborated Butyrate on Oncogenic Signaling Pathways. Nutrients 2019, 11, 1026. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Fei, N.; Bruneau, A.; Zhang, X.; Wang, R.; Wang, J.; Rabot, S.; Gérard, P.; Zhao, L. Endotoxin Producers Overgrowing in Human Gut Microbiota as the Causative Agents for Nonalcoholic Fatty Liver Disease. mBio 2020, 11, e03263-19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, Y.-W.; Zhao, Z.-H.; Yang, R.-X.; Xin, F.-Z.; Liu, X.-L.; Pan, Q.; Zhou, H.; Fan, J.-G. Sodium Butyrate Reduces High-Fat Diet-Induced Non-Alcoholic Steatohepatitis through Upregulation of Hepatic GLP-1R Expression. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Svegliati-Baroni, G.; Saccomanno, S.; Rychlicki, C.; Agostinelli, L.; De Minicis, S.; Candelaresi, C.; Faraci, G.; Pacetti, D.; Vivarelli, M.; Nicolini, D.; et al. Glucagon-like Peptide-1 Receptor Activation Stimulates Hepatic Lipid Oxidation and Restores Hepatic Signalling Alteration Induced by a High-Fat Diet in Nonalcoholic Steatohepatitis. Liver Int. 2011, 31, 1285–1297. [Google Scholar] [CrossRef]
- Thing, M.; Werge, M.P.; Kimer, N.; Hetland, L.E.; Rashu, E.B.; Nabilou, P.; Junker, A.E.; Galsgaard, E.D.; Bendtsen, F.; Laupsa-Borge, J.; et al. Targeted Metabolomics Reveals Plasma Short-Chain Fatty Acids Are Associated with Metabolic Dysfunction-Associated Steatotic Liver Disease. BMC Gastroenterol. 2024, 24, 43. [Google Scholar] [CrossRef]
- Baumann, A.; Jin, C.J.; Brandt, A.; Sellmann, C.; Nier, A.; Burkard, M.; Venturelli, S.; Bergheim, I. Oral Supplementation of Sodium Butyrate Attenuates the Progression of Non-Alcoholic Steatohepatitis. Nutrients 2020, 12, 951. [Google Scholar] [CrossRef]
- Zhao, S.; Jang, C.; Liu, J.; Uehara, K.; Gilbert, M.; Izzo, L.; Zeng, X.; Trefely, S.; Fernandez, S.; Carrer, A.; et al. Dietary Fructose Feeds Hepatic Lipogenesis via Microbiota-Derived Acetate. Nature 2020, 579, 586–591. [Google Scholar] [CrossRef]
- Xue, H.; Gao, Y.; Shi, Z.; Gao, H.; Xie, K.; Tan, J. Interactions between Polyphenols and Polysaccharides/Proteins: Mechanisms, Effect Factors, and Physicochemical and Functional Properties: A Review. Int. J. Biol. Macromol. 2025, 142793. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A Major Update of the Phenol-Explorer Database to Incorporate Data on the Effects of Food Processing on Polyphenol Content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Li, H.; Liang, J.; Han, M.; Gao, Z. Polyphenols Synergistic Drugs to Ameliorate Non-Alcoholic Fatty Liver Disease via Signal Pathway and Gut Microbiota: A Review. J. Adv. Res. 2025, 68, 43–62. [Google Scholar] [CrossRef]
- Agrinier, A.-L.; Morissette, A.; Daoust, L.; Gignac, T.; Marois, J.; Varin, T.V.; Pilon, G.; Larose, É.; Gagnon, C.; Desjardins, Y.; et al. Camu-Camu Decreases Hepatic Steatosis and Liver Injury Markers in Overweight, Hypertriglyceridemic Individuals: A Randomized Crossover Trial. Cell Rep. Med. 2024, 5, 101682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, Z.; Shi, L.; Yan, S.; Huang, Z.; Lu, B.; Wang, Z.; Ji, L. 2,3,5,4’-Tetrahydroxy-Stilbene-2-O-β-D-Glucoside Ameliorates NAFLD via Attenuating Hepatic Steatosis through Inhibiting Mitochondrial Dysfunction Dependent on SIRT5. Phytomedicine 2022, 99, 153994. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Guo, W.; Wang, J.; Liu, S.; Li, Z.; Li, Y. Probiotic Consumption and Hepatic Steatosis: Results from the NHANES 2011–2016 and Mendelian Randomization Study. Front. Nutr. 2024, 11. [Google Scholar] [CrossRef]
- Gérard, P. Metabolism of Cholesterol and Bile Acids by the Gut Microbiota. Pathogens 2013, 3, 14–24. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Yang, Z.-X.; Shen, W.; Sun, H. Effects of Nuclear Receptor FXR on the Regulation of Liver Lipid Metabolism in Patients with Non-Alcoholic Fatty Liver Disease. Hepatol. Int. 2010, 4, 741–748. [Google Scholar] [CrossRef]
- Ma, K.; Saha, P.K.; Chan, L.; Moore, D.D. Farnesoid X Receptor Is Essential for Normal Glucose Homeostasis. J. Clin. Invest. 2006, 116, 1102–1109. [Google Scholar] [CrossRef]
- de Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; van der Meer, R. Saturated Fat Stimulates Obesity and Hepatic Steatosis and Affects Gut Microbiota Composition by an Enhanced Overflow of Dietary Fat to the Distal Intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Mason, A.; Luketic, V.; Lindor, K.; Gordon, S.C.; Mayo, M.; Kowdley, K.V.; Vincent, C.; Bodhenheimer, H.C.; Parés, A.; et al. Efficacy of Obeticholic Acid in Patients With Primary Biliary Cirrhosis and Inadequate Response to Ursodeoxycholic Acid. Gastroenterology 2015, 148, 751–761.e8. [Google Scholar] [CrossRef]
- Lai, J.; Luo, L.; Zhou, T.; Feng, X.; Ye, J.; Zhong, B. Alterations in Circulating Bile Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Systematic Review and Meta-Analysis. Biomolecules 2023, 13, 1356. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.R.; Cremon, C.; Marasco, G.; Savarino, E.; Guglielmetti, S.; Bonomini, F.; Palombo, M.; Fuschi, D.; Rotondo, L.; Mantegazza, G.; et al. Molecular Mechanisms Underlying Loss of Vascular and Epithelial Integrity in Irritable Bowel Syndrome. Gastroenterology 2024, 167, 1152–1166. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Kinoshita, M.; Harada, K.; Mizutani, M.; Masahata, K.; Kayama, H.; Takeda, K. Commensal Bacteria-Dependent Indole Production Enhances Epithelial Barrier Function in the Colon. PLOS ONE 2013, 8, e80604. [Google Scholar] [CrossRef]
- Ritze, Y.; Bárdos, G.; Hubert, A.; Böhle, M.; Bischoff, S.C. Effect of Tryptophan Supplementation on Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice. Br. J. Nutr. 2014, 112, 1–7. [Google Scholar] [CrossRef]
- Ma, L.; Li, H.; Hu, J.; Zheng, J.; Zhou, J.; Botchlett, R.; Matthews, D.; Zeng, T.; Chen, L.; Xiao, X.; et al. Indole Alleviates Diet-Induced Hepatic Steatosis and Inflammation in a Manner Involving Myeloid Cell 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 3. Hepatology 2020, 72, 1191. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef]
- Crane, J.D.; Palanivel, R.; Mottillo, E.P.; Bujak, A.L.; Wang, H.; Ford, R.J.; Collins, A.; Blümer, R.M.; Fullerton, M.D.; Yabut, J.M.; et al. Inhibiting Peripheral Serotonin Synthesis Reduces Obesity and Metabolic Dysfunction by Promoting Brown Adipose Tissue Thermogenesis. Nat. Med. 2015, 21, 166–172. [Google Scholar] [CrossRef]
- Arto, C.; Rusu, E.C.; Clavero-Mestres, H.; Barrientos-Riosalido, A.; Bertran, L.; Mahmoudian, R.; Aguilar, C.; Riesco, D.; Chicote, J.U.; Parada, D.; et al. Metabolic Profiling of Tryptophan Pathways: Implications for Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease. Eur. J. Clin. Investig. 2024, 54, e14279. [Google Scholar] [CrossRef]
- Noga, A.A.; Vance, D.E. A Gender-Specific Role For Phosphatidylethanolamine N-Methyltransferase-Derived Phosphatidylcholine in the Regulation of Plasma High Density and Very Low Density Lipoproteins in Mice. J. Biol. Chem. 2003, 278, 21851–21859. [Google Scholar] [CrossRef]
- Li, Z.; Agellon, L.B.; Vance, D.E. Phosphatidylcholine Homeostasis and Liver Failure *. J. Biol. Chem. 2005, 280, 37798–37802. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Rolo, A.P.; Duarte, F.V.; Simões, A.M.; Palmeira, C.M. Differential Alterations in Mitochondrial Function Induced by a Choline-Deficient Diet: Understanding Fatty Liver Disease Progression. Mitochondrion 2008, 8, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Giudetti, A.M.; Bellanti, F.; Priore, P.; Rollo, T.; Tamborra, R.; Siculella, L.; Vendemiale, G.; Altomare, E.; Gnoni, G.V. Oxidation of Hepatic Carnitine Palmitoyl Transferase-I (CPT-I) Impairs Fatty Acid Beta-Oxidation in Rats Fed a Methionine-Choline Deficient Diet. PLoS ONE 2011, 6, e24084. [Google Scholar] [CrossRef] [PubMed]
- Hebbard, L.; George, J. Animal Models of Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 35–44. [Google Scholar] [CrossRef]
- Arao, Y.; Kawai, H.; Kamimura, K.; Kobayashi, T.; Nakano, O.; Hayatsu, M.; Ushiki, T.; Terai, S. Effect of Methionine/Choline-Deficient Diet and High-Fat Diet-Induced Steatohepatitis on Mitochondrial Homeostasis in Mice. Biochem. Biophys. Res. Commun. 2020, 527, 365–371. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Machado, M.V.; Cortez-Pinto, H. Diet, Microbiota, Obesity, and NAFLD: A Dangerous Quartet. Int. J. Mol. Sci. 2016, 17, 481. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 5214. [Google Scholar] [CrossRef]
- Metabolic Profiling Reveals a Contribution of Gut Microbiota to Fatty Liver Phenotype in Insulin-Resistant Mice | PNAS. Available online: https://www.pnas.org/doi/full/10.1073/pnas.0601056103 (accessed on 29 November 2024).
- Perva, I.T.; Simina, I.E.; Bende, R.; Motofelea, A.C.; Chirita Emandi, A.; Andreescu, N.; Sima, A.; Vlad, A.; Sporea, I.; Zimbru, C.; et al. Use of a Micronutrient Cocktail to Improve Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in Adults with Obesity: A Randomized, Double-Blinded Pilot Clinical Trial. Medicine 2024, 60, 1366. [Google Scholar] [CrossRef]
- Nian, F.; Chen, Y.; Xia, Q.; Zhu, C.; Wu, L.; Lu, X. Gut Microbiota Metabolite Trimethylamine N-Oxide Promoted NAFLD Progression by Exacerbating Intestinal Barrier Disruption and Intrahepatic Cellular Imbalance. Int. Immunopharmacol. 2024, 142, 113173. [Google Scholar] [CrossRef]
- Theofilis, P.; Vordoni, A.; Kalaitzidis, R.G. Trimethylamine N-Oxide Levels in Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Metabolites 2022, 12, 1243. [Google Scholar] [CrossRef] [PubMed]
- Cope, K.; Risby, T.; Diehl, A.M. Increased Gastrointestinal Ethanol Production in Obese Mice: Implications for Fatty Liver Disease Pathogenesis. Gastroenterology 2000, 119, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of Gut Microbiomes in Nonalcoholic Steatohepatitis (NASH) Patients: A Connection Between Endogenous Alcohol and NASH. Hepatology 2013, 57, 601. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Li, H.; Zhao, J.; Wei, X.; Lin, W.; Zhao, X.; Jiang, A.; Yuan, J. Endogenous Ethanol Produced by Intestinal Bacteria Induces Mitochondrial Dysfunction in Non-Alcoholic Fatty Liver Disease. J. Gastroenterol. Hepatol. 2020, 35, 2009–2019. [Google Scholar] [CrossRef]
- Baker, S.S.; Baker, R.D.; Liu, W.; Nowak, N.J.; Zhu, L. Role of Alcohol Metabolism in Non-Alcoholic Steatohepatitis. PLoS ONE 2010, 5, e9570. [Google Scholar] [CrossRef]
- Shen, Z.; Ajmo, J.M.; Rogers, C.Q.; Liang, X.; Le, L.; Murr, M.M.; Peng, Y.; You, M. Role of SIRT1 in Regulation of LPS- or Two Ethanol Metabolites-Induced TNF-α Production in Cultured Macrophage Cell Lines. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 296, G1047–G1053. [Google Scholar] [CrossRef]
- Perazza, F.; Leoni, L.; Colosimo, S.; Musio, A.; Bocedi, G.; D’Avino, M.; Agnelli, G.; Nicastri, A.; Rossetti, C.; Sacilotto, F.; et al. Metformin and the Liver: Unlocking the Full Therapeutic Potential. Metabolites 2024, 14, 186. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling Type 2 Diabetes and Metformin Treatment Signatures in the Human Gut Microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia Muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Yan, C.; Li, C.; Zhang, L.; Zhang, L.; Liang, E.; Liu, T.; Mao, J. Effect of Metformin on Nonalcoholic Fatty Liver Based on Meta-Analysis and Network Pharmacology. Medicine 2022, 101, e31437. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Fu, J. Efficacy of Various Hypoglycemic Agents in the Treatment of Patients With Nonalcoholic Liver Disease With or Without Diabetes: A Network Meta-Analysis. Front. Endocrinol. (Lausanne) 2021, 12, 649018. [Google Scholar] [CrossRef] [PubMed]
- Sawangjit, R.; Chongmelaxme, B.; Phisalprapa, P.; Saokaew, S.; Thakkinstian, A.; Kowdley, K.V.; Chaiyakunapruk, N. Comparative Efficacy of Interventions on Nonalcoholic Fatty Liver Disease (NAFLD): A PRISMA-Compliant Systematic Review and Network Meta-Analysis. Medicine 2016, 95, e4529. [Google Scholar] [CrossRef]
- Haukeland, J.W.; Konopski, Z.; Eggesbø, H.B.; von Volkmann, H.L.; Raschpichler, G.; Bjøro, K.; Haaland, T.; Løberg, E.M.; Birkeland, K. Metformin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Controlled Trial. Scand. J. Gastroenterol. 2009, 44, 853–860. [Google Scholar] [CrossRef]
- Anushiravani, A.; Haddadi, N.; Pourfarmanbar, M.; Mohammadkarimi, V. Treatment Options for Nonalcoholic Fatty Liver Disease: A Double-Blinded Randomized Placebo-Controlled Trial. Eur. J. Gastroenterol. Hepatol. 2019, 31, 613. [Google Scholar] [CrossRef]
- Shibuya, T.; Fushimi, N.; Kawai, M.; Yoshida, Y.; Hachiya, H.; Ito, S.; Kawai, H.; Ohashi, N.; Mori, A. Luseogliflozin Improves Liver Fat Deposition Compared to Metformin in Type 2 Diabetes Patients with Non-Alcoholic Fatty Liver Disease: A Prospective Randomized Controlled Pilot Study. Diabetes Obes. Metab. 2018, 20, 438–442. [Google Scholar] [CrossRef]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef]
- Gkiourtzis, N.; Michou, P.; Moutafi, M.; Glava, A.; Cheirakis, K.; Christakopoulos, A.; Vouksinou, E.; Fotoulaki, M. The Benefit of Metformin in the Treatment of Pediatric Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Pediatr. 2023, 182, 4795–4806. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Farhadi, A.; Gundlapalli, S.; Shaikh, M.; Frantzides, C.; Harrell, L.; Kwasny, M.M.; Keshavarzian, A. Susceptibility to Gut Leakiness: A Possible Mechanism for Endotoxaemia in Non-Alcoholic Steatohepatitis. Liver Int. 2008, 28, 1026–1033. [Google Scholar] [CrossRef]
- Gangarapu, V.; Ince, A.T.; Baysal, B.; Kayar, Y.; Kılıç, U.; Gök, Ö.; Uysal, Ö.; Şenturk, H. Efficacy of Rifaximin on Circulating Endotoxins and Cytokines in Patients with Nonalcoholic Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; Bellini, M.; Corsetti, M.; Di Nardo, G.; Falangone, F.; Fuccio, L.; Galeazzi, F.; Iovino, P.; Sarnelli, G.; et al. Italian Guidelines for the Management of Irritable Bowel Syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig. Liver Dis. 2023, 55, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gao, L.; Yin, Z.; Ye, S.; Zhao, H.; Peng, Q. Probiotics and Rifaximin for the Prevention of Travelers’ Diarrhea: A Systematic Review and Network Meta-Analysis. Medicine 2022, 101, e30921. [Google Scholar] [CrossRef]
- Didyk, O.K.; Chernyavskyi, V.V.; Shypulin, V.P.; Tishchenko, V.V. Effectiveness of Rifaximin and Probiotics for the Correction of Intestinal Permeability in Patients with Metabolic-Associated Fatty Liver Disease in Combination with Type 2 Diabetes Mellitus. Wiad. Lek. 2024, 77, 732–738. [Google Scholar] [CrossRef]
- A, A.-R.; N, M.; W, S.; M, R.; R, E.; R, E.; K, Z.; M, A.; Aa, E.; M, A.; et al. Rifaximin in Nonalcoholic Fatty Liver Disease: Hit Multiple Targets with a Single Shot. Eur. J. Gastroenterol. Hepatol. 2018, 30. [Google Scholar] [CrossRef]
- Vinelli, V.; Biscotti, P.; Martini, D.; Del Bo’, C.; Marino, M.; Meroño, T.; Nikoloudaki, O.; Calabrese, F.M.; Turroni, S.; Taverniti, V.; et al. Effects of Dietary Fibers on Short-Chain Fatty Acids and Gut Microbiota Composition in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 2559. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving Our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. The Control and Consequences of Bacterial Fermentation in the Human Colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Guo, M. Chapter 3 - DIETARY FIBER AND DIETARY FIBER RICH FOODS. In Functional Foods; Woodhead Publishing Series in Food Science, Technology and Nutrition; Guo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 63–111. ISBN 978-1-84569-592-7. [Google Scholar]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant Starch: Promise for Improving Human Health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Ni, Y.; Qian, L.; Siliceo, S.L.; Long, X.; Nychas, E.; Liu, Y.; Ismaiah, M.J.; Leung, H.; Zhang, L.; Gao, Q.; et al. Resistant Starch Decreases Intrahepatic Triglycerides in Patients with NAFLD via Gut Microbiome Alterations. Cell Metab. 2023, 35, 1530–1547.e8. [Google Scholar] [CrossRef] [PubMed]
- Fackelmann, G.; Manghi, P.; Carlino, N.; Heidrich, V.; Piccinno, G.; Ricci, L.; Piperni, E.; Arrè, A.; Bakker, E.; Creedon, A.C.; et al. Gut Microbiome Signatures of Vegan, Vegetarian and Omnivore Diets and Associated Health Outcomes across 21,561 Individuals. Nat. Microbiol. 2025, 10, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Burcelin, R.; Ruidavets, J.B.; Cani, P.D.; Fauvel, J.; Alessi, M.C.; Chamontin, B.; Ferriéres, J. Energy Intake Is Associated with Endotoxemia in Apparently Healthy Men. Am. J. Clin. Nutr. 2008, 87, 1219–1223. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.-J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef]
- Lerma-Aguilera, A.M.; Pérez-Burillo, S.; Navajas-Porras, B.; León, E.D.; Ruíz-Pérez, S.; Pastoriza, S.; Jiménez-Hernández, N.; Cämmerer, B.-M.; Rufián-Henares, J.Á.; Gosalbes, M.J.; et al. Effects of Different Foods and Cooking Methods on the Gut Microbiota: An in Vitro Approach. Front. Microbiol. 2024, 14, 1334623. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A Randomised Trial of the Effect of Omega-3 Polyunsaturated Fatty Acid Supplements on the Human Intestinal Microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]
- Vijay, A.; Astbury, S.; Le Roy, C.; Spector, T.D.; Valdes, A.M. The Prebiotic Effects of Omega-3 Fatty Acid Supplementation: A Six-Week Randomised Intervention Trial. Gut Microbes 2021, 13, 1863133. [Google Scholar] [CrossRef]
- Gilsenan, S.; Leong, D.; Cotter, P.D.; Brennan, L.; Nilaweera, K.N. Digging Deep for Nutrients and Metabolites Derived from High Dietary Protein Intake and Their Potential Functions in Metabolic Health. Nutr. Res. Rev. 2024, 1–13. [Google Scholar] [CrossRef]
- Ma, R.; Shi, G.; Li, Y.; Shi, H. Trimethylamine N-Oxide, Choline and Its Metabolites Are Associated with the Risk of Non-Alcoholic Fatty Liver Disease. Br. J. Nutr. 2024, 131, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary Emulsifiers Directly Alter Human Microbiota Composition and Gene Expression Ex Vivo Potentiating Intestinal Inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Panyod, S.; Wu, W.-K.; Chang, C.-T.; Wada, N.; Ho, H.-C.; Lo, Y.-L.; Tsai, S.-P.; Chen, R.-A.; Huang, H.-S.; Liu, P.-Y.; et al. Common Dietary Emulsifiers Promote Metabolic Disorders and Intestinal Microbiota Dysbiosis in Mice. Commun. Biol. 2024, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology 2022, 162, 743–756. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zilberman-Schapira, G.; Segal, E.; Elinav, E. Non-Caloric Artificial Sweeteners and the Microbiome: Findings and Challenges. Gut Microbes 2015, 6, 149–155. [Google Scholar] [CrossRef]
- Gauthier, E.; Milagro, F.I.; Navas-Carretero, S. Effect of Low-and Non-Calorie Sweeteners on the Gut Microbiota: A Review of Clinical Trials and Cross-Sectional Studies. Nutrition 2024, 117, 112237. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Sokal-Dembowska, A.; Filip, R. Effects of Selected Food Additives on the Gut Microbiome and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Medicina 2025, 61, 192. [Google Scholar] [CrossRef]
- García, S.; Monserrat-Mesquida, M.; Ugarriza, L.; Casares, M.; Gómez, C.; Mateos, D.; Angullo-Martínez, E.; Tur, J.A.; Bouzas, C. Ultra-Processed Food Consumption and Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD): A Longitudinal and Sustainable Analysis. Nutrients 2025, 17, 472. [Google Scholar] [CrossRef]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-Processed Food Intake and Obesity: What Really Matters for Health—Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef]
- Lodge, M.; Dykes, R.; Kennedy, A. Regulation of Fructose Metabolism in Nonalcoholic Fatty Liver Disease. Biomolecules 2024, 14, 845. [Google Scholar] [CrossRef]
- Stanhope, K.L. Role of Fructose-Containing Sugars in the Epidemics of Obesity and Metabolic Syndrome. Annu. Rev. Med. 2012, 63, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Mateos, D.; Casares, M.; Gómez, C.; Ugarriza, L.; Tur, J.A.; Sureda, A. Effects of a Two-Year Lifestyle Intervention on Intrahepatic Fat Reduction and Renal Health: Mitigation of Inflammation and Oxidative Stress, a Randomized Trial. Antioxidants 2024, 13, 754. [Google Scholar] [CrossRef] [PubMed]
- Brichacek, A.L.; Florkowski, M.; Abiona, E.; Frank, K.M. Ultra-Processed Foods: A Narrative Review of the Impact on the Human Gut Microbiome and Variations in Classification Methods. Nutrients 2024, 16, 1738. [Google Scholar] [CrossRef]
- Manzhalii, E.; Virchenko, O.; Falalyeyeva, T.; Beregova, T.; Stremmel, W. Treatment Efficacy of a Probiotic Preparation for Non-Alcoholic Steatohepatitis: A Pilot Trial. J. Dig. Dis. 2017, 18, 698–703. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium Longum with Fructo-Oligosaccharides in Patients with Non Alcoholic Steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef]
- Sepideh, A.; Karim, P.; Hossein, A.; Leila, R.; Hamdollah, M.; Mohammad E, G.; Mojtaba, S.; Mohammad, S.; Ghader, G.; Seyed Moayed, A. Effects of Multistrain Probiotic Supplementation on Glycemic and Inflammatory Indices in Patients with Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial. J. Am. Coll. Nutr. 2016, 35, 500–505. [Google Scholar] [CrossRef]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; Alshathry, A.; Childs, C.E.; Del Fabbro, S.; Bilson, J.; Moyses, H.E.; et al. Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1597–1610.e7. [Google Scholar] [CrossRef]
- Tian, S.; Lei, Y.; Zhao, F.; Che, J.; Wu, Y.; Lei, P.; Kang, Y.E.; Shan, Y. Improving Insulin Resistance by Sulforaphane via Activating the Bacteroides and Lactobacillus SCFAs-GPR-GLP1 Signal Axis. Food Funct. 2024, 15, 8644–8660. [Google Scholar] [CrossRef]
- Paravati, M.R.; Procopio, A.C.; Milanović, M.; Scarlata, G.G.M.; Milošević, N.; Ružić, M.; Milić, N.; Abenavoli, L. Onion Polyphenols as Multi-Target-Directed Ligands in MASLD: A Preliminary Molecular Docking Study. Nutrients 2024, 16, 1226. [Google Scholar] [CrossRef]
- Handu, D.; Stote, K.; Piemonte, T. Evaluating Bioactive-Substance-Based Interventions for Adults with MASLD: Results from a Systematic Scoping Review. Nutrients 2025, 17, 453. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary Supplementation with Inulin-Propionate Ester or Inulin Improves Insulin Sensitivity in Adults with Overweight and Obesity with Distinct Effects on the Gut Microbiota, Plasma Metabolome and Systemic Inflammatory Responses: A Randomised Cross-over Trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Reshef, N.; Gophna, U.; Reshef, L.; Konikoff, F.; Gabay, G.; Zornitzki, T.; Knobler, H.; Maor, Y. Prebiotic Treatment in Patients with Nonalcoholic Fatty Liver Disease (NAFLD)-A Randomized Pilot Trial. Nutrients 2024, 16, 1571. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.Y.L.; Orr, D.; Plank, L.D.; Vatanen, T.; O’Sullivan, J.M.; Murphy, R. Randomised Double-Blind Placebo-Controlled Trial of Inulin with Metronidazole in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 937. [Google Scholar] [CrossRef]
- Calabrese, F.M.; Disciglio, V.; Franco, I.; Sorino, P.; Bonfiglio, C.; Bianco, A.; Campanella, A.; Lippolis, T.; Pesole, P.L.; Polignano, M.; et al. A Low Glycemic Index Mediterranean Diet Combined with Aerobic Physical Activity Rearranges the Gut Microbiota Signature in NAFLD Patients. Nutrients 2022, 14, 1773. [Google Scholar] [CrossRef]
- Jian, C.; Luukkonen, P.; Sädevirta, S.; Yki-Järvinen, H.; Salonen, A. Impact of Short-Term Overfeeding of Saturated or Unsaturated Fat or Sugars on the Gut Microbiota in Relation to Liver Fat in Obese and Overweight Adults. Clin. Nutr. 2021, 40, 207–216. [Google Scholar] [CrossRef]
- He, K.; Guo, L.-L.; Tang, H.; Peng, X.; Li, J.; Feng, S.; Bie, C.; Chen, W.; Li, Y.; Wang, M.; et al. A Freshwater Fish-Based Diet Alleviates Liver Steatosis by Modulating Gut Microbiota and Metabolites: A Clinical Randomized Controlled Trial in Chinese Participants With Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2022, 117, 1621–1631. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, R.; Yang, X.; Dai, J.; Huang, M.; Ji, X.; Li, Y.; Okekunle, A.P.; Gao, G.; Onwuka, J.U.; et al. Yogurt Improves Insulin Resistance and Liver Fat in Obese Women with Nonalcoholic Fatty Liver Disease and Metabolic Syndrome: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2019, 109, 1611–1619. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, J.; Zhang, Q.; Liu, L.; Lu, M.; Meng, G.; Yao, Z.; Wu, H.; Xia, Y.; Bao, X.; et al. Association between Habitual Yogurt Consumption and Newly Diagnosed Non-Alcoholic Fatty Liver Disease. Eur. J. Clin. Nutr. 2020, 74, 491–499. [Google Scholar] [CrossRef]
- Bakhshimoghaddam, F.; Shateri, K.; Sina, M.; Hashemian, M.; Alizadeh, M. Daily Consumption of Synbiotic Yogurt Decreases Liver Steatosis in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. J. Nutr. 2018, 148, 1276–1284. [Google Scholar] [CrossRef]
- Nabavi, S.; Rafraf, M.; Somi, M.H.; Homayouni-Rad, A.; Asghari-Jafarabadi, M. Effects of Probiotic Yogurt Consumption on Metabolic Factors in Individuals with Nonalcoholic Fatty Liver Disease. J. Dairy. Sci. 2014, 97, 7386–7393. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).